Development of an All-Marine 3D Printed Bioactive Hydrogel Dressing for Treatment of Hard-to-Heal Wounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Ink Formulation and Optimization
2.2. Ink Rheology
2.3. Three-Dimensional Printing of Hydrogels
2.4. Release Profile
2.5. Chemical Characterization
2.6. Bioburden
2.7. Bioactivity and Biocompatibility
2.7.1. Cytotoxicity
2.7.2. Cell Cultivation
2.7.3. Pro-collagen I Alpha 1 Assay
3. Results and Discussion
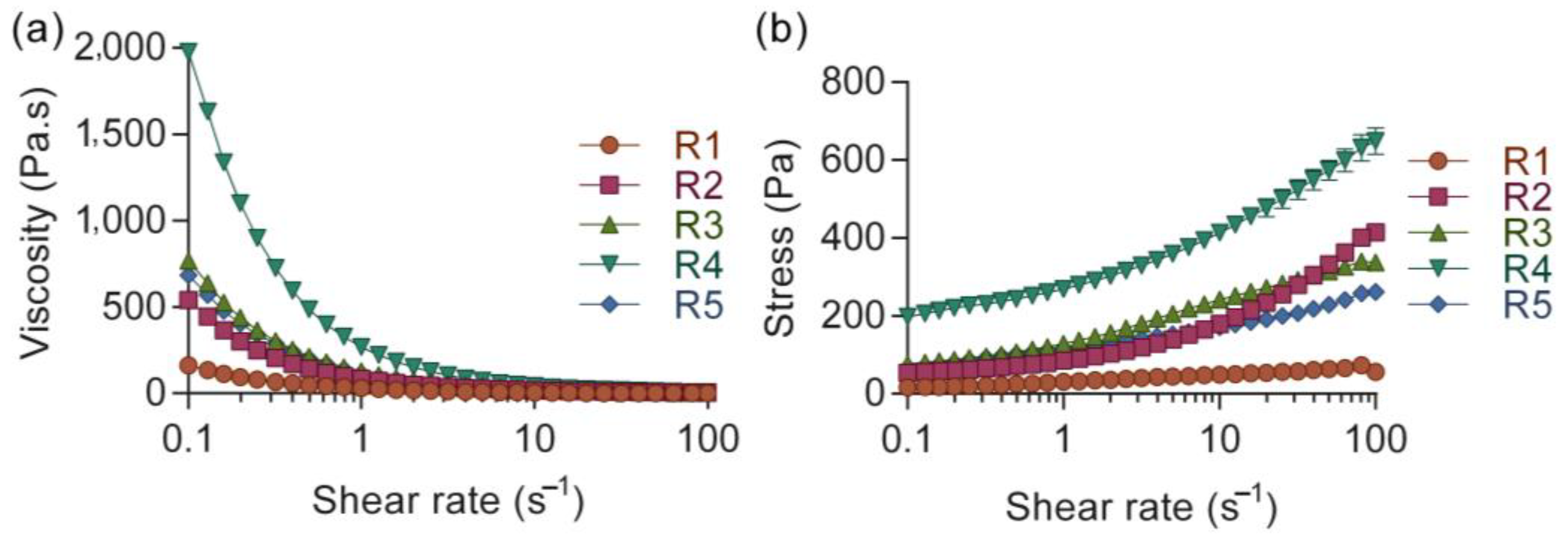
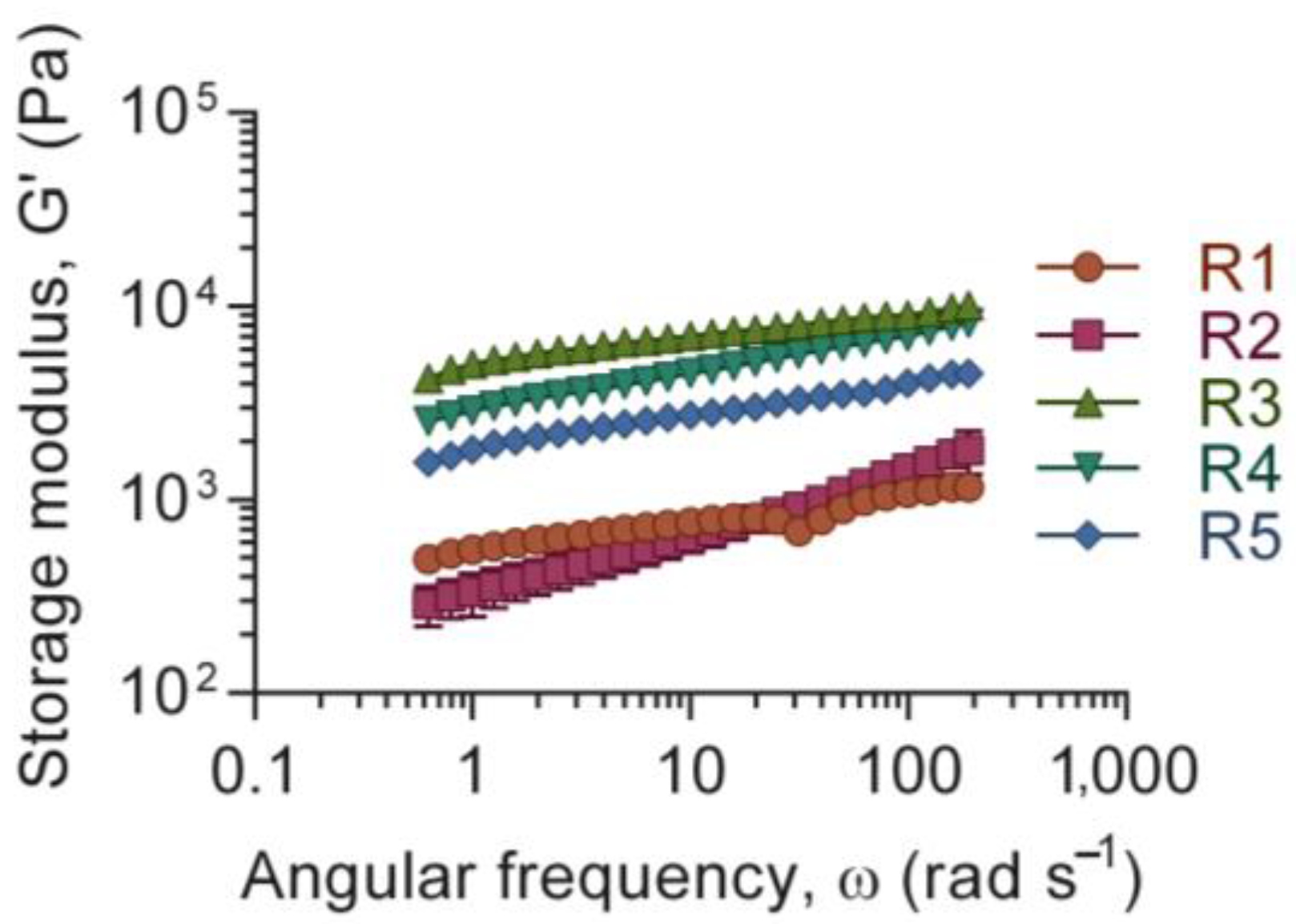
3.1. Ink Rheology
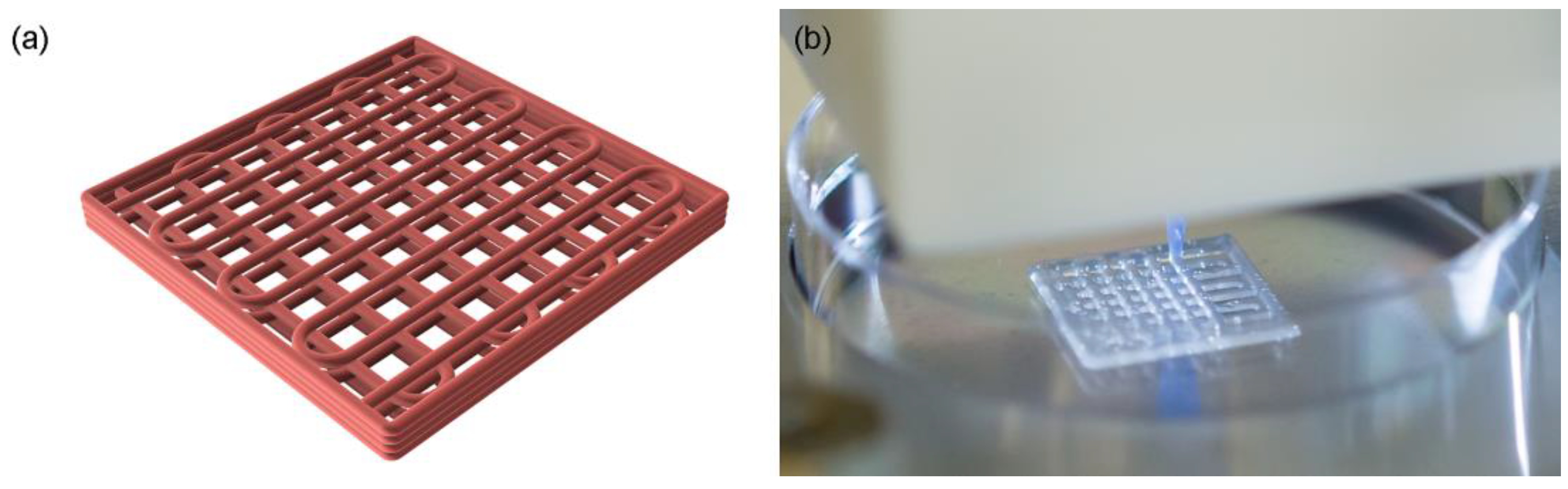
3.2. Three-Dimensional Printing and Structural Characterization
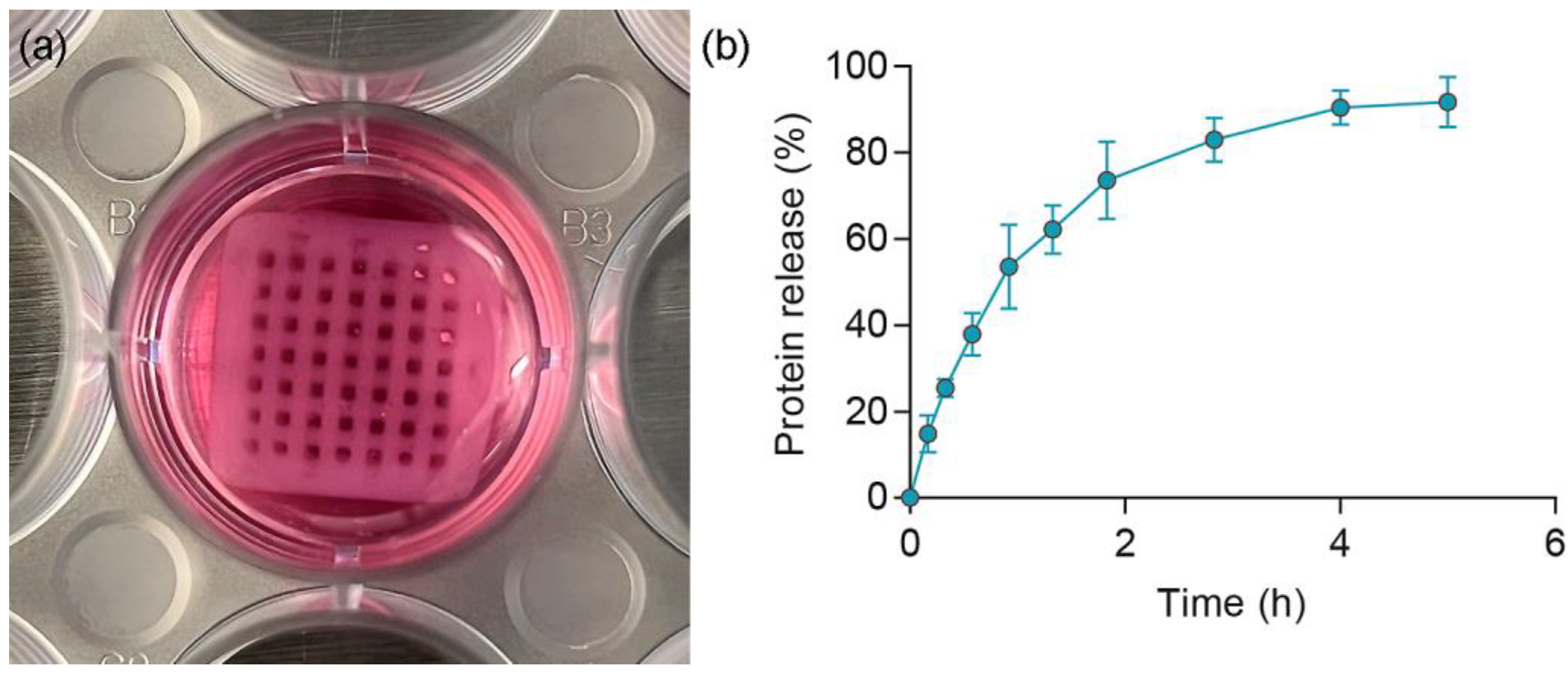
3.3. Release Profile
3.4. Chemical Characterization
3.5. Bioburden Analysis
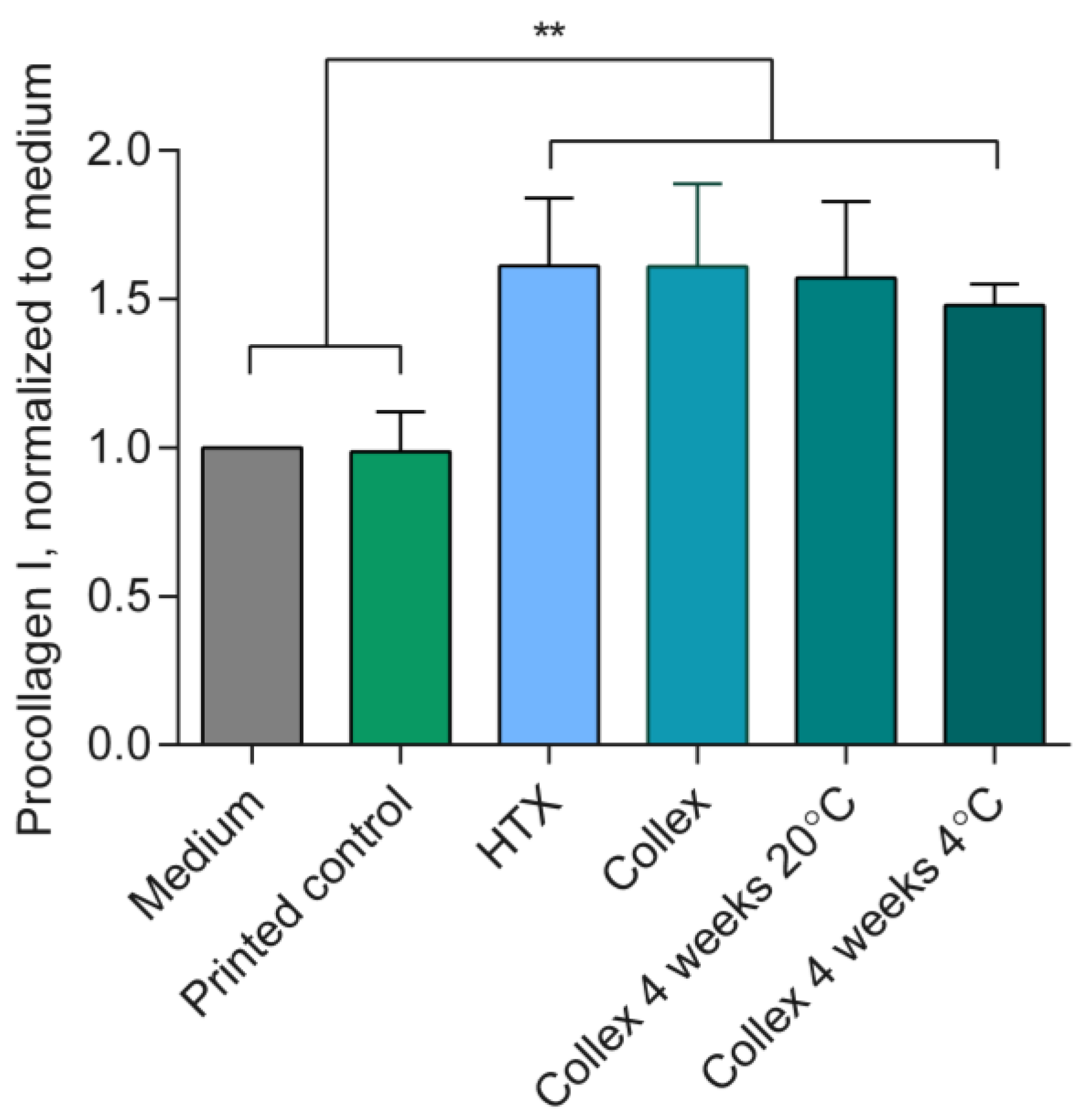
3.6. Bioactivity and Biocompatibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reinke, J.M.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Xiaojie, W.; Banda, J.; Qi, H.; Chang, A.K.; Bwalya, C.; Chao, L.; Li, X. Scarless wound healing: Current insights from the perspectives of TGF-beta, KGF-1, and KGF-2. Cytokine Growth Factor Rev. 2022, 66, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Xue, M.; Zhao, R.; Lin, H.; Jackson, C. Delivery systems of current biologicals for the treatment of chronic cutaneous wounds and severe burns. Adv. Drug Deliv. Rev. 2018, 129, 219–241. [Google Scholar] [CrossRef]
- Ogawa, R. Keloid and Hypertrophic Scars Are the Result of Chronic Inflammation in the Reticular Dermis. Int. J. Mol. Sci. 2017, 18, 606. [Google Scholar] [CrossRef] [PubMed]
- Chappidi, S.; Buddolla, V.; Ankireddy, S.R.; Lakshmi, B.A.; Kim, Y.J. Recent trends in diabetic wound healing with nanofibrous scaffolds. Eur. J. Pharmacol. 2023, 945, 175617. [Google Scholar] [CrossRef]
