A Highly Sensitive, Ultra-Durable, Eco-Friendly Ionic Skin for Human Motion Monitoring
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Experiment Method
2.2. Evaluation Method of Parameters and Performance of S−iSkin
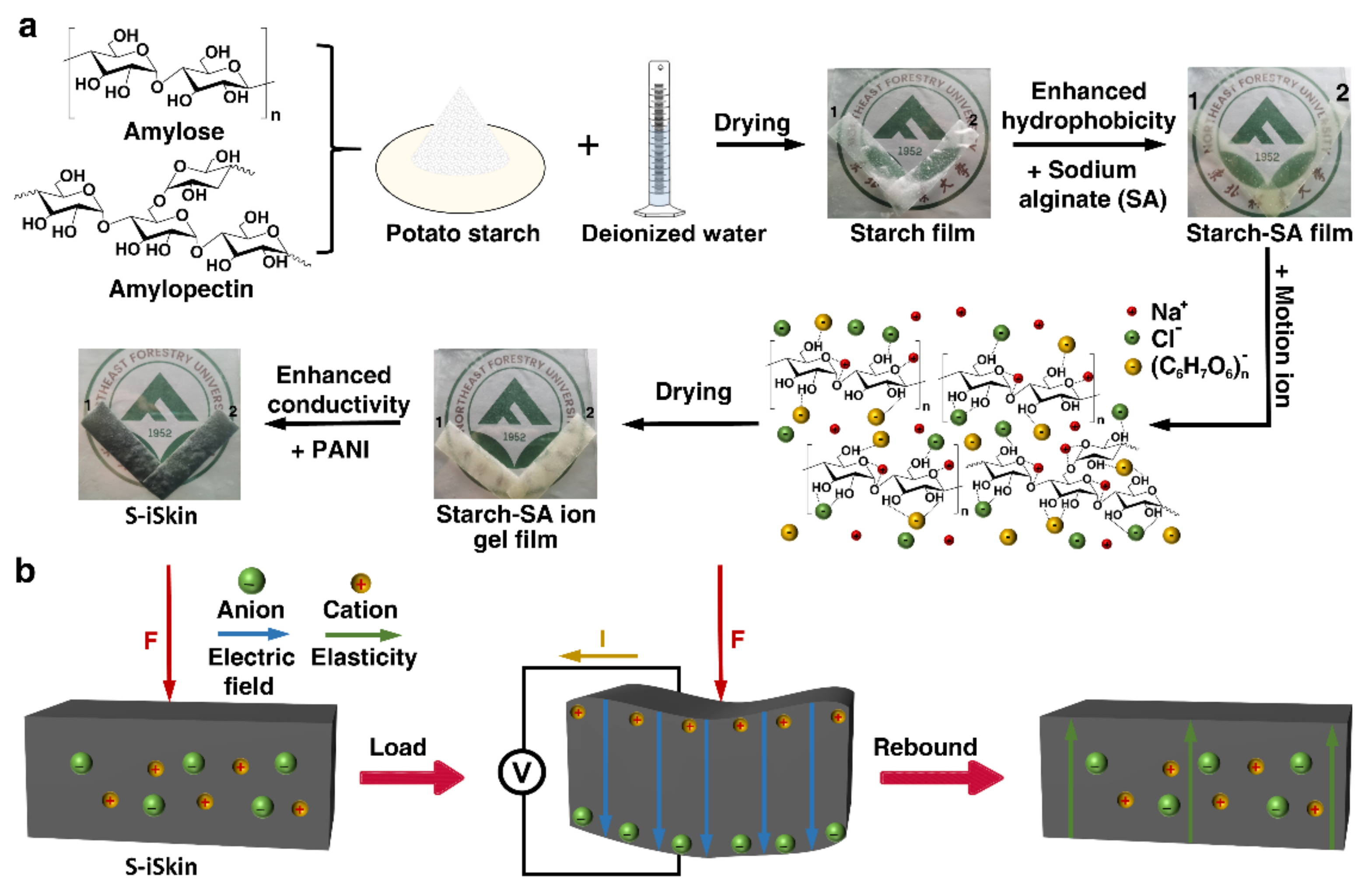
2.3. Fabrication Process of S−iSkin
3. Results and Discussion
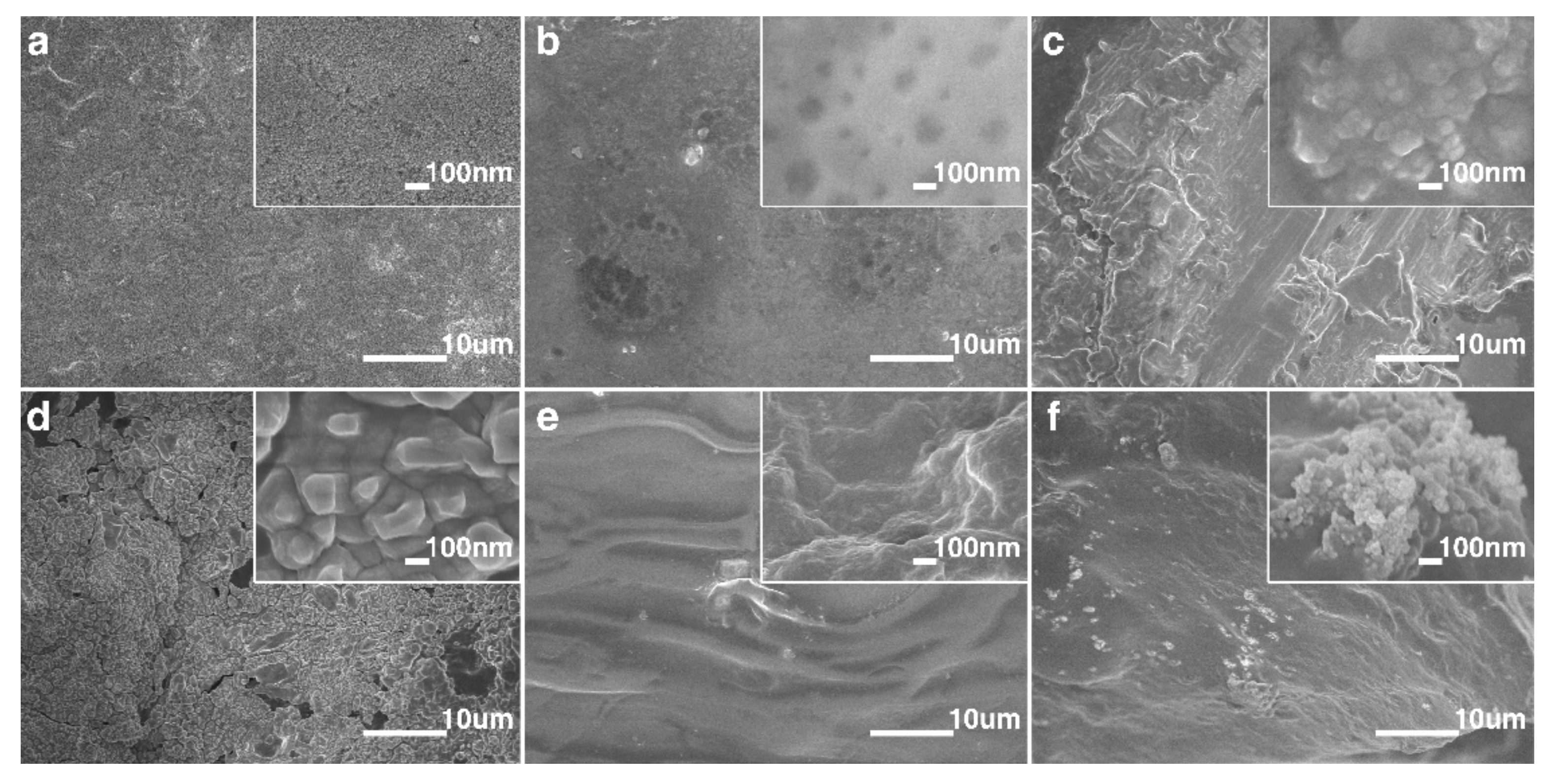
3.1. Structure and Morphology of S−iSkin
3.2. Structural Characterization and Mechanical Properties of S−iSkin
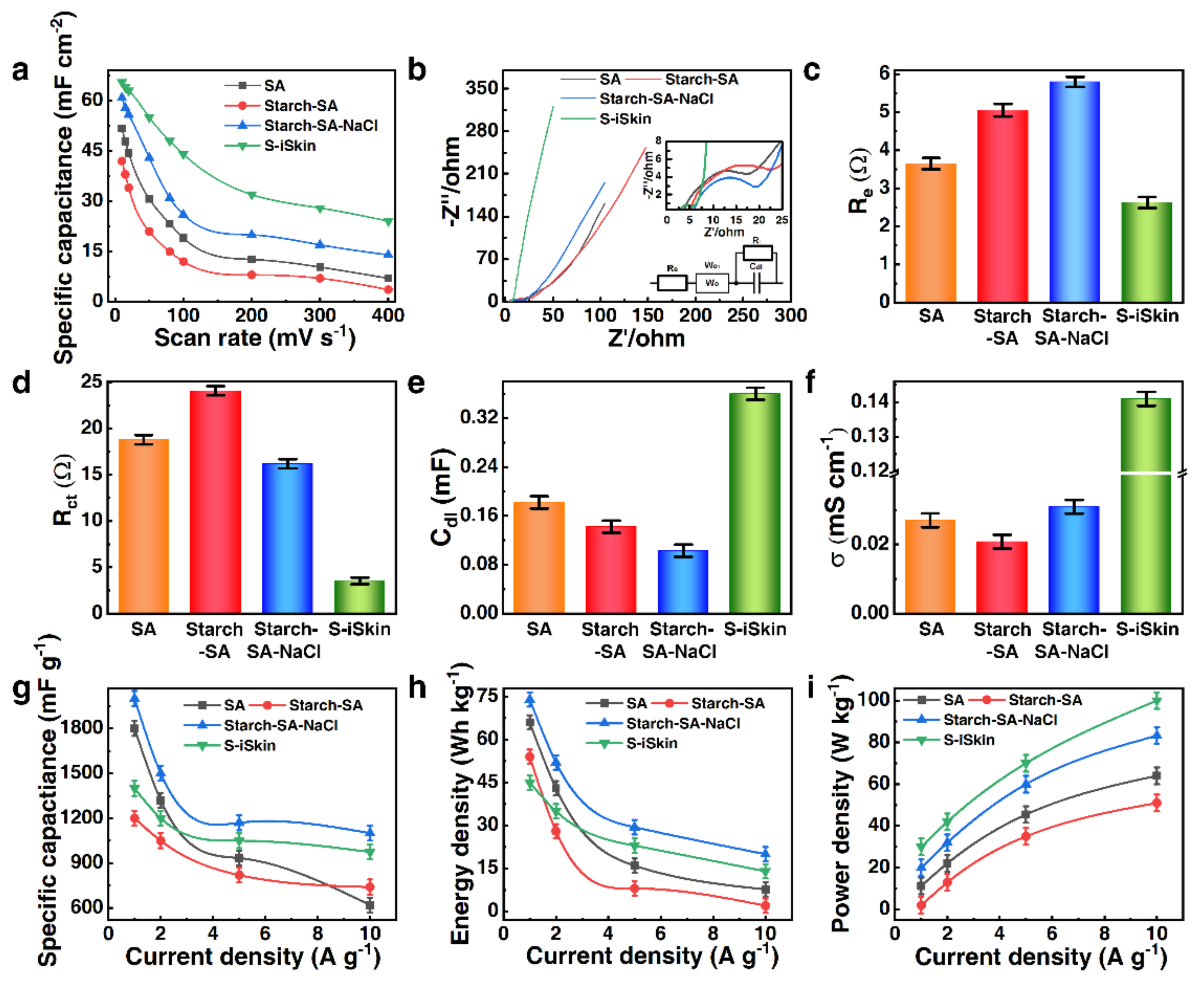
3.3. Electrochemical Performance of S−iSkin
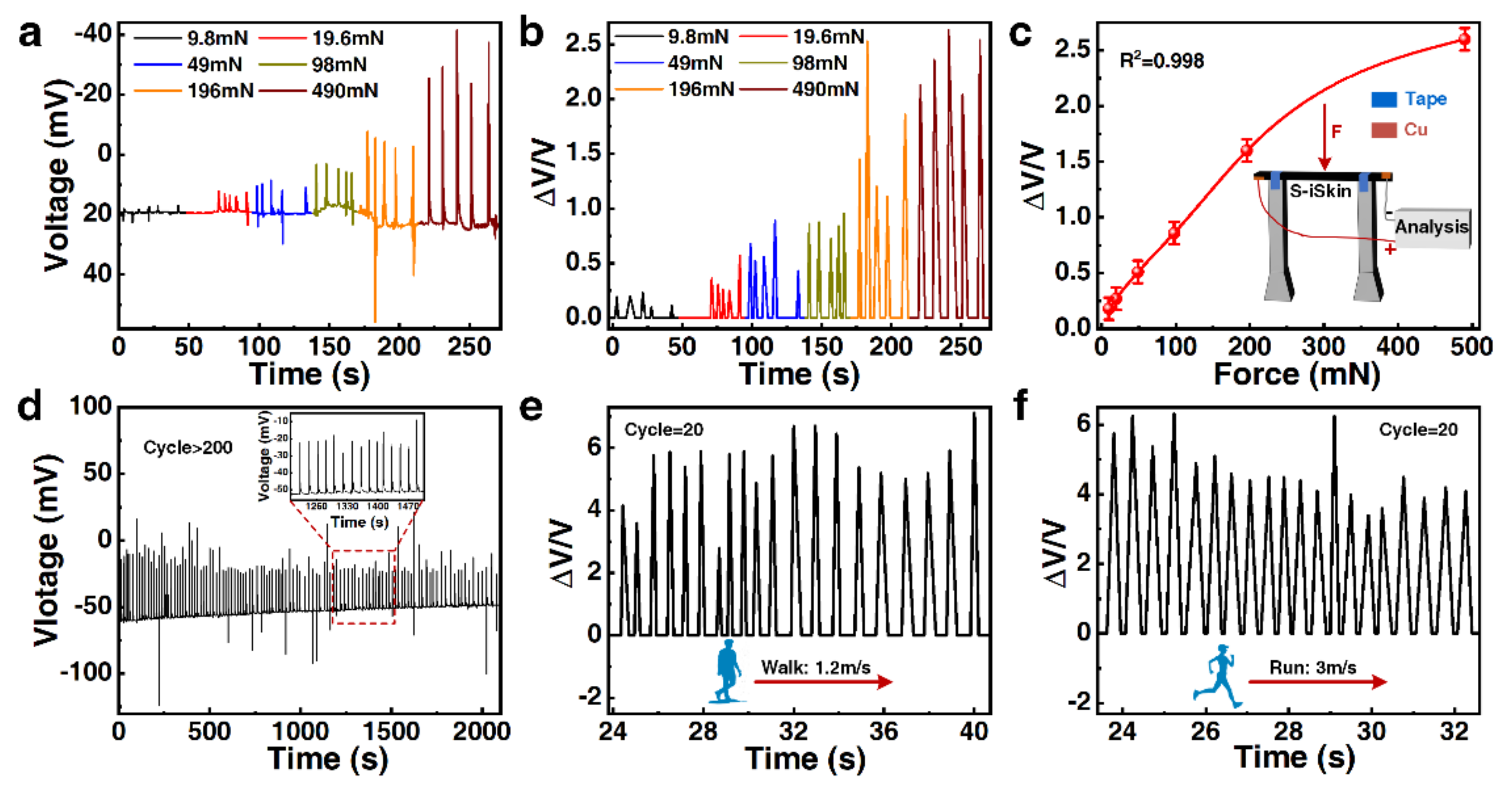
