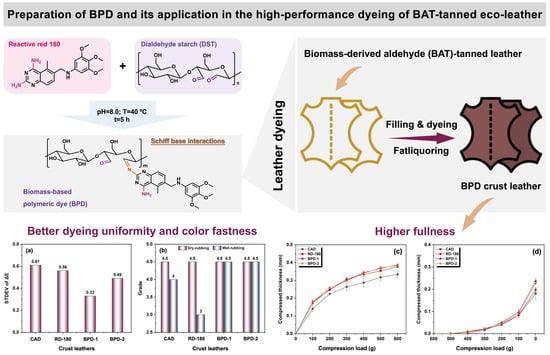
Novel Biomass-Based Polymeric Dyes: Preparation and Performance Assessment in the Dyeing of Biomass-Derived Aldehyde-Tanned Leather
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. BAT Tanning of Pickled Sheepskin
2.3. Preparation of BPD
2.4. Dyeing Trials for the BAT-Tanned Leather
2.5. Characterizations
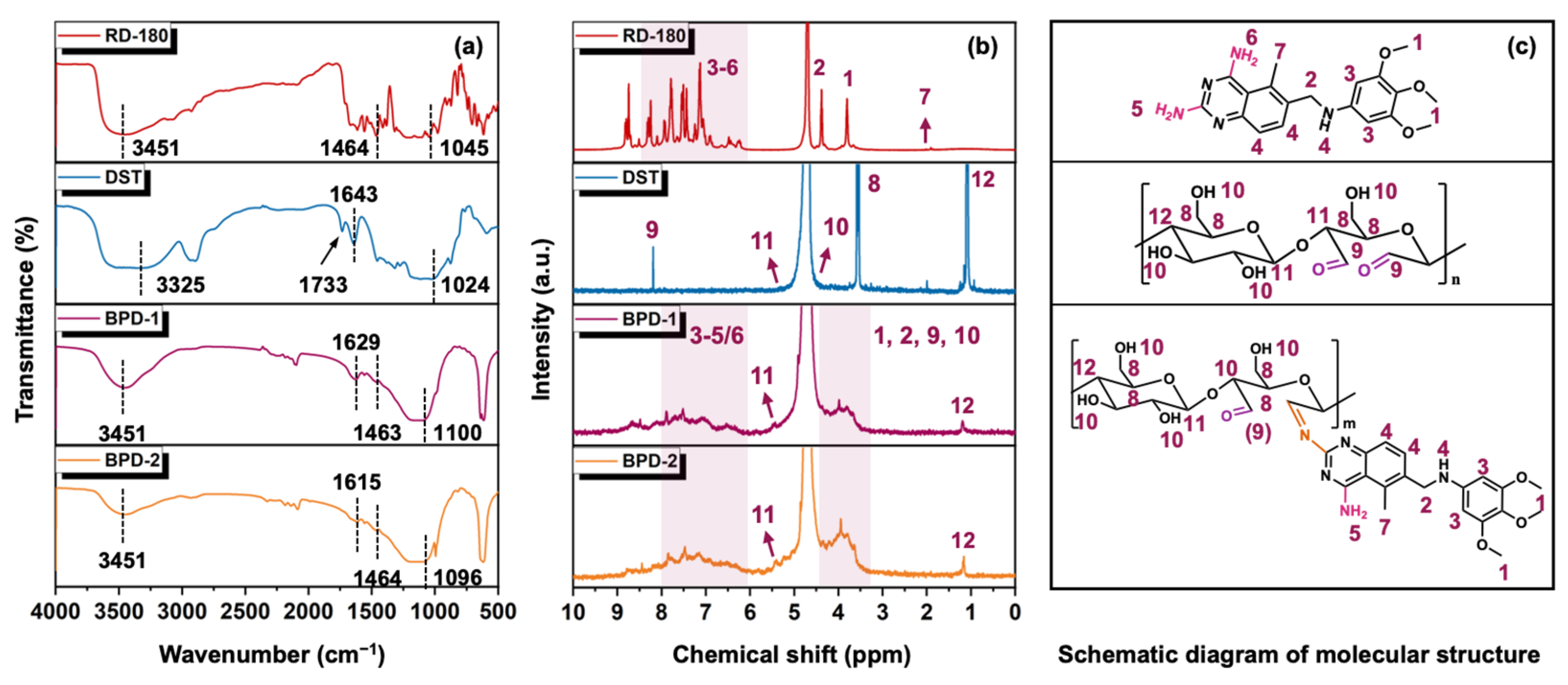
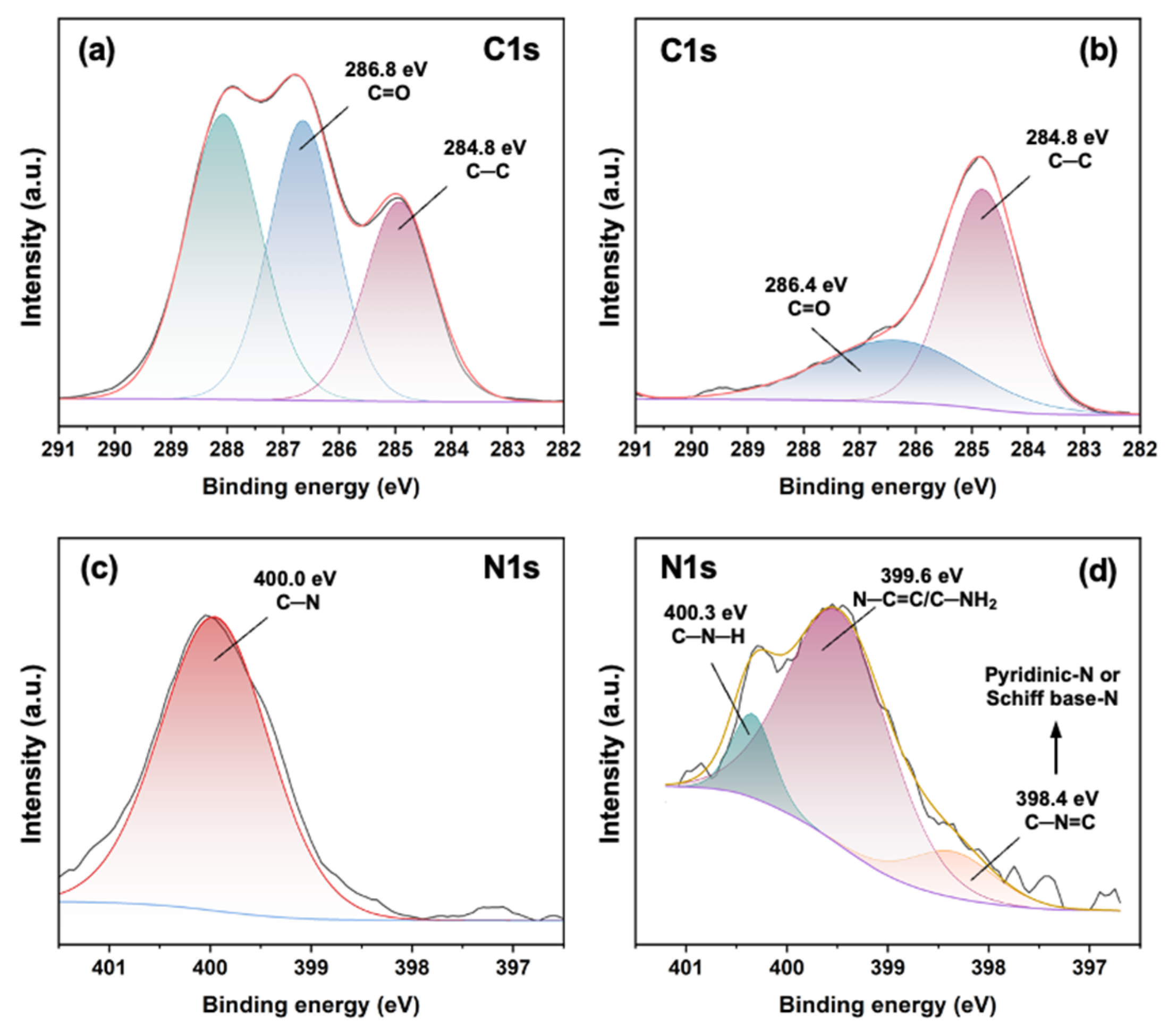
2.5.1. Structural Characterization
2.5.2. Uptake Ratio of Dye
2.5.3. Determination of the Physical Property of Crust Leather
3. Results and Discussion
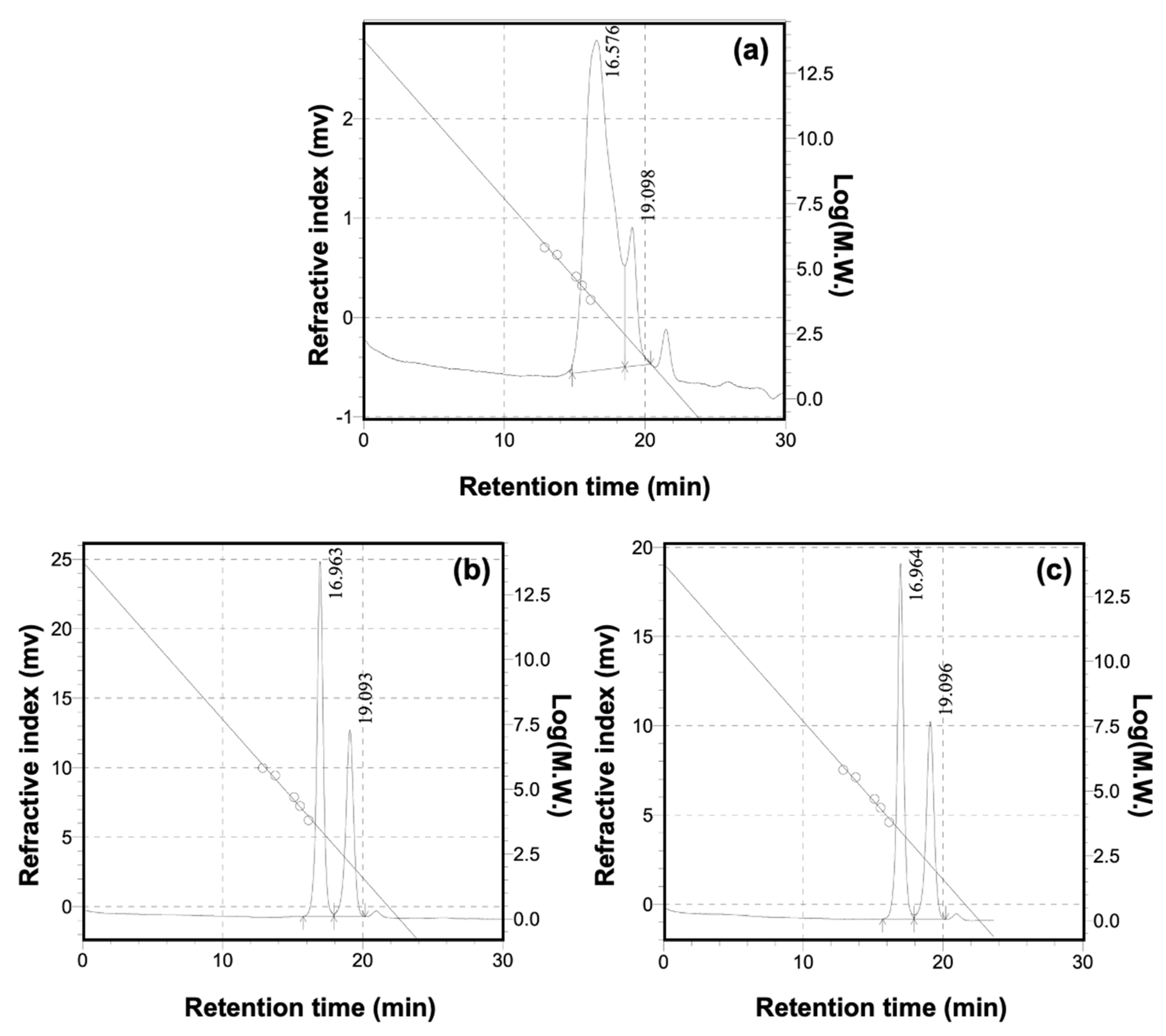
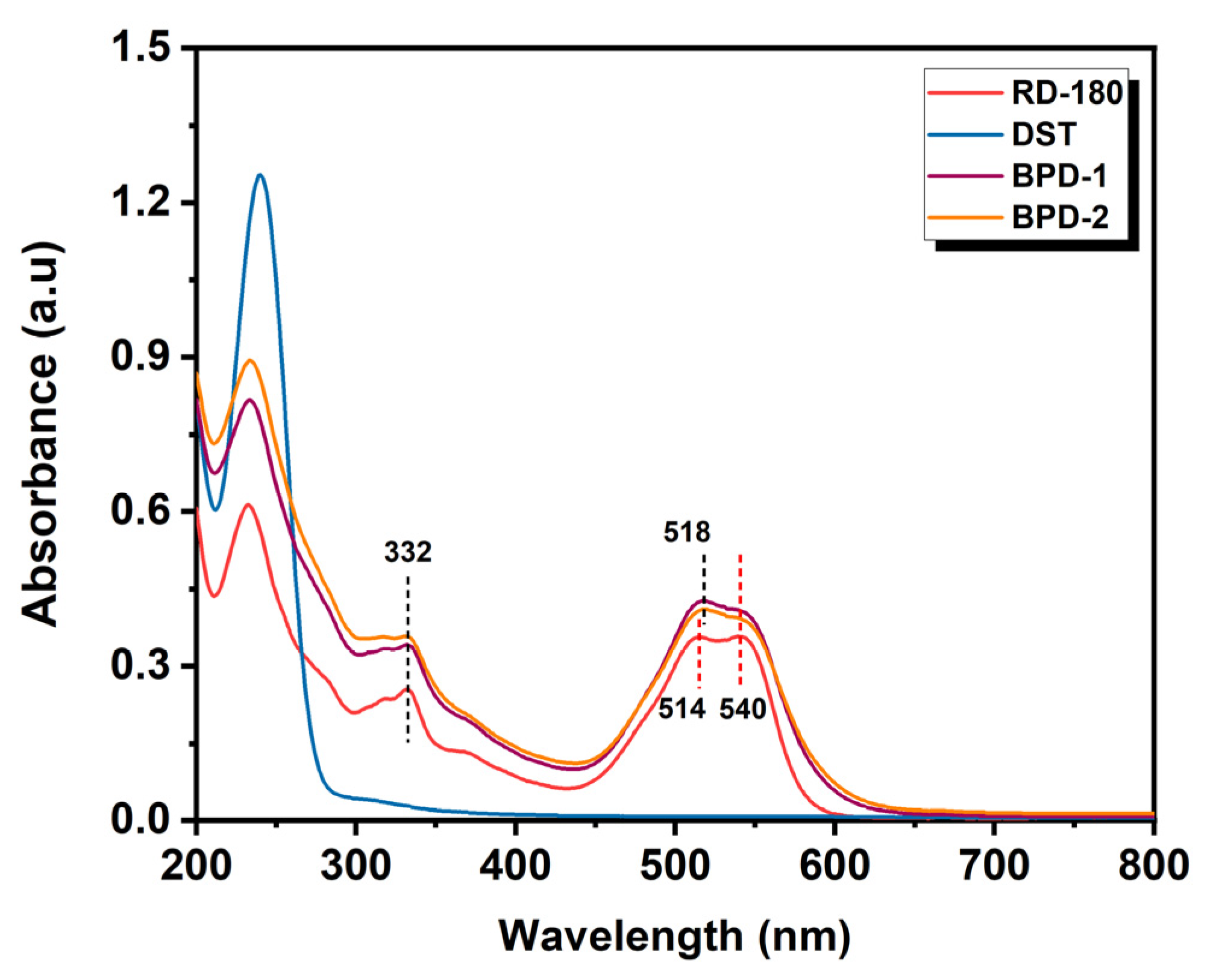
3.1. Structural Features of BPD
3.2. Color Properties of BPD
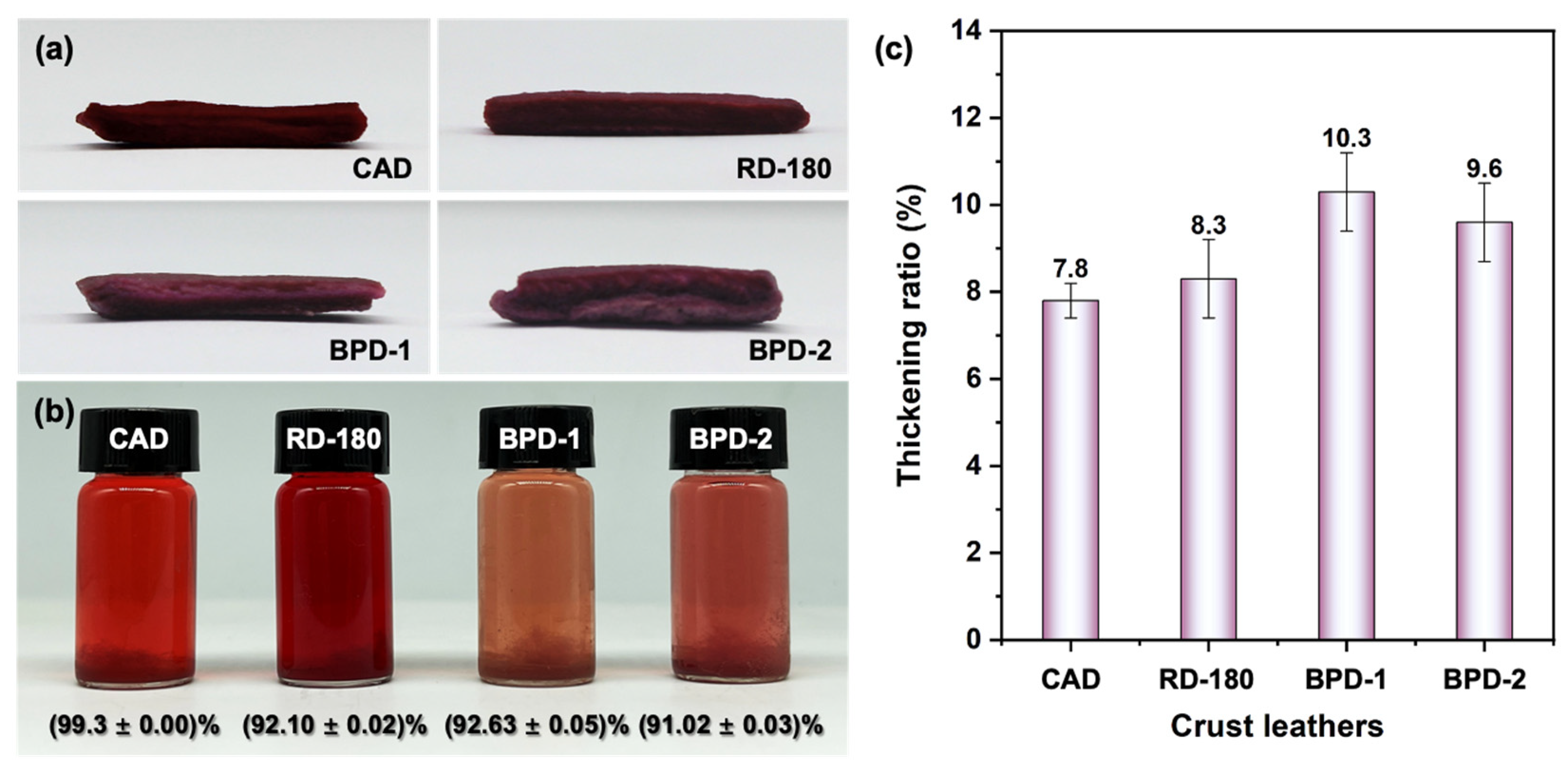
3.2.1. Penetration and Uptake of BPD
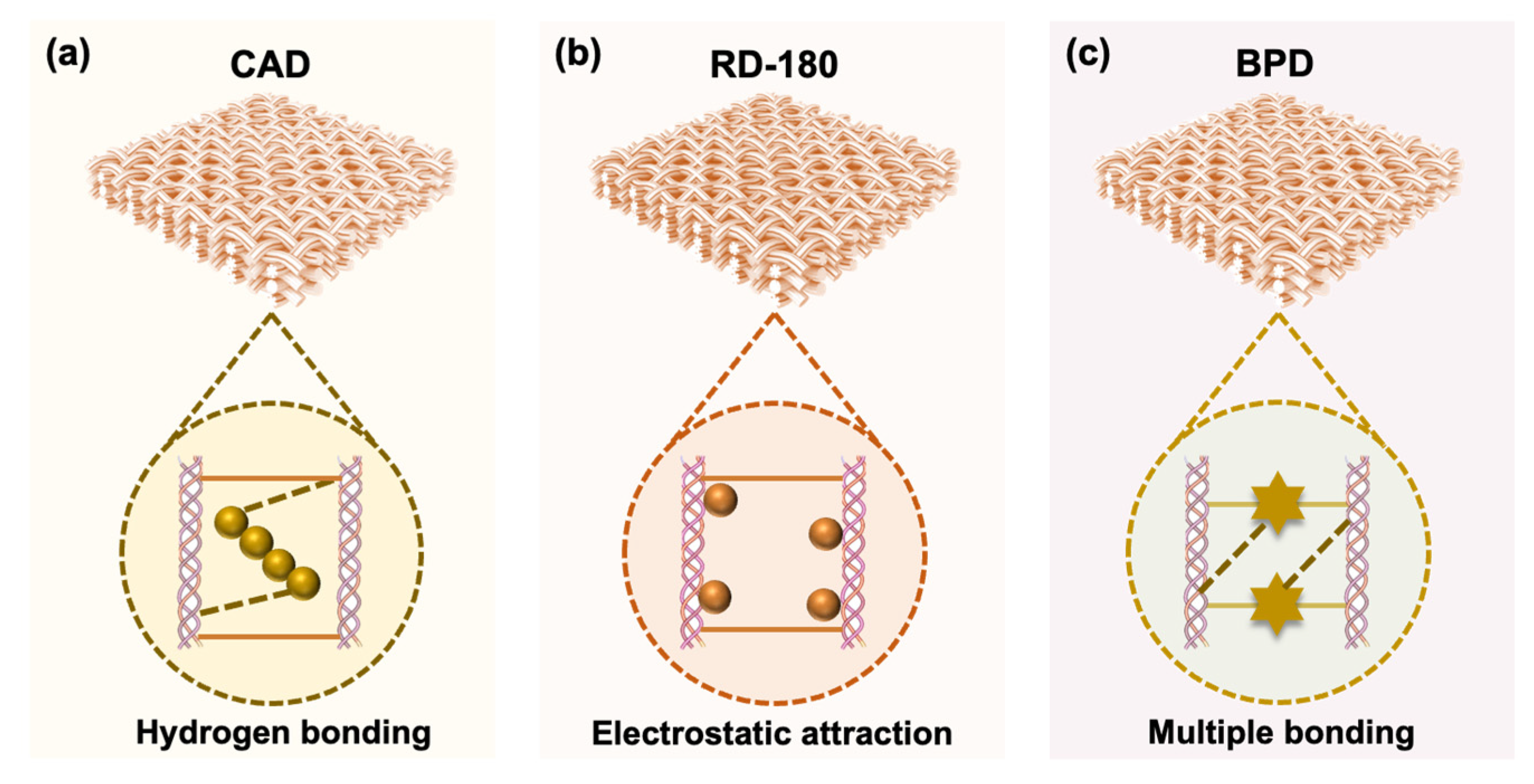
3.2.2. Dyeing Performance of BPD
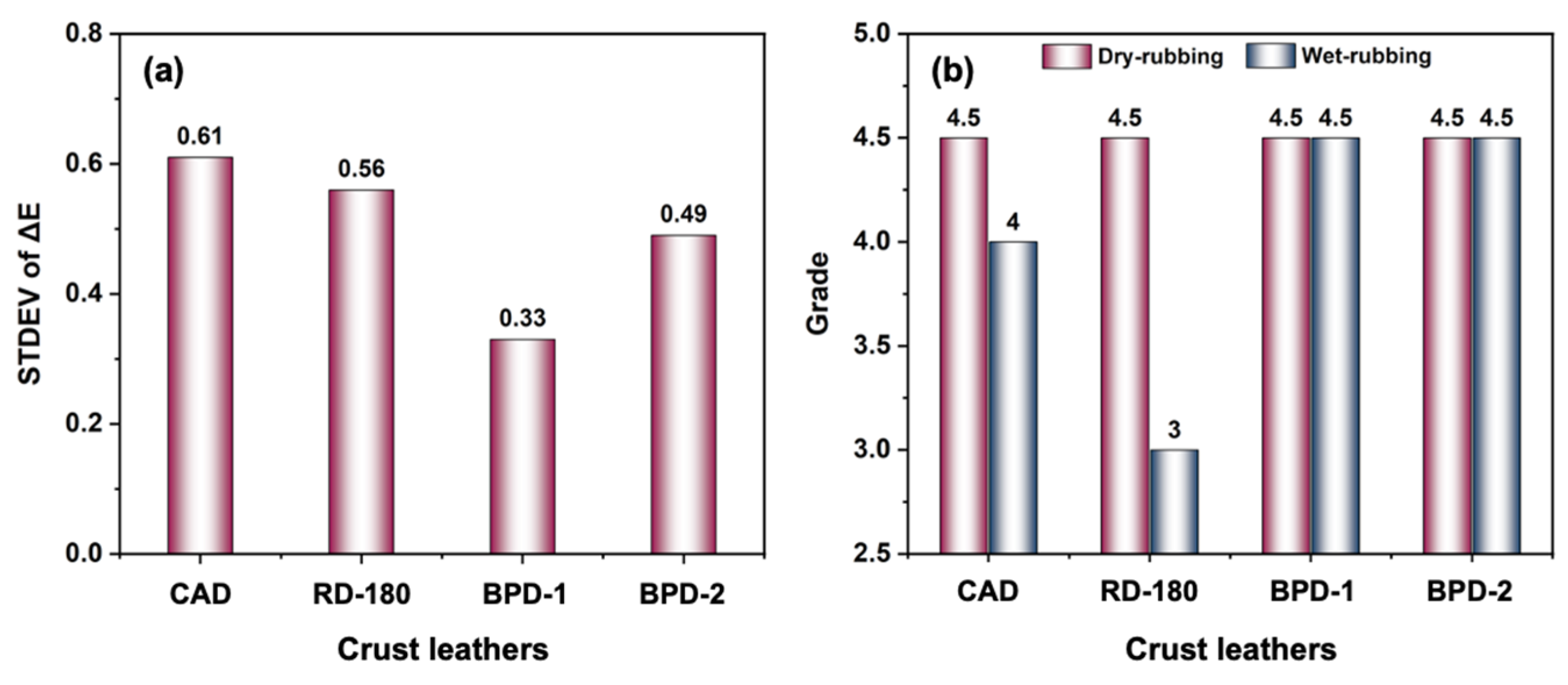
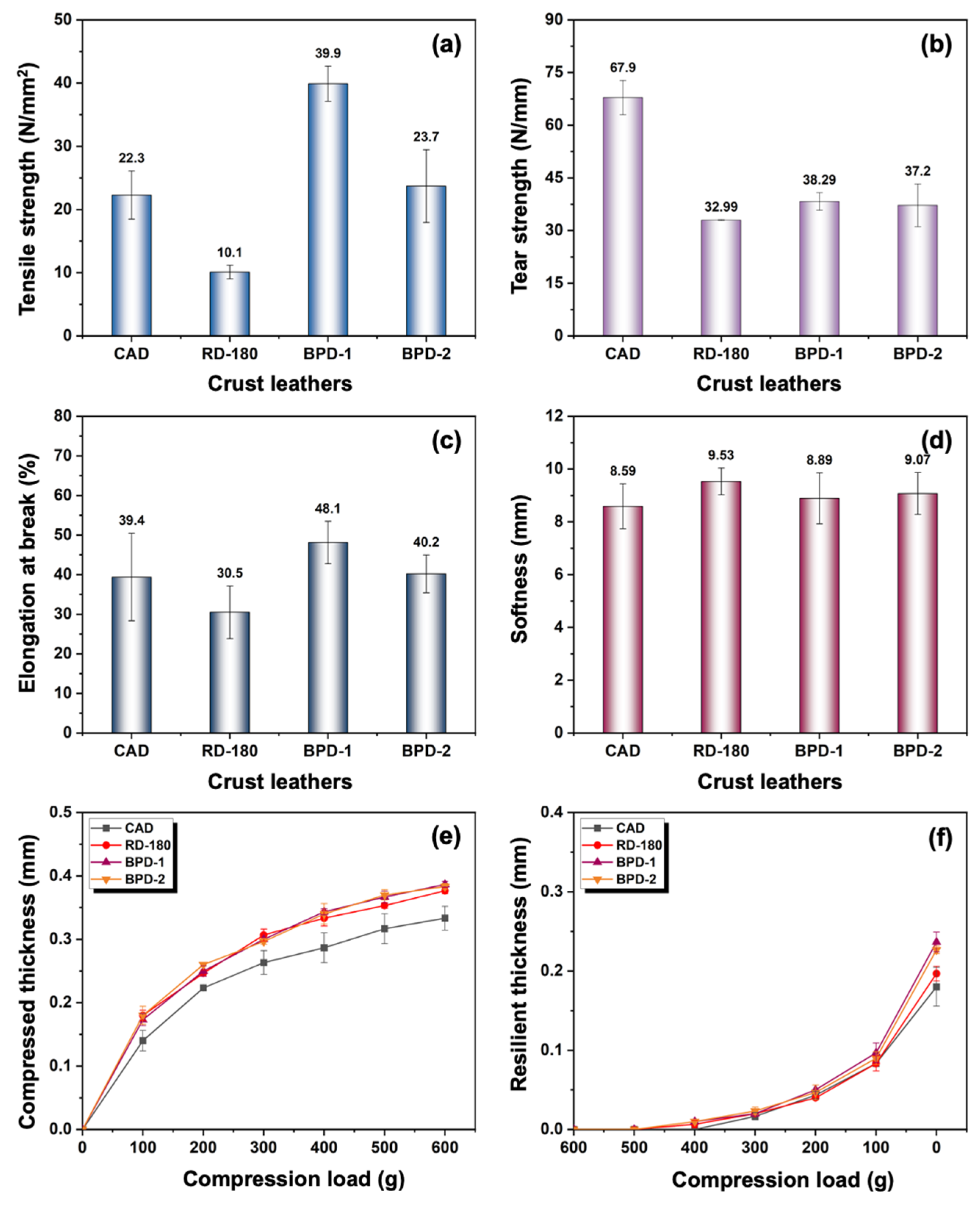
3.2.3. Physical and Organoleptic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BPD | biobased polymeric dye |
| DST | dialdehyde starch |
| RD-180 | reactive red 180 |
| BAT | biomass-derived aldehyde tanning agent |
| CAD | conventional anionic dye |
| CCLW | chromium-containing leather waste |
| CFTAs | chrome-free tanning agents |
| IEP | isoelectric point |
| CAPMs | conventional anionic post-tanning materials |
| AWPUD | amino-terminated waterborne polyurethane-based polymeric dye |
| DAP | dialdehyde polysaccharides |
| FTIR | Fourier transform infrared spectroscopy |
| NMR | nuclear magnetic resonance |
| XPS | X-ray photoelectron spectroscopy |
| GPC | gel permeation chromatography |
| ΔE | total color difference |
| STDEV | standard deviation |
| CF | collagen fiber |
References
- Kanagaraj, J.; Panda, R.C.; Prasanna, R. Sustainable chrome tanning system using protein-based product developed from leather waste: Wealth from waste. Polym. Bull. 2022, 79, 10201–10228. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, W. A rapid and cleaner chrome tanning technology based on ultrasound and microwave. J. Clean. Prod. 2019, 247, 119452. [Google Scholar] [CrossRef]