- Oncul, U.; Dalgıç, N.; Demir, M.; Karadeniz, P.; Karadağ, Ç.A. Use of procalcitonin as a biomarker for sepsis in pediatric burns. Eur. J. Pediatr. 2023, 182, 1561–1567. [Google Scholar] [CrossRef]
- Yunoki, S.; Kohta, M.; Ohyabu, Y.; Iwasaki, T. In Vitro Parallel Evaluation of Antibacterial Activity and Cytotoxicity of Commercially Available Silver-Containing Wound Dressings. Plast. Surg. Nurs. 2015, 35, 203–211. [Google Scholar] [CrossRef]
- Terzioğlu, E.; Arslan, M.; Balaban, B.G.; Çakar, Z.P. Microbial silver resistance mechanisms: Recent developments. World J. Microbiol. Biotechnol. 2022, 38, 158. [Google Scholar] [CrossRef]
- NDSR Food and Nutrient Database. Developed by the Nutrition Coordinating Center (NCC), University of Minnesota, Minneapolis, MN. 2022. Available online: http://www.ncc.umn.edu/ndsr-database-page/ (accessed on 10 March 2023).
- Schakel, S.F.; Sievert, Y.A.; Buzzard, I.M. Sources of data for developing and maintaining a nutrient database. J. Am. Diet. Assoc. 1988, 88, 1268–1271. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, S. Immune-Relevant and Antioxidant Activities of Vitellogenin and Yolk Proteins in Fish. Nutrients 2015, 7, 8818–8829. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Mlyniec, K.; Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-kappaB signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef]
- Birjandi Nejad, H.; Blasco, L.; Moran, B.; Cebrian, J.; Woodger, J.; Gonzalez, E.; Pritts, C.; Milligan, J. Bio-based Algae Oil: An oxidation and structural analysis. Int. J. Cosmet. Sci. 2020, 42, 237–247. [Google Scholar] [CrossRef]
