Different Roles between PEDOT:PSS as Counter Electrode and PEDOT:Carrageenan as Electrolyte in Dye-Sensitized Solar Cell Applications: A Systematic Literature Review
Abstract
1. Introduction
2. Methods
3. Synthesis and Characteristics of PEDOT:PSS and PEDOT:Carrageenan
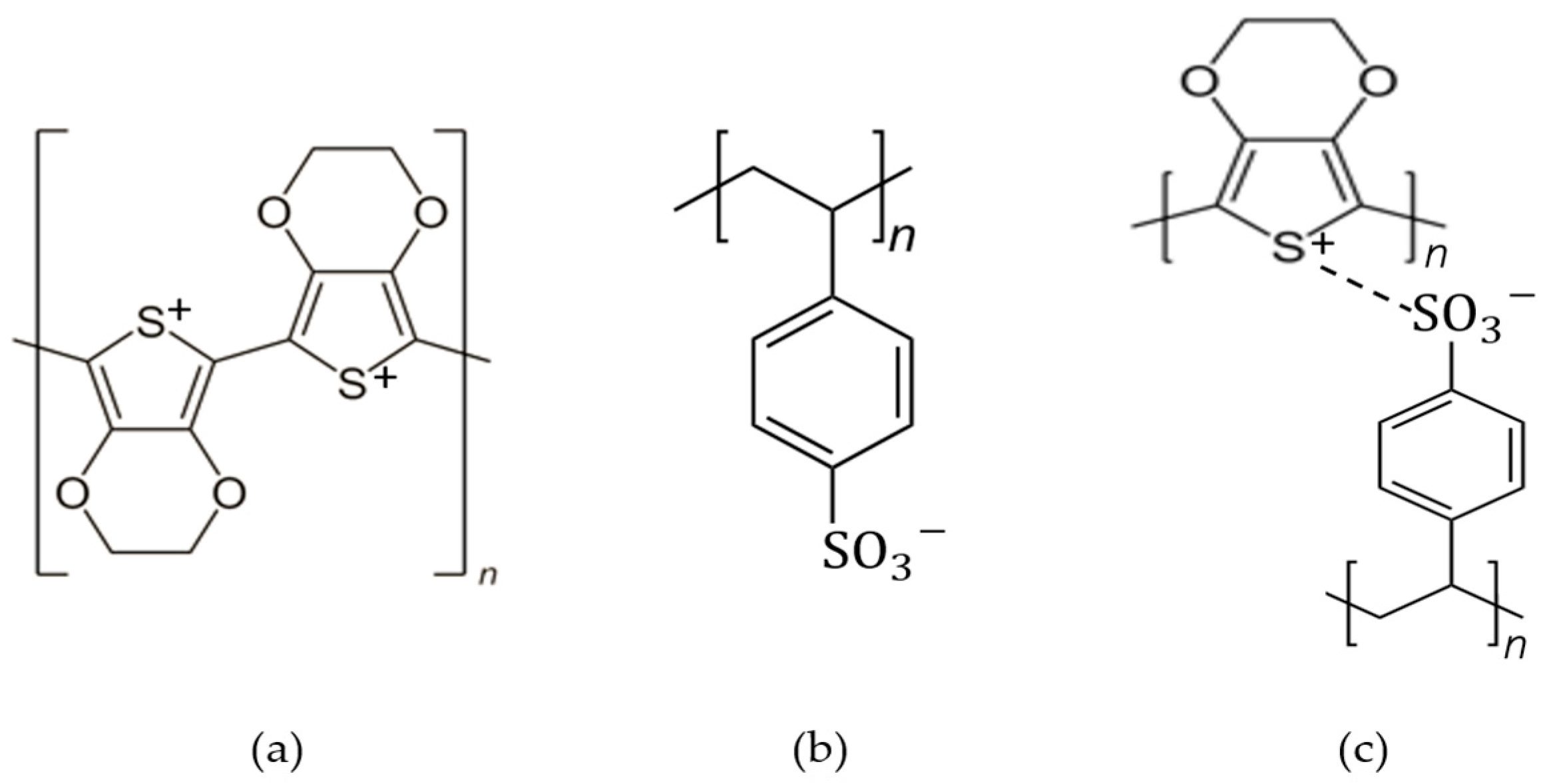
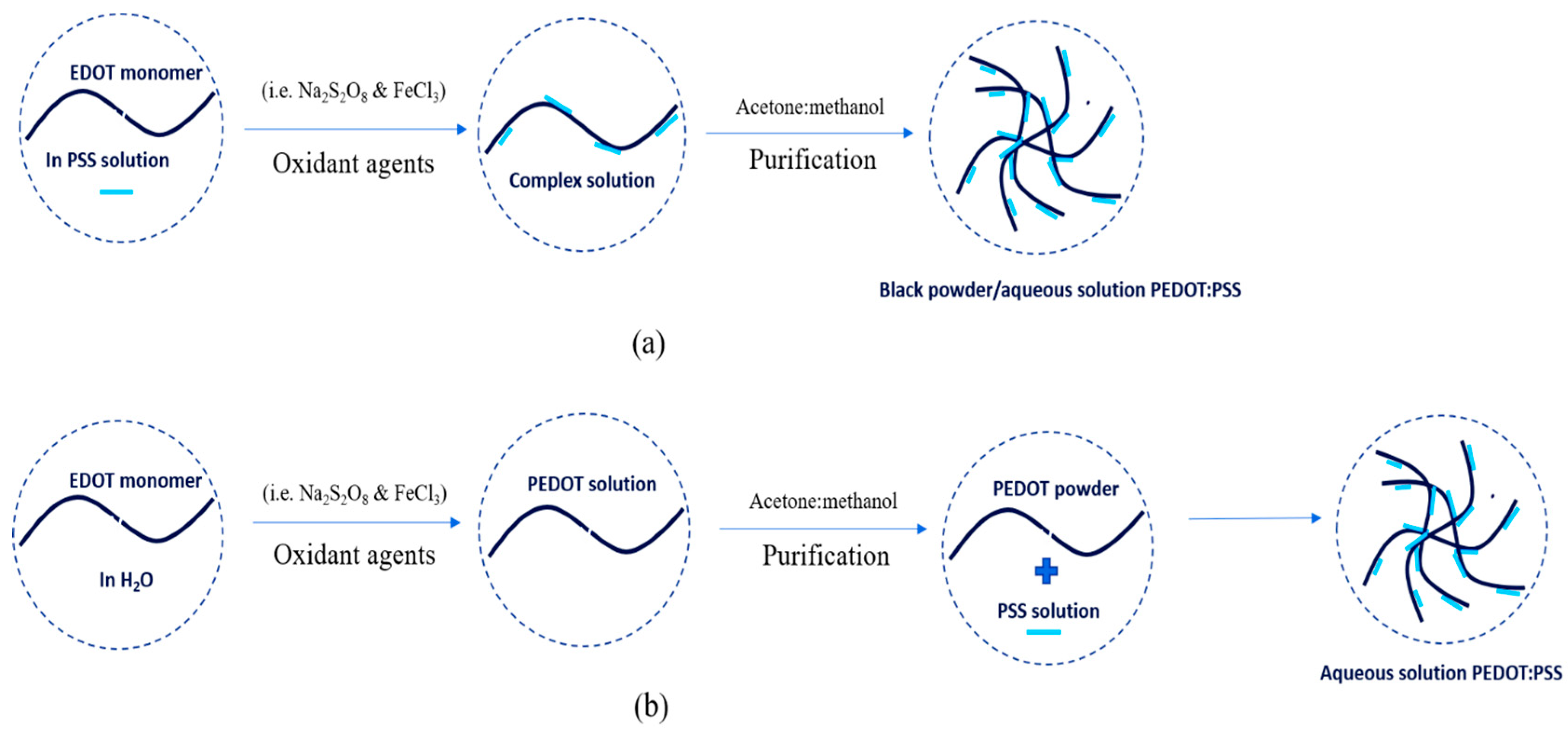
3.1. Synthesis and Characteristics of PEDOT:PSS
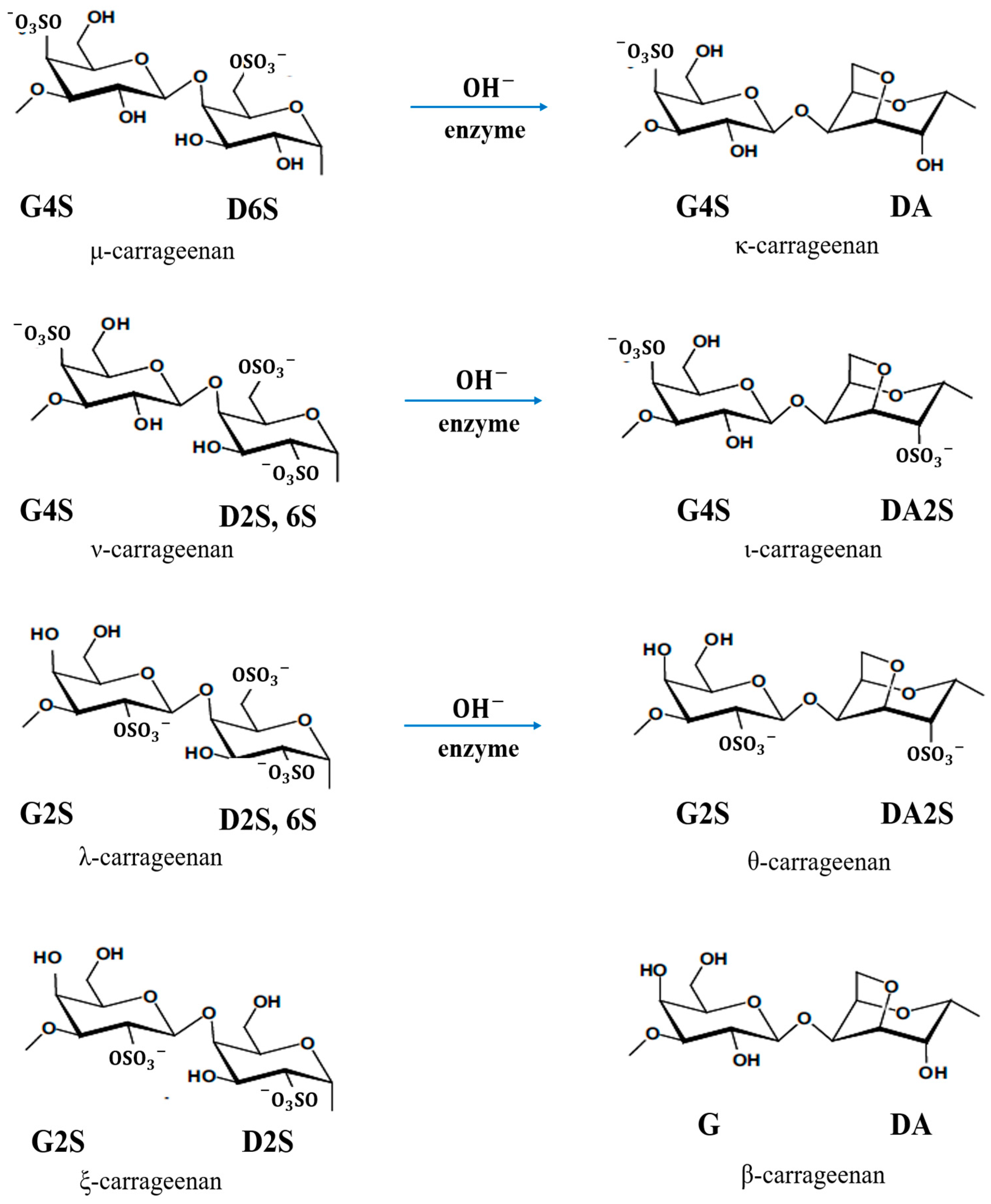
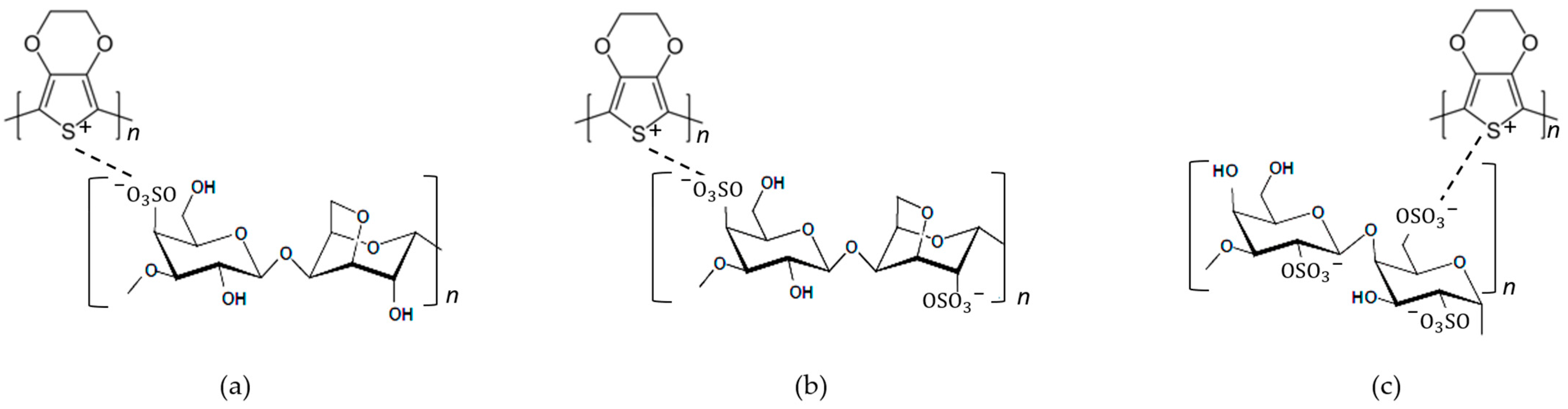
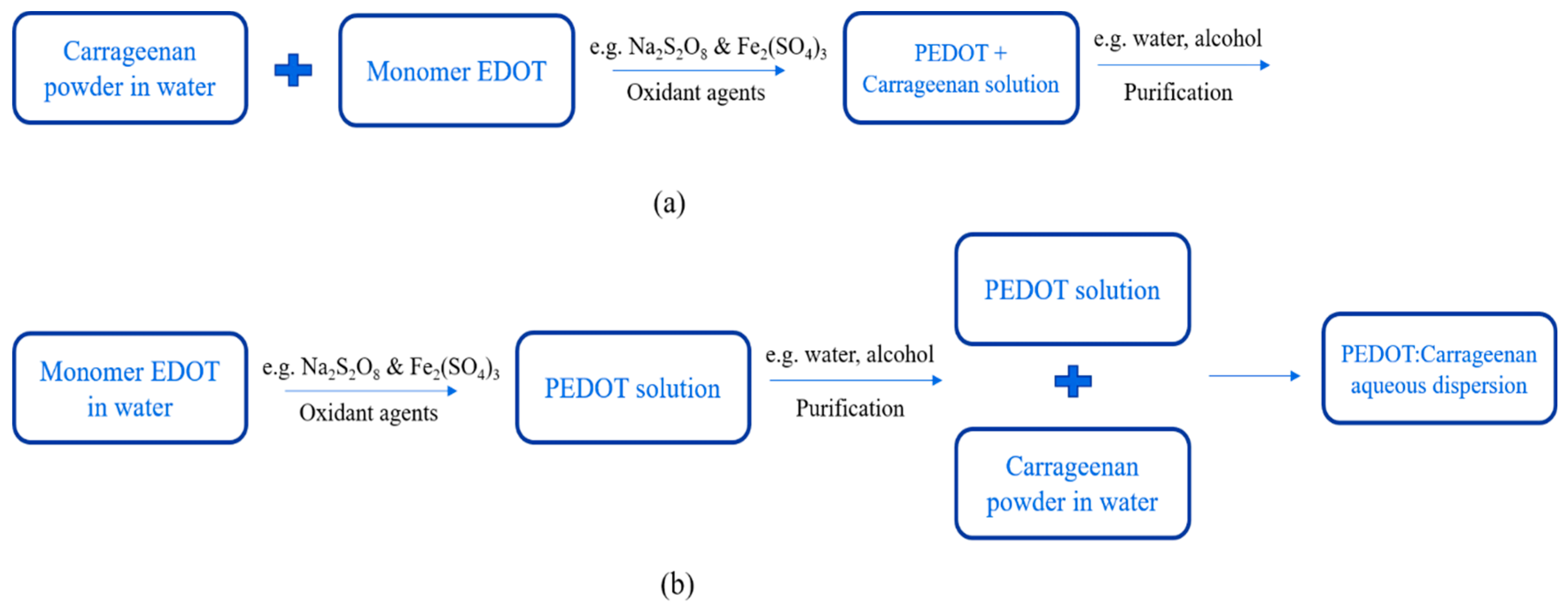
3.2. Synthesis and Characteristics of PEDOT:Carrageenan
4. Results and Discussions
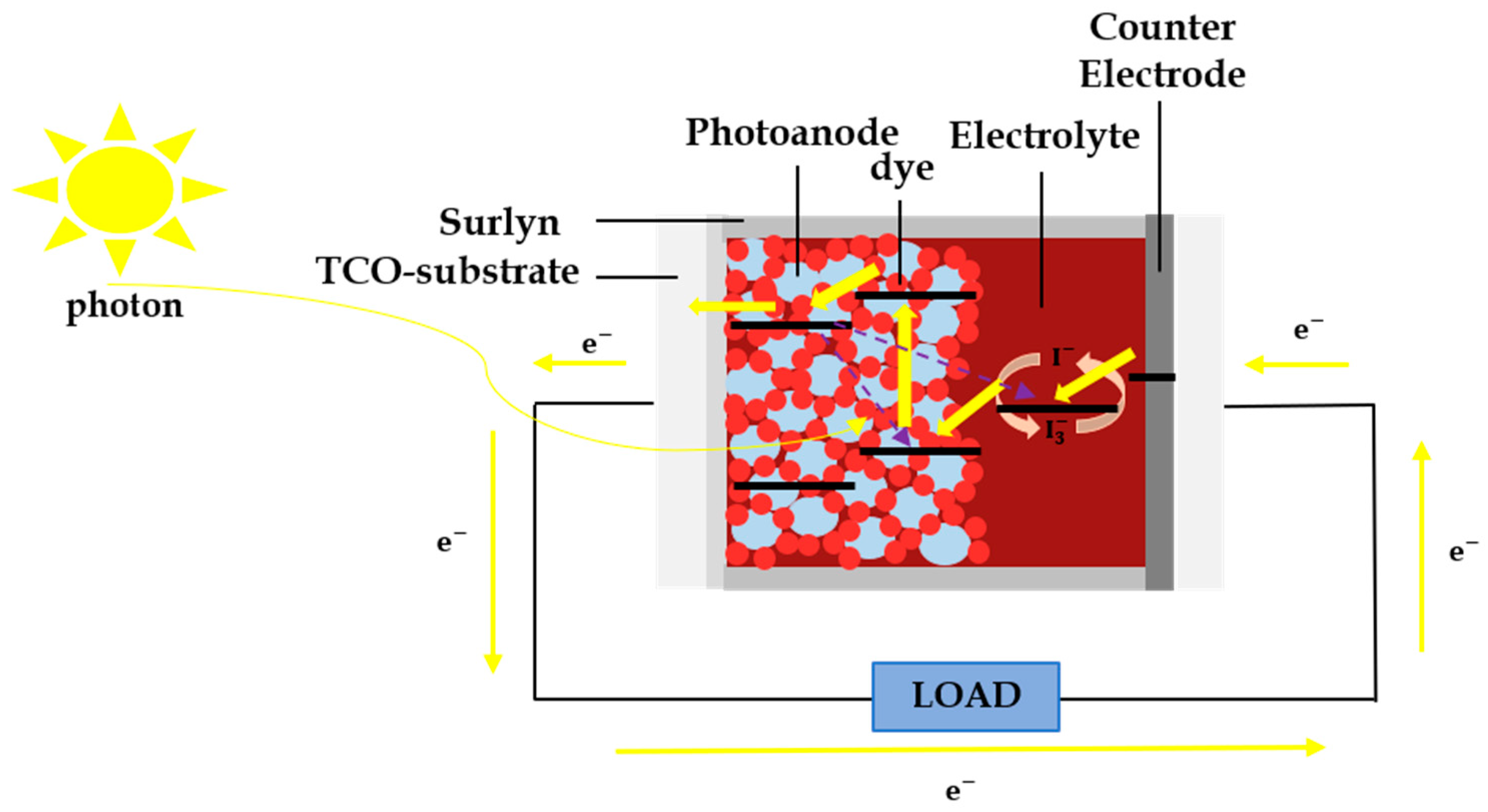
4.1. Conductive Polymer for DSSC
4.2. PEDOT:PSS as Counter Electrode
4.3. PEDOT:Carrageenan as Electrolyte
5. Author’s Perspective
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balraju, P.; Kumar, M.; Roy, M.S.; Sharma, G.D. Dye sensitized solar cells (DSSCs) based on modified iron phthalocyanine nanostructured TiO2 electrode and PEDOT:PSS counter electrode. Synth. Met. 2009, 159, 1325–1331. [Google Scholar] [CrossRef]
- Chiang, C.H.; Wu, C.G. High-efficient dye-sensitized solar cell based on highly conducting and thermally stable PEDOT:PSS/glass counter electrode. Org. Electron. 2013, 14, 1769–1776. [Google Scholar] [CrossRef]
- Yue, G.; Wu, J.; Xiao, Y.; Lin, J.; Huang, M.; Fan, L.; Yao, Y. A dye-sensitized solar cell based on PEDOT:PSS counter electrode. Chin. Sci. Bull. 2013, 58, 559–566. [Google Scholar] [CrossRef]
- Anil, A.; Sambhudevan, S.; Sreekala, C.O.; Shankar, B. Effect of silver nanoparticle in the PEDOT:PSS counter electrode of dye sensitized solar cell. AIP Conf. Procedings 2019, 2162, 020123. [Google Scholar]
- Pujiarti, H.; Arsyad, W.S.; Shobih; Muliani, L.; Hidayat, R. Efficient and Stable Photovoltaic Characteristics of Quasi-Solid State DSSC using Polymer Gel Electrolyte Based on Ionic Liquid in Organosiloxane Polymer Gels. IOP Conf. Ser. J. Phys. Conf. Ser. 2018, 1011, 012020. [Google Scholar] [CrossRef]
- Ito, B.I.; de Freitas, J.N.; Paoli, M.A.D.; Nogueira, A.F. Application of a Composite Polymer Electrolyte Based on Montmorillonite in Dye-Sensitized Solar Cells. J. Braz. Chem. Soc. 2008, 19, 688–696. [Google Scholar] [CrossRef]
