Iron-Coordinated L-Lysine–Based Nanozymes with High Peroxidase-like Activity for Sensitive Hydrogen Peroxide and Glucose Detection
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Preparation of N-Acryloyl-l-lysine
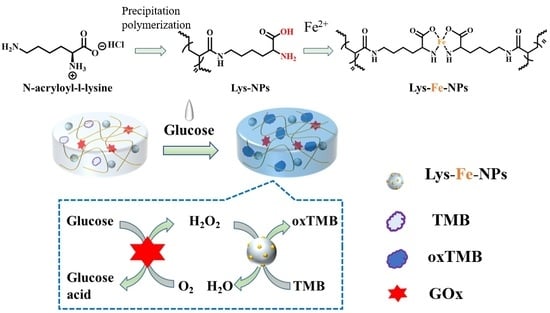
2.3. Preparation of Lys-NPs and Lys-Fe-NPs
2.4. Measurement of Peroxidase-like Activity of Lys-Fe-NPs
2.5. Steady-State Kinetic Assay
2.6. Experimental Method for the Measurement of H2O2 and Glucose
2.7. Preparation of Integrated Agarose-Based Hydrogel Film
3. Results and Discussion
3.1. Preparation of Lys-Fe-NPs
3.2. Peroxidase-like Catalytic Activity of Lys-Fe-NPs
3.3. Kinetic Data and Reaction Mechanism of Lys-Fe-NPs
3.4. Colorimetric Assays for H2O2 and Glucose
3.5. Lys-Fe-NPs-GOx-TMB Agarose-Based Hydrogel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- López-Lázaro, M. Dual role of hydrogen peroxide in cancer: Possible relevance to cancer chemoprevention and therapy. Cancer Lett. 2007, 252, 1–8. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, R.X.; Yue, X.M. Synthesis of diboronic acid-based fluorescent probes for the sensitive detection of glucose in aqueous media and biological matrices. ACS Sens. 2021, 6, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shen, X.; Baimanov, D. Immobilized ferrous Ion and glucose oxidase on graphdiyne and its application on one-step glucose detection. ACS Appl. Mater. Interfaces 2019, 11, 2647–2654. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.P.; Zhang, F.; Ding, X.Y. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- Huang, D.; Wang, X.; Zhang, C. Sorptive removal of ionizable antibiotic sulfamethazine from aqueous solution by graphene oxide-coated biochar nanocomposites: Influencing factors and mechanism. Chemosphere 2017, 186, 414–421. [Google Scholar] [CrossRef]
- Bommer, C.; Heesemann, E.; Sagalova, V. he global economic burden of diabetes in adults aged 20–79 years: A cost-of-illness study. Lancet Diabetes Endocrinol. 2017, 5, 423–430. [Google Scholar] [CrossRef]
- Jiang, L.C.; Zhang, W.D. Electrodeposition of TiO2 nanoparticles on multiwalled carbon nanotube arrays for hydrogen peroxide sensing. Electroanalysis 2009, 21, 988–993. [Google Scholar] [CrossRef]
- Fan, X.; Tian, R.; Wang, T. An ultrathin iron-porphyrin based nanocapsule with high peroxidase-like activity for highly sensitive glucose detection. Nanoscale 2018, 10, 22155–22160. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhu, C.; Su, S. Self-catalyzed, Self-limiting growth of glucose oxidase-mimicking gold nanoparticles. Acs Nano 2010, 4, 7451–7458. [Google Scholar] [CrossRef]
- Liu, B. Surface modification of nanozymes. Nano Res. 2017, 10, 24. [Google Scholar] [CrossRef]
- Fidelli, A.M.; Bahar, K.; Howarth, A.J. Green and rapid mechanosynthesis of high-porosity NU- and UiO-type metal-organic frameworks. Chem. Commun. 2018, 54, 6999–7002. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Quan, W. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.Y. Nanozyme: A new type of artificial enzyme. Prog. Biochem. Biophys. 2018, 45, 101–104. [Google Scholar]
- Wang, G.; Yan, L.; Ren, H. Functional cell surface displaying of acetylcholinesterase for spectrophotometric sensing organophosphate pesticide. Sens. Actuators B Chem. 2019, 279, 483–489. [Google Scholar]
