Moisture Adsorption–Desorption Behaviour in Nanocomposite Copolymer Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymerisation of Poly(Vinyl Alcohol-co-Acrylic Acid), P(VA-AA)
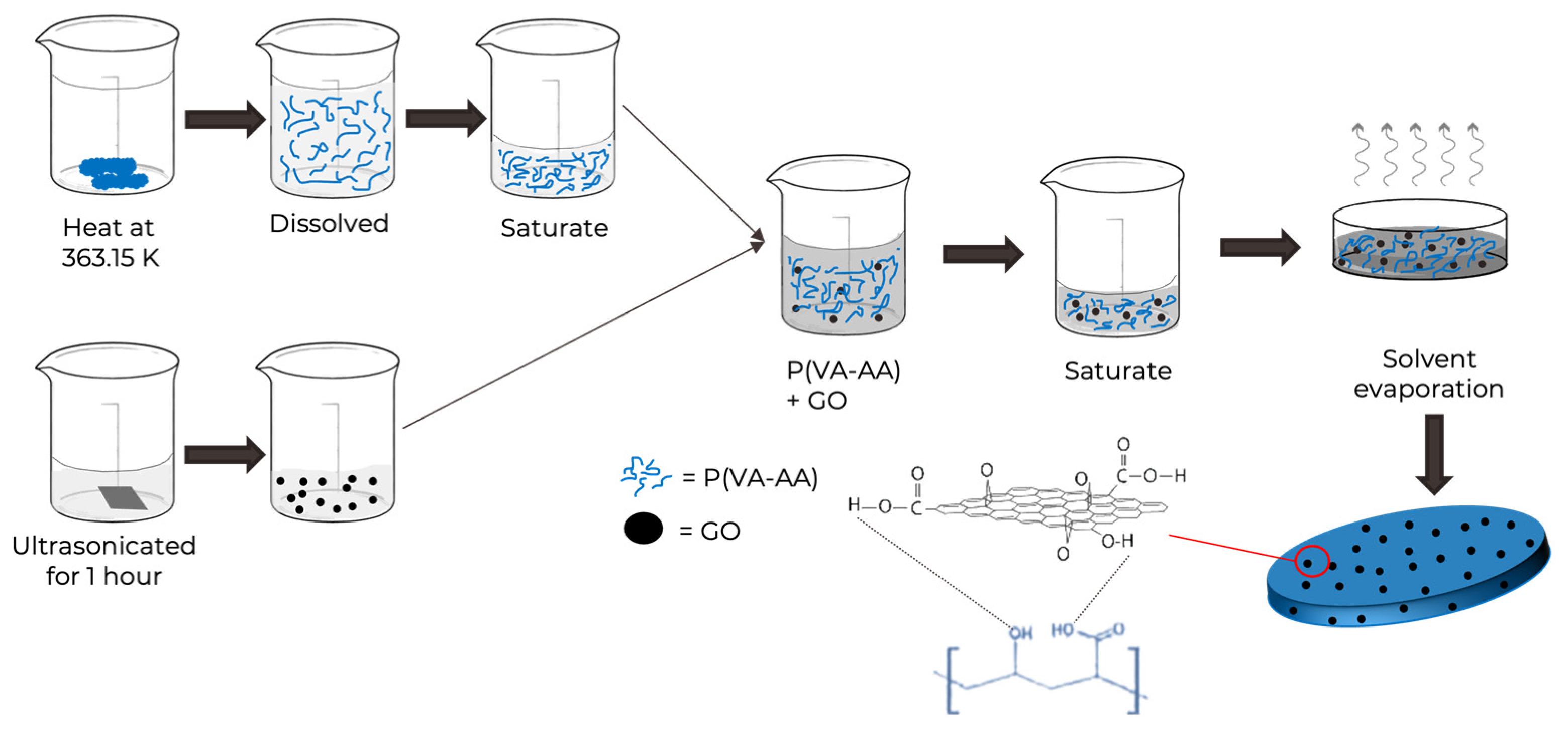
2.3. Preparation of Copolymer/GO Composite Films via Solvent Casting
2.4. Fourier Transform Infrared (FT-IR)
2.5. Field Emission Spectroscopy (FESEM)
2.6. Adsorption Isotherms
2.7. Adsorption Kinetics
2.8. Desorption Kinetics
2.9. Cycling Stability
3. Results and Discussion
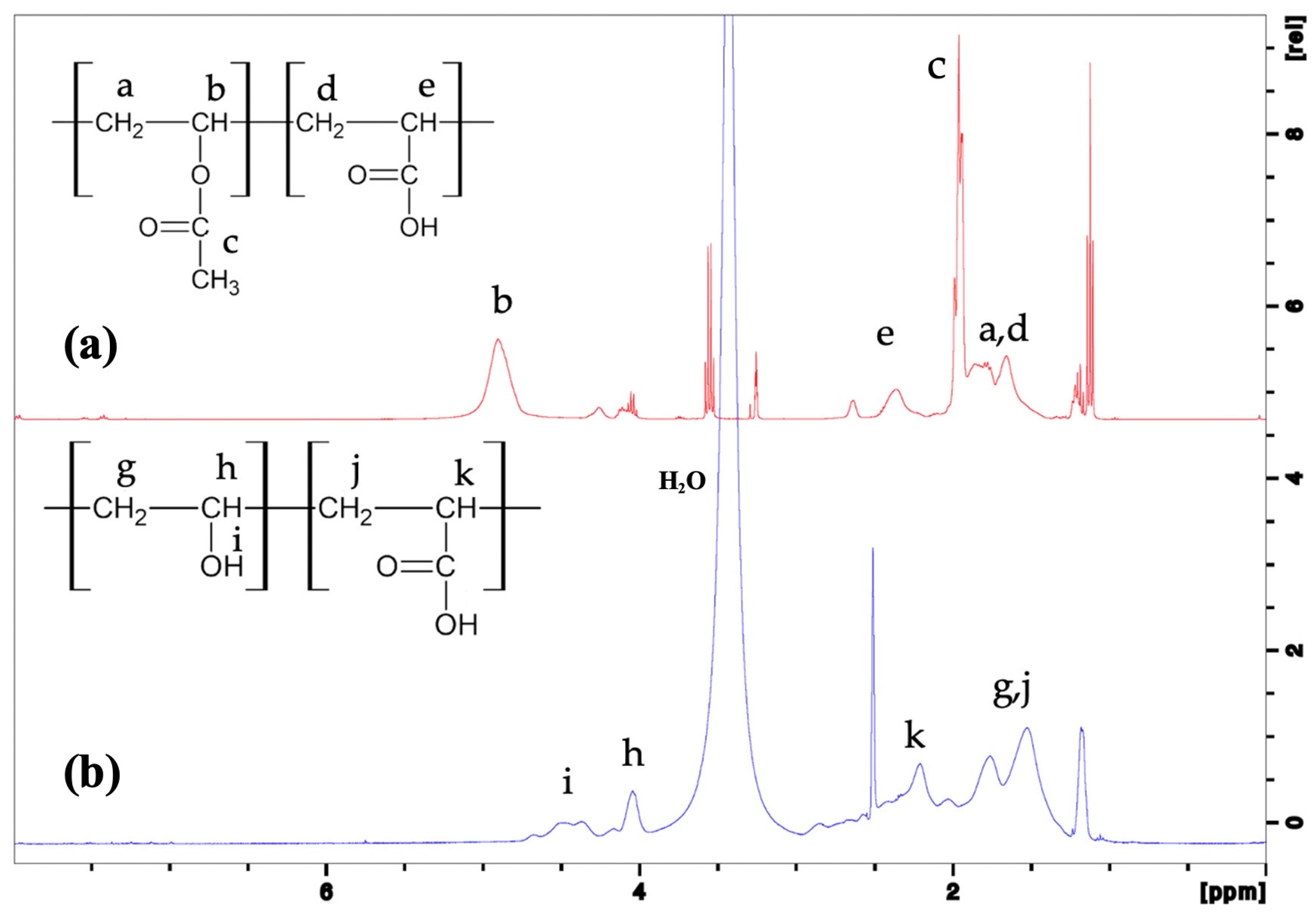
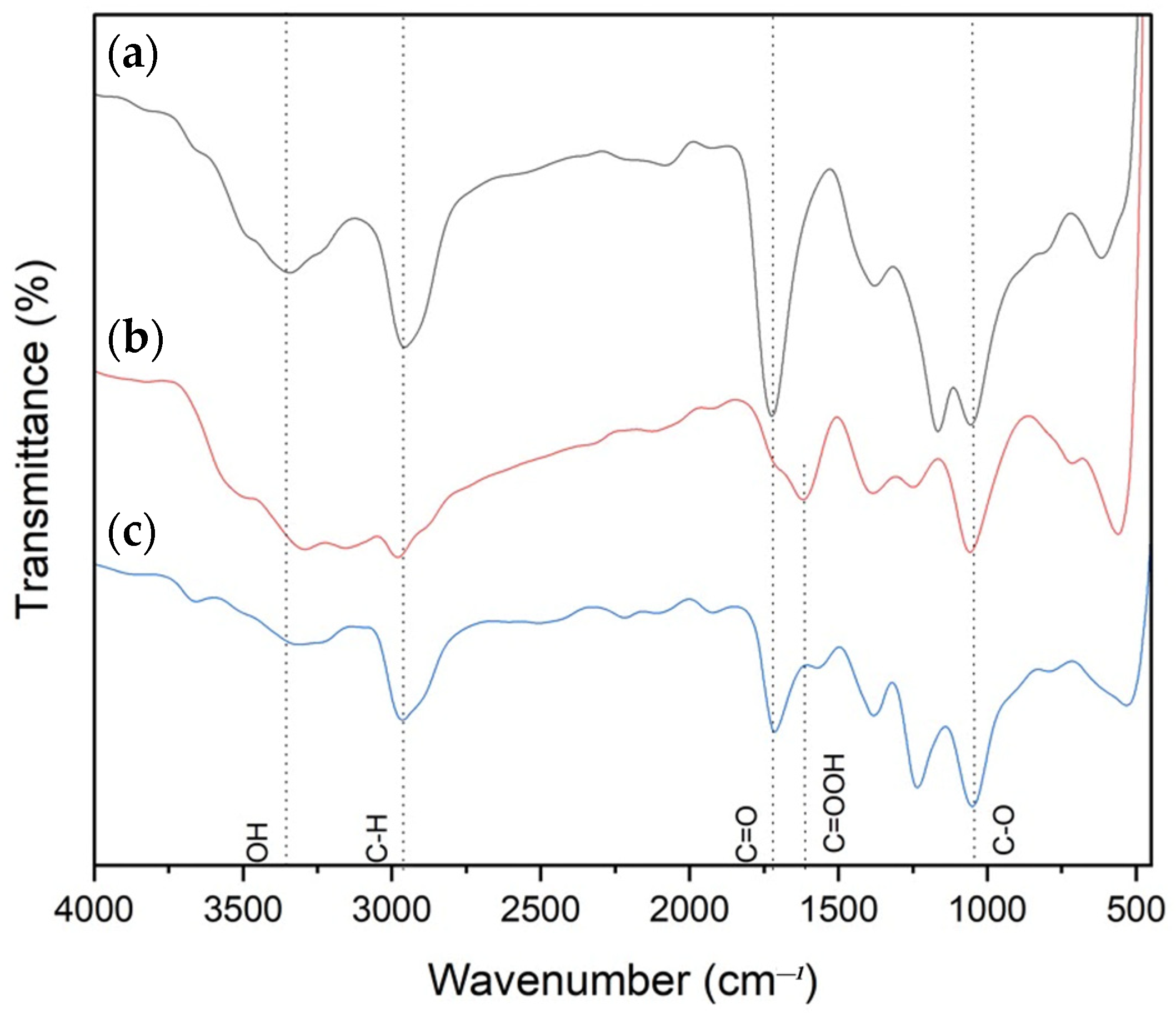
3.1. Chemical Structure
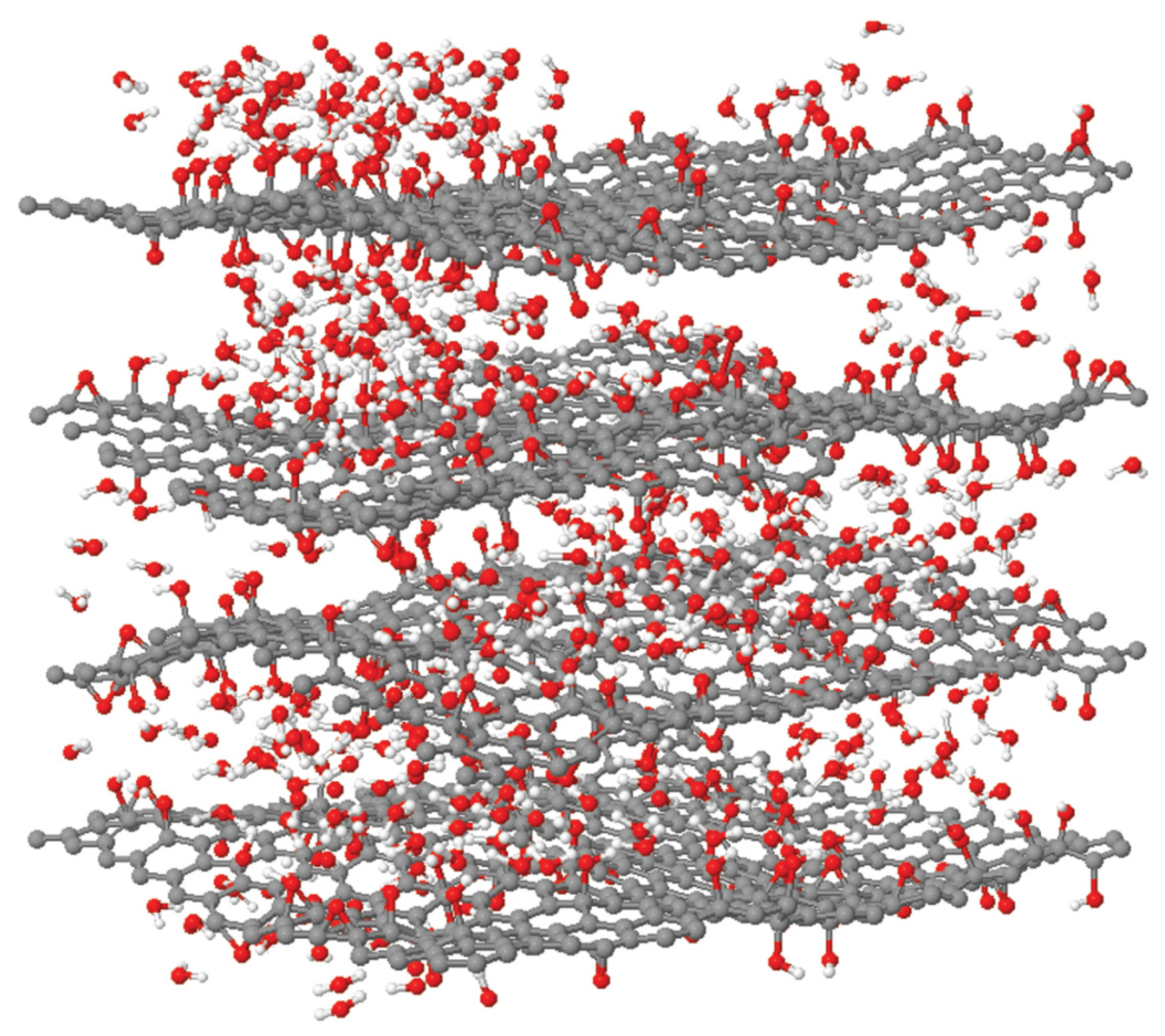
3.2. Surface Morphology
3.3. Contact Angle Measurements
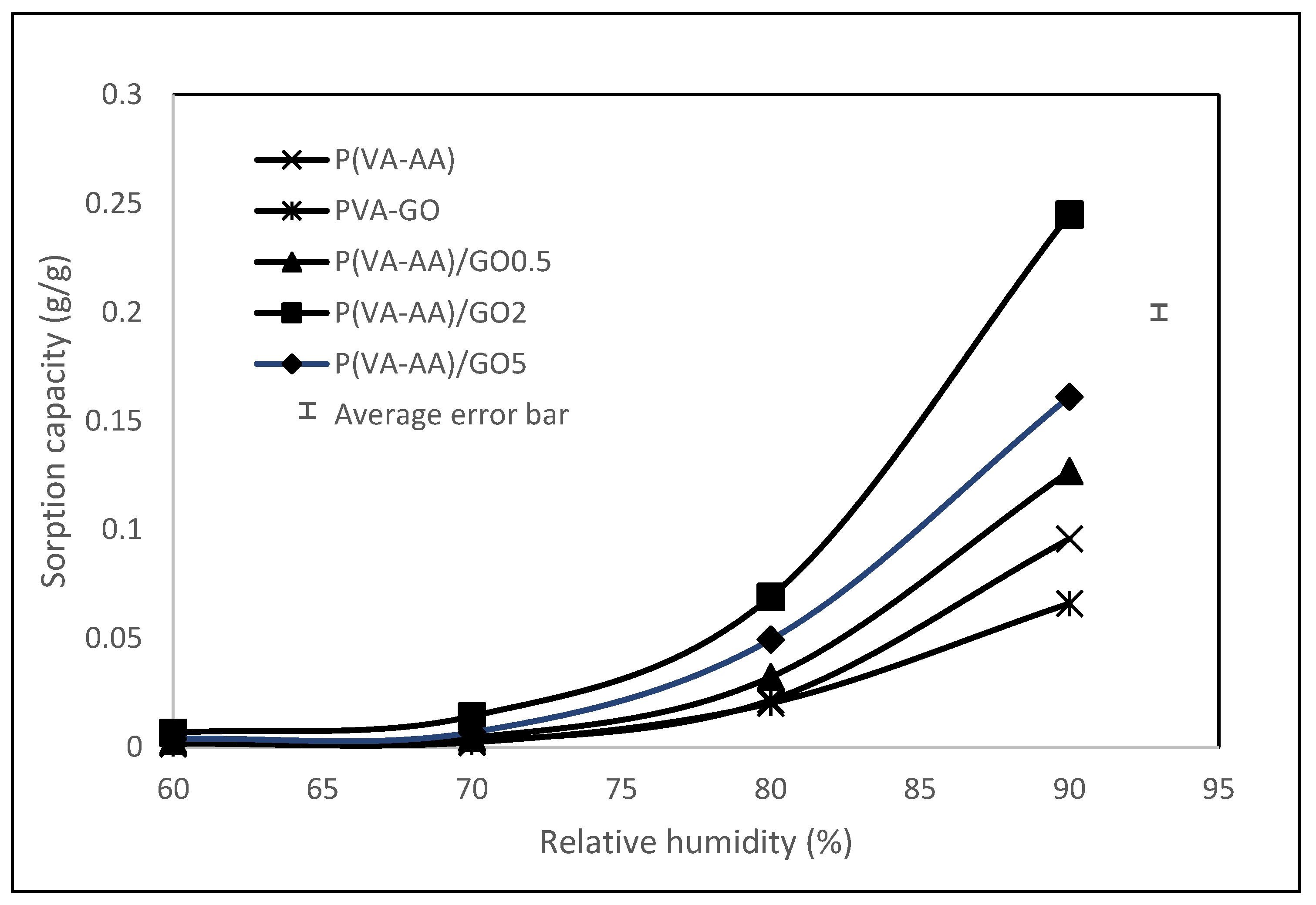
3.4. Adsorption Isotherms
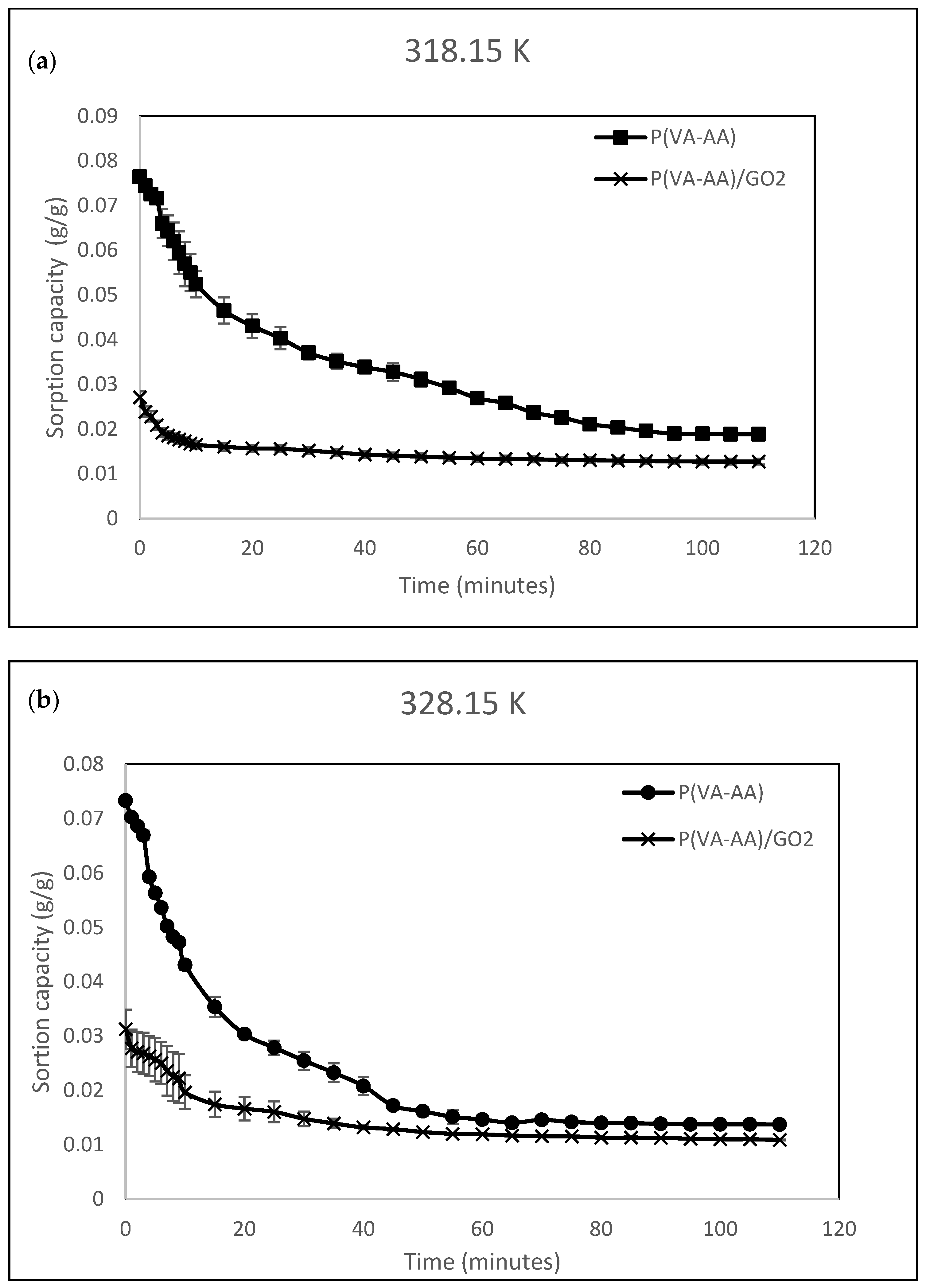
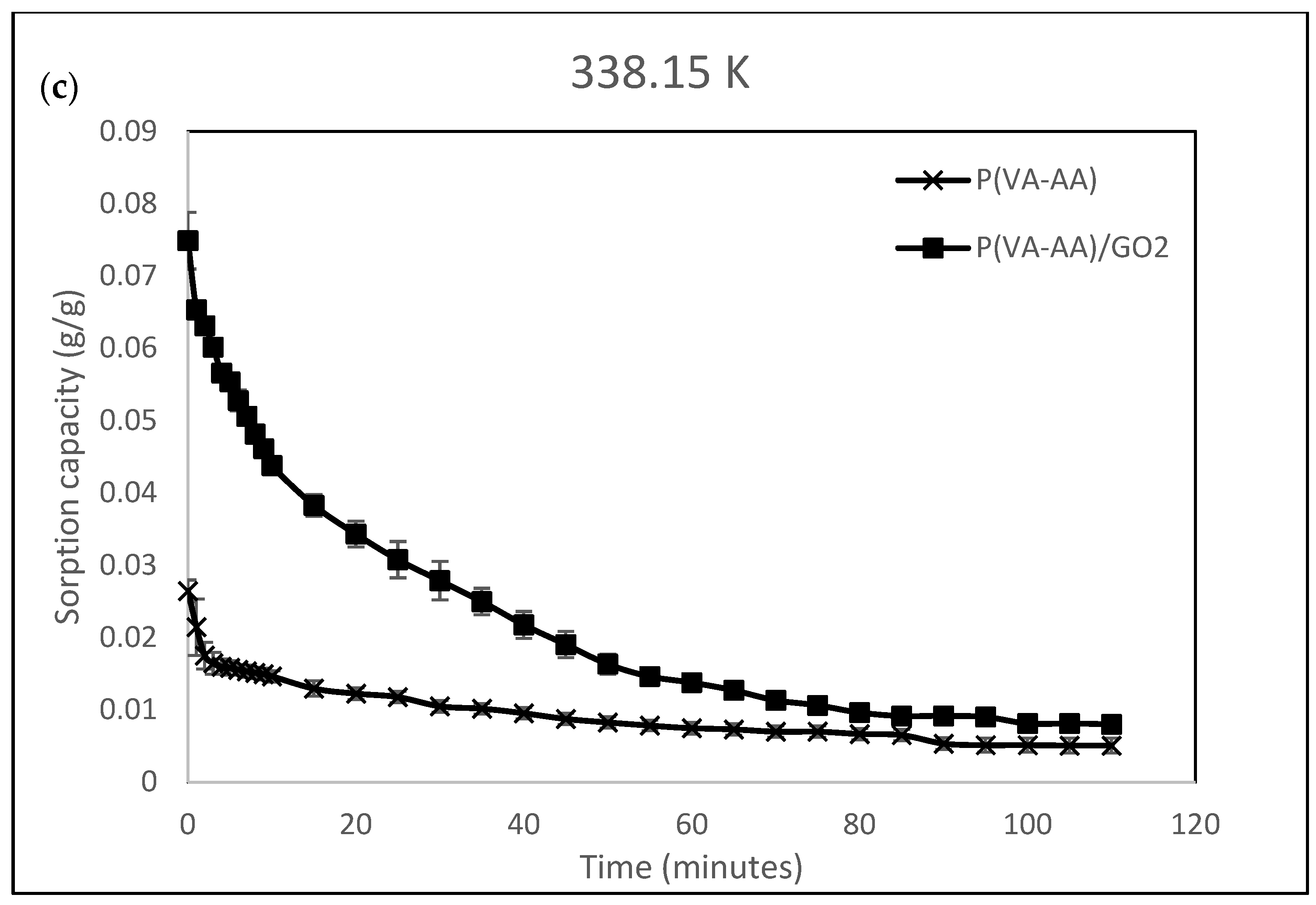
3.5. Adsorption Kinetic
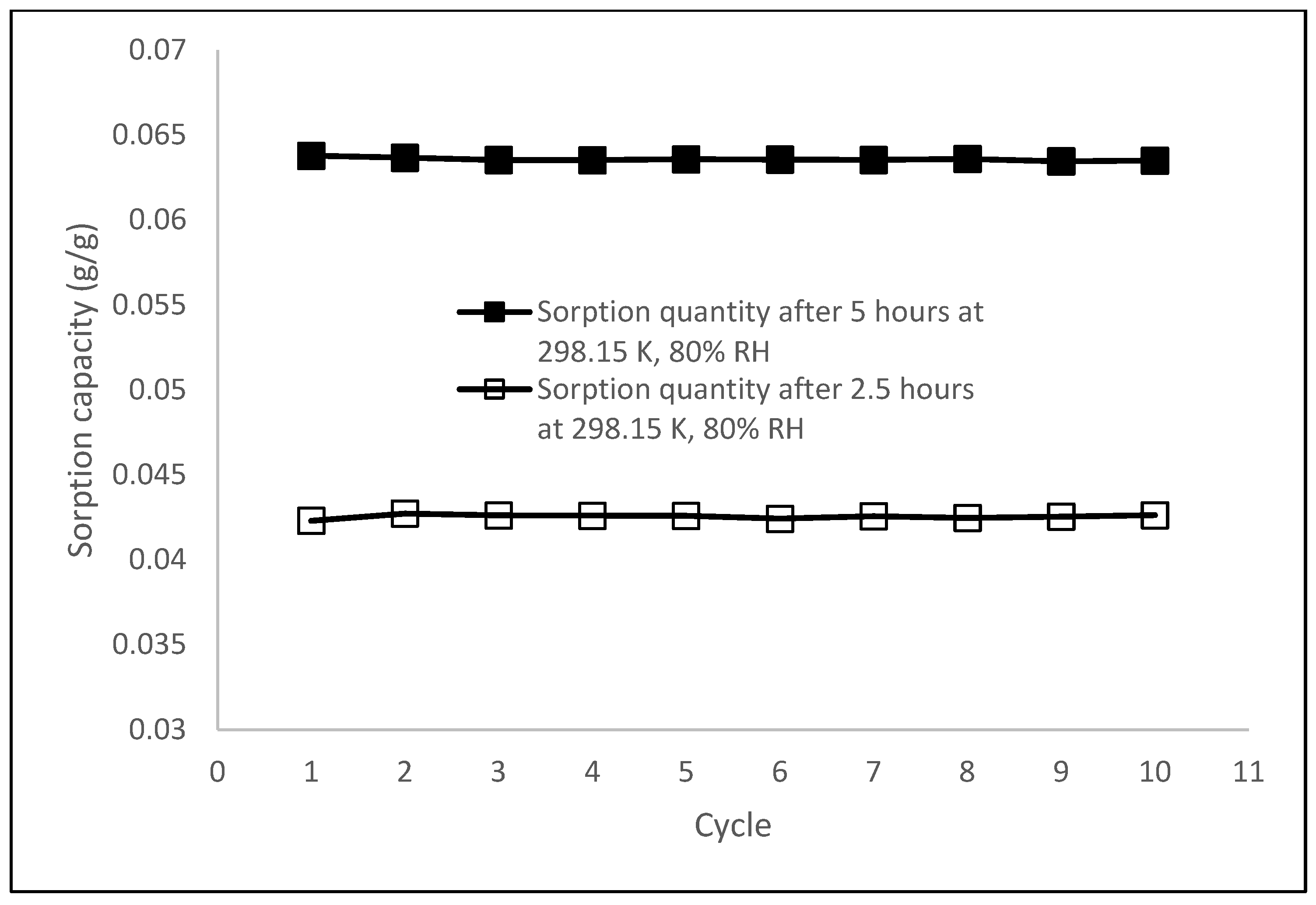
3.6. Cycling Stability
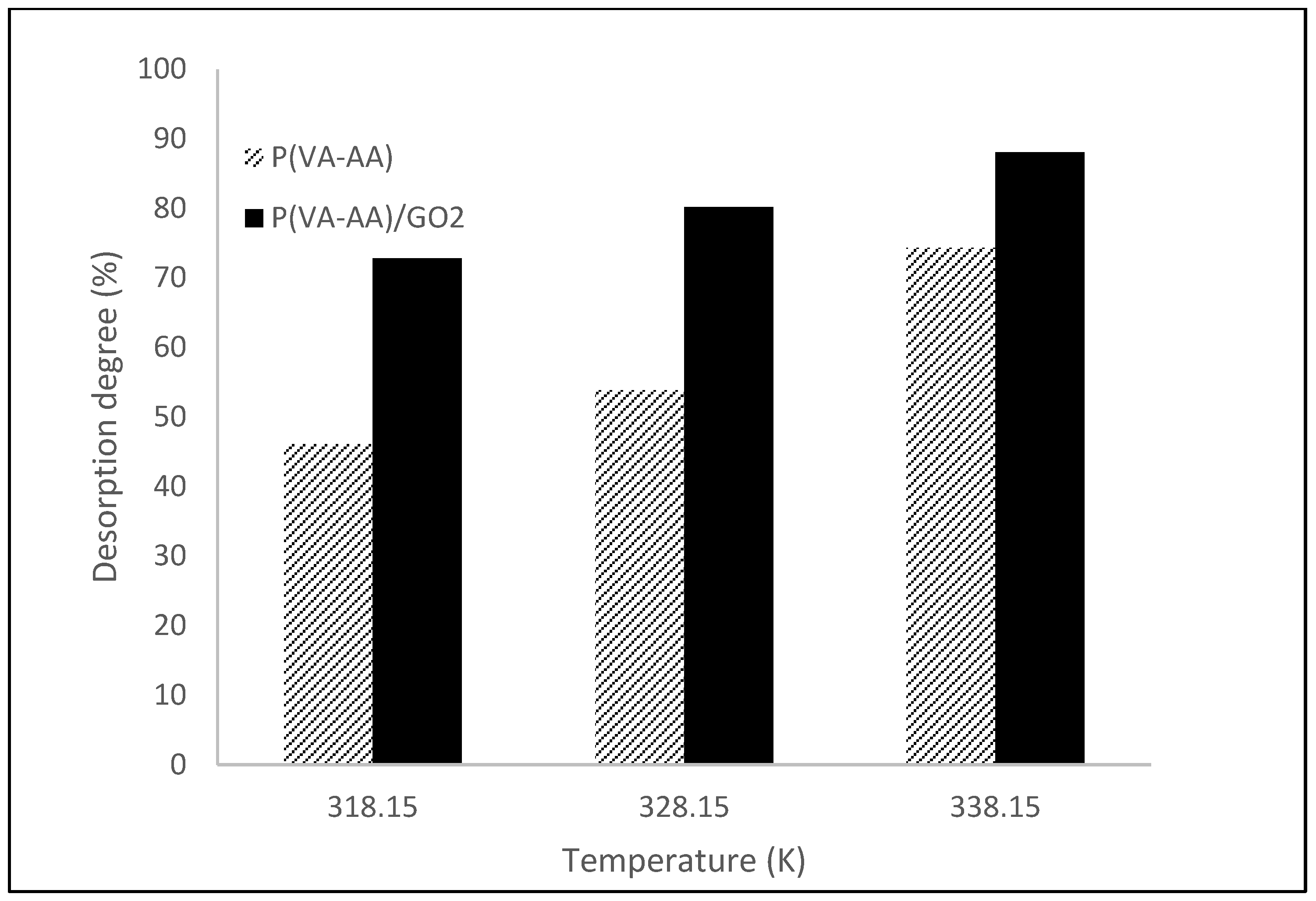
3.7. Desorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicol, J.F.; Humphreys, M.A. Adaptive Thermal Comfort and Sustainable Thermal Standards for Buildings. Energy Build. 2002, 34, 563–572. [Google Scholar] [CrossRef]
- Wen, T.; Lu, L. A Review of Correlations and Enhancement Approaches for Heat and Mass Transfer in Liquid Desiccant Dehumidification System. Appl. Energy 2019, 239, 757–784. [Google Scholar] [CrossRef]
- Dai, L.; Yao, Y.; Jiang, F.; Yang, X.; Zhou, X.; Xiong, P. Sorption and Regeneration Performance of Novel Solid Desiccant Based on PVA-LiCl Electrospun Nanofibrous Membrane. Polym. Test. 2017, 64, 242–249. [Google Scholar] [CrossRef]
- Ge, G.; Xiao, F.; Niu, X. Control Strategies for a Liquid Desiccant Air-Conditioning System. Energy Build. 2011, 43, 1499–1507. [Google Scholar] [CrossRef]
- Qi, R.; Lu, L.; Yang, H. Development of Simplified Prediction Model for Internally Cooled/Heated Liquid Desiccant Dehumidification System. Energy Build. 2013, 59, 133–142. [Google Scholar] [CrossRef]