- Sarwar, S.; Lee, M.; Park, S.; Dao, T.T.; Ullah, A.; Hong, S.; Han, C.H. Transformation of a liquid electrolyte to a gel inside dye sensitized solar cells for better stability and performance. Thin Solid Film. 2020, 704, 138024. [Google Scholar] [CrossRef]
- Gemeiner, P.; Peřinka, N.; Švorc, L.; Hatala, M.; Gál, L.; Belovičová, M.; Syrový, T.; Mikula, M. Pt–free counter electrodes based on modified screen–printed PEDOT:PSS catalytic layers for dye–sensitized solar cells. Mater. Sci. Semicond. Process. 2017, 66, 162–169. [Google Scholar] [CrossRef]
- Moolsarn, K.; Tangtrakarn, A.; Pimsawat, A.; Duangsa, K.; Mongkolkachit, C.; Maiaugree, W.; Amornkitbamrung, V. A Dye-Sensitized Solar Cell Using a Composite of PEDOT:PSS and Carbon Derived from Human Hair for a Counter Electrode. Int. J. Photoenergy 2017, 2017, 1064868. [Google Scholar] [CrossRef]
- Bu, C.; Tai, Q.; Liu, Y.; Guo, S.; Zhao, X. A transparent and stable polypyrrole counter electrode for dye-sensitized solar cell. J. Power Sources 2013, 221, 78–83. [Google Scholar] [CrossRef]
- Diah, A.W.M.; Saehana, S.; Holdsworth, C.I. Potency of Carrageenan as the doping agent for poly(3,4-ethylenedioxythiophene) conductive polymer. IOP Conf. Ser. J. Phys. Conf. Ser. 2019, 1242, 012007. [Google Scholar] [CrossRef]
- Bella, F.; Bongiovanni, R. Photoinduced polymerization: An innovative, powerful and environmentally friendly technique for the preparation of polymer electrolytes for dye-sensitized solar cells. J. Photochem. Photobiol. C Photochem. Rev. 2013, 16, 1–21. [Google Scholar] [CrossRef]
- Wen, Y.; Xu, J. Scientific Importance of Water-Processable PEDOT–PSS and Preparation, Challenge and New Application in Sensors of Its Film Electrode: A Review. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1121–1150. [Google Scholar] [CrossRef]
- Diah, A.W.M.; Wirayudha, A.; Kandolia, T.V.; Pujianti, N.K.T.; Widyakirana, I.; Hasriani, N.A.S.; Saehana, S.; Holdsworth, C.I. The Effect of Synthetic Conditions on the Characteristics of Carrageenan-Doped Poly(3,4-ethylenedioxythiophene). Macromol. Symp. 2020, 391, 1900162. [Google Scholar] [CrossRef]
- Fagiolari, L.; Varaia, E.; Mariotti, N.; Bonomo, M.; Barolo, C.; Bella, F. Poly(3,4-ethylenedioxythiophene) in Dye-Sensitized Solar Cells: Toward Solid-State and Platinum-Free Photovoltaics. Adv. Sustain. Syst. 2021, 5, 2100025. [Google Scholar] [CrossRef]