- Hedberg, Y.S. Chromium and leather: A review on the chemistry of relevance for allergic contact dermatitis to chromium. J. Leather Sci. Eng. 2020, 2, 1–15. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, Y.; Wang, F.; Zheng, X.; Liu, J.; Tang, K. Pyrolysis of sulfuric acid-treated chrome-tanned leather wastes: Kinetics, mechanism and evolved gas analysis. Waste Manag. 2022, 143, 105–115. [Google Scholar] [CrossRef]
- Hsini, A.; Naciri, Y.; Laabd, M.; Bouziani, A.; Navío, J.; Puga, F.; Boukherroub, R.; Lakhmiri, R.; Albourine, A. Development of a novel PANI@WO3 hybrid composite and its application as a promising adsorbent for Cr(VI) ions removal. J. Environ. Chem. Eng. 2021, 9, 105885. [Google Scholar] [CrossRef]
- Laabd, M.; Imgharn, A.; Hsini, A.; Naciri, Y.; Mobarak, M.; Szunerits, S.; Boukherroub, R.; Albourine, A. Efficient detoxification of Cr(VI)-containing effluents by sequential adsorption and reduction using a novel cysteine-doped PANi@faujasite composite: Experimental study supported by advanced statistical physics prediction. J. Hazard. Mater. 2021, 422, 126857. [Google Scholar] [CrossRef]
- Verma, S.K.; Sharma, P.C. Current trends in solid tannery waste management. Crit. Rev. Biotechnol. 2022, 1–18. [Google Scholar] [CrossRef]
- Yu, Y.; Lin, Y.; Zeng, Y.; Wang, Y.-N.; Zhang, W.; Zhou, J.; Shi, B. Life Cycle Assessment for Chrome Tanning, Chrome-Free Metal Tanning, and Metal-Free Tanning Systems. ACS Sustain. Chem. Eng. 2021, 9, 6720–6731. [Google Scholar] [CrossRef]
- Hao, D.; Wang, X.; Yue, O.; Liang, S.; Bai, Z.; Yang, J.; Liu, X.; Dang, X. A “wrench-like” green amphoteric organic chrome-free tanning agent provides long-term and effective antibacterial protection for leather. J. Clean. Prod. 2023, 404, 136917. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Wang, J.; Xie, T.; Sun, L.; Li, Z. A chrome-free combination tanning strategy: Based on silicic acid and plant tannin. J. Leather Sci. Eng. 2021, 3, 15. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, H.; Tang, K.; Liu, J.; Zheng, X.; Pei, Y.; Zhong, J. Optimization of dialdehyde soluble soybean polysaccharide: Preparation by response surface methodology for cleaner leather tanning. RSC Adv. 2022, 12, 7506–7515. [Google Scholar] [CrossRef] [PubMed]