- Rihan, Y.; Abd El-Bary, B. Characteristics of Desiccant Polymers for Air Conditioning Systems. J. Radiat. Res. Appl. Sci. 2009, 2, 449–465. [Google Scholar]
- Zheng, X.; Ge, T.S.; Wang, R.Z. Recent Progress on Desiccant Materials for Solid Desiccant Cooling Systems. Energy 2014, 74, 280–294. [Google Scholar] [CrossRef]
- Subramanyam, N.; Maiya, M.P.; Murthy, S.S. Parametric Studies on a Desiccant Assisted Air-Conditioner. Appl. Therm. Eng. 2004, 24, 2679–2688. [Google Scholar] [CrossRef]
- Wang, W.; Wu, L.; Li, Z.; Fang, Y.; Ding, J.; Xiao, J. An Overview of Adsorbents in the Rotary Desiccant Dehumidifier for Air Dehumidification. Dry. Technol. 2013, 31, 1334–1345. [Google Scholar] [CrossRef]
- Zhang, L.-Z.; Fu, H.-X.; Yang, Q.-R.; Xu, J.-C. Performance Comparisons of Honeycomb-Type Adsorbent Beds (Wheels) for Air Dehumidification with Various Desiccant Wall Materials. Energy 2014, 65, 430–440. [Google Scholar] [CrossRef]
- Jia, X.; Li, Y.; Cheng, Q.; Zhang, S.; Zhang, B. Preparation and Properties of Poly(Vinyl Alcohol)/Silica Nanocomposites Derived from Copolymerization of Vinyl Silica Nanoparticles and Vinyl Acetate. Eur. Polym. J. 2007, 43, 1123–1131. [Google Scholar] [CrossRef]
- Mittal, H.; Al Alili, A.; Alhassan, S.M. Adsorption isotherm and kinetics of water vapors on novel superporous hydrogel composites. Microporous Mesoporous Mater. 2020, 299, 110106. [Google Scholar] [CrossRef]
- Mou, X.; Chen, Z. Experimental and Predictive Study on the Performance and Energy Consumption Characteristics for the Regeneration of Activated Alumina Assisted by Ultrasound. Ultrason. Sonochemistry 2021, 70, 105314. [Google Scholar] [CrossRef]
- Mittal, H.; Al Alili, A.; Alhassan, S.M. Capturing Water Vapors from Atmospheric Air Using Superporous Gels. Sci. Rep. 2022, 12, 5626. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-C.; Chen, C.-H.; Chiang, Y.-C.; Chen, S.-L. Circulating Inclined Fluidized Beds with Application for Desiccant Dehumidification Systems. Appl. Energy 2016, 175, 199–211. [Google Scholar] [CrossRef]
