Fractionation of Aspen Wood to Produce Microcrystalline, Microfibrillated and Nanofibrillated Celluloses, Xylan and Ethanollignin
Abstract
1. Introduction
2. Materials
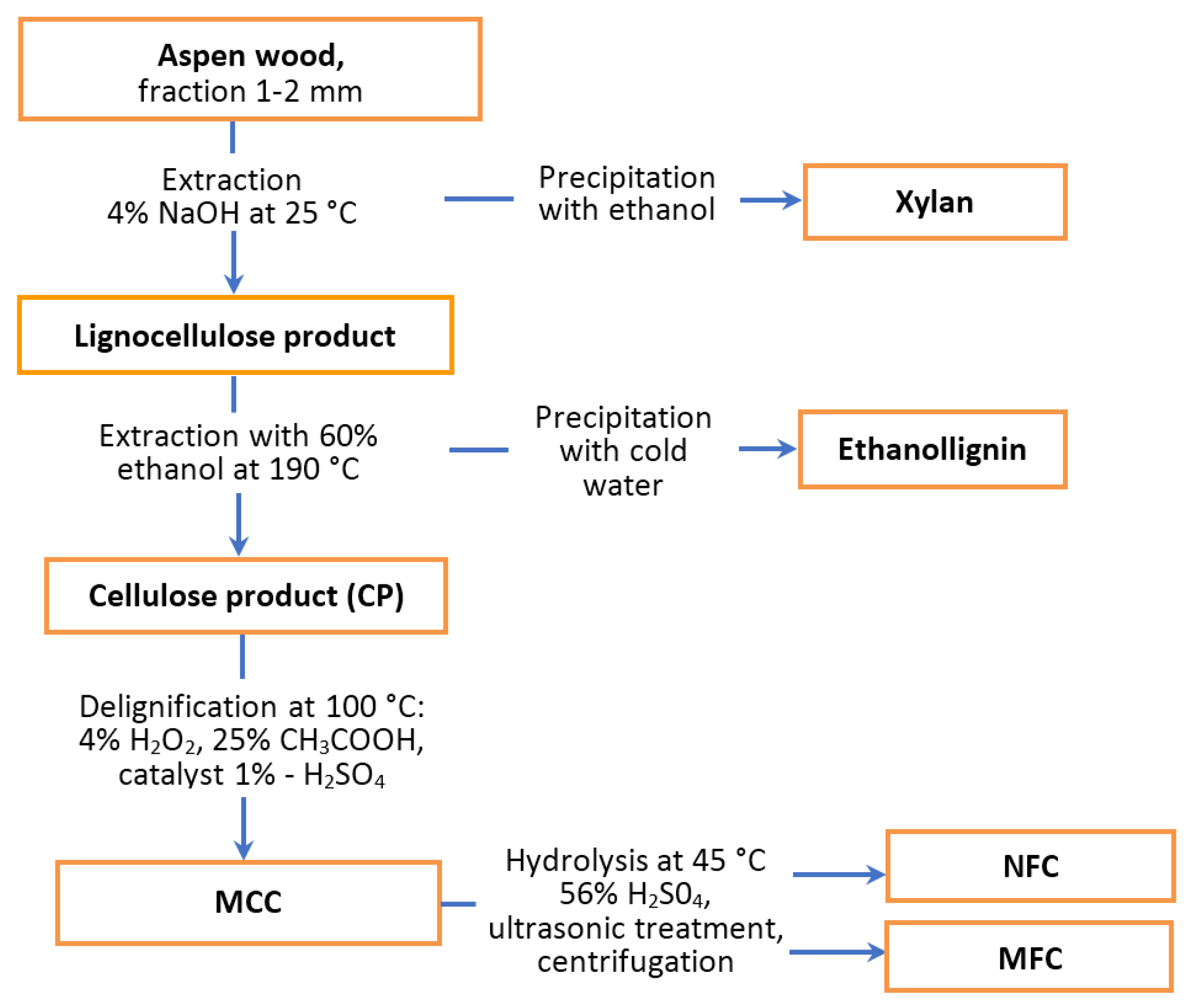
2.1. Preparation of Chemicals from Aspen Wood
2.2. Product Characterization
3. Results and Discussions
3.1. Extractive Catalytic Fractionation of Aspen Wood
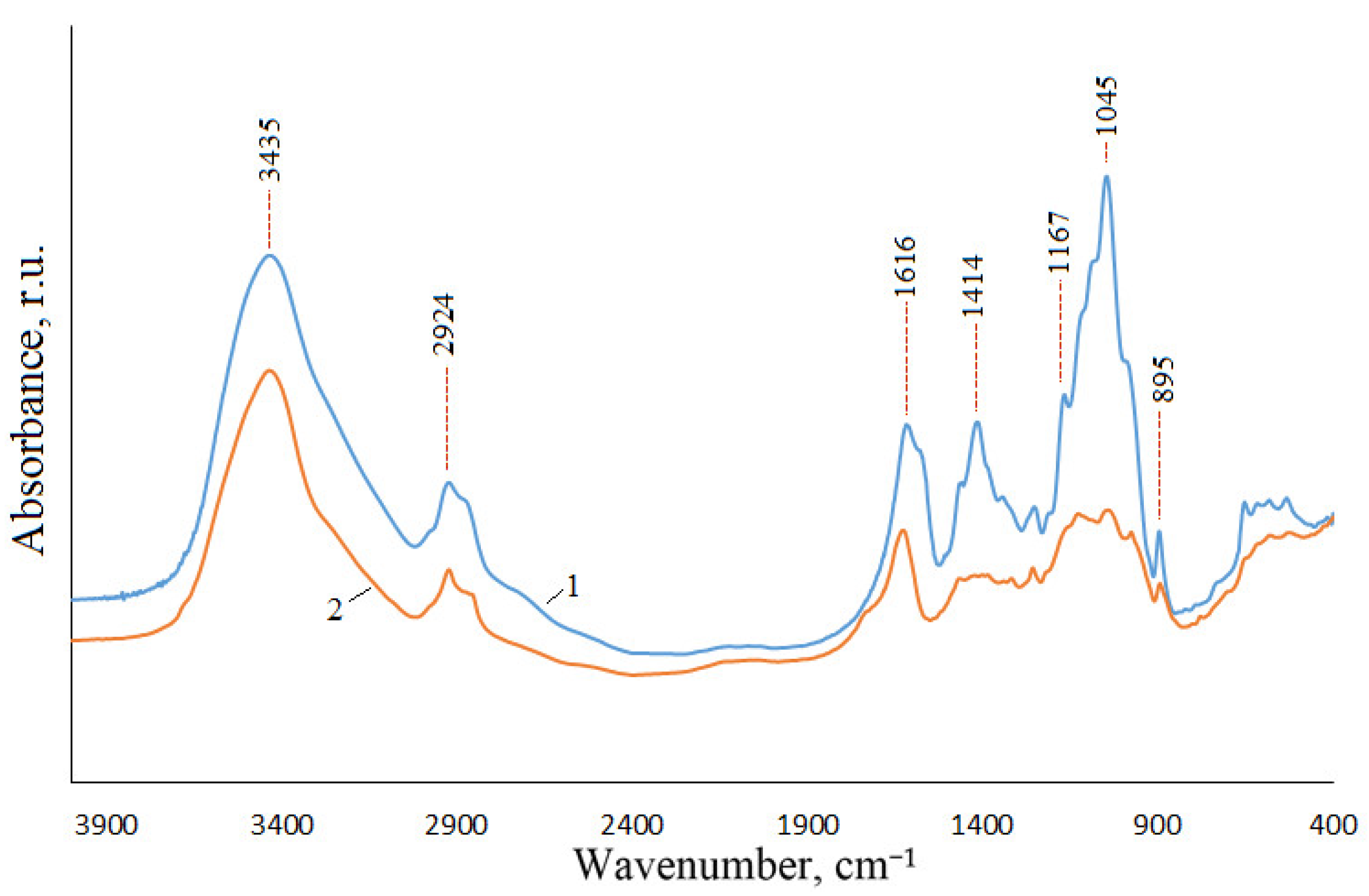
3.2. Isolation, Composition, and Structure of Xylan
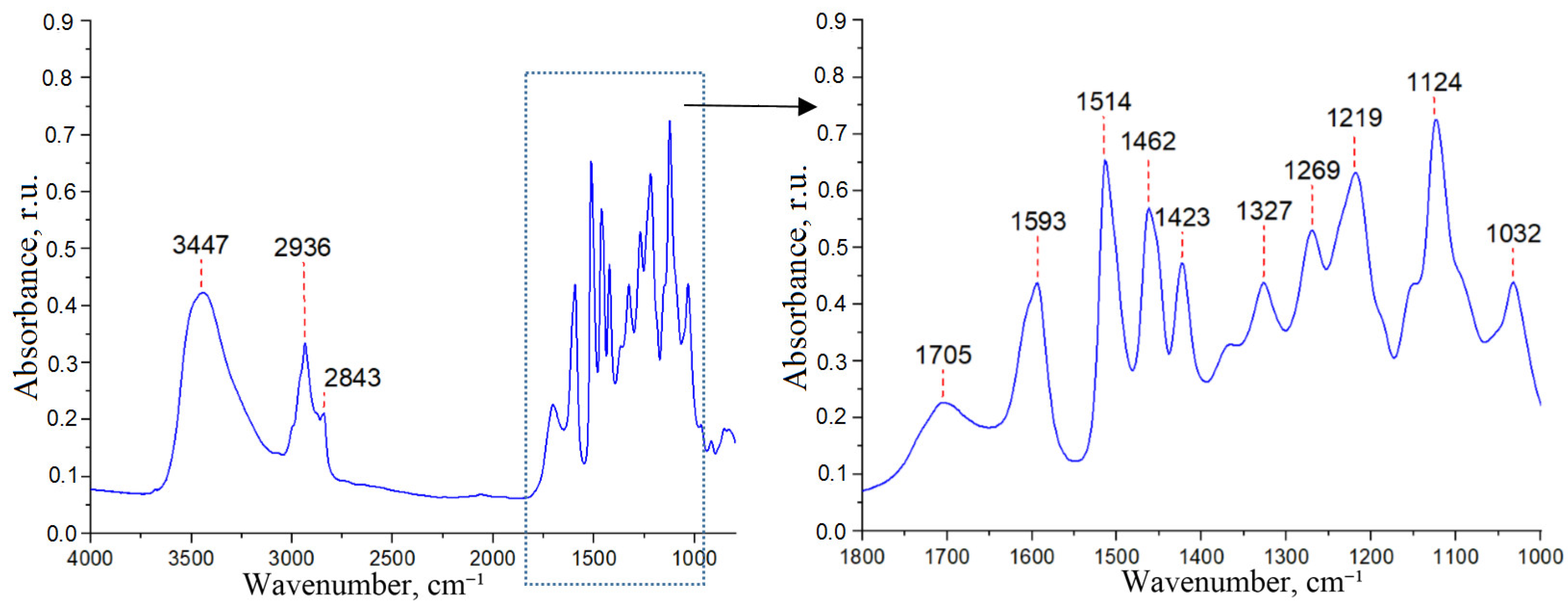
3.3. Isolation, Composition and Structure of Ethanollignin
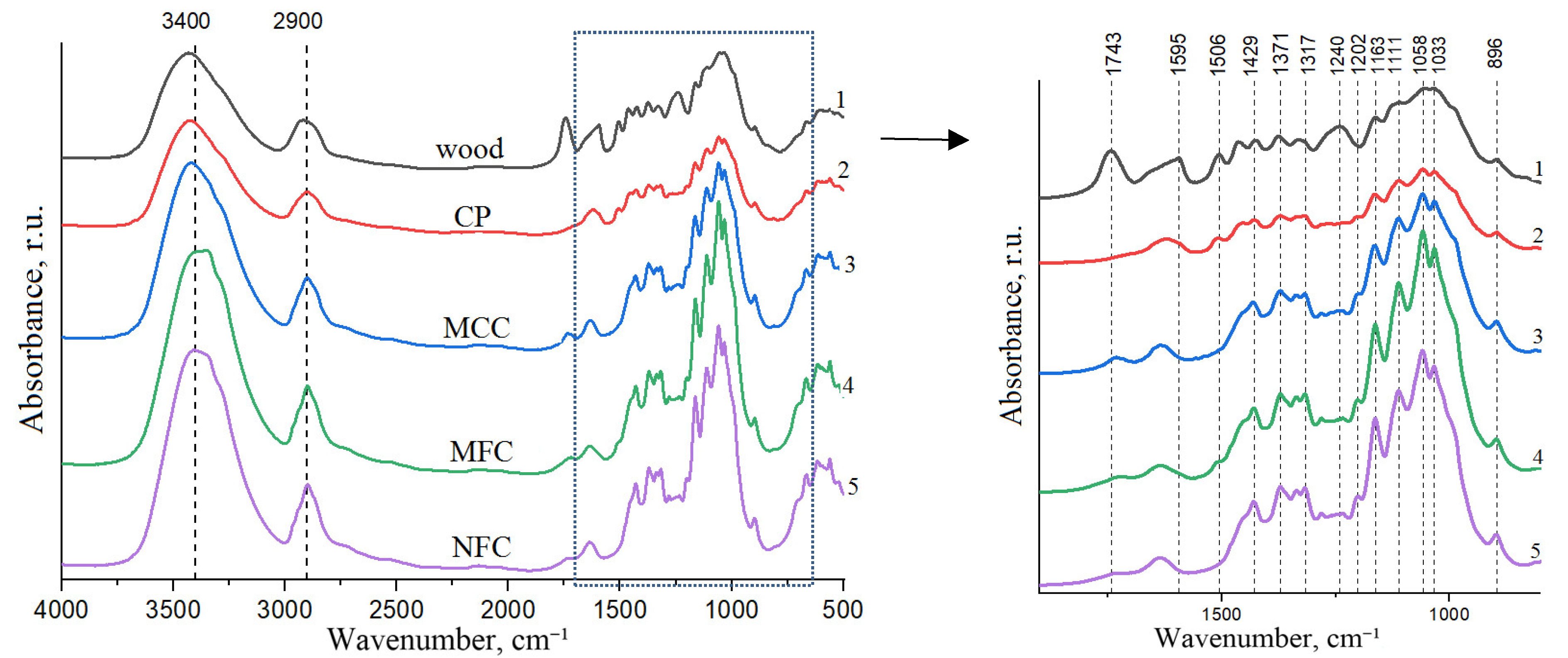
3.4. The Production, Composition, Structure and Thermochemical Properties of Cellulose Products
3.5. Structure of the NFC Film
3.6. Thermal Properties of MCC, MFC, and NFC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, W.; Feng, Y.; Fu, J.; Guo, H.; Guo, Y.; Han, B.; Jiang, Z.; Kong, L.; Li, C.; Liu, H.; et al. Catalytic conversion of lignocellulosic biomass into chemicals and fuels. Green Energy Environ. 2023, 8, 10–114. [Google Scholar] [CrossRef]
- Ding, L.; Han, X.; Chen, L.; Jiang, S. Preparation and properties of hydrophobic and transparent wood. J. Bioresour. Bioprod. 2022, 7, 295–305. [Google Scholar] [CrossRef]
- Han, X.; Wu, W.; Wang, J.; Tian, Z.; Jiang, S. Hydrogen-Bonding-Aided Fabrication of Wood Derived Cellulose Scaffold/Aramid Nanofiber into High-Performance Bulk Material. Materials 2021, 14, 5444. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Han, X.; Wu, W.; Wang, X.; Ding, L.; Wang, Y.; Li, S.; Hu, J.; Yang, W.; Zhang, C.; et al. Oxidation of cellulose molecules toward delignified oxidated hot-pressed wood with improved mechanical properties. Int. J. Biol. Macromol. 2023, 231, 123343. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Sudakova, I.G.; Garyntseva, N.V.; Tarabanko, V.E.; Yatsenkova, O.V.; Djakovitch, L.; Rataboul, F. Processes of catalytic oxidation for the production of chemicals from softwood biomass. Catal. Today 2021, 375, 132–144. [Google Scholar] [CrossRef]
- Fan, Y.; Li, H.; Su, S.; Chen, J.; Liu, C.; Wang, S.; Xu, X.; Song, G. Integration of Ru/C and base for reductive catalytic fractionation of triploid poplar. Chin. J. Catal. 2022, 43, 802–810. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Sudakova, I.G.; Garyntseva, N.V.; Kondrasenko, A.A.; Pestunov, A.V.; Djakovitch, L.; Pinel, C. Catalytic peroxide fractionation processes for the green biorefinery of wood. React. Kinet. Mech. Catal. 2019, 126, 717–735. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Garyntseva, N.V.; Sudakova, I.G.; Skripnikov, A.M.; Pestunov, A.V. Heterogeneous catalytic fractionation of birch-wood biomass into microcrystalline cellulose, xylose and enterosorbents. Chem. Plant Raw Mater. 2021, 4, 105–118. [Google Scholar] [CrossRef]
