High Wear Resistance of POSS Grafted-Polyimide/Silica Composites under Atomic Oxygen Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
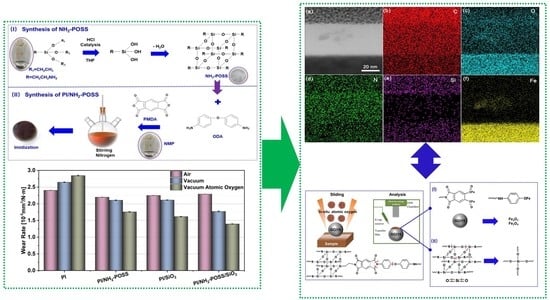
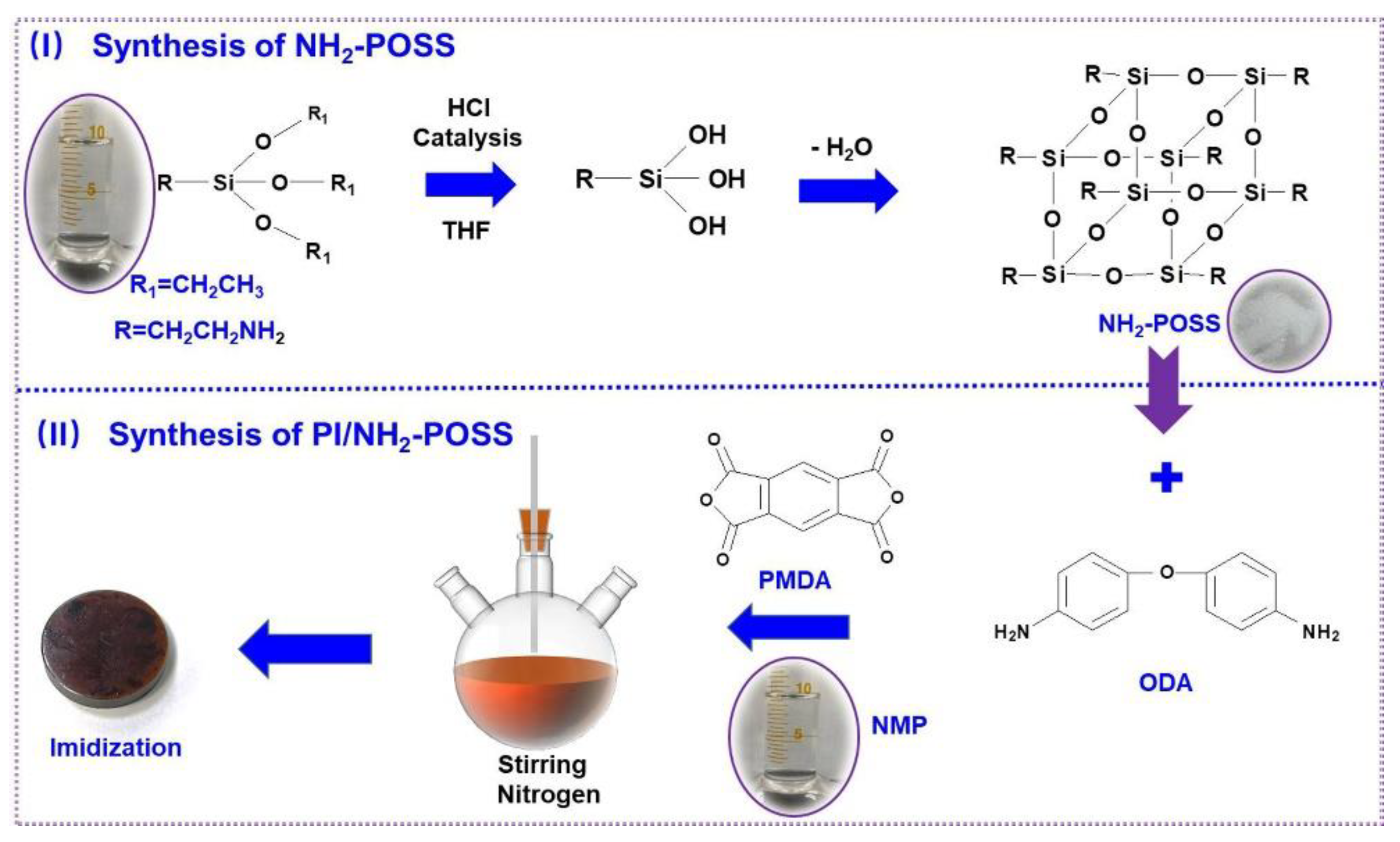
2.2. Synthesis of Polyimide Composites
2.3. Atomic Oxygen Irradiation and Tribology Tests
2.4. Characterization of Polyimide Composites
2.5. Characterization of Worn Surfaces and Tribofilms
3. Results and Discussions
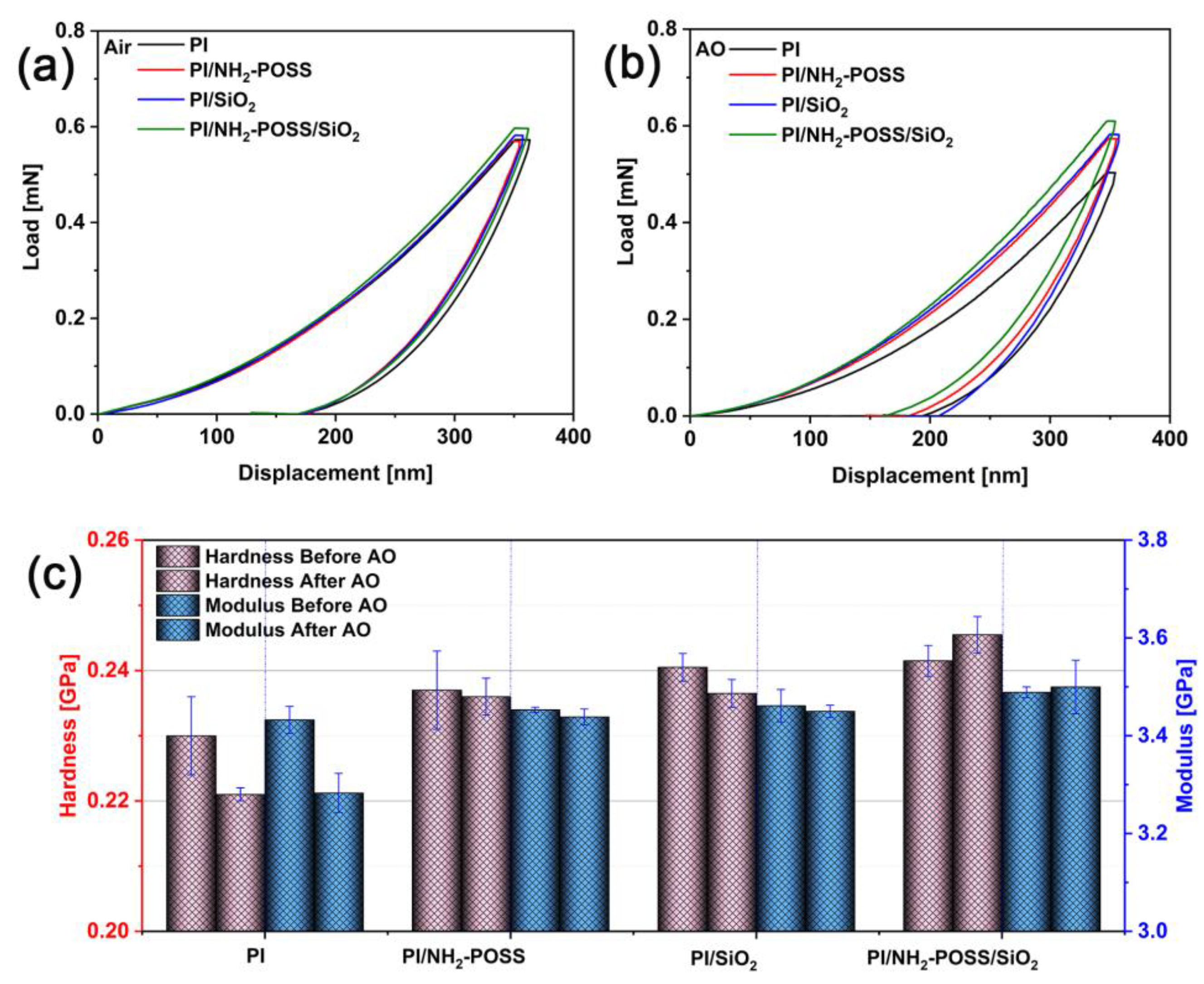
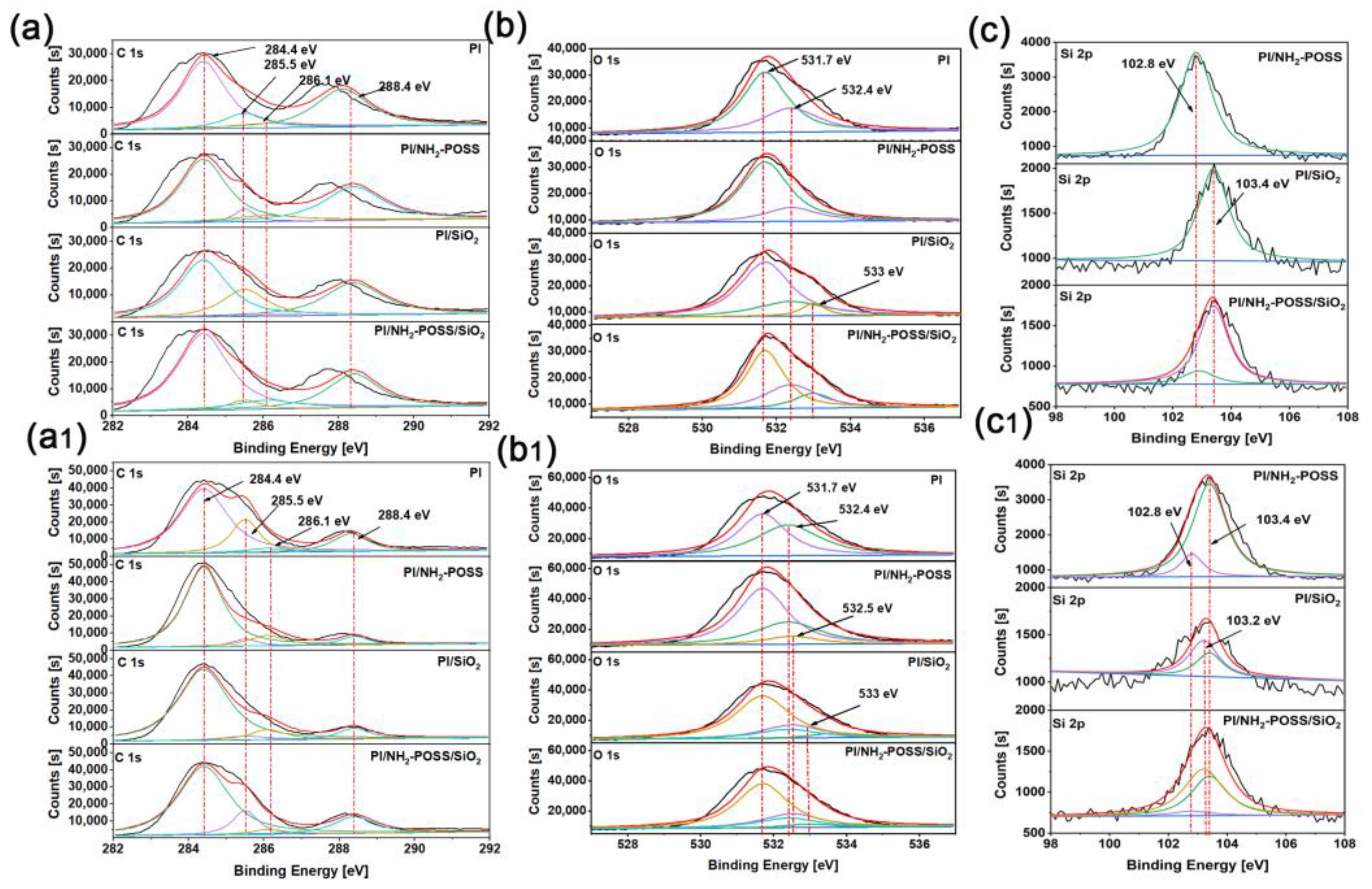
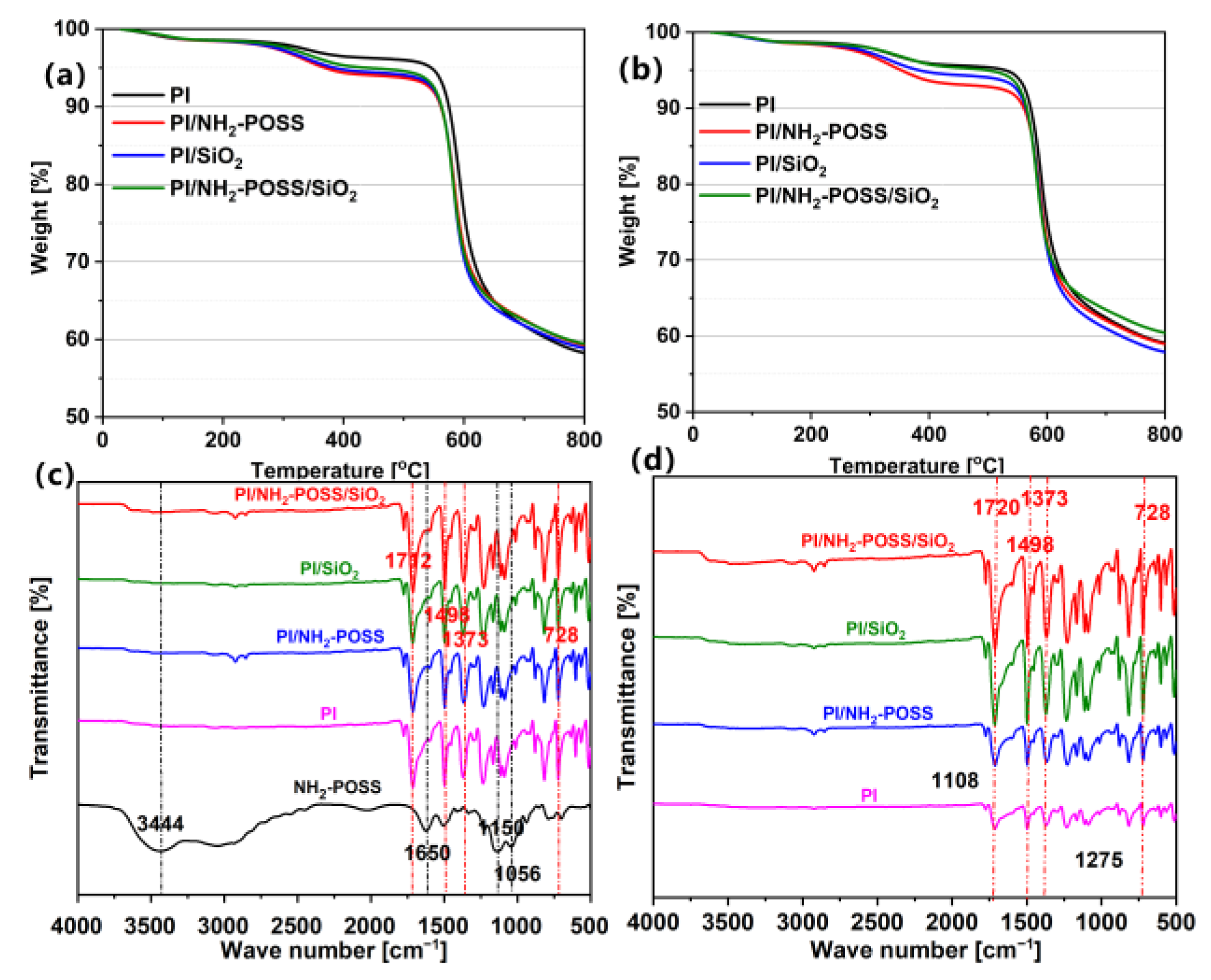
3.1. Mechanical and Structure Analysis
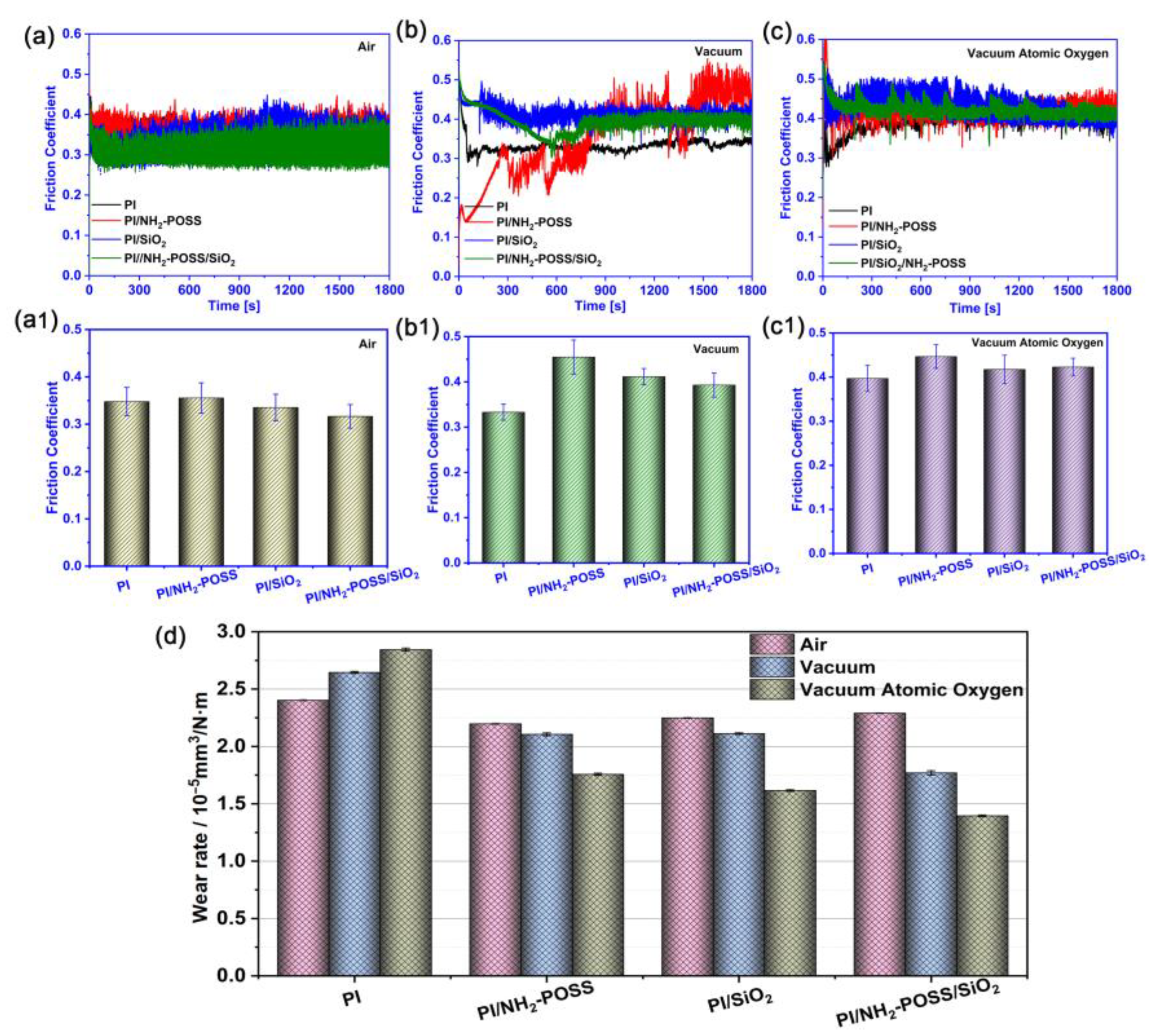
3.2. Tribological Behaviors
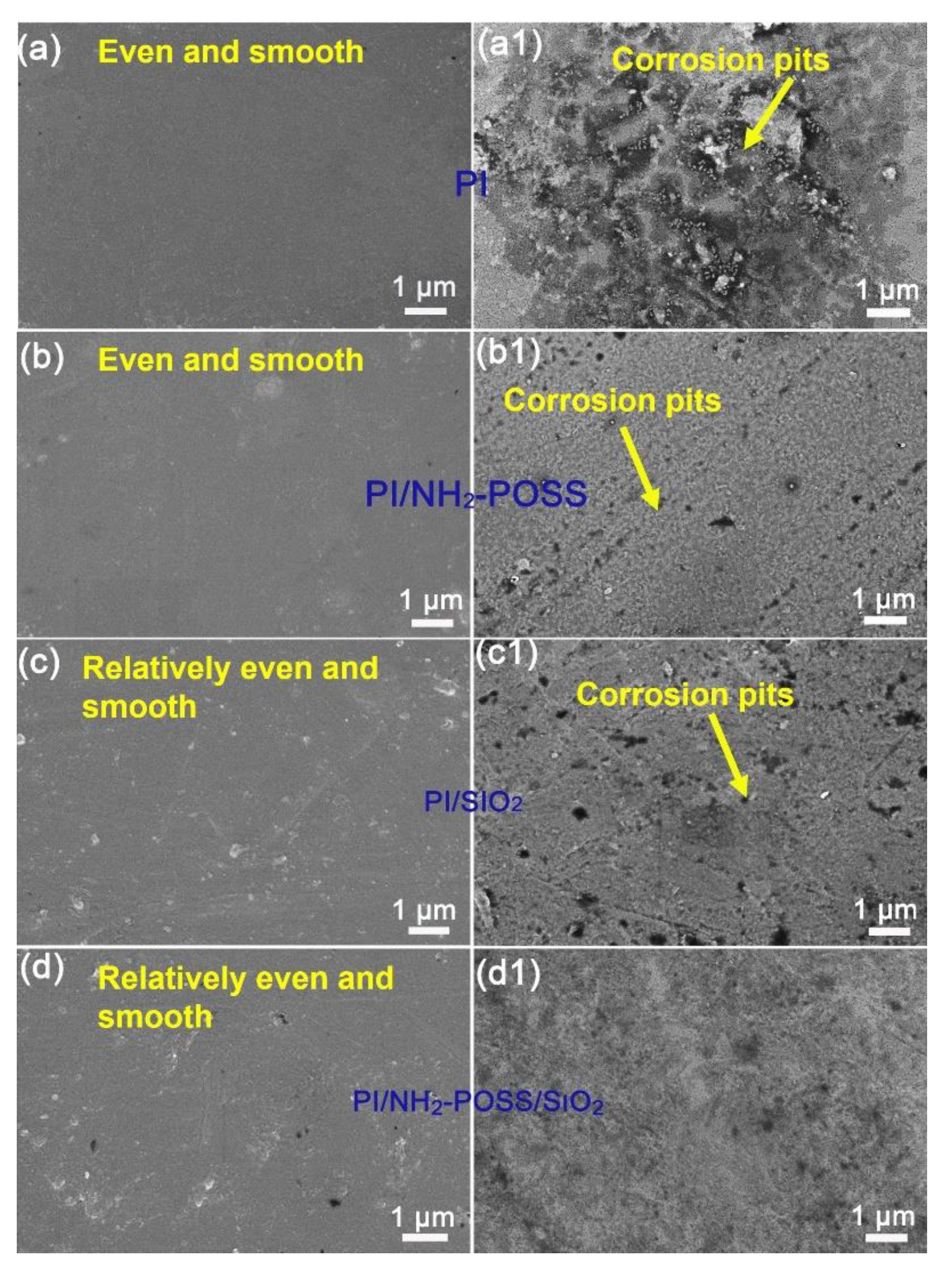
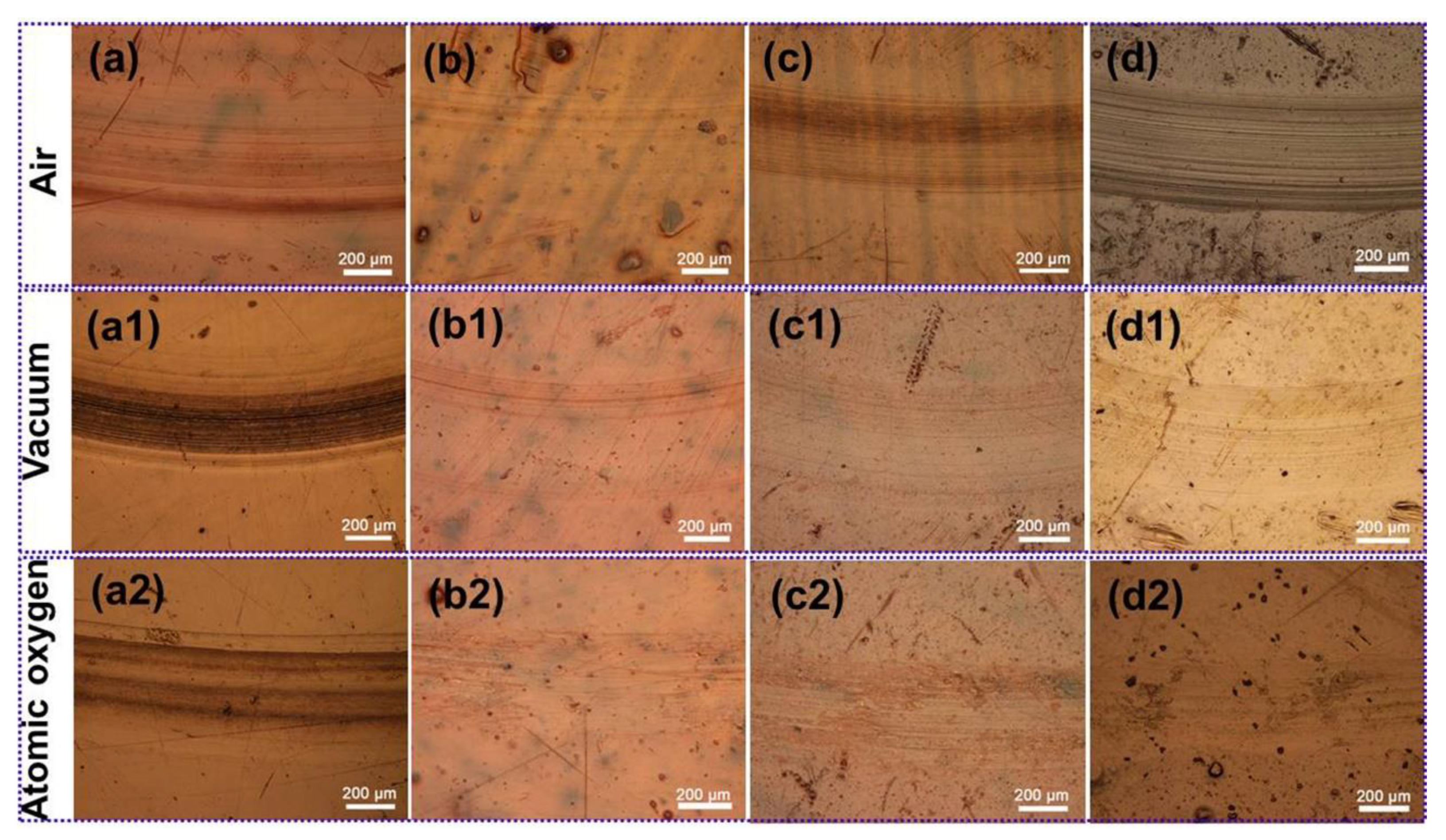
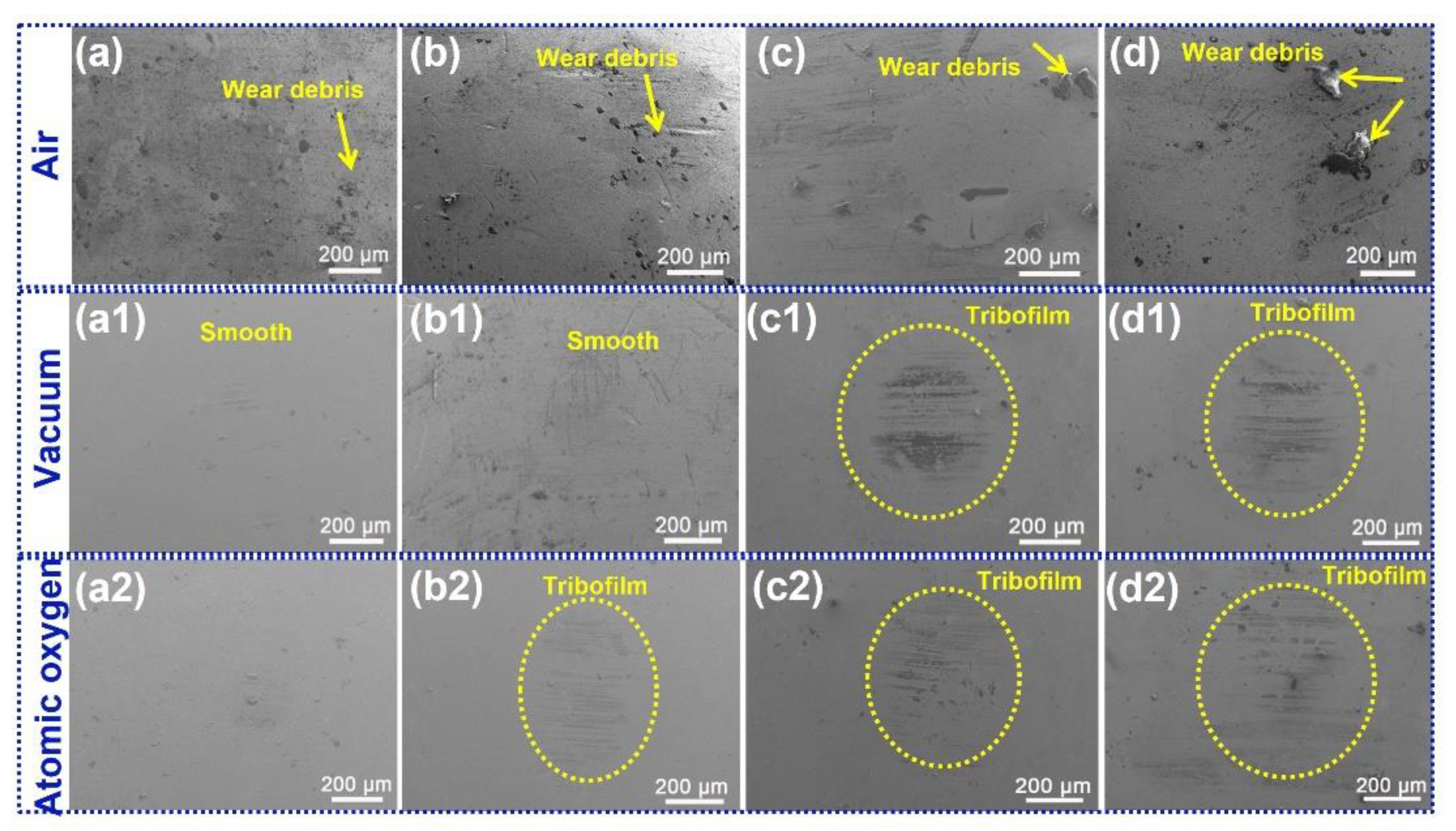
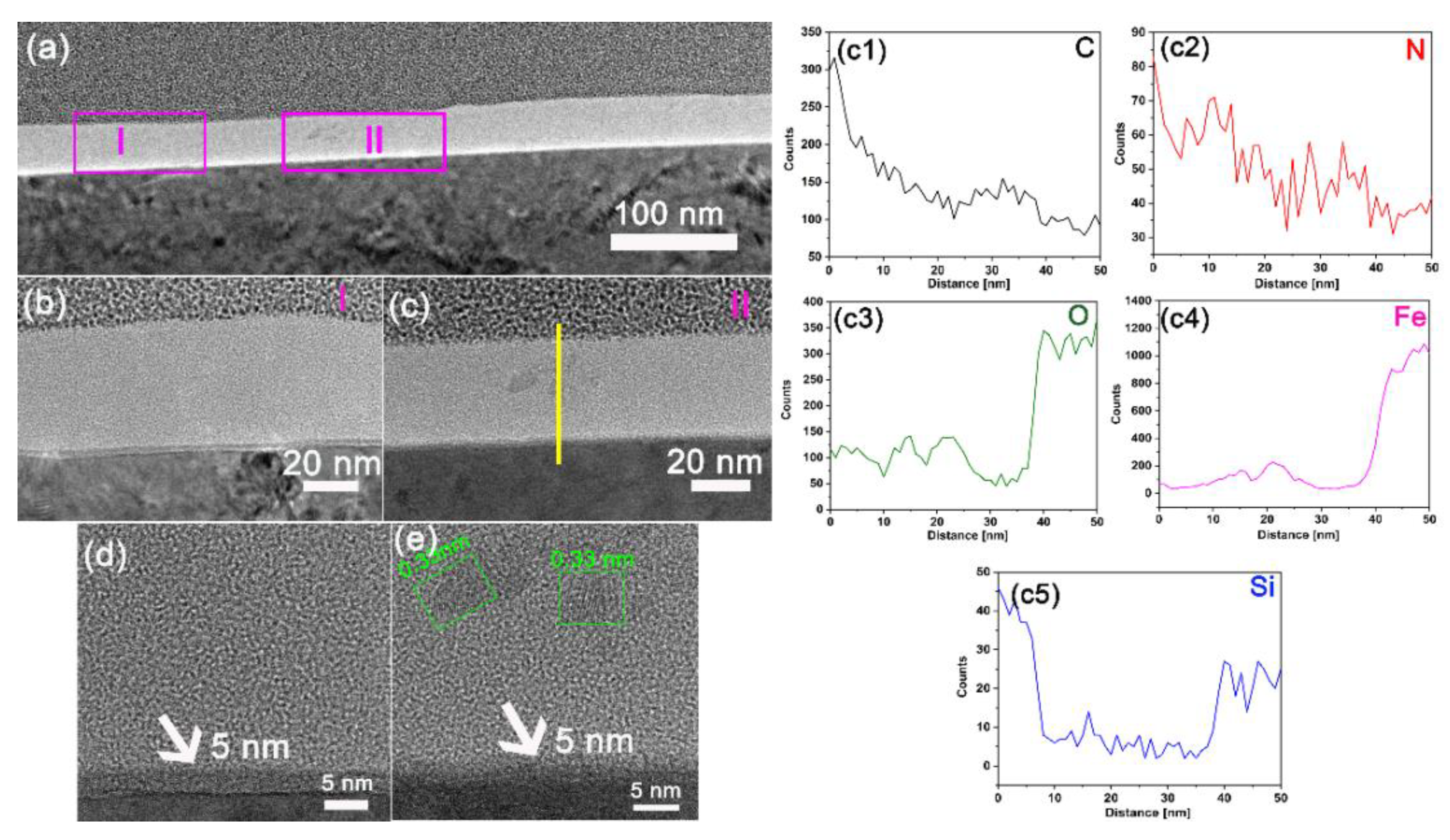
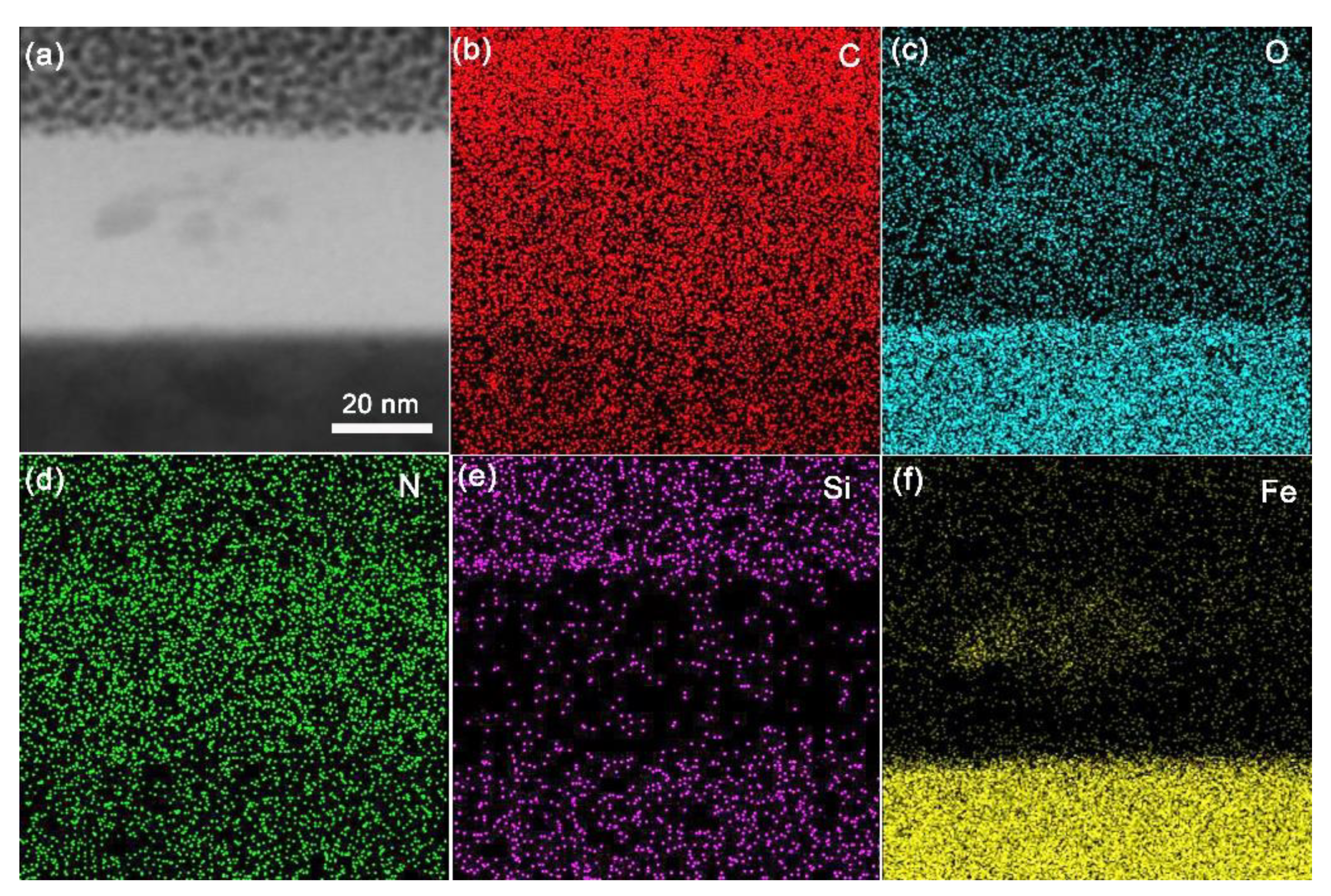
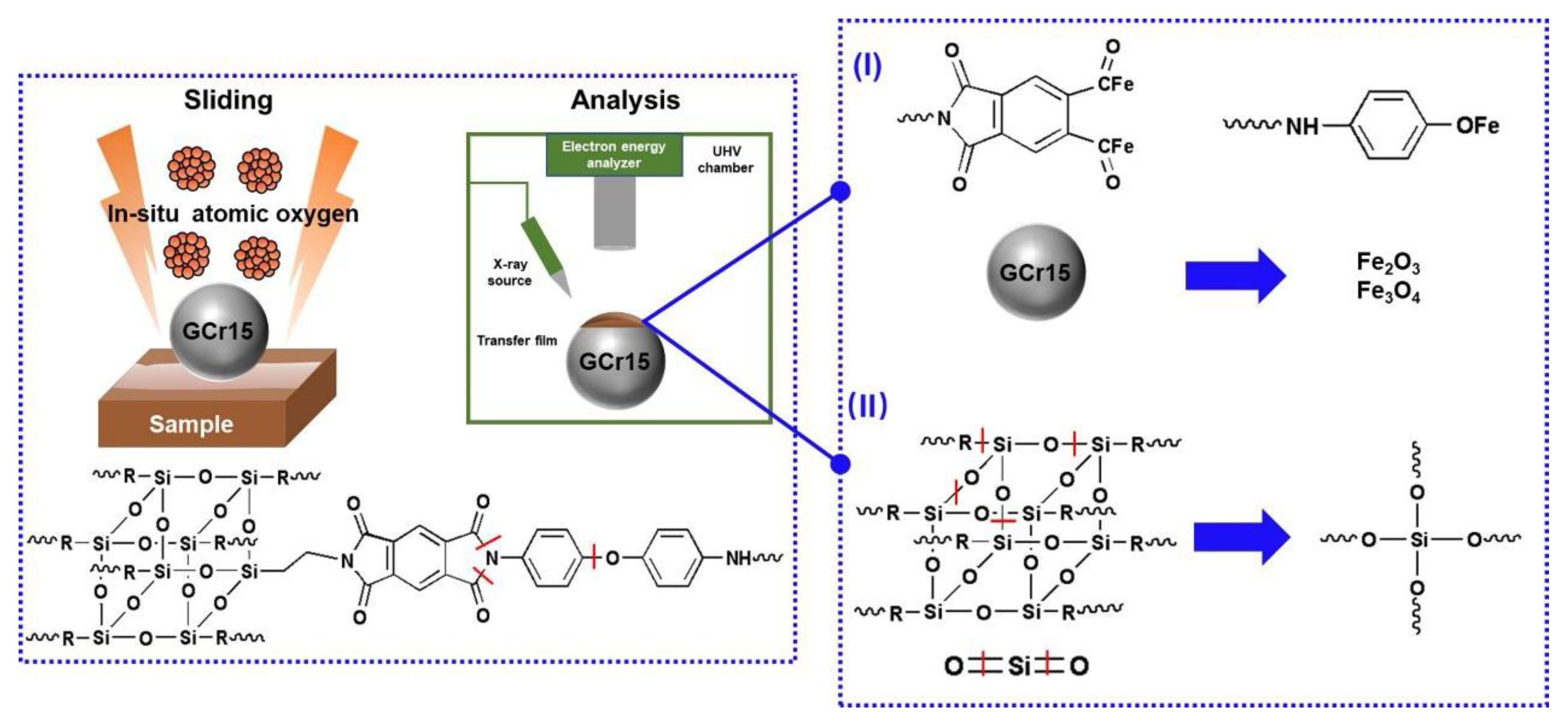
3.3. Analysis of Tribological Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minton, T.K.; Wright, M.E.; Tomczak, S.J.; Marquez, S.A.; Shen, L.; Brunsvold, A.L.; Cooper, R.; Zhang, J.; Vij, V.; Guenthner, A.J.; et al. Atomic oxygen effects on POSS polyimides in low earth orbit. ACS Appl. Mater. Interfaces 2012, 4, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, H.; Liu, C.; Zhang, L.; Wang, Q.; Hu, H.; Zheng, J. Influence of bias patterns on the tribological properties of highly hydrogenated PVD a-C:H films. Surf. Coat. Technol. 2022, 442, 128234. [Google Scholar] [CrossRef]