- Michalak, M.; Pierzak, M.; Krecisz, B.; Suliga, E. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Et Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Ishak, W.M.W.; Katas, H.; Yuen, N.P.; Abdullah, M.A.; Zulfakar, M.H. Topical application of omega-3-, omega-6-, and omega-9-rich oil emulsions for cutaneous wound healing in rats. Drug Deliv. Transl. Res. 2019, 9, 418–433. [Google Scholar] [CrossRef]
- Stone, R., 2nd; Saathoff, E.C.; Larson, D.A.; Wall, J.T.; Wienandt, N.A.; Magnusson, S.; Kjartansson, H.; Natesan, S.; Christy, R.J. Accelerated Wound Closure of Deep Partial Thickness Burns with Acellular Fish Skin Graft. Int. J. Mol. Sci. 2021, 22, 1590. [Google Scholar] [CrossRef]
- Jais, A.M.; Matori, M.F.; Kittakoop, P.; Sowanborirux, K. Fatty acid compositions in mucus and roe of Haruan, Channa striatus, for wound healing. Gen. Pharmacol. 1998, 30, 561–563. [Google Scholar] [CrossRef]
- Junker, J.P.; Kamel, R.A.; Caterson, E.J.; Eriksson, E. Clinical Impact Upon Wound Healing and Inflammation in Moist, Wet, and Dry Environments. Adv. Wound Care 2013, 2, 348–356. [Google Scholar] [CrossRef]
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound healing and treating wounds: Chronic wound care and management. J. Am. Acad. Dermatol. 2016, 74, 607–625, quiz 625–606. [Google Scholar] [CrossRef] [PubMed]
- Ivone, R.; Yang, Y.; Shen, J. Recent Advances in 3D Printing for Parenteral Applications. AAPS J. 2021, 23, 87. [Google Scholar] [CrossRef] [PubMed]
- Tabriz, A.G.; Douroumis, D. Recent advances in 3D printing for wound healing: A systematic review. J. Drug Deliv. Sci. Technol. 2022, 74, 103564. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, K.; Kumar, P.; Choonara, Y.E.; du Toit, L.C.; Pillay, V. Three-dimensional printing of extracellular matrix (ECM)-mimicking scaffolds: A critical review of the current ECM materials. J. Biomed. Mater. Res. Part A 2020, 108, 2324–2350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, X. Alginate hydrogel dressings for advanced wound management. Int. J. Biol. Macromol. 2020, 162, 1414–1428. [Google Scholar] [CrossRef]