- Asim, N.; Amin, M.H.; Alghoul, M.A.; Badiei, M.; Mohammad, M.; Gasaymeh, S.S.; Amin, N.; Sopian, K. Key Factors of Desiccant-Based Cooling Systems: Materials. Appl. Therm. Eng. 2019, 159, 113946. [Google Scholar] [CrossRef]
- Deshpande, D.S.; Bajpai, R.; Bajpai, A.K. Synthesis and Characterization of Polyvinyl Alcohol Based Semi Interpenetrating Polymeric Networks. J. Polym. Res. 2012, 19, 9938. [Google Scholar] [CrossRef]
- Popescu, M.-C. Structure and Sorption Properties of CNC Reinforced PVA Films. Int. J. Biol. Macromol. 2017, 101, 783–790. [Google Scholar] [CrossRef]
- Pan, Y.; Sahoo, N.G.; Li, L. The Application of Graphene Oxide in Drug Delivery. Expert Opin. Drug Deliv. 2012, 9, 1365–1376. [Google Scholar] [CrossRef]
- Chung, C.; Kim, Y.-K.; Shin, D.; Ryoo, S.-R.; Hong, B.H.; Min, D.-H. Biomedical Applications of Graphene and Graphene Oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef]
- Lian, B.; De Luca, S.; You, Y.; Alwarappan, S.; Yoshimura, M.; Sahajwalla, V.; Smith, S.C.; Leslie, G.; Joshi, R.K. Extraordinary Water Adsorption Characteristics of Graphene Oxide. Chem. Sci. 2018, 9, 5106–5111. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Wu, H.A.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A.K. Unimpeded Permeation of Water through Helium-Leak-Tight Graphene-Based Membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.I.; Ingole, P.G.; Jeon, J.; Hong, S.U.; Choi, W.K.; Lee, H.K. Water Vapor Transport Properties of Interfacially Polymerized Thin Film Nanocomposite Membranes Modified with Graphene Oxide and GO-TiO2 Nanofillers. Chem. Eng. J. 2019, 373, 1190–1202. [Google Scholar] [CrossRef]
- Chen, C.-H.; Hsu, C.-Y.; Chen, C.-C.; Chiang, Y.-C.; Chen, S.-L. Silica Gel/Polymer Composite Desiccant Wheel Combined with Heat Pump for Air-Conditioning Systems. Energy 2016, 94, 87–99. [Google Scholar] [CrossRef]
- Ge, T.S.; Dai, Y.J.; Wang, R.Z.; Peng, Z.Z. Experimental Comparison and Analysis on Silica Gel and Polymer Coated Fin-Tube Heat Exchangers. Energy 2010, 35, 2893–2900. [Google Scholar] [CrossRef]