- Zhang, X.; Lin, S.; Liu, S. Advances in organometallic/organic nanozymes and their applications. Coord. Chem. Rev. 2020, 429, 213652. [Google Scholar] [CrossRef]
- Han, L.; Shi, J.; Liu, A. Novel biotemplated MnO2 1D nanozyme with controllable peroxidase-like activity and unique catalytic mechanism and its application for glucose sensing. Sens. Actuators B Chem. 2017, 252, 919–926. [Google Scholar] [CrossRef]
- Fan, S.Q.; Liang, B.; Xiao, X.X. Controllable display of sequential enzymes on yeast surface with enhanced biocatalytic activity toward efficient enzymatic biofuel cells. J. Am. Chem. Soc. 2020, 142, 3222–3230. [Google Scholar] [CrossRef]
- Hu, Y.; Dai, L.; Liu, D. Progress & prospect of metal-organic frameworks (MOFs) for enzyme immobilization (enzyme/MOFs). Renew. Sustain. Energy Rev. 2018, 91, 793–801. [Google Scholar]
- Johnson, L.K. Witness testimony from the Church Committee hearings on covert action, 1975. Intell. Natl. Secur. 2019, 34, 899–913. [Google Scholar] [CrossRef]
- Liao, J. Molecular recognition of protein kinase binding pockets for design of potent and selective kinase inhibitors. J. Med. Chem. 2007, 50, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhuang, J.; Nie, L. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Qin, Y.; Huang, Y. A label-free fluorescent assay for hydrogen peroxide and glucose based on bifunctional MIL-53(Fe) nanozyme. Chem. Commun. 2018, 54, 1762–1765. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Xu, W.; Yan, H. Fe-N-C Single-Atom Nanozyme for the Intracellular Hydrogen Peroxide Detection. Anal. Chem. 2019, 91, 11994–11999. [Google Scholar] [CrossRef]
- Chen, Q.; Liang, C. High oxidase-mimic activity of Fe nanoparticles embedded in an N-rich porous carbon and their application for sensing of dopamine. Talanta Int. J. Pure Appl. Anal. Chem. 2018, 182, 476–483. [Google Scholar] [CrossRef]
- Tan, D.H.; Ma, C.; Gao, L. Metal-organic framework-derived copper nanoparticle@carbon nanocomposites as peroxidase mimics for colorimetric sensing of ascorbic acid. Chem.-A Eur. J. 2014, 20, 16377–16383. [Google Scholar] [CrossRef]
- Oh, J.K.; Drumright, R.; Matyjaszewski, K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Jin, S.; Pan, Y.J.; Wang, C.C. Reflux Precipitation Polymerization: A New Technology for Preparation of Monodisperse Polymer Nanohydrogels. Acta Chim. Sin. 2013, 71, 1500–1504. [Google Scholar] [CrossRef]
- Kai, L.; Stöver, H. Synthesis of monodisperse poly(divinylbenzene) microspheres. J. Polym. Sci. Part A Polym. Chem. 2010, 31, 3257–3263. [Google Scholar]
- Wang, F.; Zhang, Y.T.; Yang, P. Fabrication of polymeric microgels using reflux-precipitation polymerization and its application for phosphoprotein enrichment. J. Mater. Chem. B 2013, 2, 2575–2582. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Pan, H.; Sheng, H.D. Nanogel Multienzyme Mimics Synthesized by Biocatalytic ATRP and Metal Coordination for Bioresponsive Fluorescence Imaging. Angew. Chem. 2020, 59, 11748–11753. [Google Scholar] [CrossRef]
- Feng, J.; Huang, P.; Wu, F.Y. Gold-platinum bimetallic nanoclusters with enhanced peroxidase-like activity and their integrated agarose hydrogel-based sensing platform for the colorimetric analysis of glucose levels in serum. Analyst 2017, 142, 4106–4115. [Google Scholar] [CrossRef]
