Effect of the Filler Modification on the Thermal and Mechanical Properties of Composite Polypropylene/Wollastonite Drawn Fibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
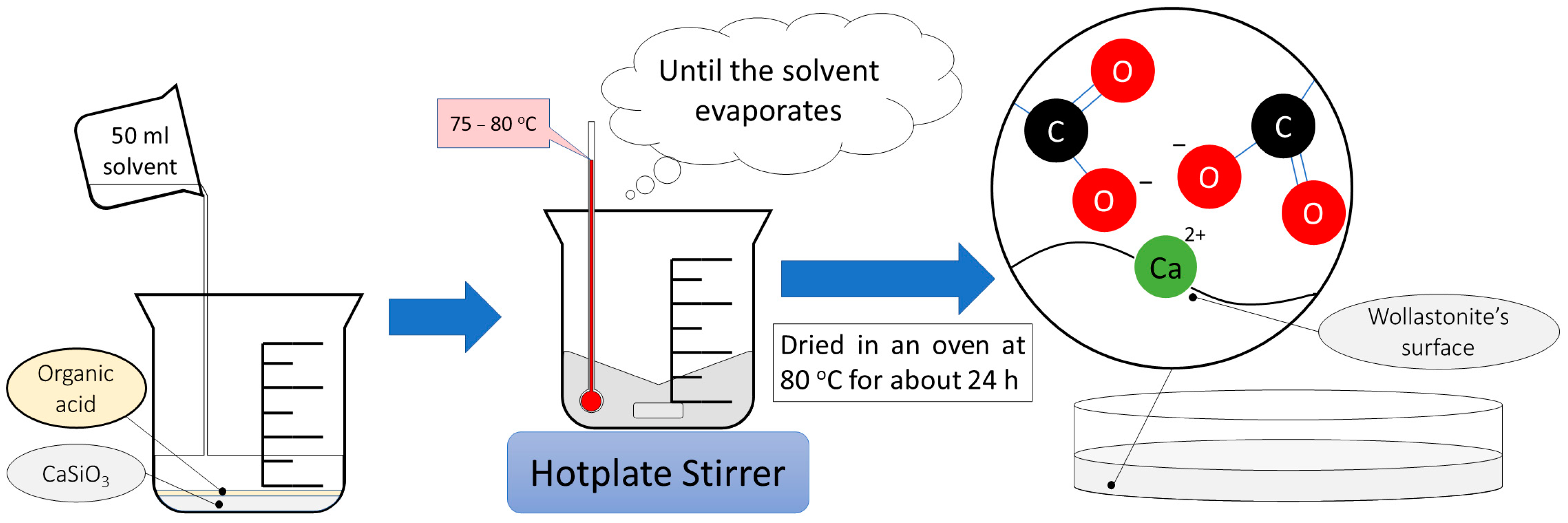
2.2. Wollastonite Modification
2.3. Fiber Production and Drawing
2.4. Characterization
3. Results and Discussion
3.1. Effect of the Compatibilizer
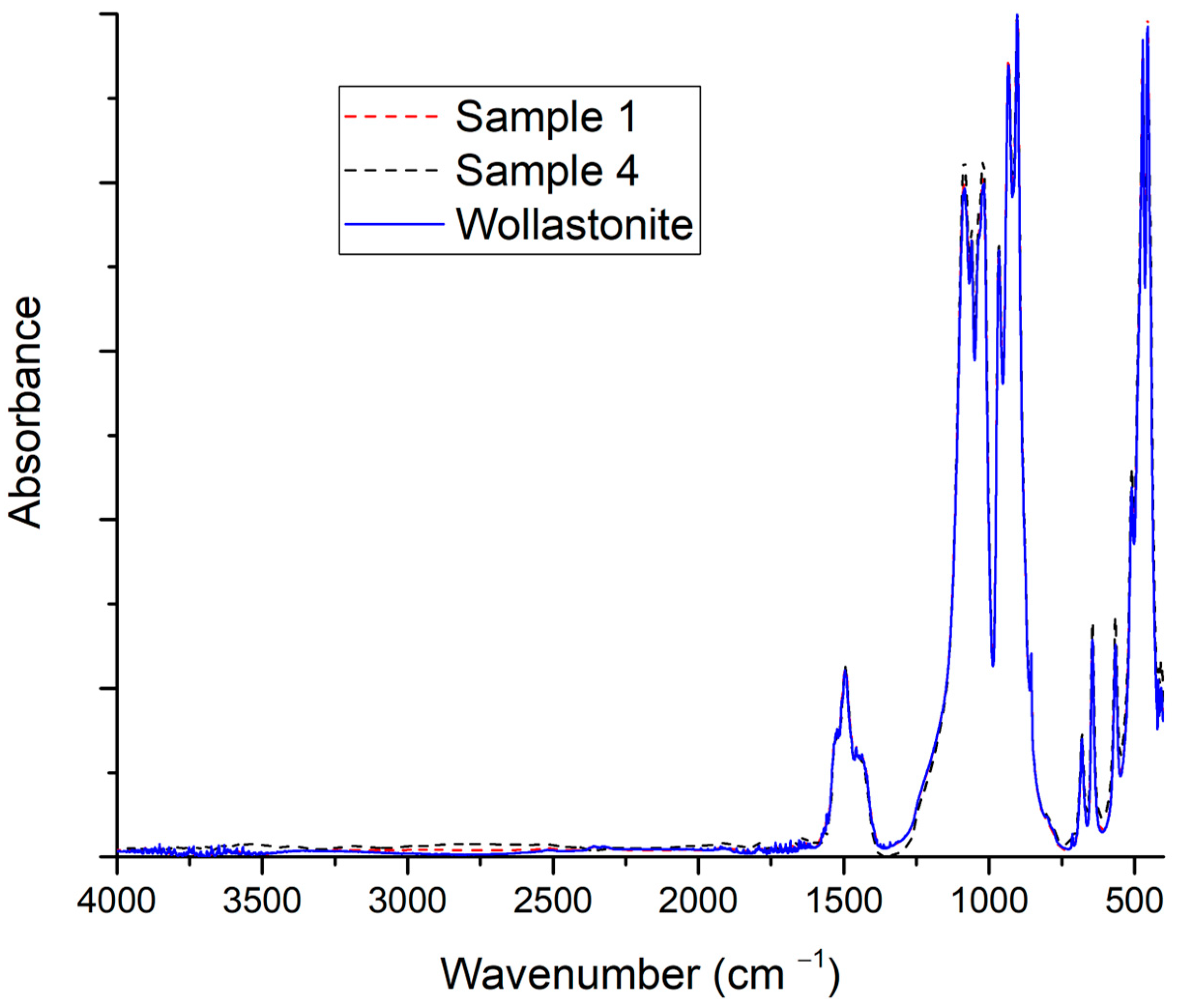
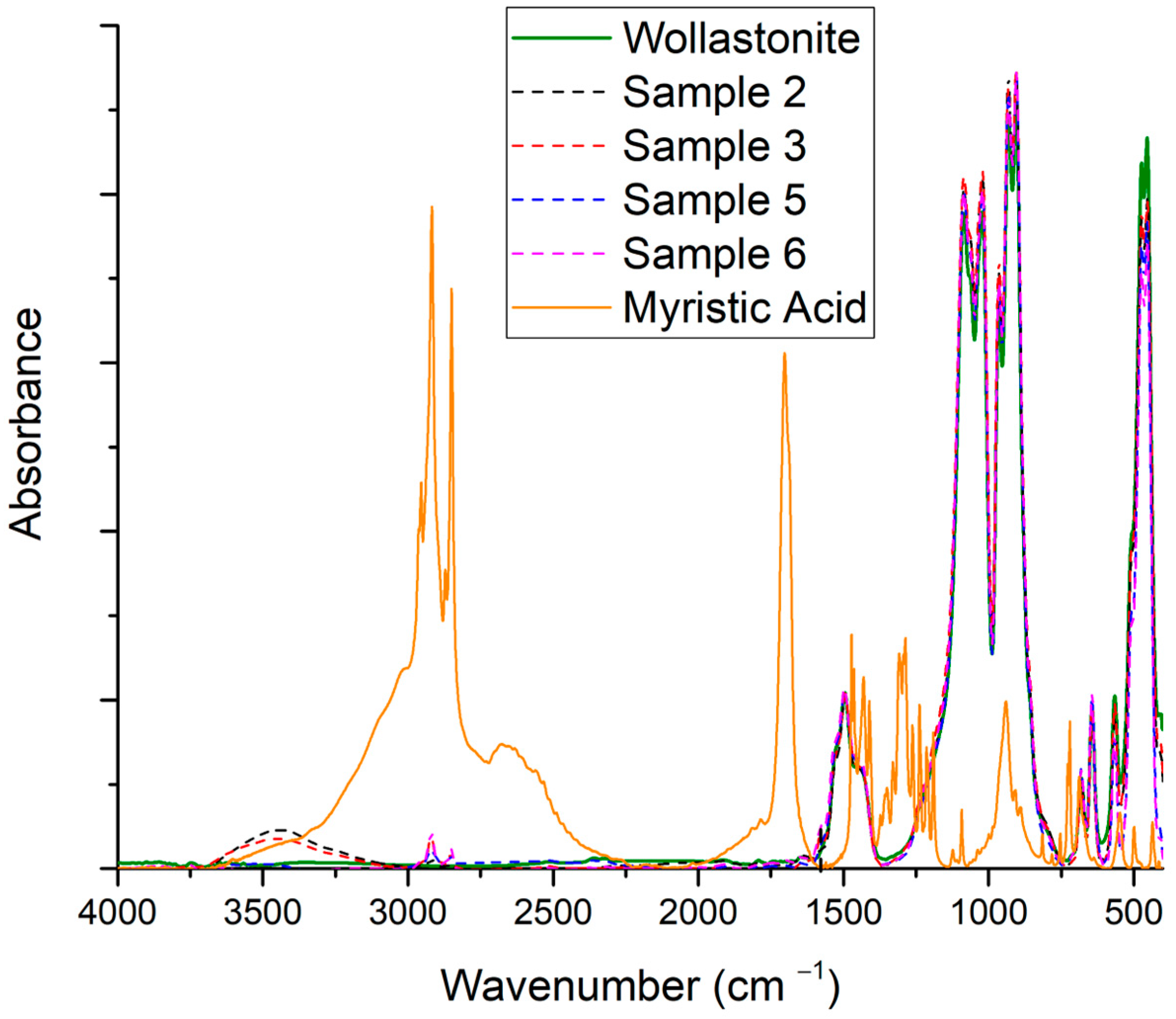
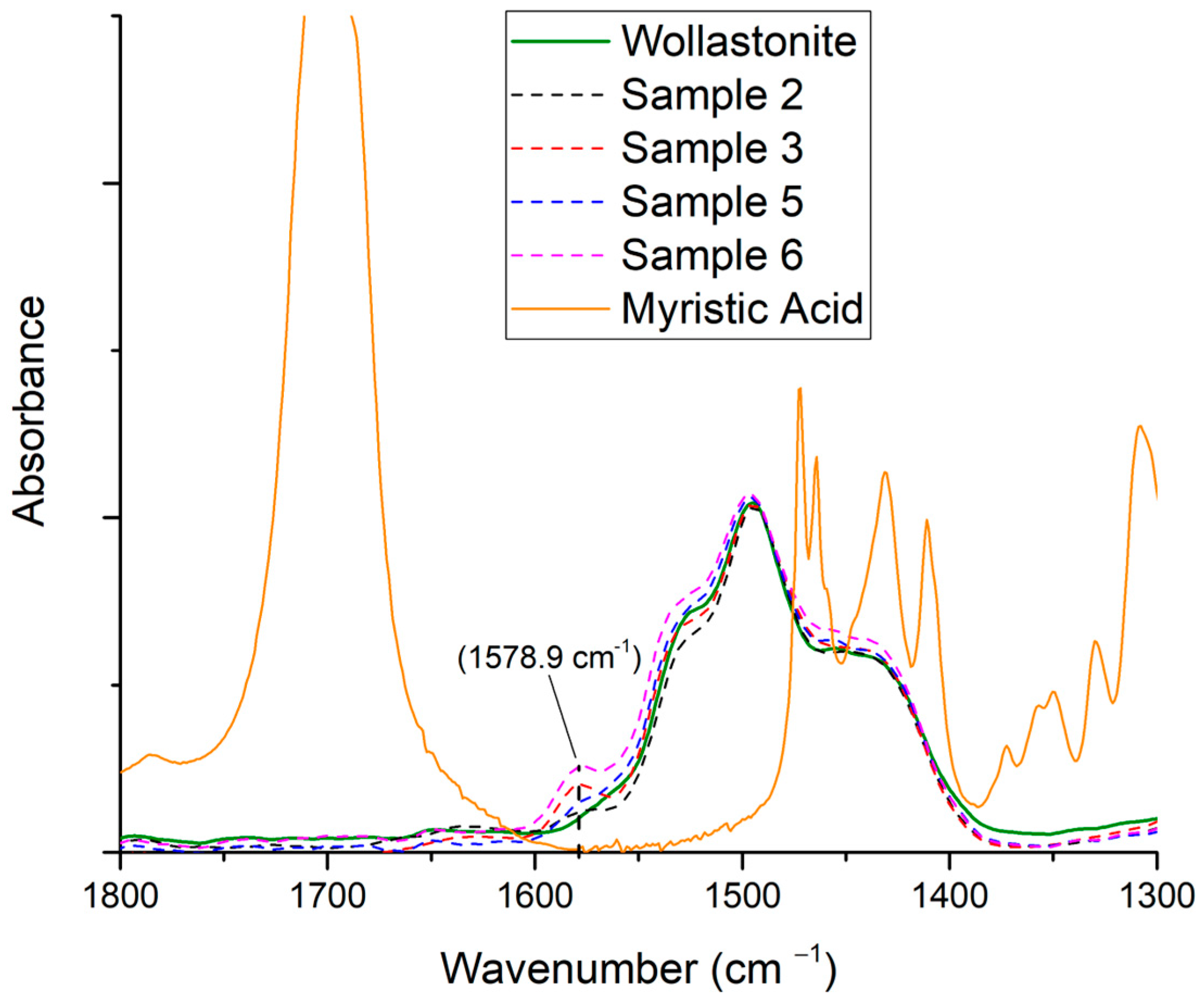
3.2. Effect of the Solvent
3.3. Effect of Organic Acid Used for Wollastonite’s Surface Modification
3.3.1. Myristic Acid
3.3.2. Maleic Acid
3.3.3. Malonic Acid
3.3.4. Glutaric Acid
3.3.5. Pimelic Acid
3.3.6. Suberic Acid
3.4. Characterization Results of PP–Modified Wollastonite Drawn Fibers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- United Nations Environment Programme; Technical University of Denmark (DTU). Mapping of Global Plastics Value Chain and Plastics Losses to the Environment: With a Particular Focus on Marine; United Nations Environment Programme: Nairobi, Kenya, 2018. [Google Scholar]
- Behfarnia, K.; Behravan, A. Application of high performance polypropylene fibers in concrete lining of water tunnels. Mater. Des. 2014, 55, 274–279. [Google Scholar] [CrossRef]
- Moore, E.P., Jr. Polypropylene Handbook; Hanser/Gardner Publications, Inc.: Cincinnati, OH, USA, 1996. [Google Scholar]
- Dragaun, H.; Hubeny, H.; Muschik, H. Shear-induced β-form crystallization in isotactic polypropylene. J. Polym. Sci. Polym. Phys. Ed. 1977, 15, 1779–1789. [Google Scholar] [CrossRef]
- Moitzi, J.; Skalicky, P. Shear induced crystallization of isotactic polypropylene melts: Isothermal WAXS experiments with synchrotron radiation. Polymer 1993, 34, 3168–3172. [Google Scholar] [CrossRef]
- Stocker, W.; Schumacher, M.; Graff, S.; Thierry, A.; Wittmann, J.-C.; Lotz, B. Epitaxial Crystallization and AFM Investigation of a Frustrated Polymer Structure: Isotactic Poly(propylene), β Phase. Macromolecules 1998, 31, 807–814. [Google Scholar] [CrossRef]
- Lovinger, A.J.; Chua, J.O.; Gryte, C.C. Studies on the α and β forms of isotactic polypropylene by crystallization in a temperature gradient. J. Polym. Sci. Polym. Phys. Ed. 1977, 15, 641–656. [Google Scholar] [CrossRef]
- Padden Jr, F.J.; Keith, H.D. Spherulitic crystallization in polypropylene. J. Appl. Phys. 1959, 30, 1479–1484. [Google Scholar] [CrossRef]
- Luujsterburg, B.; Jobse, P.; Merino, D.H.; Peijs, T.; Goossens, H. Solid-State Drawing of b-Nucleated Polypropylene: Effect of Additives on Drawability and Mechanical Properties. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 1071–1082. [Google Scholar] [CrossRef]
- Luo, F.; Geng, C.; Wang, K.; Deng, H.; Chen, F.; Fu, Q.; Na, B. New Understanding in Tuning Toughness of β-Polypropylene: The Role of β-Nucleated Crystalline Morphology. Macromolecules 2009, 42, 9325–9331. [Google Scholar] [CrossRef]
- Tsioptsias, C.; Leontiadis, K.; Messaritakis, S.; Terzaki, A.; Xidas, P.; Mystikos, K.; Tzimpilis, E.; Tsivintzelis, I. Experimental Investigation of Polypropylene Composite Drawn Fibers with Talc, Wollastonite, Attapulgite and Single-Wall Carbon Nanotubes. Polymers 2022, 14, 260. [Google Scholar] [CrossRef]
- Leontiadis, K.; Tsioptsias, C.; Messaritakis, S.; Terzaki, A.; Xidas, P.; Mystikos, K.; Tzimpilis, E.; Tsivintzelis, I. Optimization of Thermal and Mechanical Properties of Polypropylene-Wollastonite Composite Drawn Fibers Based on Surface Response Analysis. Polymers 2022, 14, 924. [Google Scholar] [CrossRef] [PubMed]
- Larin, B.; Lyashenko, T.; Harel, H.; Marom, G. Flow induced orientated morphology and properties of nanocomposites of polypropylene/vapor grown carbon fibers. Compos. Sci. Technol. 2011, 71, 177–182. [Google Scholar] [CrossRef]