- Ariram, N.; Madhan, B. Development of bio-acceptable leather using bagasse. J. Clean. Prod. 2019, 250, 119441. [Google Scholar] [CrossRef]
- Maćczak, P.; Kaczmarek, H.; Ziegler-Borowska, M.; Węgrzynowska-Drzymalska, K.; Burkowska-But, A. The Use of Chitosan and Starch-Based Flocculants for Filter Backwash Water Treatment. Materials 2022, 15, 1056. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Li, L.; Xing, J.; Cheng, C.; Hu, M.; Luo, Y.; Shi, S.; Liu, Y.; Cui, Z.; Yu, X. Cross-linking porcine peritoneum by oxidized konjac glucomannan: A novel method to improve the properties of cardiovascular substitute material. Collagen Leather 2023, 5, 5. [Google Scholar] [CrossRef]
- Wang, W.; Huang, W.-C.; Zheng, J.; Xue, C.; Mao, X. Preparation and comparison of dialdehyde derivatives of polysaccharides as cross-linking agents. Int. J. Biol. Macromol. 2023, 236, 123913. [Google Scholar] [CrossRef]
- Wang, L.; Mo, H.; Li, H.; Xu, D.; Gao, D.; Liu, Z.; Zhang, J.; Yao, L.; Hu, L. Preparation and application of tremella polysaccharide based chrome free tanning agent for sheepskin processing. Int. J. Biol. Macromol. 2023, 241, 124493. [Google Scholar] [CrossRef]
- Wang, X.; Sun, S.; Zhu, X.; Guo, P.; Liu, X.; Liu, C.; Lei, M. Application of amphoteric polymers in the process of leather post-tanning. J. Leather Sci. Eng. 2021, 3, 9. [Google Scholar] [CrossRef]
- Ding, W.; Remón, J.; Gao, M.; Li, S.; Liu, H.; Jiang, Z.; Ding, Z. A novel synergistic covalence and complexation bridging strategy based on multi-functional biomass-derived aldehydes and Al(III) for engineering high-quality eco-leather. Sci. Total. Environ. 2023, 862, 160713. [Google Scholar] [CrossRef]
- Ding, W.; Guo, S.; Liu, H.; Pang, X.; Ding, Z. Synthesis of an amino-terminated waterborne polyurethane-based polymeric dye for high-performance dyeing of biomass-derived aldehyde-tanned chrome-free leather. Mater. Today Chem. 2021, 21, 100508. [Google Scholar] [CrossRef]