- Lee, J.; Lee, D.-Y. Sorption Characteristics of a Novel Polymeric Desiccant. Int. J. Refrig. 2012, 35, 1940–1949. [Google Scholar] [CrossRef]
- Jayakrishnan, P.; Ramesan, M.T. Synthesis, Characterization and Electrical Properties of Fe3O4/Poly (Vinyl Alcohol-Co-Acrylic Acid) Nanocomposites. AIP Conf. Proc. 2014, 1620, 165–172. [Google Scholar] [CrossRef]
- Minhas, M.U.; Ahmad, M.; Ali, L.; Sohail, M. Synthesis of Chemically Cross-Linked Polyvinyl Alcohol-Co-Poly (Methacrylic Acid) Hydrogels by Copolymerization; a Potential Graft-Polymeric Carrier for Oral Delivery of 5-Fluorouracil. DARU J. Pharm. Sci. 2013, 21, 44. [Google Scholar] [CrossRef]
- Liu, A.; Honma, I.; Ichihara, M.; Zhou, H. Poly(Acrylic Acid)-Wrapped Multi-Walled Carbon Nanotubes Composite Solubilization in Water: Definitive Spectroscopic Properties. Nanotechnology 2006, 17, 2845–2849. [Google Scholar] [CrossRef]
- Cherifi, B.I.; Belbachir, M.; Rahmouni, A. Green Anionic Polymerization of Vinyl Acetate Using Maghnite-Na+ (Algerian MMT): Synthesis Characterization and Reactional Mechanism. Discov. Chem. Eng. 2021, 1, 5. [Google Scholar] [CrossRef]
- Bleszynski, M. The Modification of Polyvinyl Alcohol for Ice Nucleation Based upon the Structures of Antifreeze Glycoproteins Found in Antarctic Fish. Biophysica 2022, 2, 417–427. [Google Scholar] [CrossRef]
- Islam, M.T.; Haldorai, Y.; Nguyen, V.T.; Islam, M.N.; Ra, C.S.; Shim, J.-J. Controlled Radical Polymerization of Vinyl Acetate in Supercritical CO2 Catalyzed by CuBr/Terpyridine. Korean J. Chem. Eng. 2014, 31, 1088–1094. [Google Scholar] [CrossRef]
- Korbag, I.; Mohamed Saleh, S. Studies on the Formation of Intermolecular Interactions and Structural Characterization of Polyvinyl Alcohol/Lignin Film. Int. J. Environ. Stud. 2016, 73, 226–235. [Google Scholar] [CrossRef]
- Shahabuddin, S.; Sarih, N.; Afzal Kamboh, M.; Rashidi Nodeh, H.; Mohamad, S. Synthesis of Polyaniline-Coated Graphene Oxide@SrTiO3 Nanocube Nanocomposites for Enhanced Removal of Carcinogenic Dyes from Aqueous Solution. Polymers 2016, 8, 305. [Google Scholar] [CrossRef]