- Jin, L.; Bower, C.; Zhou, O. Alignment of carbon nanotubes in a polymer matrix by mechanical stretching. Appl. Phys. Lett. 1998, 73, 1197–1199. [Google Scholar] [CrossRef]
- Leontiadis, K.; Tsioptsias, C.; Messaritakis, S.; Terzaki, A.; Xidas, P.; Mystikos, K.; Tzimpilis, E.; Tsivintzelis, I. Surface Response Analysis for the Optimization of Mechanical and Thermal Properties of Polypropylene Composite Drawn Fibers with Talc and Carbon Nanotubes. Polymers 2022, 14, 1329. [Google Scholar] [CrossRef]
- Kaci, M.; Touati, N.; Bourmaud, A.; Grohens, Y. Use of Nanoindentation to Mechanically Characterized Polypropylene/Cloisite 15A Nanocomposites Films Exposed to Gamma-Irradiation. Macromol. Symp. 2021, 396, 2000219. [Google Scholar] [CrossRef]
- Farahani, A.; Parvareh, A.; Moraveji, M.K.; Soudbar, D. Study on thermal and mechanical behaviors of polypropylene grad 552R/Cloisite 15A nanocomposites suitable for yarn applications. Polym. Polym. Compos. 2021, 29, S325–S334. [Google Scholar]
- Chen, M.; Wan, C.; Shou, W.; Zhang, Y.; Zhang, Y.; Zhang, J. Effects of Interfacial Adhesion on Properties of Polypropylene/Wollastonite Composites. J. Appl. Polym. Sci. 2008, 107, 1718–1723. [Google Scholar] [CrossRef]
- Tambe, P.B.; Bhattacharyya, A.R.; Kamath, S.S.; Kulkarni, A.R.; Sreekumar, T.V.; Srivastav, A.; Bhasker Rao, K.U.; Liu, Y.; Kumar, S. Structure–Property Relationship Studies in Amine Functionalized Multiwall Carbon Nanotubes Filled Polypropylene Composite Fiber. Polym. Eng. Sci. 2011, 52, 1183–1194. [Google Scholar] [CrossRef]
- Joshi, M.; Viswanathan, V. High-Performance Filaments from Compatibilized Polypropylene/Clay Nanocomposites. J. Appl. Polym. Sci. 2006, 102, 2164–2174. [Google Scholar] [CrossRef]
- Lorenzi, D.; Sartori, G.; Ferrara, G.; Fambri, L. Spinnability of Nanofilled Polypropylene. Macromol. Symp. 2011, 301, 73–81. [Google Scholar] [CrossRef]
- Mohan, T.P.; Kanny, K. Preparation and characteristics of polypropylene-clay nanocomposite fibers. J. Polym. Eng. 2015, 35, 773–784. [Google Scholar] [CrossRef]
- Borsig, E.; Ujhelyiová, A.; Mlynarčíková, Z.; Kaemfer, D.; Mülhaupt, R.; Marcinčin, A.; Berek, D. DSC study of syndiotactic polypropylene/organoclay nanocomposite fibers: Crystallization and melting behavior. Int. J. Polym. Mater. Polym. Biomater. 2007, 56, 771–788. [Google Scholar] [CrossRef]
- Fambri, L.; Dabrowska, I.; Ceccato, R.; Pegoretti, A. Effects of fumed silica and draw ratio on nanocomposite polypropylene fibers. Polymers 2017, 9, 41. [Google Scholar] [CrossRef]
- Luyt, A.S.; Dramicanin, M.D.; Antic, Ž.; Djokovic, V. Morphology, mechanical and thermal properties of composites of polypropylene and nanostructured wollastonite filler. Polym. Test. 2009, 28, 348–356. [Google Scholar] [CrossRef]
- Trotignon, J.P.; Demdoum, L.; Verdu, J. Effect of mineral fillers in low concentration on the mechanical properties of polymeric materials. Part 1: Static and fatigue fracture of polypropylene, qualitative aspects. Composites 1992, 23, 313–318. [Google Scholar] [CrossRef]
- Hadal, R.; Dasari, A.; Rohrmann, J.; Misra, R.D.K. Effect of wollastonite and talc on the micromechanisms of tensile deformation in polypropylene composites. Mater. Sci. Eng. A 2004, 372, 296–315. [Google Scholar] [CrossRef]
- Meng, M.-R.; Dou, Q. Effect of pimelic acid on the crystallization, morphology and mechanical properties of polypropylene/wollastonite composites. Mater. Sci. Eng. A 2008, 492, 177–184. [Google Scholar] [CrossRef]
- Dasari, A.; Misra, R. The role of micrometric wollastonite particles on stress whitening behavior of polypropylene composites. Acta Mater. 2004, 52, 1683–1697. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, Z.; Dai, X.; Li, M.; Mai, K. Crystalline Morphology and Mechanical Properties of Isotactic Polypropylene Composites Filled by Wollastonite With β-Nucleating Surface. Polym. Compos. 2014, 35, 1445–1452. [Google Scholar] [CrossRef]