- Wei, W.; Wang, H.; Hu, Y.H. A review on PEDOT-based counter electrodes for dye-sensitized solar cells. Int. J. Energy Res. 2014, 38, 1099–1111. [Google Scholar] [CrossRef]
- Kayser, L.V.; Lipomi, D.J. Stretchable Conductive Polymers and Composites Based on PEDOT and PEDOT:PSS. Adv. Mater. 2018, 31, 1806133. [Google Scholar] [CrossRef]
- Sakunpongpitiporn, P.; Phasuksom, K.; Paradee, N.; Sirivat, A. Facile synthesis of highly conductive PEDOT:PSS via surfactant templates. RSC Adv. 2019, 9, 6363. [Google Scholar] [CrossRef]
- Louwet, F.; Grooenendal, L.; Dhaen, J.; Manca, J.; Luppen, J.V.; Verdonck, E.; Leenders, L. PEDOT/PSS: Synthesis, characterization, properties and application. Synth. Met. 2003, 135–136, 115–117. [Google Scholar] [CrossRef]
- Panigrahy, S.; Kandasubramanian, B. Polymeric thermoelectric PEDOT: PSS & composites: Synthesis, progress, and applications. Eur. Polym. J. 2020, 132, 109726. [Google Scholar]
- Mochizuki, Y.; Horii, T.; Okuzaki, H. Effect of pH on Structure and Conductivity of PEDOT/PSS. Trans. Mater. Res. Soc. Jpn. 2012, 37, 307–310. [Google Scholar] [CrossRef]
- Dong, J.; Portale, G. Role of the Processing Solvent on the Electrical Conductivity of PEDOT:PSS. Adv. Mater. Interfaces 2020, 7, 2000641. [Google Scholar] [CrossRef]
- Horii, T.; Hikawa, H.; Katsunuma, M.; Okuzaki, H. Synthesis of highly conductive PEDOT:PSS and correlation with hierarchical structure. Polymer 2018, 140, 33–38. [Google Scholar] [CrossRef]
- van de Velde, F.; Antipova, A.S.; Rollema, H.S.; Burova, T.V.; Grinberg, N.V.; Pereira, L.; Gilsenan, P.M.; Tromp, R.H.; Rudolph, B.; Grinberg, V.Y. The structure of κ/i-hybrid carrageenans II. Coil–helix transition as a function of chain composition. Carbohydr. Res. 2005, 340, 1113–1129. [Google Scholar] [CrossRef]
- Thrimawithana, T.R.; Young, S.; Dunstan, D.E.; Alany, R.G. Texture and rheological characterization of kappa and iota carrageenan in the presence of counter ions. Carbohydr. Polym. 2010, 82, 69–77. [Google Scholar] [CrossRef]