- Yin, W.-Z.; Xiao, L.-P.; Zou, S.-L.; Li, W.-X.; Wang, H.; Sun, R.-C. Valorization of lignin through reductive catalytic fractionation of fermented corn stover residues. Bioresour. Technol. 2023, 373, 128752. [Google Scholar] [CrossRef]
- Oh, S.; Gu, S.; Choi, J.-W.; Suh, D.J.; Lee, H.; Kim, C.S.; Kim, K.H.; Yoo, C.-J.; Choi, J.; Ha, J.-M. Metal/acid bifunctional catalysts for the reductive catalytic fractionation of lignocellulose into phenols and holocellulose. J. Environ. Chem. Eng. 2022, 10, 108085. [Google Scholar] [CrossRef]
- Thoresen, P.P.; Lange, H.; Rova, U.; Christakopoulos, P.; Matsakas, L. Covalently bound humin-lignin hybrids as important novel substructures in organosolv spruce lignins. Int. J. Biol. Macromol. 2023, 233, 123471. [Google Scholar] [CrossRef] [PubMed]
- Pongchaiphol, S.; Suriyachai, N.; Hararak, B.; Raita, M.; Laosiripojana, N.; Champreda, V. Physicochemical characteristics of organosolv lignins from different lignocellulosic agricultural wastes. Int. J. Biol. Macromol. 2022, 216, 710–727. [Google Scholar] [CrossRef] [PubMed]
- Galkin, M.V.; Samec, J.S. Lignin valorization through catalytic lignocellulose fractionation: A fundamental platform for the future biorefinery. ChemSusChem 2016, 9, 1544–1558. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Huang, X.; Hendriks, B.M.; Boot, M.D.; Hensen, E.J. Coupling organosolv fractionation and reductive depolymerization of woody biomass in a two-step catalytic process. Green Chem. 2018, 20, 2308–2319. [Google Scholar] [CrossRef]
- Bae, S.; Choi, J.-H.; Ahn, M.; Kim, R.; Kim, H. Ethanol organosolv lignin as a substitute for commercial antioxidants, focusing on the structural properties and synergistic effect with myricetin. Food Chem. 2023, 418, 136009. [Google Scholar] [CrossRef] [PubMed]
- da Mata, A.K.A.; de Andrade Felipe, V.T.; Mazzetto, S.E.; Lomonaco, D.; Avelino, F. Development of an eco-friendly acetosolv protocol for tuning the acetylation of coconut shell lignin: Structural, antioxidant, solubility and UV-blocking properties. Int. J. Biol. Macromol. 2022, 211, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Naidu, D.S.; Hlangothi, S.P.; John, M.J. Bio-based products from xylan: A review. Carbohydr. Polym. 2018, 179, 28–41. [Google Scholar] [CrossRef]
- Wang, S.; Gao, W.; Wang, Y.; Song, T.; Qi, H.; Xiang, Z. Emulsifying properties of naturally acetylated xylans and their application in lutein delivery emulsion. Carbohydr. Polym. 2022, 296, 119927. [Google Scholar] [CrossRef]