- Li, L.; Dou, Q. Effects of Malonic Acid Treatment on Crystal Structure, Melting Behavior, Morphology, and Mechanical Properties of Isotactic Poly(propylene)/Wollastonite Composites. Polym. Compos. 2010, 31, 966–973. [Google Scholar] [CrossRef]
- Liang, J.-Z. Impact and Flexural Properties of PP/CaSiO3 Composites. Polym. Compos. 2018, 39, 398–404. [Google Scholar] [CrossRef]
- Liang, J.; Li, B.; Ruan, J. Crystallization properties and thermal stability of polypropylene composites filled with wollastonite. Polym. Test. 2015, 42, 185–191. [Google Scholar] [CrossRef]
- Virta, R.L.; Revette, D. Wollastonite. Mining Eng. 2006, 58, 61–62. [Google Scholar]
- Tsioptsias, C.; Leontiadis, K.; Tzimpilis, E.; Tsivintzelis, I. Polypropylene nanocomposite fibers: A review of current trends and new developments. J. Plast. Film Sheeting 2021, 37, 283–311. [Google Scholar] [CrossRef]
- Salas-Papayanopolos, H.; Morales, A.; Lozano, T.; Laria, J.; Sanchez, S.; Rodriguez, F.; Martinez, G.; Cerino, F. Improvement of Toughness Properties of Polypropylene/Wollastonite Composites Using an Interface Modifier. Polym. Compos. 2014, 35, 1184–1192. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, Z.; Wang, C.; Jiang, J.; Dai, X.; Mai, K. Preparation and characterization of wollastonite with a β-nucleating surface and its filled isotactic polypropylene composites. J. Mater. Sci. 2013, 48, 5225–5235. [Google Scholar] [CrossRef]
- Dou, Q. Effect of Calcium Salts Aliphatic Dicarboxylic Acids on the Formation of β crystalline Form in Isotactic Poly (propylene). Adv. Mater. Res. 2012, 391–392, 875–882. [Google Scholar] [CrossRef]
- Rao, K.H.; Forssberg, K.S.E.; Forsling, W. Interfacial interactions and mechanical properties of mineral filled polymer composites: Wollastonite in PMMA polymer matrix. Colloids Surf. A Physicochem. Eng. Asp. 1998, 133, 107–117. [Google Scholar]
- Chatterjee, A.; Khobragade, P.S.; Mishra, S. Physicomechanical properties of wollastonite (CaSiO3)/styrene butadiene rubber (SBR) nanocomposites. J. Appl. Polym. Sci. 2015, 132, 42811. [Google Scholar] [CrossRef]
- Pugh, R.J.; Boutonnet-Kizling, M.; Palm, C.-O. Grinding of wollastonite under gaseous environments: Influence on Acidic/Basic Surface Sites. Miner. Eng. 1995, 8, 1239–1246. [Google Scholar] [CrossRef]
- Li, Z.; Shen, S.Y.; Peng, J.R.; Yang, C.R. Mechanochemical Modification of Wollastonite and Its Application to Polypropylene. Key Eng. Mater. 2003, 249, 409–412. [Google Scholar] [CrossRef]
- Acierno, S.; Barretta, R.; Luciano, R.; Marotti de Sciarra, F.; Russo, P. Experimental evaluations and modeling of the tensile behavior of polypropylene/single-walled carbon nanotubes fibers. Compos. Struct. 2017, 174, 12–18. [Google Scholar] [CrossRef]
- Coppola, B.; Di Maio, L.; Incarnato, L.; Tulliani, J.-M. Preparation and Characterization of Polypropylene/Carbon Nanotubes (PP/CNTs) Nanocomposites as Potential Strain Gauges for Structural Health Monitoring. Nanomaterials 2020, 10, 814. [Google Scholar] [CrossRef] [PubMed]
- Ran, S.; Zong, X.; Fang, D.; Hsiao, B.S.; Chu, B. Structural and Morphological Studies of Isotactic Polypropylene Fibers during Heat/Draw Deformation by in-Sity Synchrotron SAXS/WAXD. Macromolecules 2001, 34, 2569–2578. [Google Scholar] [CrossRef]
- Lefevre, G.; Preocanin, T.; Lutzenkirchen, J. Attenuated Total Reflection—Infrared Spectroscopy Applied to the Study of Mineral—Aqueous Electrolyte Solution Interfaces: A general Overview and Case Study. In Infrared Spectroscopy—Materials Science, Engineering and Technology; Theophanides, T., Ed.; IntechOpen: London, UK, 2012; pp. 97–122. [Google Scholar]
- Chiral Chromatography: Separating Twins. Available online: https://blogs.ntu.edu.sg/cy1101-1819s1-g09/2018/10/440/ (accessed on 5 May 2022).