- Pereira, L.; Amado, A.M.; Critchley, A.T.; van de Velde, F.; Rebeiro-Claro, P.J.A. Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocoll. 2009, 23, 1903–1909. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B., Jr.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Volery, P.; Besson, R.; Schaffer-Lequart, C. Characterization of Commercial Carrageenans by Fourier Transform Infrared Spectroscopy Using Single-Reflection Attenuated Total Reflection. J. Agric. Food Chem. 2004, 52, 7457–7463. [Google Scholar] [CrossRef]
- Webber, V.; de Carvalho, S.M.; Ogliari, P.J.; Hayashi, L.; Barreto, P.L.M. Optimization of the extraction of carrageenan from Kappaphycus alvarezii using response surface methodology. Ciênc. Tecnol. Aliment. Camp. 2012, 32, 812–828. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Rupérez, P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocoll. 2011, 25, 1514–1520. [Google Scholar] [CrossRef]
- Rivnay, J.; Inal, S.; Collins, B.A.; Sessolo, M.; Stavrinidou, E.; Strakosas, X.; Tassone, C.; Delongchamp, D.M.; Malliaras, G.G. Structural control of mixed ionic and electronic transport in conducting polymers. Nat. Commun. 2016, 7, 11287. [Google Scholar] [CrossRef] [PubMed]
- Imae, I.; Yamane, H.; Imato, K.; Ooyama, Y. Thermoelectric properties of PEDOT:PSS/SWCNT composite films with controlled carrier density. Compos. Commun. 2021, 27, 100897. [Google Scholar] [CrossRef]
- Ng, C.A.; Camacho, D.H. Polymer electrolyte system based on carrageenan-poly(3,4-ethylenedioxythiophene) (PEDOT) composite for dye sensitized solar cell. IOP Conf. Ser. Mater. Sci. Eng. 2015, 79, 012020. [Google Scholar] [CrossRef]
- Wu, J.; Lan, Z.; Lin, J.; Huang, M.; Huang, Y.; Fan, L.; Luo, G.; Lin, Y.; Xie, Y.; Wei, Y. Counter electrodes in dye-sensitized solar cells. Chem. Soc. Rev. 2017, 46, 5975. [Google Scholar] [CrossRef]
- Oktaviani, E.; Nursam, N.M.; Prastomo, N.; Shobih. Effect of counter electrode materials on the performance of dye-sensitized solar cell. AIP Conf. Proc. 2020, 2232, 050001. [Google Scholar]
- Theerthagiri, J.; Senthil, A.R.; Madhavan, J.; Maiyalagan, T. Recent Progress in Non-Platinum Counter Electrode Materials for Dye-Sensitized Solar Cells. Chem. Elect. Chem. 2015, 2, 928–945. [Google Scholar] [CrossRef]
- Saranya, K.; Rameez, M.; Subramania, A. Developments in conducting polymer based counter electrodes for dye sensitized solar cells—An overview. Eur. Polym. J. 2015, 66, 207–227. [Google Scholar] [CrossRef]