- Zha, Z.; Wang, X.; Wang, G.; Yin, H.; Wang, H. Synthesis and structural characterization of xylan acetate ester and its antinephritic effects in rats with experimental chronic kidney disease. Int. J. Biol. Macromol. 2023, 240, 124413. [Google Scholar] [CrossRef]
- Gutiérrez-Hernández, J.M.; Castorena-Alejandro, C.; Escobar-García, D.M.; Escalante, A.; Flores, H.; Pozos-Guillen, A.; Gatenholm, P.; Toriz, G. In vitro evaluation of spruce xylan/MWCNTs hydrogel scaffolds for bone regeneration. Mater. Today Commun. 2023, 35, 106070. [Google Scholar] [CrossRef]
- Zeybek, N.; Büyükkileci, A.O.; Güleç, S.; Polat, M.; Polat, H. Designing robust xylan/chitosan composite shells around drug-loaded MSNs: Stability in upper GIT and degradation in the colon microbiota. J. Drug Deliv. Sci. Technol. 2023, 79, 103983. [Google Scholar] [CrossRef]
- Costa Urtiga, S.C.; Marcelino, H.R.; Egito ES, T.; Oliveira, E.E. Xylan in drug delivery: A review of its engineered structures and biomedical applications. Eur. J. Pharm. Biopharm. 2020, 151, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zou, Y.; Gao, W.; Tan, Z.; Shen, W.; Wei, P. Xylan assisted construction of highly hydrophobic wood surface. Prog. Org. Coat. 2023, 174, 107328. [Google Scholar] [CrossRef]
- Tedeschi, G.; Guzman-Puyol, S.; Ceseracciu, L.; Paul, U.C.; Picone, P.; Di Carlo, M.; Athanassiou, A.; Heredia-Guerrero, J.A. Multifunctional Bioplastics Inspired by Wood Composition: Effect of Hydrolyzed Lignin Addition to Xylan–Cellulose Matrices. Biomacromolecules 2020, 21, 910–920. [Google Scholar] [CrossRef]
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, Structures, and Advanced Applications of Nanocomposites from Biorenewable Resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [CrossRef]
- Lin, H.; Ma, M.; Chu, Q.; Xu, L.; Chen, S.; Shi, Y.; He, H.; Wang, X. Multifunctional nanofibrillated cellulose/ZnO@rGO composite films for thermal conductivity, electrical insulation, and antibacterial applications. Compos. Struct. 2023, 312, 116896. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites—A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef]
- Zhao, Y.; Moser, C.; Lindstrom, M.E.; Henriksson, G.; Li, J. Cellulose Nanofibers from Softwood, Hardwood, and Tunicate: Preparation-Structure-Film Performance Interrelation. ACS Appl. Mater. Interfaces 2017, 9, 13508–13519. [Google Scholar] [CrossRef]
- Tahir, D.; Karim, M.R.A.; Hu, H.; Naseem, S.; Rehan, M.; Ahmad, M.; Zhang, M. Sources, Chemical Functionalization, and Commercial Applications of Nanocellulose and Nanocellulose-Based Composites: A Review. Polymers 2022, 14, 4468. [Google Scholar] [CrossRef]