- Wang, T.; Yi, W.; Zhang, Y.; Wu, H.; Fan, H.; Zhao, J.; Wang, S. Sodium alginate hydrogel containing platelet-rich plasma for wound healing. Colloids Surf. B Biointerfaces 2023, 222, 113096. [Google Scholar] [CrossRef]
- Bowers, S.; Franco, E. Chronic Wounds: Evaluation and Management. Am. Fam. Physician 2020, 101, 159–166. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Gombotz, W.R.; Wee, S. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 1998, 31, 267–285. [Google Scholar] [CrossRef]
- Tudoroiu, E.E.; Dinu-Pirvu, C.E.; Albu Kaya, M.G.; Popa, L.; Anuta, V.; Prisada, R.M.; Ghica, M.V. An Overview of Cellulose Derivatives-Based Dressings for Wound-Healing Management. Pharmaceuticals 2021, 14, 1215. [Google Scholar] [CrossRef]
- Wang, C.; Bai, J.; Tian, P.; Xie, R.; Duan, Z.; Lv, Q.; Tao, Y. The Application Status of Nanoscale Cellulose-Based Hydrogels in Tissue Engineering and Regenerative Biomedicine. Front. Bioeng. Biotechnol. 2021, 9, 732513. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, J.; Svanstrom, A.; Berglin, M.; Petronis, S.; Bogestal, Y.; Stenlund, P.; Standoft, S.; Stahlberg, A.; Landberg, G.; Chinga-Carrasco, G.; et al. 3D Printed Nanocellulose Scaffolds as a Cancer Cell Culture Model System. Bioengineering 2021, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Chinga-Carrasco, G. Potential and Limitations of Nanocelluloses as Components in Biocomposite Inks for Three-Dimensional Bioprinting and for Biomedical Devices. Biomacromolecules 2018, 19, 701–711. [Google Scholar] [CrossRef]