- Rovira, J.; Domingo, J.L. Human health risks due to exposure to inorganic and organic chemicals from textiles: A review. Environ. Res. 2018, 168, 62–69. [Google Scholar] [CrossRef]
- Ding, W.; Liu, H.; Li, S.; Remón, J.; Pang, X.; Ding, Z. Providing Natural Organic Pigments with Excellent Tanning Capabilities: A Novel “One-Pot” Tanning–Dyeing Integration Strategy for Sustainable Leather Manufacturing. ACS Sustain. Chem. Eng. 2022, 10, 17346–17354. [Google Scholar] [CrossRef]
- Ding, W.; Remón, J.; Jiang, Z. Biomass-derived aldehyde tanning agents with in situ dyeing properties: A ‘Two Birds with One Stone’ strategy for engineering chrome-free and dye-free colored leather. Green Chem. 2022, 24, 3750–3758. [Google Scholar] [CrossRef]
- Ciardelli, F.; Ruggeri, G.; Pucci, A. Dye-containing polymers: Methods for preparation of mechanochromic materials. Chem. Soc. Rev. 2012, 42, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Zhang, S.; Yang, J.; Liu, F. Synthesis of a novel water-soluble crosslinking polymeric dye with good dyeing properties. Dye. Pigment. 2006, 68, 69–73. [Google Scholar] [CrossRef]
- Skariyachan, S.; Taskeen, N.; Kishore, A.P.; Krishna, B.V. Recent advances in plastic degradation – From microbial consortia-based methods to data sciences and computational biology driven approaches. J. Hazard. Mater. 2021, 426, 128086. [Google Scholar] [CrossRef] [PubMed]
- Cywar, R.M.; Rorrer, N.A.; Hoyt, C.B.; Beckham, G.T.; Chen, E.Y.-X. Bio-based polymers with performance-advantaged properties. Nat. Rev. Mater. 2021, 7, 83–103. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Z.; Long, Z.; Shan, Z. Development of Aldehyde and Similar-to-Aldehyde Tanning Agents. Text. Res. J. 2022, 92, 3387–3397. [Google Scholar] [CrossRef]
- Hu, M.; Peng, X.; Shi, S.; Wan, C.; Cheng, C.; Yu, X. Dialdehyde xanthan gum and curcumin synergistically crosslinked bioprosthetic valve leaflets with anti-thrombotic, anti-inflammatory and anti-calcification properties. Carbohydr. Polym. 2023, 310, 120724. [Google Scholar] [CrossRef]