- Esmaeili, Y.; Bidram, E.; Zarrabi, A.; Amini, A.; Cheng, C. Graphene Oxide and Its Derivatives as Promising In-Vitro Bio-Imaging Platforms. Sci. Rep. 2020, 10, 18052. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Liu, W.; Schwenzer, B.; Manandhar, S.; Chase-Woods, D.; Engelhard, M.H.; Devanathan, R.; Fifield, L.S.; Bennett, W.D.; Ginovska, B.; et al. Graphene Oxide Membranes with High Permeability and Selectivity for Dehumidification of Air. Carbon 2016, 106, 164–170. [Google Scholar] [CrossRef]
- Arabkhani, P.; Asfaram, A.; Ateia, M. Easy-To-Prepare Graphene Oxide/Sodium Montmorillonite Polymer Nanocomposite with Enhanced Adsorption Performance. J. Water Process Eng. 2020, 38, 101651. [Google Scholar] [CrossRef]
- Wu, G.M.; Lin, S.J.; Yang, C.C. Preparation and Characterization of PVA/PAA Membranes for Solid Polymer Electrolytes. J. Membr. Sci. 2006, 275, 127–133. [Google Scholar] [CrossRef]
- Jayakrishnan, P.; Ramesan, M.T. Studies on the Effect of Magnetite Nanoparticles on Magnetic, Mechanical, Thermal, Temperature Dependent Electrical Resistivity and DC Conductivity Modeling of Poly (Vinyl Alcohol-Co-Acrylic Acid)/Fe3O4 Nanocomposites. Mater. Chem. Phys. 2017, 186, 513–522. [Google Scholar] [CrossRef]
- Diken, M.E.; Kizilduman, B.K.; Yilmaz Kardaş, B.; Doğan, E.E.; Doğan, M.; Turhan, Y.; Doğan, S. Synthesis, Characterization, and Their Some Chemical and Biological Properties of PVA/PAA/NPS Hydrogel Nanocomposites: Hydrogel and Wound Dressing. J. Bioact. Compat. Polym. 2020, 35, 203–215. [Google Scholar] [CrossRef]
- Chee, B.S.; de Lima, G.G.; de Lima, T.A.M.; Seba, V.; Lemarquis, C.; Pereira, B.L.; Bandeira, M.; Cao, Z.; Nugent, M. Effect of Thermal Annealing on a Bilayer Polyvinyl Alcohol/Polyacrylic Acid Electrospun Hydrogel Nanofibres Loaded with Doxorubicin and Clarithromycin for a Synergism Effect against Osteosarcoma Cells. Mater. Today Chem. 2021, 22, 100549. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, C.; Ma, S. Advanced Porous Organic Polymer Membranes: Design, Fabrication, and Energy-Saving Applications. EnergyChem 2022, 4, 100079. [Google Scholar] [CrossRef]