- Leppiniemi, J.; Lahtinen, P.; Paajanen, A.; Mahlberg, R.; Metsa-Kortelainen, S.; Pinomaa, T.; Pajari, H.; Vikholm-Lundin, I.; Pursula, P.; Hytonen, V.P. 3D-Printable Bioactivated Nanocellulose-Alginate Hydrogels. ACS Appl. Mater. Interfaces 2017, 9, 21959–21970. [Google Scholar] [CrossRef] [PubMed]
- Gilljam, K.M.; Stenlund, P.; Standoft, S.; Andersen, S.B.; Kaaber, K.; Lund, H.; Bryn, K.R.K. Alginate and nanocellulose dressings with extract from salmon roe reduce inflammation and accelerate healing of porcine burn wounds. J. Burn Care Res. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Paxton, N.; Smolan, W.; Bock, T.; Melchels, F.; Groll, J.; Jungst, T. Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability. Biofabrication 2017, 9, 044107. [Google Scholar] [CrossRef]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef]
- Ma, J.; Lin, Y.; Chen, X.; Zhao, B.; Zhang, J. Flow behavior, thixotropy and dynamical viscoelasticity of sodium alginate aqueous solutions. Food Hydrocoll. 2014, 38, 119–128. [Google Scholar] [CrossRef]
- Pääkkö, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; et al. Enzymatic Hydrolysis Combined with Mechanical Shearing and High-Pressure Homogenization for Nanoscale Cellulose Fibrils and Strong Gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef]
- Leonardo, M.; Prajatelistia, E.; Judawisastra, H. Alginate-based bioink for organoid 3D bioprinting: A review. Bioprinting 2022, 28, e00246. [Google Scholar] [CrossRef]
- Kavanagh, G.M.; Ross-Murphy, S.B. Rheological characterisation of polymer gels. Prog. Polym. Sci. 1998, 23, 533–562. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Xu, C. Nanocellulose-Based Inks for 3D Bioprinting: Key Aspects in Research Development and Challenging Perspectives in Applications—A Mini Review. Bioengineering 2020, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, P.; Siqueira, É.; de