- Hu, H.; Liu, X.; Zhang, K.; Cao, Z.; Gou, S.; Zheng, Y.; Feng, X.; Hao, H.; Zhou, H. Research on the damage mechanism of low current 50 keV electron beam on the micro structure, composition and vacuum tribological performance of MoS2-Ti films deposited by unbalanced magnetron sputtering. Surf. Coat. Technol. 2021, 406, 126708. [Google Scholar] [CrossRef]
- Liu, X.; Gong, P.; Hu, H.; Zhao, M.; Zhang, K.; Zhou, H. Study on the tribological properties of PVD polymer-like carbon films in air/vacuum/N2 and cycling environments. Surf. Coat. Technol. 2021, 406, 126677. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, D.; Wang, D.; Yu, Q.; Bai, Y.; Cai, M.; Weng, L.; Zhou, F.; Liu, W. MoS2 Lubricating Film Meets Supramolecular Gel: A Novel Composite Lubricating System for Space Applications. ACS Appl. Mater. Interfaces 2021, 13, 58036–58047. [Google Scholar] [CrossRef]
- Zhao, L.; Leng, X.; Bai, B.; Zhao, R.; Cai, Z.; He, J.; Li, J.; Chen, H. Effect of Coating Thickness on the Atomic Oxygen Resistance of Siloxane Coatings Synthesized by Plasma Polymerization Deposition Technique. Coatings 2023, 13, 153. [Google Scholar] [CrossRef]
- Feng, X.; Wang, R.; Wei, G.; Zheng, Y.; Hu, H.; Yang, L.; Zhang, K.; Zhou, H. Effect of a micro-textured surface with deposited MoS2-Ti film on long-term wear performance in vacuum. Surf. Coat. Technol. 2022, 445, 128722. [Google Scholar] [CrossRef]