- Padaki, M.; Emadzadeh, D.; Masturra, T.; Ismail, A.F. Antifouling Properties of Novel PSf and TNT Composite Membrane and Study of Effect of the Flow Direction on Membrane Washing. Desalination 2015, 362, 141–150. [Google Scholar] [CrossRef]
- Toribio, F.; Bellat, J.P.; Nguyen, P.H.; Dupont, M. Adsorption of Water Vapor by Poly(Styrenesulfonic Acid), Sodium Salt: Isothermal and Isobaric Adsorption Equilibria. J. Colloid Interface Sci. 2004, 280, 315–321. [Google Scholar] [CrossRef]
- Ganji, F.; Vasheghani, F.S.; Vasheghani, F.E. Theoretical Description of Hydrogel Swelling: A Review. Iran Polym. J. 2010, 19, 375–398. [Google Scholar]
- Mittal, H.; Alili, A.A.; Alhassan, S.M. Hybrid Super-Porous Hydrogel Composites with High Water Vapor Adsorption Capacity—Adsorption Isotherm and Kinetics Studies. J. Environ. Chem. Eng. 2021, 9, 106611. [Google Scholar] [CrossRef]
- Abd Elwadood, S.N.; Dumée, L.F.; Al Wahedi, Y.; Al Alili, A.; Karanikolos, G.N. Aluminophosphate-Based Adsorbents for Atmospheric Water Generation. J. Water Process Eng. 2022, 49, 103099. [Google Scholar] [CrossRef]
- Mittal, H.; Al Alili, A.; Alhassan, S.M. Solid Polymer Desiccants Based on Poly(Acrylic Acid-Co-Acrylamide) and Laponite RD: Adsorption Isotherm and Kinetics Studies. Colloids Surf. A Physicochem. Eng. Asp. 2020, 599, 124813. [Google Scholar] [CrossRef]
- Belguith, S.; Meddeb, Z.; Ben Slama, R. Performance Analysis of Desiccant Cooling System Using Polyacrylic Acid Sodium Salt Desiccant Wheel. Sci. Technol. Built Environ. 2021, 27, 1368–1380. [Google Scholar] [CrossRef]
- Yu, Y.; De Andrade, L.C.X.; Fang, L.; Ma, J.; Zhang, W.; Tang, Y. Graphene Oxide and Hyperbranched Polymer-Toughened Hydrogels with Improved Absorption Properties and Durability. J. Mater. Sci. 2015, 50, 3457–3466. [Google Scholar] [CrossRef]
- Hu, J.; Song, J.; Du, J.; Gao, X.; Chen, A. Nitrogen-Doped Carbon Nanosheets Derived from GO for Enhancement of Supercapacitor Performance. Diam. Relat. Mater. 2022, 128, 109272. [Google Scholar] [CrossRef]