- Maiaugree, W.; Pimanpang, S.; Towannang, M.; Saekow, S.; Jarernboon, W.; Amornkitbamrung, V. Optimization of TiO2 nanoparticle mixed PEDOT–PSS counter electrodes for high efficiency dye sensitized solar cell. J. Non-Cryst. Solids 2012, 358, 2489–2495. [Google Scholar] [CrossRef]
- Su’ait, M.S.; Rahman, M.Y.A.; Ahmad, A. Review on polymer electrolyte in dye-sensitized solar cells (DSSCs). Sol. Energy 2015, 115, 452–470. [Google Scholar] [CrossRef]
- Teo, L.P.; Buraidah, M.H.; Arof, A.K. Polyacrylonitrile-based gel polymer electrolytes for dye-sensitized solar cells: A review. Ionics 2020, 26, 4215–4238. [Google Scholar] [CrossRef]
- Ngai, K.S.; Ramesh, S.; Ramesh, K.; Juan, J.C. A review of polymer electrolytes: Fundamental, approaches and applications. Ionics 2016, 22, 1259–1279. [Google Scholar] [CrossRef]
- Putra, K.K.; Rosa, E.S.; Shobih; Prastomo, N. Development of monolithic dye sensitized solar cell fabrication with polymer-based counter electrode. IOP Conf. Ser. J. Phys. Conf. Ser. 2019, 1191, 012029. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, L. Polyethylene glycol-modified poly(3,4-ethylene dioxythiophene):poly(styrene sulfonate) counter electrodes for dye-sensitized solar cell. J. Appl. Electrochem. 2013, 43, 605–610. [Google Scholar] [CrossRef]
- Maiaugree, W.; Towannang, M.; Thiangkaew, A.; Harnchana, V.; Jarernboon, W.; Pimanpang, S.; Amornkitbamrung, V. Composited NiSO4 and PEDOT:PSS counter electrode for efficient dye-sensitized solarcell based on organic T2/T− electrolyte. Mater. Lett. 2013, 111, 197–200. [Google Scholar] [CrossRef]
- Maiaugree, W.; Pimparue, P.; Jarernboon, W.; Pimanpang, S.; Amornkitbamrung, V.; Swatsitang, E. NiS(NPs)-PEDOT-PSS composite counter electrode for a high efficiency dye sensitized solar cell. Mater. Sci. Eng. B 2017, 220, 66–72. [Google Scholar] [CrossRef]
- Wan, L.; Wang, B.; Wang, S.; Wang, X.; Guo, Z.; Dong, B.; Zhao, L.; Li, J.; Zhang, Q.; Luo, T. Well-dispersed PEDOT:PSS/graphene nanocomposites synthesized by in situ polymerization as counter electrodes for dye-sensitized solar cells. J. Mater. Sci. 2015, 50, 2148–2157. [Google Scholar] [CrossRef]