- Meng, Y.; Xu, J.; Jin, Z.; Prakash, B.; Hu, Y. A review of recent advances in tribology. Friction 2020, 8, 221–300. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, C.; Yu, Q.; Zhang, J.; Zhang, M.; Cai, M.; Weng, L.; Liang, Y.; Zhou, F.; Liu, W. Supramolecular PFPE gel lubricant with anti-creep capability under irradiation conditions at high vacuum. Chem. Eng. J. 2021, 409, 128120. [Google Scholar] [CrossRef]
- Bai, Y.; Yu, Q.; Zhang, J.; Cai, M.; Liang, Y.; Zhou, F.; Liu, W. Soft-nanocomposite lubricants of supramolecular gel with carbon nanotubes. J. Mater. Chem. A 2019, 7, 7654–7663. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A.V. Approaches for Achieving Superlubricity in Two-Dimensional Materials. ACS Nano 2018, 12, 2122–2137. [Google Scholar] [CrossRef]
- Samyn, P.; Schoukens, G.; De Baets, P. Micro- to nanoscale surface morphology and friction response of tribological polyimide surfaces. Appl. Surf. Sci. 2010, 256, 3394–3408. [Google Scholar] [CrossRef]
- Roy, A.; Mu, L.; Shi, Y. Tribological properties of polyimide-graphene composite coatings at elevated temperatures. Prog. Org. Coat. 2020, 142, 105602. [Google Scholar] [CrossRef]
- Hamisa, A.H.; Azmi, W.H.; Ismail, M.F.; Rahim, R.A.; Ali, H.M. Tribology Performance of Polyol-Ester Based TiO2, SiO2, and Their Hybrid Nanolubricants. Lubricants 2023, 11, 18. [Google Scholar] [CrossRef]