- Beluns, S.; Gaidukovs, S.; Platnieks, O.; Gaidukova, G.; Mierina, I.; Grase, L.; Starkova, O.; Brazdausks, P.; Thakur, V.K. From Wood and Hemp Biomass Wastes to Sustainable Nanocellulose Foams. Ind. Crops Prod. 2021, 170, 113780. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Gopakumar, D.; Ahmad, I.; Thomas, S.; Dufresne, A.; Huang, J.; Lin, N. Advances in cellulose nanomaterials. Cellulose 2018, 25, 2151–2189. [Google Scholar] [CrossRef]
- Terzopoulou, P.; Kamperidou, V. Chemical characterization of Wood and Bark biomass of the invasive species of Tree-of-heaven (Ailanthus altissima (Mill.) Swingle), focusing on its chemical composition horizontal variability assessment. Wood Mater. Sci. Eng. 2022, 17, 469–477. [Google Scholar] [CrossRef]
- Sanchez-Salvador, J.L.; Campano, C.; Balea, A.; Tarrés, Q.; Delgado-Aguilar, M.; Mutjé, P.; Blanco, A.; Negro, C. Critical comparison of the properties of cellulose nanofibers produced from softwood and hardwood through enzymatic, chemical and mechanical processes. Int. J. Biol. Macromol. 2022, 205, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Sasaki, C.; Asada, C.; Nakamura, Y. Production of cellulose nanofibers from Aspen and Bode chopsticks using a high temperature and high pressure steam treatment combined with milling. Carbohydr. Polym. 2018, 194, 303–310. [Google Scholar] [CrossRef]
- Shao, Z.; Fu, Y.; Wang, P.; Zhang, Y.; Qin, M.; Li, X.; Zhang, F. Modification of the aspen lignin structure during integrated fractionation process of autohydrolysis and formic acid delignification. Int. J. Biol. Macromol. 2020, 165, 1727–1737. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Sharypov, V.I.; Baryshnikov, S.V.; Miroshnikova, A.V.; Taran, O.P.; Yakovlev, V.A.; Lavrenov, A.V.; Djakovitch, L. Catalytic hydrogenolysis of native and organosolv lignins of aspen wood to liquid products in supercritical ethanol medium. Catal. Today 2021, 379, 114–123. [Google Scholar] [CrossRef]
- Torgashov, V.I.; Solovieva, L.V.; Zubets, O.V.; Kaputsky, F.N. Obtaining xylan of pharmaceutical quality from birch wood. BSU Bulletin. 2014, 1, 21–26. [Google Scholar]
- Quesada-Medina, J.; López-Cremades, F.J.; Olivares-Carrillo, P. Organosolv extraction of lignin from hydrolyzed almond shells and application of the δ-value theory. Bioresour. Technol. 2010, 101, 8252–8260. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. In Laboratory Analytical Procedure (LAP) National Renewable Energy Laboratory; US Department of Energy: Washington, DC, USA, 2008. Available online: http://www.nrel.gov/biomass/analytical_procedures.html (accessed on 14 April 2023).