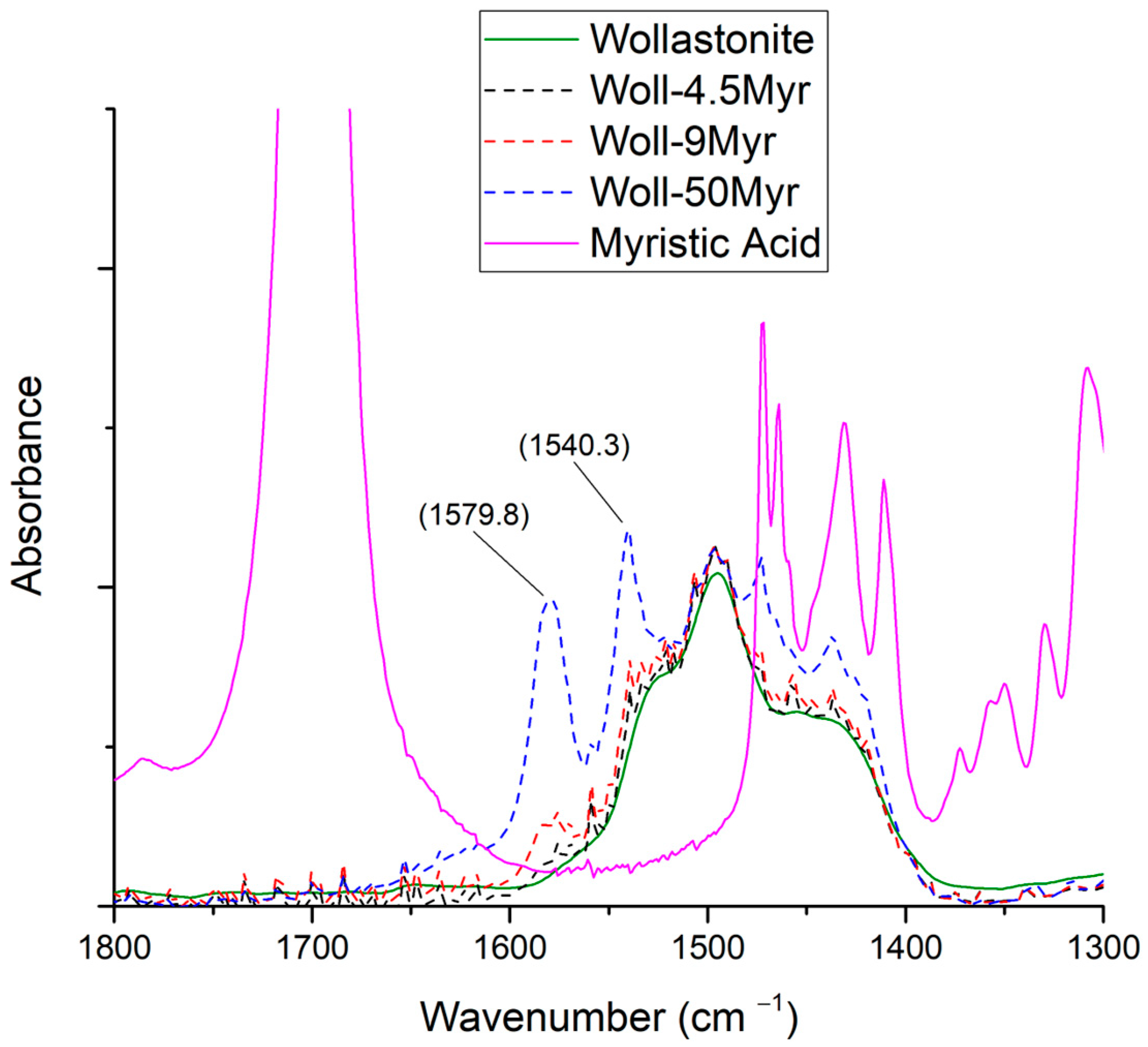
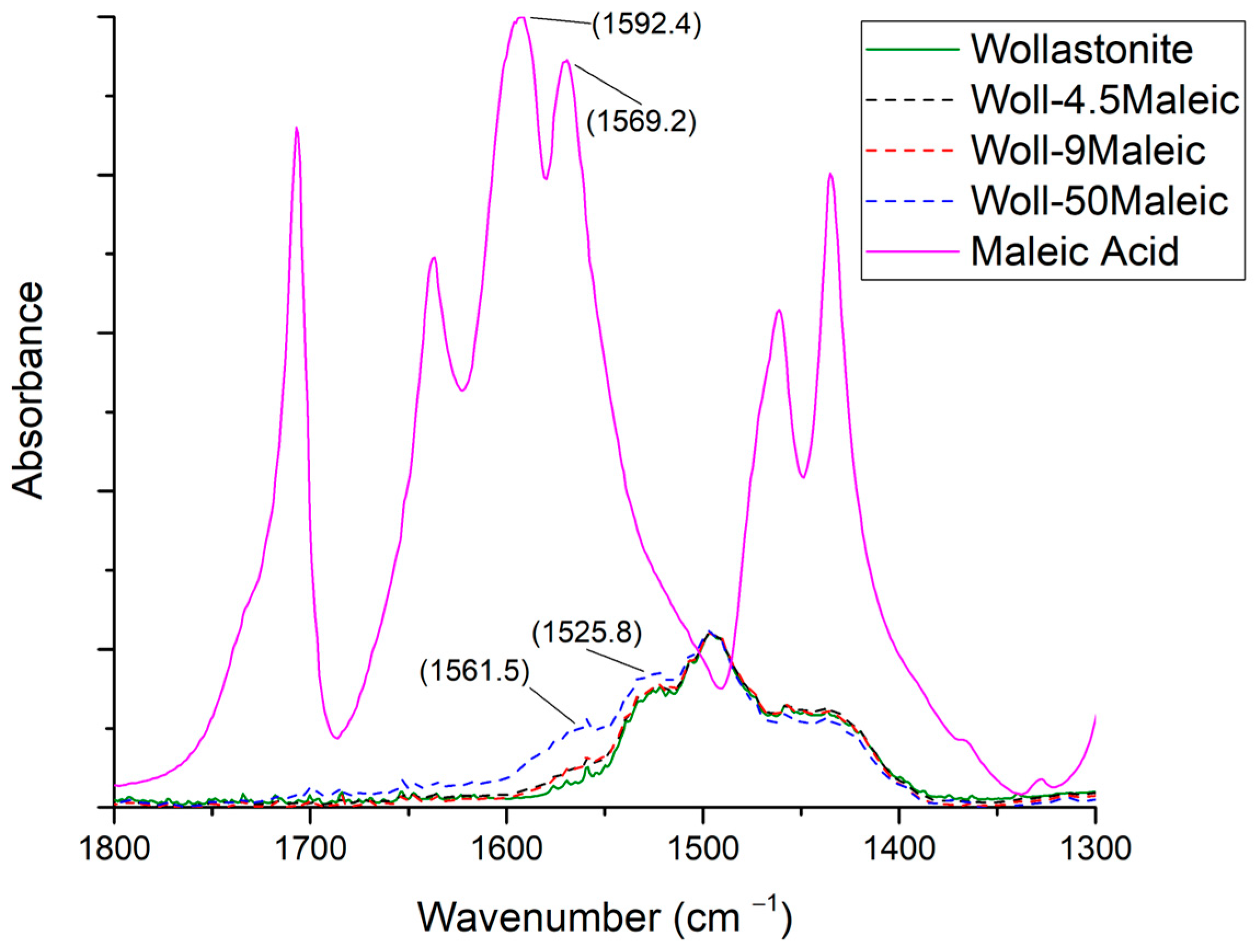
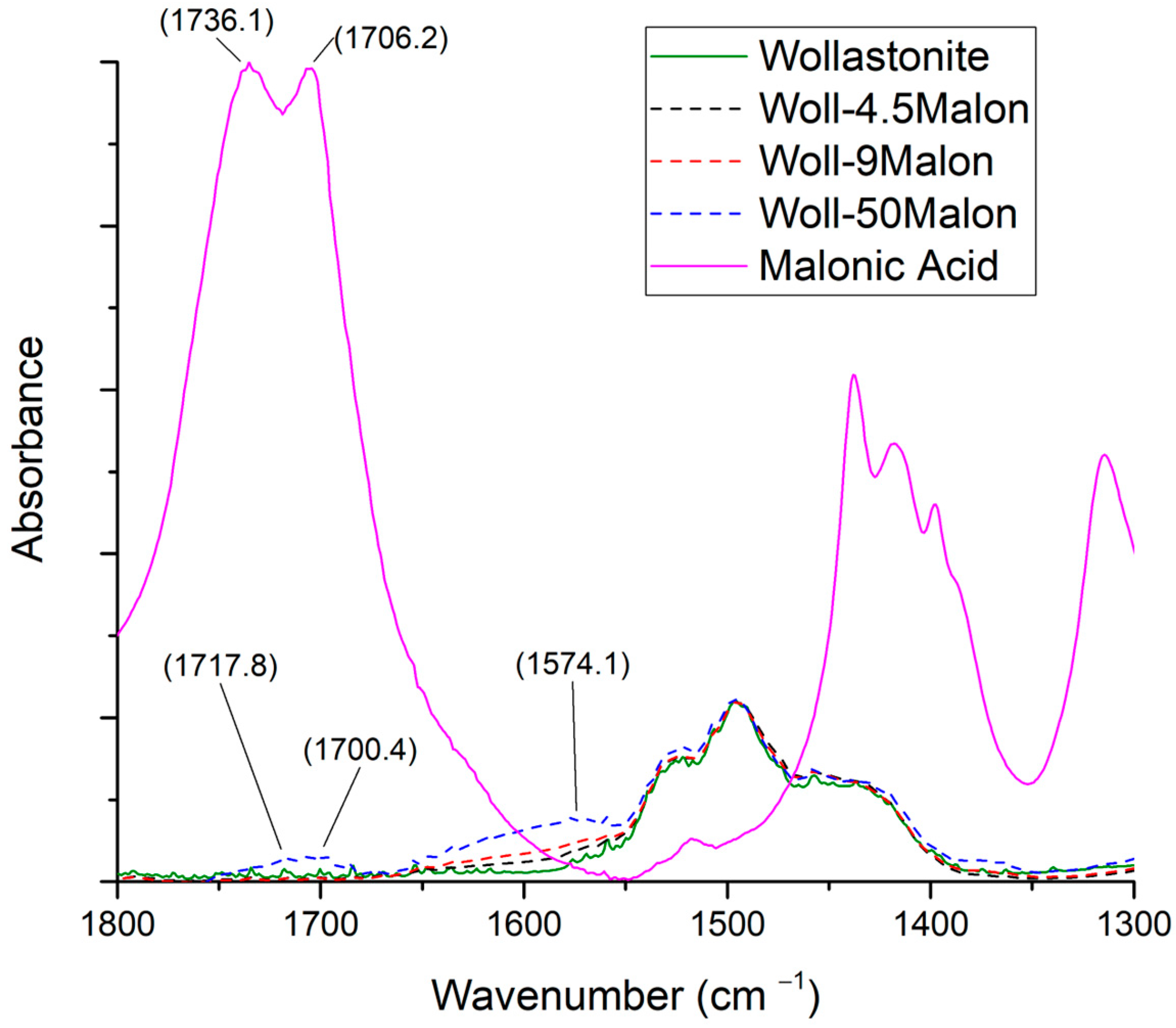
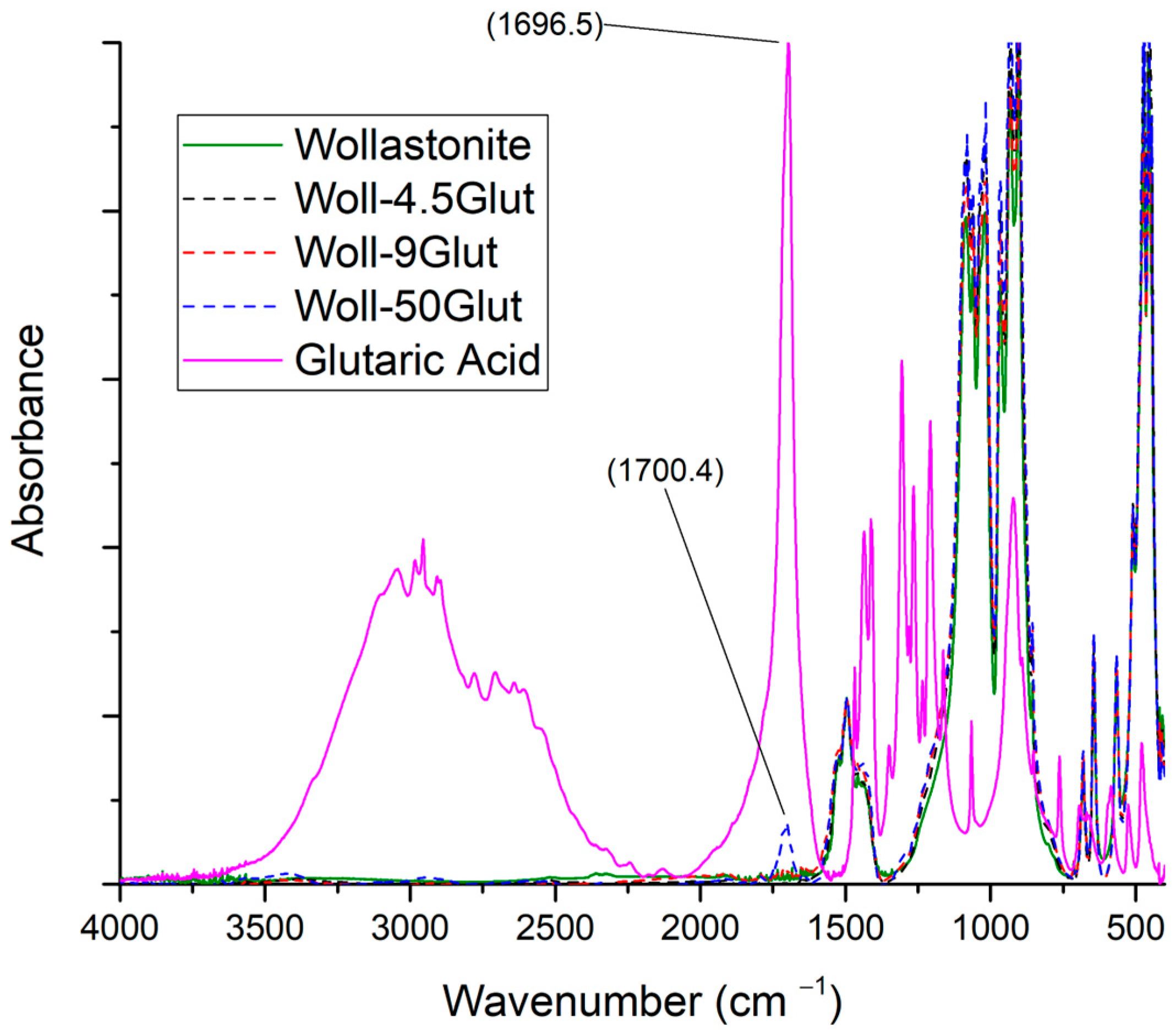
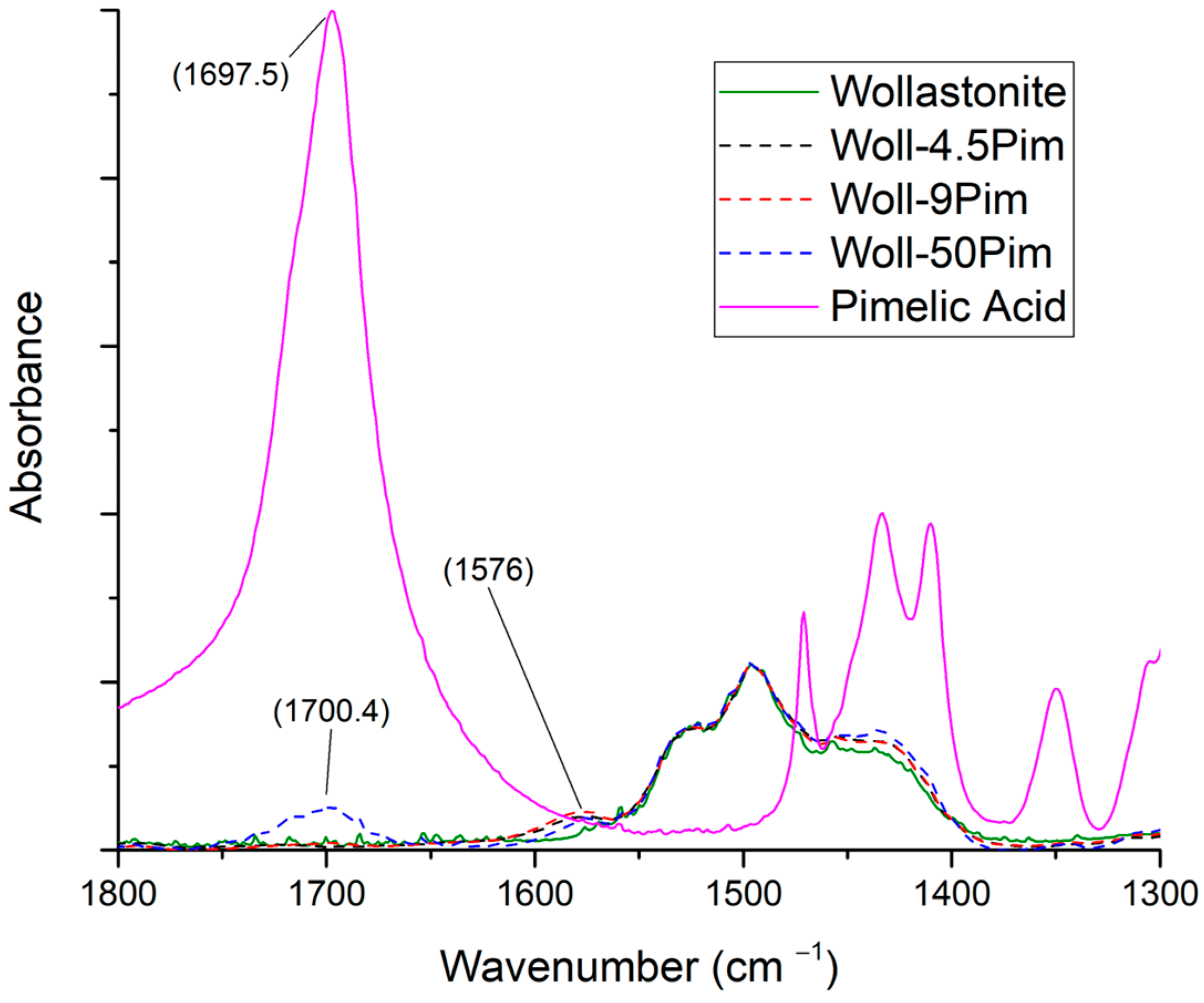
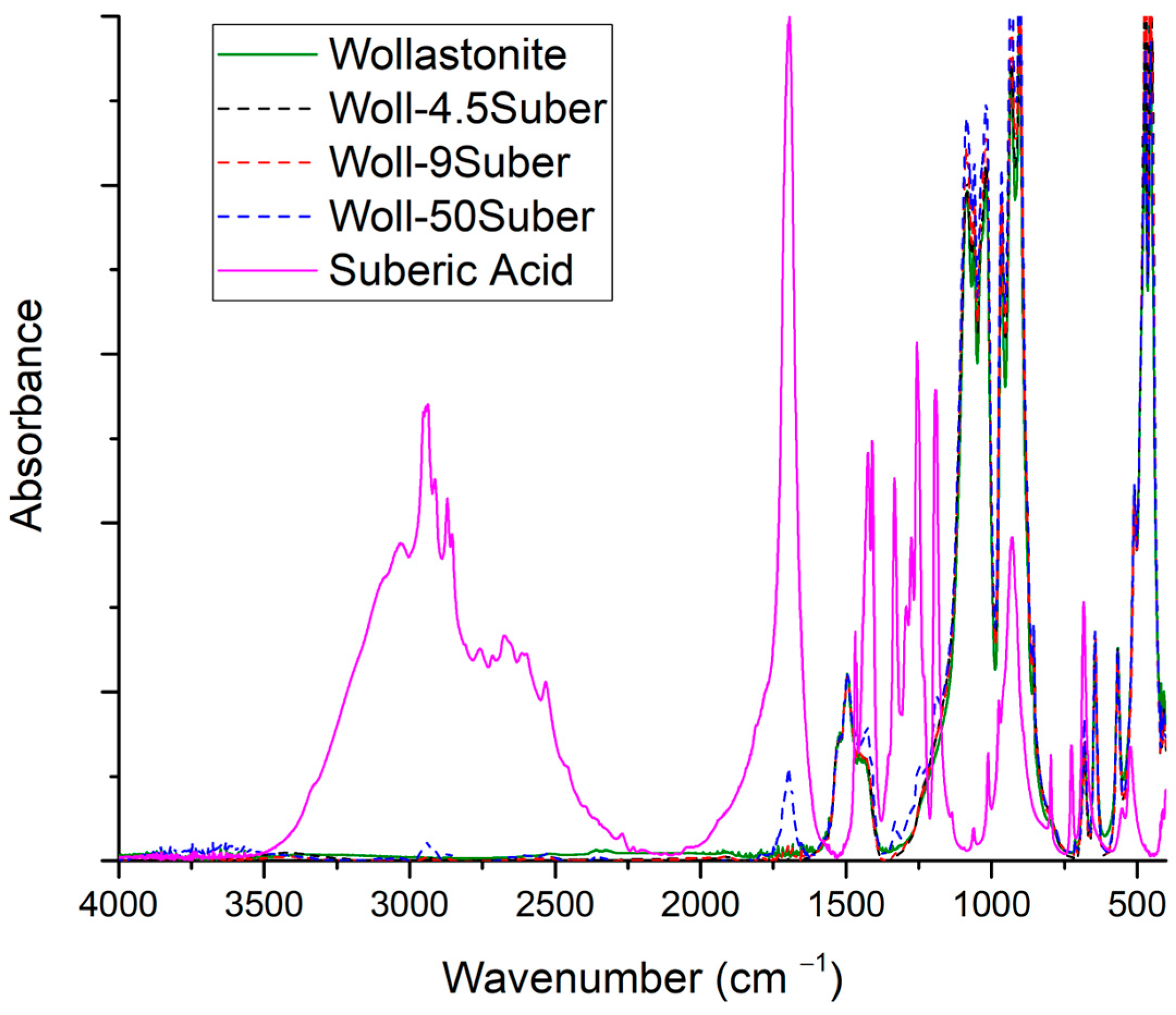
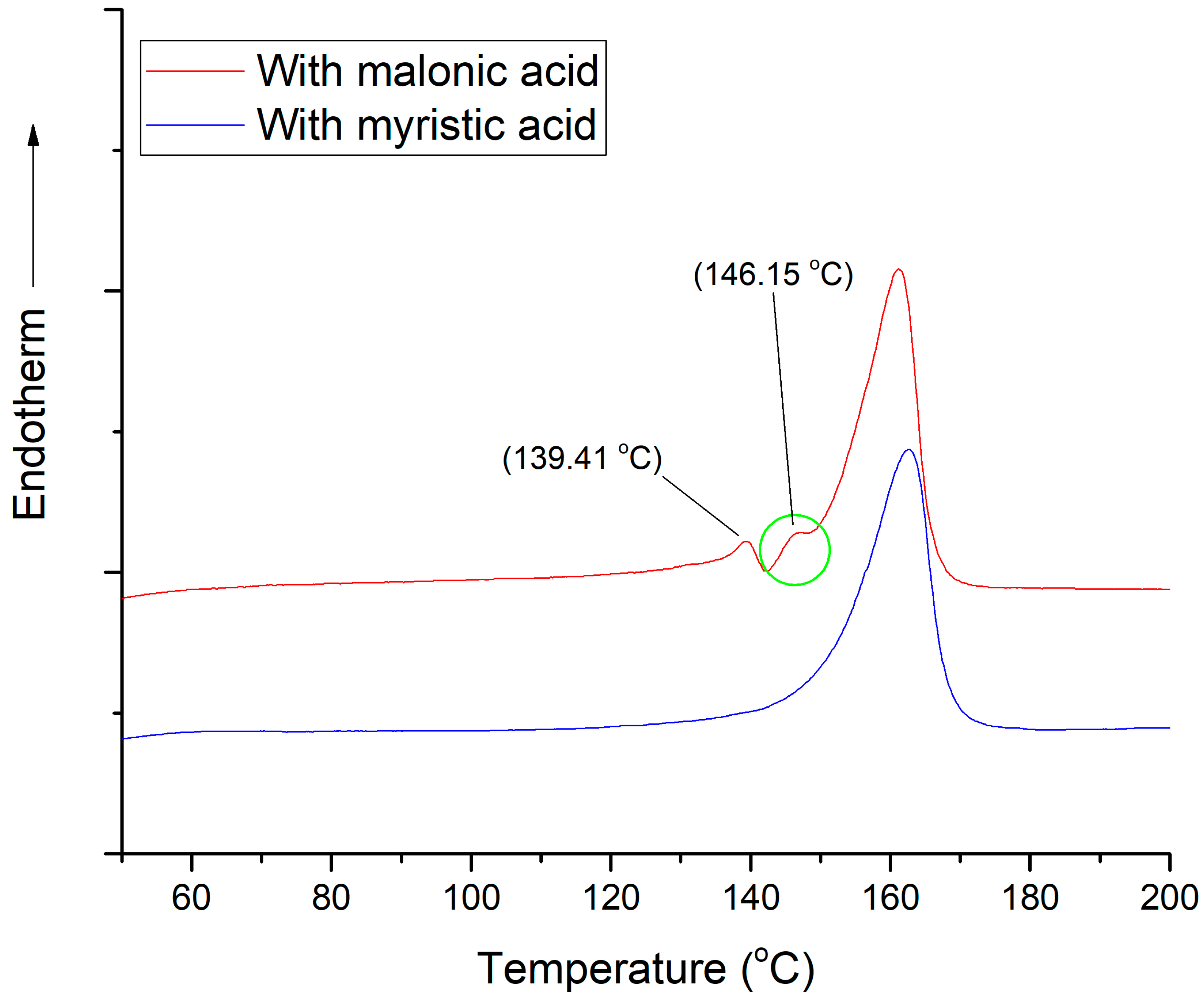
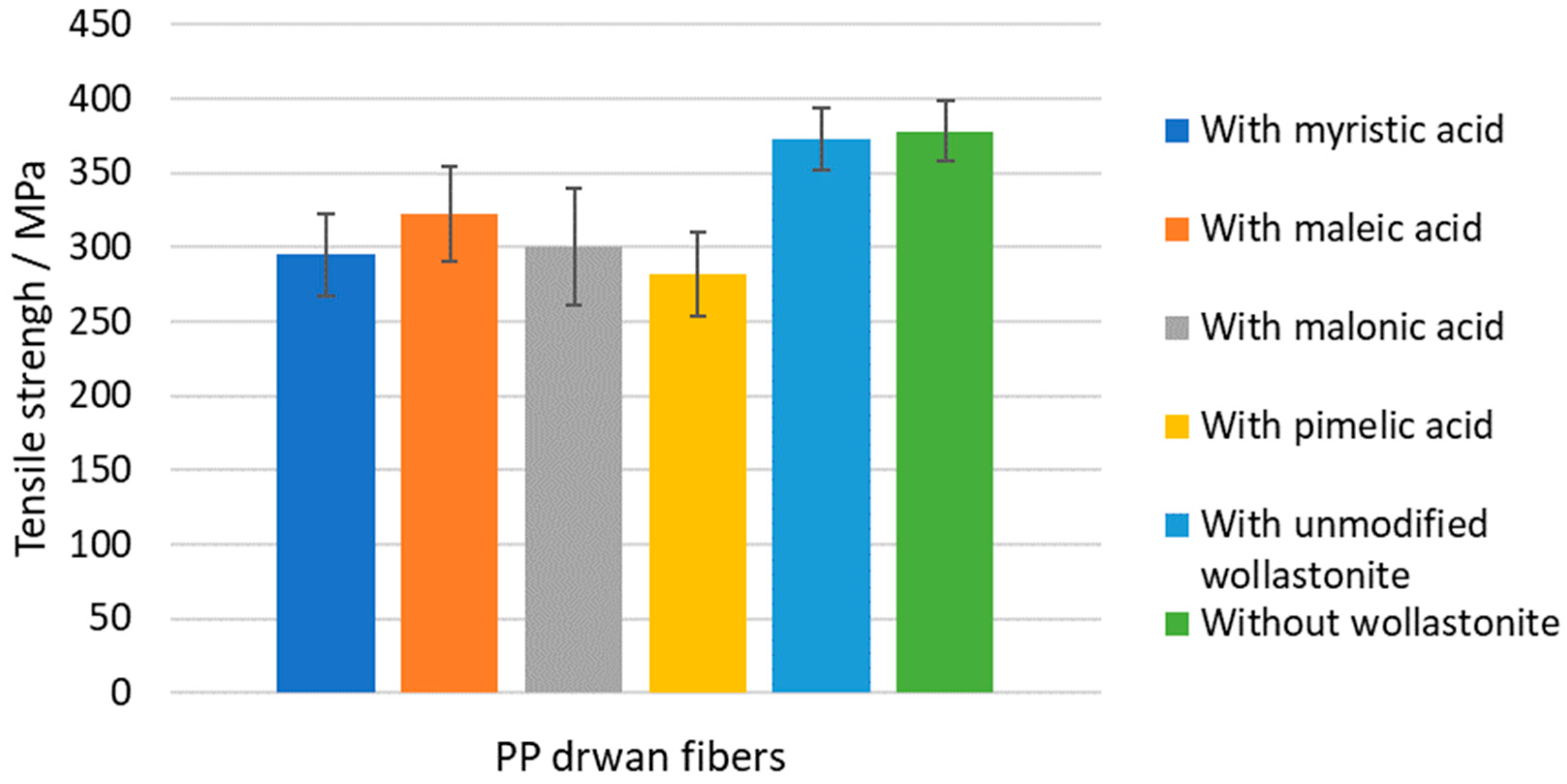

| Sample Name | Myristic Acid/Wollastonite (g/g) | Wollastonite Content (%) | Compatibilizer Content (%) |
|---|---|---|---|
| 0_0 | 0 | 0 ± 0 | 4.00 ± 0.07 |
| 0_2 | 0 | 2.00 ± 0.07 | 4.00 ± 0.08 |
| 1_2 | 0.998 | 2.00 ± 0.07 | 4.00 ± 0.08 |
| 2_2 | 1.974 | 2.00 ± 0.07 | 4.00 ± 0.08 |
| 0_0_NoBR (pure PP) | 0 | 0 | 0 |
| 0_2_NoBR | 0 | 2.00 ± 0.07 | 0 |
| 1_2_NoBR | 0.998 | 2.00 ± 0.07 | 0 |
| 2_2_NoBR | 1.974 | 2.00 ± 0.07 | 0 |
| Sample Name | Young’s Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) | % Crystallinity | Onset Decomposition Temperature (°C) | |
|---|---|---|---|---|---|---|
| Before Drawing | After Drawing | |||||
| 0_0 | 2888 ± 278 | 378 ± 20 | 53 ± 10 | 38 | 50 | 275 |
| 0_2 | 3140 ± 470 | 373 ± 20 | 50 ± 5 | 42 | 52 | 280 |
| 1_2 | 2584 ± 277 | 339 ± 23 | 74 ± 5 | 40 | 49 | 288 |
| 2_2 | 2646 ± 266 | 333 ± 9 | 61 ± 7 | 42 | 48 | 294 |