- Reddy, A.C.K.; Gurulakshmi, M.; Susmitha, K.; Raghavender, M.; Thota, N.; Subbaiah, Y.P.V. A novel PEDOT:PSS/SWCNH bilayer thin film counter electrode for efficient dye-sensitized solar cells. J. Mater. Sci. Mater. Electron. 2020, 31, 4752–4760. [Google Scholar] [CrossRef]
- Yue, G.; Ma, X.; Jiang, Q.; Tan, F.; Wu, J.; Chen, C.; Li, F.; Li, Q. PEDOT:PSS and glucose assisted preparation of molybdenum disulfide/single-wall carbon nanotubes counter electrode and served in dye sensitized solar cells. Electrochim. Acta 2014, 142, 68–75. [Google Scholar] [CrossRef]
- Palamba, A.J.; Sari, N.; Diah, A.W.M.; Saehana, S. A preliminary study of DSSC with PEDOT carrageenan as electrolyte system. J. Phys. Conf. Ser. 2021, 1763, 012090. [Google Scholar] [CrossRef]
- Sari, N.; Palamba, A.J.; Saehana, S.; Diah, A.W.M. DSSC with PEDOT-Carrageenan Electrolyte as Learning Media for Photovoltaic Concept Physics. J. Phys. Conf. Ser. 2021, 2126, 012004. [Google Scholar] [CrossRef]


| Journal Database | Keyword | Results | Types of Documents |
|---|---|---|---|
| Springer | (“PEDOT:PSS” AND “counter electrode”) OR (“PEDOT:Carrageenan” OR “PEDOT:Carr” AND “electrolyte”) OR (“polymer conductive” OR “polymer electrolyte”) AND (“DSSC” OR “Dye Sensitized Solar Cell”). | 729 | Article, chapter, reference work entry, conference proceedings, conference paper, and reference work |
| IOPscience | 114 | Article and conference paper | |
| Google Scholar | 244 | Article and conference paper |
| Wavenumber (cm−1) | Types of Atom Bonding | Identifications |
|---|---|---|
| 691 | C–S | Stretching of the thiophene ring in PEDOT |
| 840 | ||
| 936 | ||
| 983 | S–O | from oxide and the S-phenyl bond in PSS |
| 1145 | Stretching in PSS | |
| 1055 | S=O | from oxidant |
| 1198 | Stretching symmetric in PSS | |
| 1092 | C–O | Stretching in PEDOT |
| 1144 | ||
| 1340 | C–C | Stretching in the thiophene rings of PEDOT |
| 1518 | C=C | Stretching in the thiophene rings of PEDOT |
| 1640 | Stretching in the aromatic rings in PSS | |
| 2921 | C–H | Stretching of PEDOT and PSS |
| 3415 | O–H | Stretching in PSS |
| Letter Code | Carrageenan | IUPAC Name |
|---|---|---|
| D | Not Found | 4-Linked α-D-galactopyranose |
| D2S | ξ | 4-Linked α-D-galactopyranose 2-sulphate |
| D2S, 6S | λ, υ | 4-Linked α-D-galactopyranose 2,6-disulphate |