- Yu, C.; Ju, P.; Wan, H.; Chen, L.; Li, H.; Zhou, H.; Chen, J. Enhanced atomic oxygen resistance and tribological properties of PAI/PTFE composites reinforced by POSS. Prog. Org. Coat. 2020, 139, 105427. [Google Scholar] [CrossRef]
- Yu, C.; Ju, P.; Wan, H.; Chen, L.; Li, H.; Zhou, H.; Chen, J. POSS-Grafted PAI/MoS2 Coatings for Simultaneously Improved Tribological Properties and Atomic Oxygen Resistance. Ind. Eng. Chem. Res. 2019, 58, 17027–17037. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, X.; Zhang, C.; Yang, Z.; Cheng, G.; Yang, Z.; Cai, M.; Zhou, F.; Liu, W. Resistance to space atomic oxygen radiation of MAC-based supramolecular gel lubricant containing POSS. Tribol. Int. 2023, 177, 107838. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, G.; Zheng, Z.; Yu, J.; Hu, C. Tribological properties of polyimide composites reinforced with fibers rubbing against Al2O3. Friction 2020, 9, 301–314. [Google Scholar] [CrossRef]
- Yan, X.; Yang, X.; Qi, X.; Lu, G.; Dong, Y.; Liu, C.; Fan, B. Tribological properties of PAO40@SiO2/PTFE/aramid fabric composites subjected to heavy-loading conditions. Tribol. Int. 2022, 166, 107336. [Google Scholar] [CrossRef]
- Ismail, M.F.; Azmi, W.H.; Mamat, R.; Ali, H.M. Thermal and Tribological Properties Enhancement of PVE Lubricant Modified with SiO2 and TiO2 Nanoparticles Additive. Nanomaterials 2022, 13, 42. [Google Scholar] [CrossRef]