| 0_0_NoBR | 2606 ± 271 | 353 ± 19 | 73 ± 15 | 38 | 47 | 277 |
| 0_2_NoBR | 2481 ± 166 | 368 ± 19 | 60 ± 6 | 41 | 47 | 281 |
| 1_2_NoBR | 2304 ± 153 | 343 ± 18 | 68 ± 4 | 40 | 49 | 287 |
| 2_2_NoBR | 2459 ± 169 | 317 ± 15 | 76 ± 8 | 40 | 52 | 280 |
| Sample | Solvent | Myristic Acid/Wollastonite (g/g) | Myristic Acid/Wollastonite (×10−5 mol/g) |
|---|---|---|---|
| 1 | Ethanol | 0 | 0 |
| 2 | Ethanol | 0.998 | 4.370 |
| 3 | Ethanol | 1.974 | 8.643 |
| 4 | Carbon Tetrachloride | 0 | 0 |
| 5 | Carbon Tetrachloride | 1.015 | 4.445 |
| 6 | Carbon Tetrachloride | 2.028 | 8.882 |
| Peak Ratios | Sample 3 (Ethanol)/ gwol/gacid | Sample 6 (Carbon Tetrachloride)/ gwol/gacid |
|---|---|---|
| (COO−, 1580 cm−1)/(Si–O–Si bending, 560 cm−1) | 0.095 | 0.112 |
| (COO−, 1580 cm−1)/(Si–O–Si bending, 970 cm−1) | 0.027 | 0.030 |
| (COO−, 1580 cm−1)/(Si–O vibration, 1010 cm−1) | 0.013 | 0.015 |
| Sample Name | Wollastonite Sample (See Table 3) | Wollastonite Content (%) | Compatibilizer Content (%) |
|---|---|---|---|
| 0_Eth | 1 | 2.00 ± 0.07 | 4.00 ± 0.08 |
| 1_Eth | 2 | 2.00 ± 0.07 | 4.00 ± 0.08 |
| 2_Eth | 3 | 2.00 ± 0.07 | 4.00 ± 0.08 |
| 0_CCl4 | 4 | 2.01 ± 0.07 | 4.00 ± 0.08 |
| 1_CCl4 | 5 | 2.00 ± 0.07 | 4.00 ± 0.08 |