- Ding, W. Bridging-induced densification strategy based on biomass-derived aldehyde tanning integrated with terminal Al(III) crosslinking towards high-performance chrome-free leather production. J. Environ. Manag. 2022, 307. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, W.; Zhang, C.; Hossain, Y.; Oli, Z.B.S.; Pervez, N.; Sarker, S.; Hoque, I.U.; Cai, Y.; Naddeo, V. Combination of wet fixation and drying treatments to improve dye fixation onto spray-dyed cotton fabric. Sci. Rep. 2021, 11, 15403. [Google Scholar] [CrossRef]
- Peng, W.; Zhang, X.; Chen, S. The Principle and Method of Testing Leather Fullness and Softness. J. Soc. Leath. Tech. Ch. 2006, 90, 117–122. [Google Scholar]
- Aravind, P.; Selvaraj, H.; Ferro, S.; Sundaram, M. An integrated (electro- and bio-oxidation) approach for remediation of industrial wastewater containing azo-dyes: Understanding the degradation mechanism and toxicity assessment. J. Hazard. Mater. 2016, 318, 203–215. [Google Scholar] [CrossRef]
- Sundaraganesan, N.; Ilakiamani, S.; Joshua, B.D. FT-Raman and FT-IR spectra, ab initio and density functional studies of 2-amino-4,5-difluorobenzoic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Chelminiak-Dudkiewicz, D.; Smolarkiewicz-Wyczachowski, A.; Wegrzynowska-Drzymalska, K.; Ziegler-Borowska, M. Effect of Irradiation on Structural Changes of Levan. Int. J. Mol. Sci. 2022, 23, 2463. [Google Scholar] [CrossRef]
- Tian, X.; Yan, D.; Lu, Q.; Jiang, X. Cationic surface modification of nanocrystalline cellulose as reinforcements for preparation of the chitosan-based nanocomposite films. Cellulose 2017, 24, 163–174. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, P.; Wang, Y.; Gao, W. Study on physico-chemical properties of dialdehyde yam starch with different aldehyde group contents. Thermochim. Acta 2011, 512, 196–201. [Google Scholar] [CrossRef]
- Liang, L.; Hou, T.; Ouyang, Q.; Xie, L.; Zhong, S.; Li, P.; Li, S.; Li, C. Antimicrobial sodium alginate dressing immobilized with polydopamine-silver composite nanospheres. Compos. Part B Eng. 2020, 188, 107877. [Google Scholar] [CrossRef]