Lima, A.E.; Siqueira, G.; Pinzón-Garcia, A.D.; Lopes, A.P.; Segura, M.E.C.; Isaac, A.; Pereira, F.V.; Botaro, V.R. Three-Dimensional Stable Alginate-Nanocellulose Gels for Biomedical Applications: Towards Tunable Mechanical Properties and Cell Growing. Nanomaterials 2019, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Cutting, K.F. Wound exudate: Composition and functions. Br. J. Community Nurs. 2003, 8, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Bradford, C.; Freeman, R.; Percival, S.L. In vitro study of sustained antimicrobial activity of a new silver alginate dressing. J. Am. Col. Certif. Wound Spec. 2009, 1, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Price, B.L.; Lovering, A.M.; Bowling, F.L.; Dobson, C.B. Development of a Novel Collagen Wound Model To Simulate the Activity and Distribution of Antimicrobials in Soft Tissue during Diabetic Foot Infection. Antimicrob. Agents Chemother. 2016, 60, 6880–6889. [Google Scholar] [CrossRef] [PubMed]
- Stechmiller, J.K. Understanding the role of nutrition and wound healing. Nutr. Clin. Pract. 2010, 25, 61–68. [Google Scholar] [CrossRef]
- Wright, J.A.; Richards, T.; Srai, S.K. The role of iron in the skin and cutaneous wound healing. Front. Pharmacol. 2014, 5, 156. [Google Scholar] [CrossRef]
- Berksoy Hayta, S.; Durmuş, K.; Altuntaş, E.E.; Yildiz, E.; Hisarciklıo, M.; Akyol, M. The reduction in inflammation and impairment in wound healing by using strontium chloride hexahydrate. Cutan. Ocul. Toxicol. 2018, 37, 24–28. [Google Scholar] [CrossRef]
- Tenaud, I.; Sainte-Marie, I.; Jumbou, O.; Litoux, P.; Dréno, B. In vitro modulation of keratinocyte wound healing integrins by zinc, copper and manganese. Br. J. Dermatol. 1999, 140, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B.; Mirastschijski, U.; Stubbs, N.; Scanlon, E.; Agren, M.S. Zinc in wound healing: Theoretical, experimental, and clinical aspects. Wound Repair Regen. 