- Shen, Y.; Lei, W.; Tang, W.; Ouyang, T.; Liang, L.; Tian, Z.Q.; Shen, P.K. Synergistic friction-reduction and wear-resistance mechanism of 3D graphene and SiO2 nanoblend at harsh friction interface. Wear 2022, 488–489, 204175. [Google Scholar] [CrossRef]
- Song, M.M.; Duo, S.W.; Liu, T.Z. Effects of Atomic Oxygen Irradiation on PDMS/POSS Hybrid Films in Low Earth Orbit Environment. Adv. Mater. Res. 2011, 239–242, 1368–1371. [Google Scholar] [CrossRef]
- Vernigorov, K.B.; Chugunova, A.A.; Alent’ev, A.Y.; Meshkov, I.B.; Muzafarov, A.M.; Novikov, L.S.; Chernik, V.N. Investigation of the structure of a polyimide modified by hyperbranched polyorganosiloxanes. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2012, 6, 760–763. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, G.-M.; Dai, X.-M.; Liu, F.-F.; Dong, Z.-X.; Qiu, X.-P. AO-resistant properties of polyimide fibers containing phosphorous groups in main chains. Chin. J. Polym. Sci. 2016, 34, 1469–1478. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, K.; Zhan, M. Atomic oxygen erosion resistance of polyimide/ZrO2 hybrid films. Appl. Surf. Sci. 2010, 256, 7384–7388. [Google Scholar] [CrossRef]
- Liu, K.; Mu, H.; Shu, M.; Li, Z.; Gao, Y. Improved adhesion between SnO2/SiO2 coating and polyimide film and its applications to atomic oxygen protection. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 356–362. [Google Scholar] [CrossRef]
- Shimamura, H.; Nakamura, T. Mechanical properties degradation of polyimide films irradiated by atomic oxygen. Polym. Degrad. Stab. 2009, 94, 1389–1396. [Google Scholar] [CrossRef]