- Yi, R.; Peng, B.; Zhao, Y.; Nie, D.; Chen, L.; Zhang, L. Quartz Crystal Microbalance Humidity Sensors Based on Structured Graphene Oxide Membranes with Magnesium Ions: Design, Mechanism and Performance. Membranes 2022, 12, 125. [Google Scholar] [CrossRef]
- Medhekar, N.V.; Ramasubramaniam, A.; Ruoff, R.S.; Shenoy, V.B. Hydrogen Bond Networks in Graphene Oxide Composite Paper: Structure and Mechanical Properties. ACS Nano 2010, 4, 2300–2306. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, M.; Ruan, W. High-Water-Content Graphene Oxide/Polyvinyl Alcohol Hydrogel with Excellent Mechanical Properties. J. Mater. Chem. A 2014, 2, 10508–10515. [Google Scholar] [CrossRef]
- Saeid, M.; Poe, W.A.; Mak, J. Handbook of Natural Gas Transmission and Processing: Principles and Practices, 4th ed.; Gulf Professional Publishing: Cambridge, MA, USA, 2019; pp. 307–348. [Google Scholar]
- Tai, Z.; Yang, J.; Qi, Y.; Yan, X.; Xue, Q. Synthesis of a Graphene Oxide–Polyacrylic Acid Nanocomposite Hydrogel and Its Swelling and Electroresponsive Properties. RSC Adv. 2013, 3, 12751. [Google Scholar] [CrossRef]
- Zheng, X.; Ge, T.S.; Wang, R.Z.; Hu, L.M. Performance Study of Composite Silica Gels with Different Pore Sizes and Different Impregnating Hygroscopic Salts. Chem. Eng. Sci. 2014, 120, 1–9. [Google Scholar] [CrossRef]
- Jia, L.; Yao, X.; Ma, J.; Long, C. Adsorption Kinetics of Water Vapor on Hypercrosslinked Polymeric Adsorbent and Its Comparison with Carbonaceous Adsorbents. Microporous Mesoporous Mater. 2017, 241, 178–184. [Google Scholar] [CrossRef]
- Zimny, T.; Finqueneisel, G.; Cossarutto, L.; Weber, J.V. Water Vapor Adsorption on Activated Carbon Preadsorbed with Naphtalene. J. Colloid Interface Sci. 2005, 285, 56–60. [Google Scholar] [CrossRef]
- Average Humidity in Kuala Lumpur (Kuala Lumpur Federal Territory). Available online: https://weather-and-climate.com/average-monthly-Humidity-perc,Kuala-Lumpur,Malaysia (accessed on 1 February 2023).