- Kürschner, K.; Hanak, A. Zur Bestimmung der sog. Rohfaser. Ein neues Verfahren der Bestimmung der Rohcellulose in Kakao. Z. Für Unters. Der Lebensm. 1930, 59, 484–494. [Google Scholar] [CrossRef]
- Obolenskaya, A.V.; Elnitskaya, Z.P.; Leonovich, A.A. Laboratory Research on Wood and Cellulose Chemistry; Ekologiya: Moscow, Russia, 1991. [Google Scholar]
- Ruiz-Matute, A.I.; Hernández-Hernández, O.; Rodríguez-Sánchez, S.; Sanz, M.L.; Martínez-Castro, I. Derivatization of carbohydrates for GC and GC–MS analyses. J. Chromatogr. B 2011, 879, 1226–1240. [Google Scholar] [CrossRef]
- Sun, S.-L.; Wen, J.-L.; Ma, M.-G.; Sun, R.-C. Successive alkali extraction and structural characterization of hemicelluloses from sweet sorghum stem. Carbohydr. Polym. 2013, 92, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Jonson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulose performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Açıkalın, K. Thermogravimetric analysis of walnut shell as pyrolysis feedstock. J. Therm. Anal. Calorim. 2011, 105, 145–150. [Google Scholar] [CrossRef]
- Ganeshan, G.; Shadangi, K.P.; Mohanty, K. Degradation kinetic study of pyrolysis and co-pyrolysis of biomass with polyethylene terephthalate (PET) using Coats–Redfern method. J. Therm. Anal. Calorim. 2018, 131, 1803–1816. [Google Scholar] [CrossRef]
- Gabrielii, I.; Gatenholm, P.; Glasser, W.G.; Jain, R.K.; Kenne, L. Separation, characterization and hydrogel-formation of hemicellulose from aspen wood. Carbohydr. Polym. 2000, 43, 367–374. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]
- Vázquez, M.J.; Alonso, J.L.; Domínguez, H.; Parajó, J.C. Xylooligosaccharides: Manufacture and applications. Trends Food Sci. Technol. 2000, 11, 387–393. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- De Freitas, C.; Carmona, E.; Brienzo, M. Xylooligosaccharides production process from lignocellulosic biomass and bioactive effects. Bioact. Carbohydr. Diet. Fibre 2019, 18, 100184. [Google Scholar] [CrossRef]
- Sheng, K.; Zhang, S.; Liu, J.E.; Jin, C.; Xu, Z.; Zhang, X. Hydrothermal carbonization of cellulose and xylan into hydrochars and application on glucose isomerization. J. Clean. Prod. 2019, 237, 117831. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Sudakova, I.G.; Chudina, A.I.; Garyntseva, N.V.; Kazachenko, A.S.; Skripnikov, A.M.; Malyar, Y.N.; Ivanov, I.P. Fractionation of birch wood biomass into valuable chemicals by the extraction and catalytic processes. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Kačuráková, M.; Wellner, N.; Ebringerová, A.; Hromádková, Z.; Wilson, R.H.; Belton, P.S. Characterisation of xylan-type polysaccharides and associated cell wall components by FT-IR and FT-Raman spectroscopies. Food Hydrocoll. 1999, 13, 35–41. [Google Scholar] [CrossRef]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Song, G.; Pan, Z.; Tu, M.; Kharaziha, M.; Zhang, X.; Show, P.-L.; Sun, F. Advances in organosolv modified components occurring during the organosolv pretreatment of lignocellulosic biomass. Bioresour. Technol. 2023, 368, 128356. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Lei, F.; Li, P.; Jian, J. Lignocellulosic biomass to biofuels and biochemicals: A comprehensive review with a focus on ethanol organosolv pretreatment technology. Biotechnol. Bioeng. 2018, 115, 2683–2702. [Google Scholar] [CrossRef]