- Li, P.; Zeng, J.; Wang, B.; Cheng, Z.; Xu, J.; Gao, W.; Chen, K. Waterborne fluorescent dual anti-counterfeiting ink based on Yb/Er-carbon quantum dots grafted with dialdehyde nano-fibrillated cellulose. Carbohydr. Polym. 2020, 247, 116721. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, H.; Ji, G. Development of Novel Graphene/g-C3N4 Composite with Broad-Frequency and Light-Weight Features. Part. Part. Syst. Char. 2016, 33, 656–663. [Google Scholar]
- Jagst, E. Surface Functional Group Characterization Using Chemical Derivatization X-ray Photoelectron Spectroscopy (CD-XPS); Bundesanstalt für Materialforschung und -Prüfung: Berlin, Germany, 2011. [Google Scholar]
- Yokwana, K.; Ray, S.C.; Khenfouch, M.; Kuvarega, A.T.; Mamba, B.B.; Mhlanga, S.D.; Nxumalo, E.N. Facile Synthesis of Nitrogen Doped Graphene Oxide from Graphite Flakes and Powders: A Comparison of Their Surface Chemistry. J. Nanosci. Nanotechnol. 2018, 18, 5470–5484. [Google Scholar] [CrossRef]
- Shah, S.S.; Alfasane, A.; Bakare, I.A.; Aziz, A.; Yamani, Z.H. Polyaniline and heteroatoms–enriched carbon derived from Pithophora polymorpha composite for high performance supercapacitor. J. Energy Storage 2020, 30, 101562. [Google Scholar] [CrossRef]
- Veelaert, S.; de Wit, D.; Gotlieb, K.; Verhé, R. Chemical and physical transitions of periodate oxidized potato starch in water. Carbohydr. Polym. 1997, 33, 153–162. [Google Scholar] [CrossRef]
- Ríos, M.-C.; Bravo, N.-F.; Sánchez, C.-C.; Portilla, J. Chemosensors based on N-heterocyclic dyes: Advances in sensing highly toxic ions such as CN− and Hg2+. RSC Adv. 2021, 11, 34206–34234. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Li, Z.; Peng, Y.-X.; Geng, J.; Qian, H.-F.; Huang, W. Post-modification of 2-formylthiophene based heterocyclic azo dyes. Dye. Pigment. 2016, 133, 143–152. [Google Scholar] [CrossRef]
- Tripathy, S.; Dash, S. Solvation studies of some tailor made α-N,N-dimethylaminostyryl-N-alkyl pyridinium dyes in binary solvent mixtures containing alcohols, hexane, 1,4-dioxane, DCM and acetone. J. Mol. Liq. 2015, 206, 29–38. [Google Scholar] [CrossRef]