2007, 15, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.R.; Aalaa, M.; Nasli-Esfahani, E.; Atlasi, R.; Sanjari, M.; Namazi, N. The effects of dietary/herbal supplements and the serum levels of micronutrients on the healing of diabetic foot ulcers in animal and human models: A systematic review. J. Diabetes Metab. Disord. 2021, 20, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, L.; Halila, R.; Ryhänen, L. Enzymes converting procollagens to collagens. J. Cell. Biochem. 1985, 28, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Gajbhiye, S.; Wairkar, S. Collagen fabricated delivery systems for wound healing: A new roadmap. Biomater. Adv. 2022, 142, 213152. [Google Scholar] [CrossRef] [PubMed]
- Tsegay, F.; Elsherif, M.; Butt, H. Smart 3D Printed Hydrogel Skin Wound Bandages: A Review. Polymers 2022, 14, 1012. [Google Scholar] [CrossRef] [PubMed]

| Ink | Alginate (%) | Nanocellulose (%) | HTX (%) |
|---|---|---|---|
| R1 | 0.5 | 1.0 | 12 |
| R2 | 2.0 | 1.0 | 12 |
| R3 | 0.5 | 1.7 | 12 |
| R4 | 2.0 | 1.7 | 12 |
| R5 | 1.0 | 1.4 | 12 |
| Micro-Organisms | American Type Culture Collection (ATCC) Number |
|---|---|
| Staphylococcus aureus | 6538 |
| Pseudomonas aeruginosa | 9027 |
| Bacillus subtilis | 6633 |
| Candida albicans | 10,231 |
| Aspergillus brasiliensis | 16,404 |
| Ink | Alginate (%) | Nanocellulose (%) | HTX (%) | Yield Stress (Pa) |
|---|---|---|---|---|
| R1 | 0.5 | 1.0 | 12 | 6.7 ± 3.1 |
| R2 | 2.0 | 1.0 | 12 | 34.7 ± 3 |
| R3 | 0.5 | 1.7 | 12 | 28.8 ± 1.9 |
| R4 | 2.0 | 1.7 | 12 | 116.2 ± 6 |
| R5 | 1.0 | 1.4 | 12 | 33.9 ± 5.3 |
| Ink | Grid Uniformity | Filament Width (mm) | Pore Size (mm) | Patch Height (mm) |
|---|---|---|---|---|