| 2_CCl4 | 6 | 2.00 ± 0.07 | 4.01 ± 0.08 |
| Sample Name | Young’s Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) | % Crystallinity | Onset Decomposition Temperature (°C) | |
|---|---|---|---|---|---|---|
| Before Drawing | After Drawing | |||||
| 0_Eth | 3140 ± 470 | 373 ± 20 | 50 ± 5 | 42 | 52 | 280 |
| 1_Eth | 2584 ± 277 | 339 ± 23 | 74 ± 5 | 40 | 49 | 288 |
| 2_Eth | 2646 ± 266 | 333 ± 9 | 61 ± 7 | 42 | 48 | 294 |
| 0_CCl4 | 3415 ± 349 | 342 ± 19 | 56 ± 6 | 39 | 50 | 282 |
| 1_CCl4 | 3576 ± 396 | 370 ± 19 | 57 ± 7 | 38 | 45 | 280 |
| 2_CCl4 | 3038 ± 391 | 330 ± 13 | 56 ± 3 | 39 | 48 | 285 |
| Organic Acid | Organic Acid/Wollastonite (×10−5 mol/g) | Sample Name |
|---|---|---|
| Myristic acid | 4.445 ± 0.005 | Woll-4.5 Myr |
| 8.882 ± 0.005 | Woll-9 Myr | |
| 43.838 ± 0.007 | Woll-50 Myr | |
| Maleic acid | 4.408 ± 0.009 | Woll-4.5 Maleic |
| 8.858 ± 0.009 | Woll-9 Maleic | |
| 42.889 ± 0.007 | Woll-50 Maleic | |
| Malonic acid | 4.507 ± 0.009 | Woll-4.5 Malon |
| 8.977 ± 0.010 | Woll-9 Malon | |
| 50.003 ± 0.015 | Woll-50 Malon | |
| Glutaric acid | 4.494 ± 0.008 | Woll-4.5 Glut |
| 9.015 ± 0.008 | Woll-9 Glut | |
| 50.145 ± 0.012 | Woll-50 Glut | |