- Bharadwaja, K.; Rao, S.S.; Baburao, T. Epoxy/SIO2 nanocomposite mechanical properties and tribological performance. Mater. Today Proc. 2022, 62, 1712–1716. [Google Scholar] [CrossRef]
- Lv, M.; Wang, Q.; Wang, T.; Liang, Y. Effects of atomic oxygen exposure on the tribological performance of ZrO2-reinforced polyimide nanocomposites for low earth orbit space applications. Compos. Part B Eng. 2015, 77, 215–222. [Google Scholar] [CrossRef]
- Song, H.; Zhang, Q.; Zhang, Y.; Wang, Y.; Zhou, Z.; Zhang, P.; Yuan, B. Waterborne polyurethane/3-amino-polyhedral oligomeric silsesquioxane (NH2-POSS) nanocomposites with enhanced properties. Adv. Compos. Hybrid Mater. 2021, 4, 629–638. [Google Scholar] [CrossRef]
- Hu, C.; Qi, H.; Song, J.; Zhao, G.; Yu, J.; Zhang, Y.; He, H.; Lai, J. Exploration on the tribological mechanisms of polyimide with different molecular structures in different temperatures. Appl. Surf. Sci. 2021, 560, 150051. [Google Scholar] [CrossRef]
- Wei, Z.; Weiping, L.; Huicong, L.; Liqun, Z. Erosion of a Polyimide Material Exposed to Simulated Atomic Oxygen Environment. Chin. J. Aeronaut. 2010, 23, 268–273. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, G.; Li, G.; Zhao, F.; Zhang, L.; Guo, F.; Zhang, G. Solvent-free ionic silica nanofluids: Smart lubrication materials exhibiting remarkable responsiveness to weak electrical stimuli. Chem. Eng. J. 2020, 383, 123202. [Google Scholar] [CrossRef]