| R1 | Non-existing | N/A | N/A | 0.5 |
| R2 | Low | 0.98 ± 0.19 | 0.77 ± 0.19 | 0.7 |
| R3 | Middle | 0.65 ± 0.08 | 1.19 ± 0.12 | 0.9 |
| R4 | High | 0.54 ± 0.05 | 1.24 ± 0.06 | 1.0 |
| R5 | Low | 0.83 ± 0.25 | 0.94 ± 0.25 | 0.7 |
| Element | Amount (ng/g Test Item (a)) | Amount (ng/g Test Item (b)) |
|---|---|---|
| Iron, Fe | 510 ± 23 | 530 ± 0 |
| Manganese, Mn | 430 ± 25 | 470 ± 6 |
| Strontium, Sr | 210 ± 6 | 230 ± 0 |
| Zinc, Zn | 3300 ± 58 | 3600 ± 58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stenlund, P.; Enstedt, L.; Gilljam, K.M.; Standoft, S.; Ahlinder, A.; Lundin Johnson, M.; Lund, H.; Millqvist Fureby, A.; Berglin, M. Development of an All-Marine 3D Printed Bioactive Hydrogel Dressing for Treatment of Hard-to-Heal Wounds. Polymers 2023, 15, 2627. https://doi.org/10.3390/polym15122627
Stenlund P, Enstedt L, Gilljam KM, Standoft S, Ahlinder A, Lundin Johnson M, Lund H, Millqvist Fureby A, Berglin M. Development of an All-Marine 3D Printed Bioactive Hydrogel Dressing for Treatment of Hard-to-Heal Wounds. Polymers. 2023; 15(12):2627. https://doi.org/10.3390/polym15122627
Chicago/Turabian StyleStenlund, Patrik, Linnea Enstedt, Karin Margaretha Gilljam, Simon Standoft, Astrid Ahlinder, Maria Lundin Johnson, Henrik Lund, Anna Millqvist Fureby, and Mattias Berglin. 2023. "Development of an All-Marine 3D Printed Bioactive Hydrogel Dressing for Treatment of Hard-to-Heal Wounds" Polymers 15, no. 12: 2627. https://doi.org/10.3390/polym15122627
APA StyleStenlund, P., Enstedt, L., Gilljam, K. M., Standoft, S., Ahlinder, A., Lundin Johnson, M., Lund, H., Millqvist Fureby, A., & Berglin, M. (2023). Development of an All-Marine 3D Printed Bioactive Hydrogel Dressing for Treatment of Hard-to-Heal Wounds. Polymers, 15(12), 2627. https://doi.org/10.3390/polym15122627