| D6S | μ | 4-Linked α-D-galactopyranose 6-sulphate |
| DA | κ, β | 4-Linked 3,6-anhydro-α-D-galactopyranose |
| DA2S | ι, θ | 4-Linked 3,6-anhydro-α-D-galactopyranose 2-sulphate |
| G | β | 3-Linked β-D-galactopyranose |
| G2S | λ, θ | 3-Linked β-D-galactopyranose 2-sulphate |
| G4S | κ, ι, μ, υ | 3-Linked β-D-galactopyranose 4-sulphate |
| S | κ, ι, λ, μ, υ, θ, ξ | Sulphate ester (O-) |
| Wavenumber (cm−1) | Bond(s)/Group(s) | Letter Code | Type of Carrageenan |
|---|---|---|---|
| 1495 | C = C of thiophene ring | - | - |
| 1371 | C–C of thiophene ring | - | - |
| 1198, 1060 | C–O–C of stretching mode of the ethylene groups | - | - |
| 892 | C–H of PEDOT chains | - | - |
| 1210–1260 | S = O of sulphate ester | S | κ, ι, λ, μ, υ, θ, ξ |
| 970–975 | Galactose | G/D | κ, ι, μ, υ, θ, β |
| 928–933, 1070 (shoulder) | C–O of 3,6-anhydro-D-galactose | DA | κ, ι, θ, β |
| 890–900 | Unsulphated β-D-galactose | G/D | β |
| 840–850 | C–O–SO3 of D-galactose-4-sulphate | G4S | κ, ι, μ, υ |
| 825–830 | C–O–SO3 of D-galactose-4-sulphate | G/D2S | λ, υ, θ, ξ |
| 820, 825 (shoulder) | C–O–SO3 of D-galactose-2,6-sulphate | D2S,6S | λ, υ |
| 810–820, 867 (shoulder) | C–O–SO3 of D-galactose-6-sulphate | G/D6S | μ |
| 800–805, 905 (shoulder) | C–O–SO3 of 3,6-anhydro-D-galactose-2-sulphate | DA2S | ι, θ |
| Properties | PEDOT:PSS | PEDOT:Carrageenan |
|---|---|---|
| Conductivity (S·cm−1) | >4000 [13] | 16.23 [33] |
| Sheet resistance (Ω·sq−1) | <100 [13] | N/A |
| Transparency (%) | 80–95 [13] | N/A |
| Ionic mobility (cm2v−1s−1) | 2.2 × 10−3 [31] | N/A |
| Electronic mobility (cm2v−1s−1) | 1.3 [31] | N/A |
| Carrier density (cm−3) | 4 × 1020 [32] | N/A |
| Materials | Photoanodes | Dyes | Electrolytes | A (cm2) | Voc (volt) | Jsc (mA·cm−2) | FF | η (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PEDOT:PSS | TiO2/ZrO2 | Z 907 (20 mg/L) | ) | 0.5 × 0.5 | 0.61 | 5.04 | 0.29 | 1.77 | [42] |
| PEG-PEDOT:PSS | TiO2 | N3 | ) | 1 × 0.2 | 0.73 | 10.11 | 0.60 | 4.39 | [43] |
| PEDOT:PSS-TiO2 | TiO2 | N719 (5 × 10−4 M) | ) | 0.5 × 0.5 | 0.73 | 17.30 | 0.67 | 8.49 | [38] |
| Ni-PEDOT:PSS | TiO2 | N719 (5 × 10−4 M) | Organic electrolyte (T2/T−) | 0.5 × 0.5 | 0.66 | 8.86 | 0.38 | 2.25 | [44] |