- Huang, W.; Song, Y.; Yu, Y.; Wang, Y.-N.; Shi, B. Interaction between retanning agents and wet white tanned by a novel bimetal complex tanning agent. J. Leather Sci. Eng. 2020, 2, 8. [Google Scholar] [CrossRef]
- Lin, L.; Xiao, L.; Li, L.; Zhang, C.; Pervez, N.; Naddeo, V.; Zhang, Y.; Islam, S.; Cai, Y.; Hassan, M.M. Sustainable and eco-friendly dyeing of traditional grass cloth with a reactive dye in palm oil medium. RSC Adv. 2022, 12, 29767–29776. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Y.N.; Zeng, Y.; Wu, H.; Shi, B. Quantitative Determinations of Isoelectric Point of Retanned Leather and Distribution of Retanning Agent. J. Am. Leath. Chem. Assoc. 2018, 113, 232–238. [Google Scholar]
- Ma, J.; Yang, N.; Li, Y.; Gao, D.; Lyu, B.; Zhang, J. A cleaner approach to tanning process of cattle hide upper suede leather: Chrome-less polycarboxylate/montmorillonite nanocomposites as tanning agent. Environ. Sci. Pollut. Res. 2021, 28, 39014–39025. [Google Scholar] [CrossRef]
- He, X.; Huang, Y.; Xiao, H.; Xu, X.; Wang, Y.; Huang, X.; Shi, B. Tanning agent free leather making enabled by the dispersity of collagen fibers combined with superhydrophobic coating. Green Chem. 2021, 23, 3581–3587. [Google Scholar] [CrossRef]
- Pan, F.; Xiao, Y.; Zhang, L.; Zhou, J.; Wang, C.; Lin, W. Leather wastes into high-value chemicals: Keratin-based retanning agents via UV-initiated polymerization. J. Clean. Prod. 2023, 383, 135492. [Google Scholar] [CrossRef]
- Xu, S.; Shi, B. A green and sustainable strategy for leather manufacturing: Endow dehydrated hide with consistent and durable hydrophobicity. J. Clean. Prod. 2023, 383, 135526. [Google Scholar] [CrossRef]
| Process | Chemicals | Dosage (%) | Temperature (°C) | Duration/min | Remark |
|---|---|---|---|---|---|
| Rewetting | Water | 400 | 40 | pH = 6.5 | |
| Degreasing agent | 0.5 | ||||
| Formic acid | 0.5 | 40 | Remark-A | ||
| Retanning | Water | 100 | 35 | pH = 6.5 | |
| Acrylic resin | 3.0 | 60 | |||
| Amino resin | 2.0 | 30 | |||
| Dyestuff | x | Remark-C | |||