- Sheng, Y.; Ma, Z.; Wang, X.; Han, Y. Ethanol organosolv lignin from different agricultural residues: Toward basic structural units and antioxidant activity. Food Chem. 2022, 376, 131895. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Chesnokov, N.V.; Sudakova, I.G.; Garyntseva, N.V.; Kuznetsova, S.A.; Malyar, Y.u.N.; Yakovlev, V.A.; Djakovitch, L. Green catalytic processing of native and organosolv lignins. Catal. Today 2018, 309, 18–30. [Google Scholar] [CrossRef]
- Mikova, N.M.; Fetisova OYu Mazurova, E.V.; Ivanchenko, N.M.; Lutoschkin, M.A.; Djakovitch, L.; Chesnokov, N.V.; Kuznetsov, B.N. Study of the Thermochemical Properties of Ethanol Lignins from Abies and Aspen Wood. J. Sib. Fed. Univ. Chem. 2018, 11, 401–417. [Google Scholar] [CrossRef]
- Himmel, M.E.; Tatsumoto, K.; Grohmann, K.; Johnson, D.K.; Chum, H.L. Molecular weight distribution of aspen lignins from conventional gel permeation chromatography, universal calibration and sedimentation equilibrium. J. Chromatogr. A 1990, 498, 93–104. [Google Scholar] [CrossRef]
- Brosse, N. Organosolv Lignins: Extraction, Characterization and Utilization for the Conception of Environmentally Friendly Materials. In Lignin: Properties and Applications in Biotechnology and Bioenergy; Paterson, R.J., Ed.; Nova Science: New York, NY, USA, 2012; pp. 381–402. [Google Scholar]
- Kuznetsov, B.N.; Chesnokov, N.V.; Ivanov, I.P.; Veprikova, E.V.; Ivanchenko, N.M. Methods of Porous Materials Obtaining from Lignin and Wood Bark. J. Sib. Fed. Univ. Chem. 2015, 2, 232–255. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Sudakova, I.G.; Yatsenkova, O.V.; Garyntseva, N.V.; Rataboul, F.; Djakovitch, L. Optimizing single-stage processes of microcrystalline cellulose production via the peroxide delignification of wood in the presence of a Titania Catalyst. Catal. Ind. 2018, 10, 360–367. [Google Scholar] [CrossRef]
- Spence, K.L.; Venditti, R.A.; Rojas, O.J.; Habibi, Y.; Pawlak, J.J. A comparative study of energy consumption and physical properties of microfibrillated cellulose produced by different processing methods. Cellulose 2011, 18, 1097–1111. [Google Scholar] [CrossRef]
- Xie, H.; Du, H.; Yang, X.; Si, C. Recent strategies in preparation of cellulose nanocrystals and cellulose nanofibrils derived from raw cellulose materials. Int. J. Polym. Sci. 2018, 2018, 7923068. [Google Scholar] [CrossRef]
- Ditzel, F.I.; Prestes, E.; Carvalho, B.M.; Demiate, I.M.; Pinheiro, L.A. Nanocrystalline cellulose extracted from pine wood and corncob. Carbohydr. Polym. 2017, 157, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Poletto, M.; Ornaghi, H.L.; Zattera, A.J. Native Cellulose: Structure, Characterization and Thermal Properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef]
- Rashid, S.; Dutta, H. Characterization of nanocellulose extracted from short, medium and long grain rice husks. Ind. Crops Prod. 2020, 154, 112627. [Google Scholar] [CrossRef]
- Chirayil, C.J.; Joy, J.; Mathew, L.; Mozetic, M.; Koetz, J.; Thomas, S. Isolation and characterization of cellulose nanofibrils from Helicteres isora plant. Ind. Crops Prod. 2014, 59, 27–34. [Google Scholar] [CrossRef]
- Cebreiros, F.; Sánchez, G.; Ferrari, M.D.; Lareo, C. Integrating the coproduction of cellulose nanofibers and biobutanol from eucalyptus pulp using an environmentally friendly process. Ind. Crops Prod. 2022, 188 Pt B, 115732. [Google Scholar] [CrossRef]