- Zhou, L.; Qi, H.; Lei, Y.; Yu, J.; Guo, B.; Zhang, D. Ti3C2 MXene induced high tribological performance of polyimide/polyurea copolymer at a wide temperature range. Appl. Surf. Sci. 2023, 608, 155157. [Google Scholar] [CrossRef]
- Lv, M.; Wang, Y.; Wang, Q.; Wang, T.; Liang, Y. Effects of individual and sequential irradiation with atomic oxygen and protons on the surface structure and tribological performance of polyetheretherketone in a simulated space environment. RSC Adv. 2015, 5, 83065–83073. [Google Scholar] [CrossRef]
- Peter, C.J.; Graat, M.A.J.S. Simultaneous determination of composition and thickness of thin iron-oxide films from XPS Fe 2p spectra. Appl. Surf. Sci. 1996, 100, 36–40. [Google Scholar]
- Hu, C.; Qi, H.; Yu, J.; Zhang, G.; Zhang, Y.; He, H. Significant improvement on tribological performance of polyimide composites by tuning the tribofilm nanostructures. J. Mater. Process. Technol. 2020, 281, 116602. [Google Scholar] [CrossRef]
- Wang, Z.; Kong, L.; Guo, Z.; Zhang, X.; Wang, X.; Zhang, X. Bamboo-like SiO/C nanotubes with carbon coating as a durable and high-performance anode for lithium-ion battery. Chem. Eng. J. 2022, 428, 131060. [Google Scholar] [CrossRef]
- Xu, Y.; Qi, H.; Li, G.; Guo, X.; Wan, Y.; Zhang, G. Significance of an in-situ generated boundary film on tribocorrosion behavior of polymer-metal sliding pair. J. Colloid Interface Sci. 2018, 518, 263–276. [Google Scholar] [CrossRef]