| Mimosa | 4.0 | 60 | |||
| Formic acid | 0.5 × 2 | 15 × 2 | pH = 4.0~4.2 | ||
| Fatliquoring | Water | 150 | |||
| Fatliquor | 8.0 | ||||
| Formic acid | 0.5 × 2 | 15 × 2 + 20 | pH = 3.6~3.8 | ||
| Washing | Water | 400 × 2 | 10 × 2 | Remark-D | |
| Remark-A | Drain | ||||
| Remark-B | Drain | ||||
| Remark-C | 2% for CAD and RD-180, 4% for BPD | ||||
| Remark-D | Drain → Natural drying → Softening → Crust leather | ||||
| Samples | Mw | Mn | Mw/Mn | Particle Size (d.nm) |
|---|---|---|---|---|
| DST | 6338 | 1869 | 3.391 | 619.0 ± 58.3 |
| BPD-1 | 2997 | 2658 | 1.127 | 452.0 ± 22.2 |
| BPD-2 | 2990 | 2641 | 1.132 | 359.0 ± 6.7 |
| Crust Leather Sample | L | a | b | ΔE |
|---|---|---|---|---|
| CAD | 51.87 ± 0.83 | 39.37 ± 0.36 | 14.26 ± 0.36 | 60.45 |
| RD-180 | 52.62 ± 0.78 | 35.47 ± 0.47 | 0.39 ± 0.31 | 55.81 |
| BPD-1 | 64.31 ± 0.69 | 13.47 ± 0.24 | 9.05 ± 0.25 | 34.65 |
| BPD-2 | 61.51 ± 0.89 | 17.18 ± 0.40 | 5.86 ± 0.19 | 38.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, W.; Zhang, Y.; Li, S.; Remón, J.; Wang, K.; Bao, L.; Pang, X. Novel Biomass-Based Polymeric Dyes: Preparation and Performance Assessment in the Dyeing of Biomass-Derived Aldehyde-Tanned Leather. Polymers 2023, 15, 2300. https://doi.org/10.3390/polym15102300
Ding W, Zhang Y, Li S, Remón J, Wang K, Bao L, Pang X. Novel Biomass-Based Polymeric Dyes: Preparation and Performance Assessment in the Dyeing of Biomass-Derived Aldehyde-Tanned Leather. Polymers. 2023; 15(10):2300. https://doi.org/10.3390/polym15102300
Chicago/Turabian StyleDing, Wei, Yinuo Zhang, Shuolin Li, Javier Remón, Kanglei Wang, Lihong Bao, and Xiaoyan Pang. 2023. "Novel Biomass-Based Polymeric Dyes: Preparation and Performance Assessment in the Dyeing of Biomass-Derived Aldehyde-Tanned Leather" Polymers 15, no. 10: 2300. https://doi.org/10.3390/polym15102300
APA StyleDing, W., Zhang, Y., Li, S., Remón, J., Wang, K., Bao, L., & Pang, X. (2023). Novel Biomass-Based Polymeric Dyes: Preparation and Performance Assessment in the Dyeing of Biomass-Derived Aldehyde-Tanned Leather. Polymers, 15(10), 2300. https://doi.org/10.3390/polym15102300