| Pimelic acid | 4.501 ± 0.007 | Woll-4.5 Pim |
| 8.984 ± 0.007 | Woll-9 Pim | |
| 50.004 ± 0.010 | Woll-50 Pim | |
| Suberic acid | 4.488 ± 0.006 | Woll-4.5 Suber |
| 8.974 ± 0.006 | Woll-9 Suber | |
| 50.058 ± 0.007 | Woll-50 Suber |
| PP Sample | Crystallinity (%) | ||
|---|---|---|---|
| After First Extrusion | After Second Extrusion | After Drawing | |
| without wollastonite | Not measured | 38 | 50 |
| with unmodified wollastonite | Not measured | 42 | 52 |
| with wollastonite modified with myristic acid (Woll-50 Myr) | 42 | 40 | 48 |
| with wollastonite modified with maleic acid (Woll-50 Maleic) | 40 | 38 | 47 |
| with wollastonite modified with malonic acid (Woll-50 Malon) | 40 | 37 (+2.7% β-crystals) | 44 |
| with wollastonite modified with pimelic acid (Woll-50 Pim) | 38 | 38 | 47 |
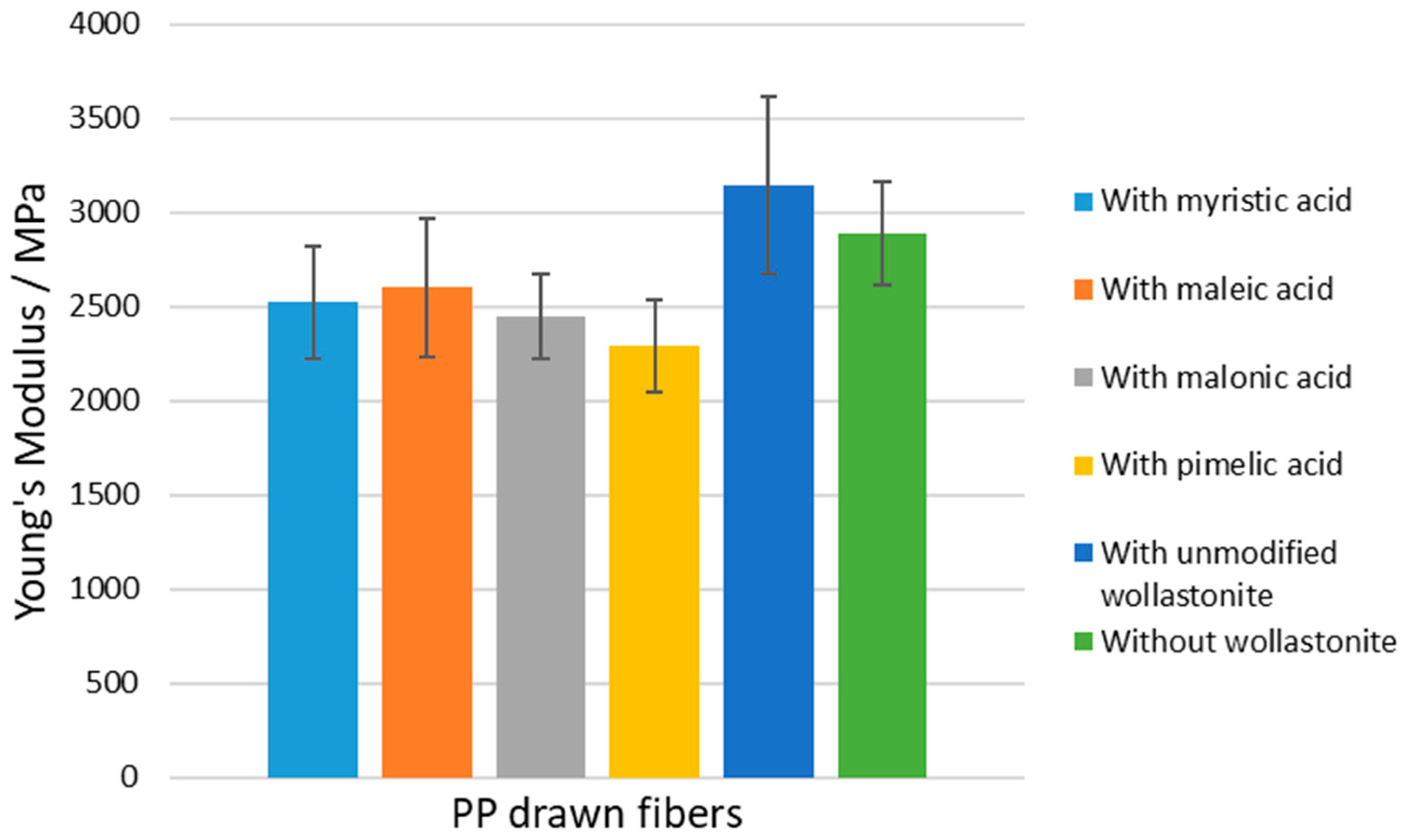
| PP Sample | Onset Decomposition Temperature (°C) | Young’s Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|
| without wollastonite | 275 | 2888 ± 278 | 378 ± 20 | 53 ± 10 |
| with unmodified wollastonite | 280 | 3140 ± 470 | 373 ± 20 | 50 ± 5 |
| with Woll-50 Myr | 287 | 2522 ± 298 | 295 ± 27 | 64 ± 9 |
| with Woll-50 Maleic | 280 | 2600 ± 367 | 322 ± 32 | 64 ± 8 |