- Winston, P.W.; Bates, D.H. Saturated Solutions for the Control of Humidity in Biological Research. Ecology 1960, 41, 232–237. [Google Scholar] [CrossRef]
- Serbezov, A. Adsorption Equilibrium of Water Vapor on F-200 Activated Alumina. J. Chem. Eng. Data 2003, 48, 421–425. [Google Scholar] [CrossRef]
- Jia, C.X.; Dai, Y.J.; Wu, J.Y.; Wang, R.Z. Use of Compound Desiccant to Develop High Performance Desiccant Cooling System. Int. J. Refrig. 2007, 30, 345–353. [Google Scholar] [CrossRef]
- Asuquo, J.E.; Udegbunam, I.S.; Etim, E.E. Effect of Temperature on the Adsorption of Metallic Soaps of Castor Seed Oil onto Haematite. Int. J. Adv. Res. Chem. Sci. 2017, 4, 345–353. [Google Scholar] [CrossRef]
- Sexton, B.A.; Hughes, A.E. A Comparison of Weak Molecular Adsorption of Organic Molecules on Clean Copper and Platinum Surfaces. Surf. Sci. 1984, 140, 227–248. [Google Scholar] [CrossRef]






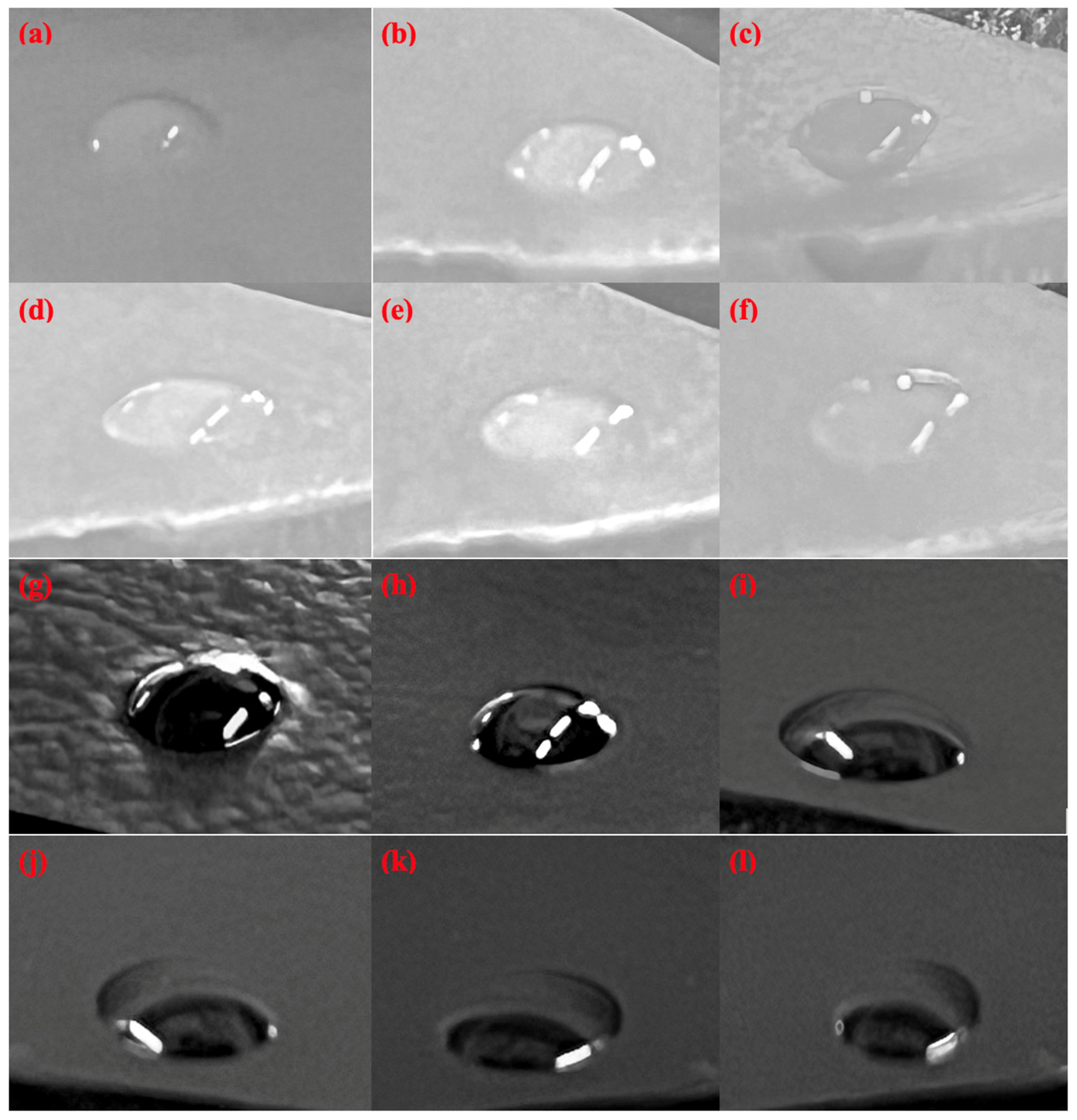


| Sample | Time (min) | Contact Angle (°) |
|---|---|---|
| P(VA-AA) | 0 | 51.5 |
| 1 | 45.5 | |
| 2 | 37.5 | |
| 3 | 32.0 | |
| 4 | 28.5 | |
| 5 | 24.5 | |
| 6 | 20.0 | |
| 7 | 20.0 | |
| P(VA-AA)/GO2 | 0 | 38.5 |
| 1 | 29.0 | |
| 2 | 21.5 | |
| 3 | 19.5 | |
| 4 | 17.0 | |
| 5 | 17.0 |
| Sample | k | R2 | |
|---|---|---|---|
| PVA/GO | 0.0081 | 0.9896 | 4.4809 × 10−05 |
| P(VA-AA) | 0.0083 | 0.9956 | 4.4652 × 10−05 |
| P(VA-AA)/GO0.5 | 0.0105 | 0.9904 | 6.6953 × 10−05 |
| P(VA-AA)/GO2 | 0.0093 | 0.9911 | 1.4528 × 10−04 |
| P(VA-AA)/GO5 | 0.0115 | 0.9809 | 1.0802 × 10−04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Md Zulkiflie, F.A.; Muhamad Sarih, N.; Hashim, N.A.; Mohd Zubir, M.N.; Abdullah, S.; Mohd Amin, A.S. Moisture Adsorption–Desorption Behaviour in Nanocomposite Copolymer Films. Polymers 2023, 15, 2998. https://doi.org/10.3390/polym15142998
Md Zulkiflie FA, Muhamad Sarih N, Hashim NA, Mohd Zubir MN, Abdullah S, Mohd Amin AS. Moisture Adsorption–Desorption Behaviour in Nanocomposite Copolymer Films. Polymers. 2023; 15(14):2998. https://doi.org/10.3390/polym15142998
Chicago/Turabian StyleMd Zulkiflie, Farah Aqilah, Norazilawati Muhamad Sarih, Nur Awanis Hashim, Mohd Nashrul Mohd Zubir, Shekh Abdullah, and Aida Sabrina Mohd Amin. 2023. "Moisture Adsorption–Desorption Behaviour in Nanocomposite Copolymer Films" Polymers 15, no. 14: 2998. https://doi.org/10.3390/polym15142998
APA StyleMd Zulkiflie, F. A., Muhamad Sarih, N., Hashim, N. A., Mohd Zubir, M. N., Abdullah, S., & Mohd Amin, A. S. (2023). Moisture Adsorption–Desorption Behaviour in Nanocomposite Copolymer Films. Polymers, 15(14), 2998. https://doi.org/10.3390/polym15142998