| NiSO4-PEDOT:PSS | TiO2 | N719 (5 × 10−4 M) | Organic electrolyte (T2/T−) | 0.5 × 0.5 | 0.65 | 8.79 | 0.54 | 3.05 | [44] |
| NiS-PEDOT:PSS | TiO2 | N719 (5 × 10−4 M) | ) | 0.5 × 0.5 | 0.76 | 16.05 | 0.67 | 8.18 | [45] |
| PEDOT:PSS-Carbon | TiO2 | N719 (0.5 mM) | ) | 0.5 × 0.5 | 0.81 | 13.6 | 0.69 | 7.60 | [3] |
| PEDOT:PSS-Graphene | TiO2 | N719 | ) | N/A | 0.73 | 11.77 | 0.55 | 4.66 | [46] |
| PEDOT:PSS/ SWCNH | TiO2 | N719 (0.3 mM) | ) | 0.5 × 0.5 | 0.68 | 14.06 | 0.52 | 5.10 | [47] |
| MoS2/SWCNT-PEDOT:PSS | TiO2 | Z 907 | ) | 0.4 × 0.7 | 0.78 | 16.21 | 0.64 | 8.14 | [48] |
| Materials | Photoanodes | Dyes | Counter electrodes | A (cm2) | Voc (volt) | Jsc (mA·cm−2) | FF | η (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PEDOT:κ-Carrageenan | TiO2 | N719 (3 × 10−4 M) | Pt | N/A | 0.57 | 3.37 | 0.22 | 0.42 | [33] |
| PEDOT:λ-Carrageenan | TiO2 | N719 | C/Pt | 2.5 × 2.8 | 90.2 × 10−3 | 2.8 × 10−3 | N/A | 0.25 × 10−3 | [49] |
| PEDOT:κ-Carrageenan | TiO2 | N719 | C/Pt | 2.2 × 2.2 | 91.8 × 10−3 | 57.6 × 10−3 | N/A | 0.33 × 10−3 | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurazizah, E.S.; Aprilia, A.; Risdiana, R.; Safriani, L. Different Roles between PEDOT:PSS as Counter Electrode and PEDOT:Carrageenan as Electrolyte in Dye-Sensitized Solar Cell Applications: A Systematic Literature Review. Polymers 2023, 15, 2725. https://doi.org/10.3390/polym15122725
Nurazizah ES, Aprilia A, Risdiana R, Safriani L. Different Roles between PEDOT:PSS as Counter Electrode and PEDOT:Carrageenan as Electrolyte in Dye-Sensitized Solar Cell Applications: A Systematic Literature Review. Polymers. 2023; 15(12):2725. https://doi.org/10.3390/polym15122725
Chicago/Turabian StyleNurazizah, Euis Siti, Annisa Aprilia, Risdiana Risdiana, and Lusi Safriani. 2023. "Different Roles between PEDOT:PSS as Counter Electrode and PEDOT:Carrageenan as Electrolyte in Dye-Sensitized Solar Cell Applications: A Systematic Literature Review" Polymers 15, no. 12: 2725. https://doi.org/10.3390/polym15122725
APA StyleNurazizah, E. S., Aprilia, A., Risdiana, R., & Safriani, L. (2023). Different Roles between PEDOT:PSS as Counter Electrode and PEDOT:Carrageenan as Electrolyte in Dye-Sensitized Solar Cell Applications: A Systematic Literature Review. Polymers, 15(12), 2725. https://doi.org/10.3390/polym15122725