- Autlov, S.A.; Bazarnova, N.G.; Kushnir, E.J. Microcrystalline cellulose: Structure, properties and applications (Review). Chem. Plant Raw Mater. 2013, 3, 33–41. [Google Scholar] [CrossRef]
- Kassab, Z.; Boujemaoui, A.; Ben Youcef, H.; Hajlane, A.; Hannache, H.; El Achaby, M. Production of cellulose nanofibrils from alfa fibers and its nanoreinforcement potential in polymer nanocomposites. Cellulose 2019, 26, 9567–9581. [Google Scholar] [CrossRef]
- Dominic, C.D.M.; Raj, V.; Neenu, K.V.; Begum, P.M.S.; Formela, K.; Saeb, M.R.; Prabhu, D.D.; Vijayan, P.P.; Ajithkumar, T.G.; Parameswaranpillai, J. Chlorine-free extraction and structural characterization of cellulose nanofibers from waste husk of millet (Pennisetum glaucum). Int. J. Biol. Macromol. 2022, 206, 92–104. [Google Scholar] [CrossRef]
- Neenu, K.V.; Midhun, C.D.D.; Begum, P.M.S.; Parameswaranpillai, J.; Kanoth, B.P.; David, D.A.; SSajadi, M.; Dhanyasree, P.; Ajithkumar, T.G.; Badawi, M. Effect of oxalic acid and sulphuric acid hydrolysis on the preparation and properties of pineapple pomace derived cellulose nanofibers and nanopapers. Int. J. Biol. Macromol. 2022, 209, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Zhang, W.; Xu, Y.; Yu, Y.; Lin, X.; Fu, M.; Liu, H.; Peng, J.; Zhao, Z. Cellulose nanofiber from pomelo spongy tissue as a novel particle stabilizer for Pickering emulsion. Int. J. Biol. Macromol. 2023, 224, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Wei, Y.; Xu, Q.; Yao, W.; Cheng, Z. Cellulose nanofibers prepared from TEMPO-oxidation of kraft pulp and its flocculation effect on kaolin clay. J. Appl. Polym. Sci. 2014, 131, 40450. [Google Scholar] [CrossRef]
- Koivuniemi, R.; Hakkarainen, T.; Kiiskinen, J.; Kosonen, M.; Vuola, J.; Valtonen, J.; Luukko, K.; Kavola, H.; Yliperttula, M. Clinical study of nanofibrillar cellulose hydrogel dressing for skin graft donor site treatment. Adv. Wound Care 2019, 9, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, H.; Athar, M.; Dehghani, M.; Garnier, G.; Batchelor, W. Recent advancements, trends, fundamental challenges and opportunities in spray deposited cellulose nanofibril films for packaging applications. Sci. Total Environ. 2022, 836, 155654. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Liu, S.; Ji, X.; Yang, G.; Chen, J. Lipase induced highly hydrophobic nanofibrillated cellulose film for strain sensor application. Carbohydr. Polym. 2022, 284, 119193. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Rajan, K.; Djioleu, A.; Kandhola, G.; Labbé, N.; Sakon, J.; Carrier, D.J. Investigating the effects of hemicellulose pre-extraction on the production and characterization of loblolly pine nanocellulose. Cellulose 2020, 27, 3693–3706. [Google Scholar] [CrossRef]
- Roman, M.; Winter, W.T. Effect of Sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Sudakova, I.G.; Garyntseva, N.V.; Skripnikov, A.M.; Pestunov, A.V.; Gnidan, E.V. Birch wood biorefinery into microcrystalline, microfibrillated, and nanocrystalline celluloses, xylose, and adsorbents. Wood Sci. Technol. 2023, 57, 173–196. [Google Scholar] [CrossRef]

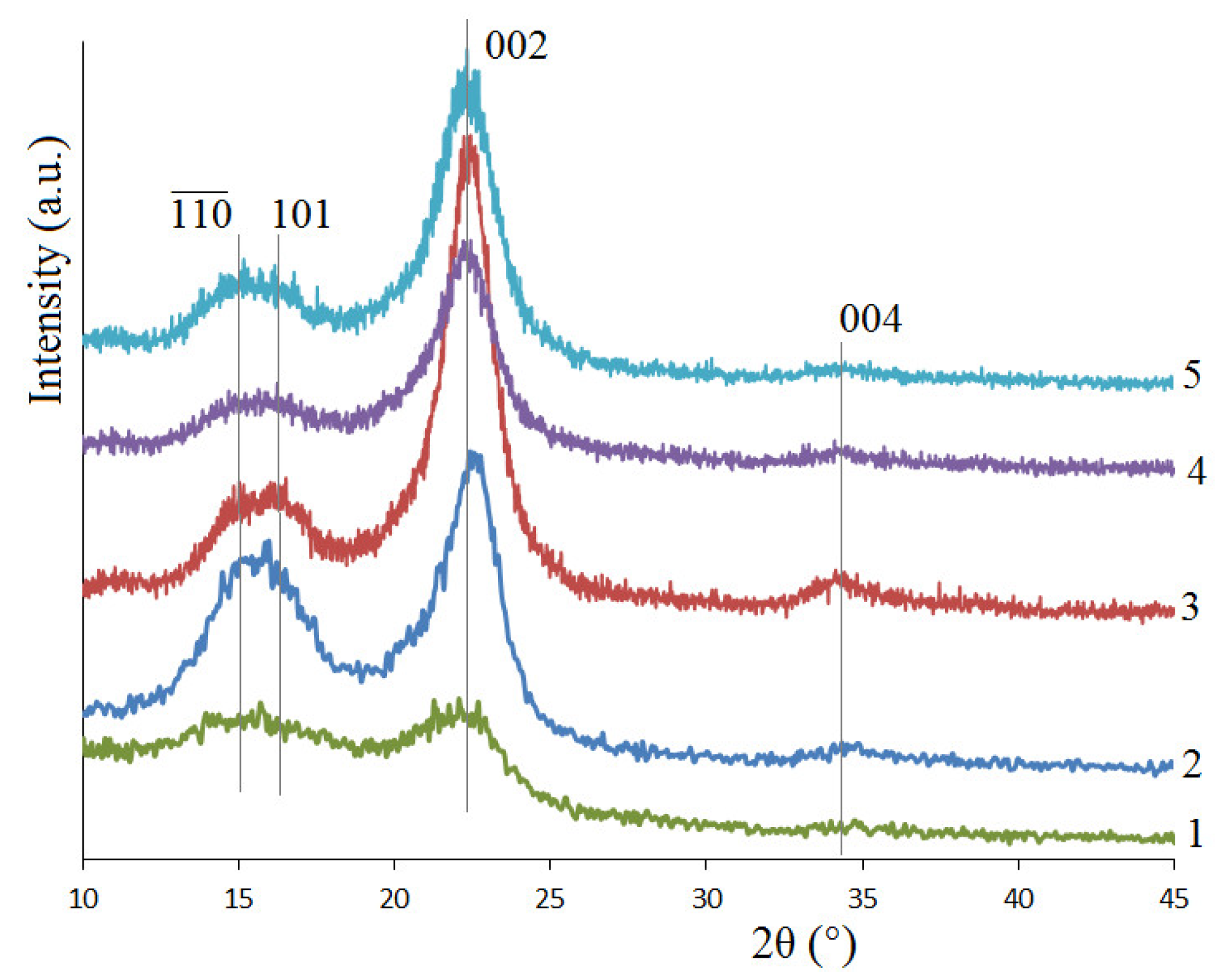

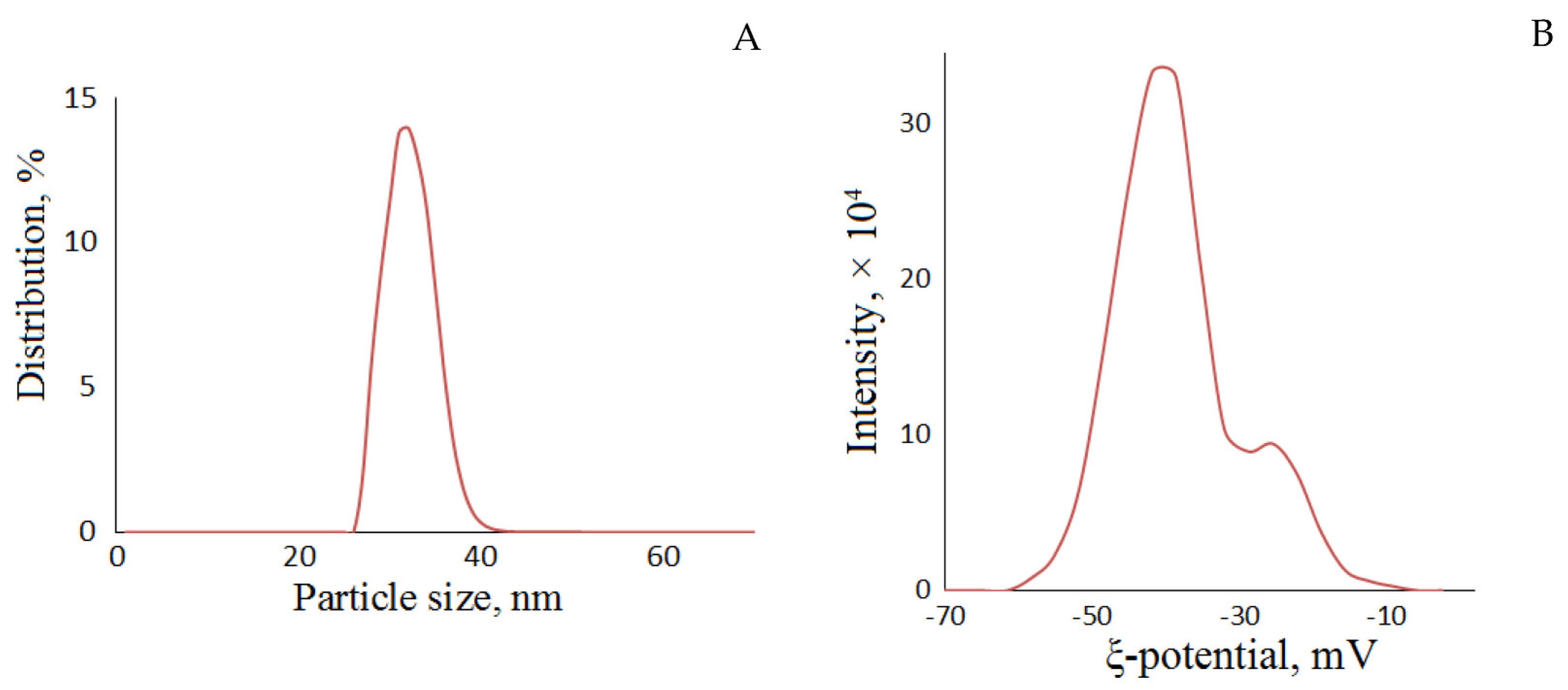

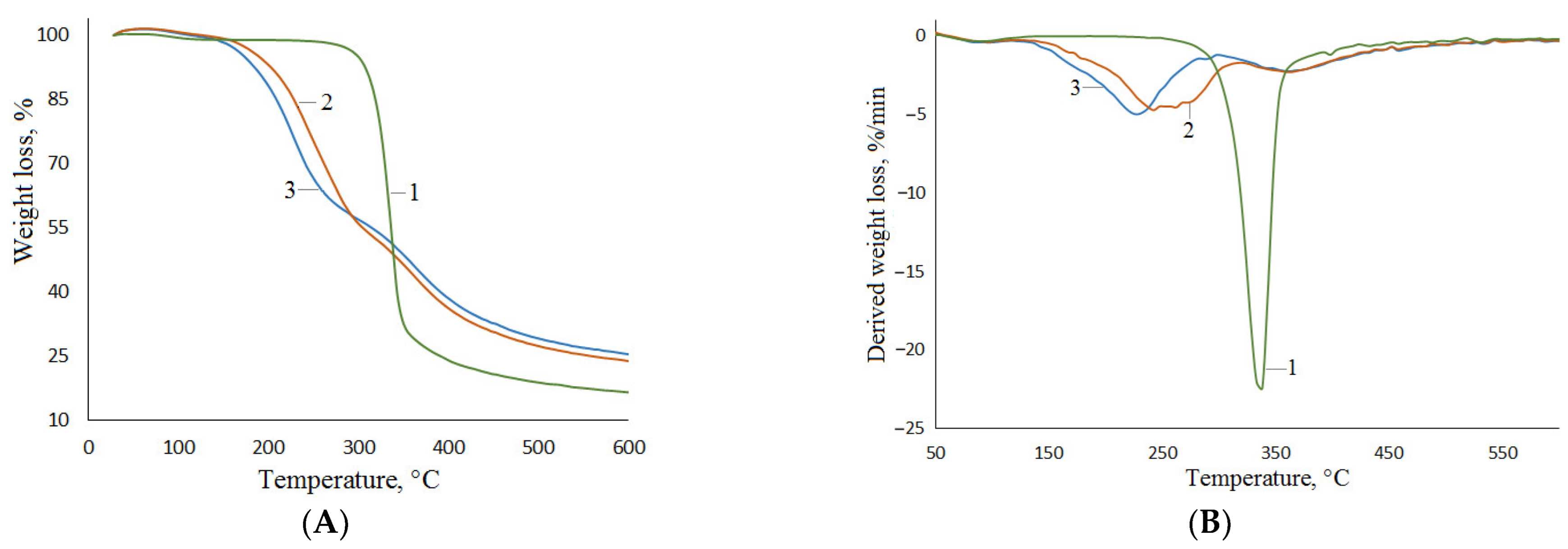