- Lian, J.J.; He, Y.L.; Li, N. Magnetic Flower-like Fe-doped CoO nanocomposites with dual enzyme-like activities for Facile and sensitive determination of H2O2 and dopamine. Inorg. Chem. 2021, 60, 1893–1901. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, M.L.; Zhao, J.N. Water-dispersible silicon dots as a peroxidase mimetic for the highly-sensitive colorimetric detection of glucose. Chem. Commun. 2014, 50, 6771–6774. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yu, H.; Wu, J. Mixed-solvent liquid exfoliated MoS2NPs as peroxidase mimetics for colorimetric detection of H2O2 and glucose. RSC Adv. 2018, 8, 7252–7259. [Google Scholar] [CrossRef]
- Li, Y.Z.; Li, T.T.; Chen, W. Co4N Nanowires: Noble-metal-free peroxidase mimetic with excellent salt- and temperature-resistant abilities. ACS Appl. Mater. Interfaces 2017, 9, 29881–29888. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, H.; Liu, X. Single Iron Site Nanozyme for Ultrasensitive Glucose Detection. Small 2020, 16, 2002343. [Google Scholar] [CrossRef]
- Bao, Y.W.; Hua, X.W.; Ran, H.H. Metal-doped carbon nanoparticles with intrinsic peroxidase-like activity for colorimetric detection of H2O2 and glucose. J. Mater. Chem. B 2018, 7, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Du, L.L.; Liao, R.X.; Zhang, H.J. Redox-activity of polydopamine for ultrafast preparation of self-healing and adhesive hydrogels. Colloids Surf. B Biointerfaces 2022, 214, 112469. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Zhang, Q.; Li, M. Horseradish peroxidase (HRP) and glucose oxidase (GOx) based dual-enzyme system: Sustainable release of H2O2 and its effect on the desirable ping pong bibi degradation mechanism. Environ. Res. 2023, 229, 115979. [Google Scholar] [CrossRef]
- Yang, X.; Pehr, E.P. Double-Stranded DNA Single-Walled Carbon Nanotube Hybrids for Optical Hydrogen Peroxide and Glucose Sensing. J. Phys. Chem. C 2007, 111, 8638–8643. [Google Scholar]






| Sample | Cross-Linker Content (wt%) | Diameter a (nm) | Diameter b (nm) | PDI b |
|---|---|---|---|---|
| Lys-NPs | 40 | 93.7 | 108.4 | 0.193 |
| Lys-Fe-NPs1 | 40 | 98.6 | 122.3 | 0.201 |
| Lys-Fe-NPs2 | 50 | 150.1 | 178.9 | 0.132 |
| Lys-Fe-NPs3 | 60 | 173.4 | 202.4 | 0.117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, X.; Wang, R.; Zhang, H.; Zhang, M.; Qu, X.; Hu, X. Iron-Coordinated L-Lysine–Based Nanozymes with High Peroxidase-like Activity for Sensitive Hydrogen Peroxide and Glucose Detection. Polymers 2023, 15, 3002. https://doi.org/10.3390/polym15143002
Hou X, Wang R, Zhang H, Zhang M, Qu X, Hu X. Iron-Coordinated L-Lysine–Based Nanozymes with High Peroxidase-like Activity for Sensitive Hydrogen Peroxide and Glucose Detection. Polymers. 2023; 15(14):3002. https://doi.org/10.3390/polym15143002
Chicago/Turabian StyleHou, Xiuqing, Ruoxue Wang, Huijuan Zhang, Meng Zhang, Xiongwei Qu, and Xiuli Hu. 2023. "Iron-Coordinated L-Lysine–Based Nanozymes with High Peroxidase-like Activity for Sensitive Hydrogen Peroxide and Glucose Detection" Polymers 15, no. 14: 3002. https://doi.org/10.3390/polym15143002
APA StyleHou, X., Wang, R., Zhang, H., Zhang, M., Qu, X., & Hu, X. (2023). Iron-Coordinated L-Lysine–Based Nanozymes with High Peroxidase-like Activity for Sensitive Hydrogen Peroxide and Glucose Detection. Polymers, 15(14), 3002. https://doi.org/10.3390/polym15143002