| PI | NH2-POSS | SiO2 | |
|---|---|---|---|
| PI | 100 | / | / |
| PI/NH2-POSS | 99 | 1 | / |
| PI/SiO2 | 99 | / | 1 |
| PI/NH2-POSS/SiO2 | 98 | 1 | 1 |
| Air | Vacuum | Vacuum Atomic Oxygen | |
|---|---|---|---|
| PI | 772.04 | 576.85 | 597.33 |
| PI/NH2-POSS | 741.85 | 645.82 | 615.79 |
| PI/SiO2 | 692.27 | 673.53 | 633.04 |
| PI/NH2-POSS/SiO2 | 846.36 | 711.45 | 720.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, H.; Lei, Y.; Lei, X.; Zhang, D.; Zhang, Y.; Yu, J.; Guo, B. High Wear Resistance of POSS Grafted-Polyimide/Silica Composites under Atomic Oxygen Conditions. Polymers 2023, 15, 2385. https://doi.org/10.3390/polym15102385
Qi H, Lei Y, Lei X, Zhang D, Zhang Y, Yu J, Guo B. High Wear Resistance of POSS Grafted-Polyimide/Silica Composites under Atomic Oxygen Conditions. Polymers. 2023; 15(10):2385. https://doi.org/10.3390/polym15102385
Chicago/Turabian StyleQi, Huimin, Yang Lei, Xuemei Lei, Ding Zhang, Yafeng Zhang, Jiaxin Yu, and Baogang Guo. 2023. "High Wear Resistance of POSS Grafted-Polyimide/Silica Composites under Atomic Oxygen Conditions" Polymers 15, no. 10: 2385. https://doi.org/10.3390/polym15102385
APA StyleQi, H., Lei, Y., Lei, X., Zhang, D., Zhang, Y., Yu, J., & Guo, B. (2023). High Wear Resistance of POSS Grafted-Polyimide/Silica Composites under Atomic Oxygen Conditions. Polymers, 15(10), 2385. https://doi.org/10.3390/polym15102385