| with Woll-50 Malon | 290 | 2447 ± 226 | 300 ± 39 | 59 ± 7 |
| with Woll-50 Pim | 283 | 2291 ± 247 | 282 ± 28 | 72 ± 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leontiadis, K.; Achilias, D.S.; Tsivintzelis, I. Effect of the Filler Modification on the Thermal and Mechanical Properties of Composite Polypropylene/Wollastonite Drawn Fibers. Polymers 2023, 15, 2986. https://doi.org/10.3390/polym15142986
Leontiadis K, Achilias DS, Tsivintzelis I. Effect of the Filler Modification on the Thermal and Mechanical Properties of Composite Polypropylene/Wollastonite Drawn Fibers. Polymers. 2023; 15(14):2986. https://doi.org/10.3390/polym15142986
Chicago/Turabian StyleLeontiadis, Konstantinos, Dimitris S. Achilias, and Ioannis Tsivintzelis. 2023. "Effect of the Filler Modification on the Thermal and Mechanical Properties of Composite Polypropylene/Wollastonite Drawn Fibers" Polymers 15, no. 14: 2986. https://doi.org/10.3390/polym15142986
APA StyleLeontiadis, K., Achilias, D. S., & Tsivintzelis, I. (2023). Effect of the Filler Modification on the Thermal and Mechanical Properties of Composite Polypropylene/Wollastonite Drawn Fibers. Polymers, 15(14), 2986. https://doi.org/10.3390/polym15142986