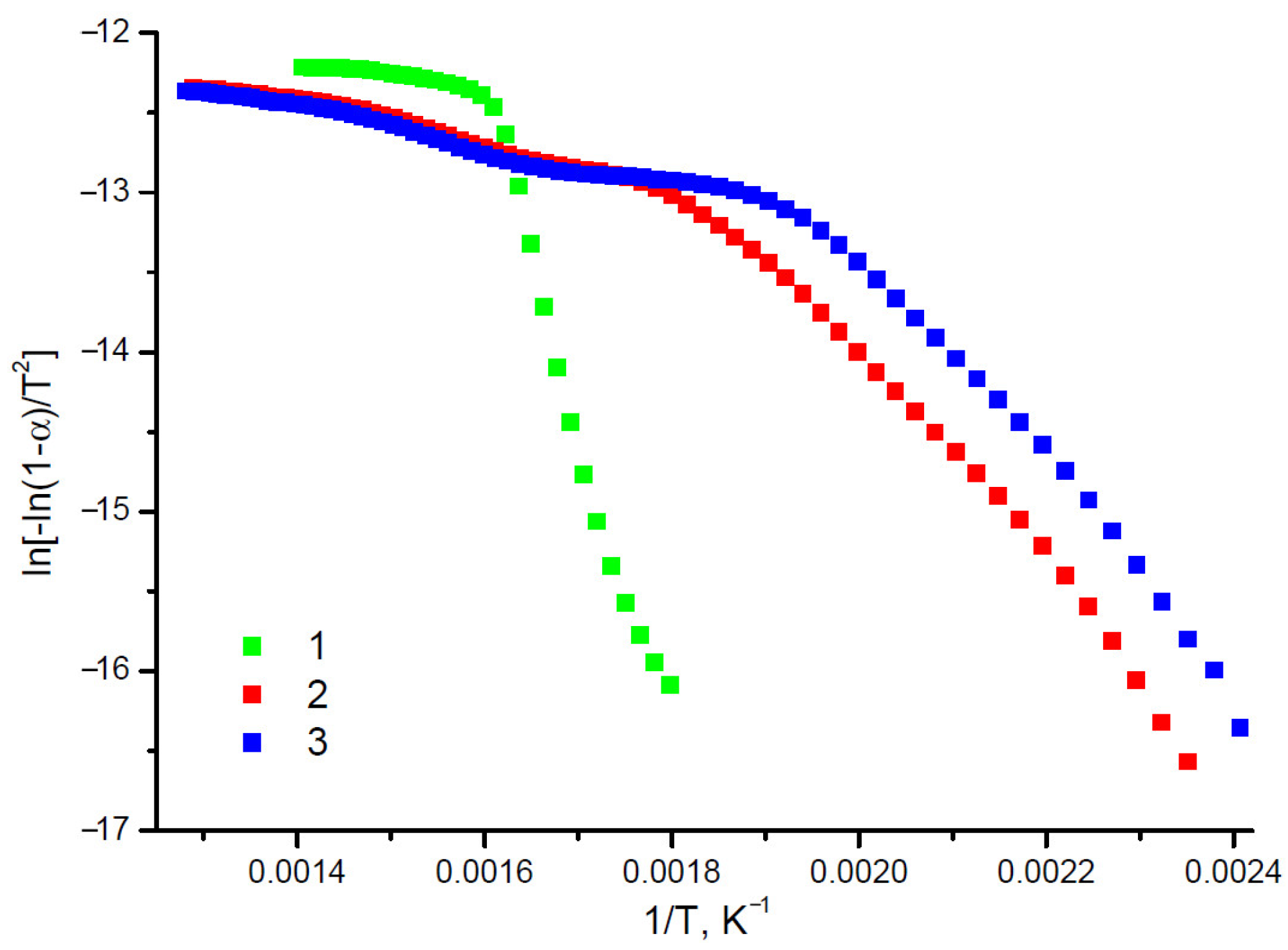
| Sample | Elemental Composition, wt.% | Atomic Ratio H/C | Atomic Ratio O/C | ||
|---|---|---|---|---|---|
| C | H | Odif | |||
| Xylan from aspen wood | 39.13 ± 0.04 | 5.89 ± 0.03 | 54.97 ± 0.07 | 1.81 | 1.05 |
| Xylan from birch wood [53] | 39.14 | 5.88 | 54.98 | 1.80 | 1.05 |
| Commercial xylan [52] | 40.68 | 6.68 | 52.65 | 1.98 | 0.97 |
| Sample * | Elemental Composition, wt.% | Atomic Ratio H/C | Atomic Ratio O/C | ||
|---|---|---|---|---|---|
| C | H | Odif | |||
| 1 | 63.67 ± 0.5 | 5.95 ± 0.04 | 30.37 ± 0.54 | 1.1 | 0.4 |
| 2 | 62.1 | 5.6 | 31.8 | 1.1 | 0.4 |
| Sample | Mn (g/mol) | Mw (g/mol) | PD |
|---|---|---|---|
| 1 | 972 ± 2 | 2704 ± 6 | 2.78 ± 0.01 |
| 2 | 659 ± 3 | 2738 ± 7 | 4.15 ± 0.01 |
| Sample | Component Content, wt.% | |||
|---|---|---|---|---|
| Cellulose | Lignin | Easy Hydrolysable Polysaccharides | Ash | |
| CP | 78.4 ± 0.8 | 8.9 ± 0.1 | 8.6 ± 0.2 | 0.7 ± 0.01 |
| MCC | 87.0 ± 0.8 | 0.4 ± 0.005 | 8.5 ± 0.2 | 0.8 ± 0.01 |
| Sample | Elemental Composition, wt.% | Atomic Ratio H/C | Atomic Ratio O/C | |||
|---|---|---|---|---|---|---|
| C | H | S | Odif | |||
| MCC | 42.6 ± 0.04 | 6.1 ± 0.01 | - | 51.2 ± 0.06 | 1.7 | 0.9 |
| MFC | 42.3 ± 0.01 | 6.1 ± 0.01 | 2.8 ± 0.09 | 48.6 ± 0.07 | 1.8 | 0.9 |
| NFC | 41.8 ± 0.06 | 6.1 ± 0.02 | 0.07 ± 0.005 | 51.9 ± 0.08 | 1.8 | 0.9 |
| Sample | CrI | Polymerization Degree |
|---|---|---|
| CP | 0.65 | 405 |
| MCC | 0.73 | 313 |
| MFC | 0.86 | 176 |
| NFC | 0.86 | 89 |
| Sample | Temperature Range, °C | Activation Energy, kJ/mol | Correlation Coefficient, R2 |
|---|---|---|---|
| MCC | 283–358 | 166 | 0.98 |
| 363–438 | 6 | 0.97 | |
| MFC | 152–302 | 50 | 0.98 |
| 307–502 | 11 | 0.97 | |
| NFC | 117–242 | 64 | 0.97 |
| 247–507 | 10 | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsov, B.N.; Chudina, A.I.; Kazachenko, A.S.; Fetisova, O.Y.; Borovkova, V.S.; Vorobyev, S.A.; Karacharov, A.A.; Gnidan, E.V.; Mazurova, E.V.; Skripnikov, A.M.; et al. Fractionation of Aspen Wood to Produce Microcrystalline, Microfibrillated and Nanofibrillated Celluloses, Xylan and Ethanollignin. Polymers 2023, 15, 2671. https://doi.org/10.3390/polym15122671
Kuznetsov BN, Chudina AI, Kazachenko AS, Fetisova OY, Borovkova VS, Vorobyev SA, Karacharov AA, Gnidan EV, Mazurova EV, Skripnikov AM, et al. Fractionation of Aspen Wood to Produce Microcrystalline, Microfibrillated and Nanofibrillated Celluloses, Xylan and Ethanollignin. Polymers. 2023; 15(12):2671. https://doi.org/10.3390/polym15122671
Chicago/Turabian StyleKuznetsov, Boris N., Anna I. Chudina, Aleksandr S. Kazachenko, Olga Yu. Fetisova, Valentina S. Borovkova, Sergei A. Vorobyev, Anton A. Karacharov, Elena V. Gnidan, Elena V. Mazurova, Andrey M. Skripnikov, and et al. 2023. "Fractionation of Aspen Wood to Produce Microcrystalline, Microfibrillated and Nanofibrillated Celluloses, Xylan and Ethanollignin" Polymers 15, no. 12: 2671. https://doi.org/10.3390/polym15122671
APA StyleKuznetsov, B. N., Chudina, A. I., Kazachenko, A. S., Fetisova, O. Y., Borovkova, V. S., Vorobyev, S. A., Karacharov, A. A., Gnidan, E. V., Mazurova, E. V., Skripnikov, A. M., & Taran, O. P. (2023). Fractionation of Aspen Wood to Produce Microcrystalline, Microfibrillated and Nanofibrillated Celluloses, Xylan and Ethanollignin. Polymers, 15(12), 2671. https://doi.org/10.3390/polym15122671