3.4. The Sensing Performance and Application of S−iSkin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lei, Z.; Wang, Q.; Sun, S.; Zhu, W.; Wu, P. A bioinspired mineral hydrogel as a self-healable, mechanically adaptable ionic skin for highly sensitive pressure sensing. Adv. Mater. 2017, 29, 1700321. [Google Scholar] [CrossRef] [PubMed]
- Amoli, V.; Kim, J.S.; Jee, E.; Chung, Y.S.; Kim, S.Y.; Koo, J.; Choi, H.; Kim, Y.; Kim, D.H. A bioinspired hydrogen bond-triggered ultrasensitive ionic mechanoreceptor skin. Nat. Commun. 2019, 10, 4019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Wang, Y.; Ren, Y.; Jin, G.; Zhang, C.; Chen, W.; Yan, F. Poly(ionic liquid) hydrogel-based anti-freezing ionic skin for a soft robotic gripper. Mater. Horiz. 2020, 7, 919–927. [Google Scholar] [CrossRef]
- Liang, S.; Zhang, Y.; Wang, H.; Xu, Z.; Chen, J.; Bao, R.; Tan, B.; Cui, Y.; Fan, G.; Wang, W.; et al. Paintable and rapidly bondable conductive hydrogels as therapeutic cardiac patches. Adv. Mater. 2018, 30, e1704235. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wang, H.; Ma, P.X.; Guo, B. Self-healing conductive hydrogels: Preparation, properties and applications. Nanoscale 2020, 12, 1224–1246. [Google Scholar] [CrossRef]
- Kong, W.; Wang, C.; Jia, C.; Kuang, Y.; Pastel, G.; Chen, C.; Chen, G.; He, S.; Huang, H.; Zhang, J.; et al. Muscle-inspired highly anisotropic, strong, ion-conductive hydrogels. Adv. Mater. 2018, 30, e1801934. [Google Scholar] [CrossRef]
- Lei, Z.; Zhu, W.; Zhang, X.; Wang, X.; Wu, P. Bio-Inspired Ionic Skin for Theranostics. Adv. Funct. Mater. 2021, 31, 2008020. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Cao, Y.; Yang, Y.; Yang, Y.; Gao, Y.; Ma, Z.; Wang, J.; Wang, W.; Wu, D. Freezing-tolerant, itive strain and pressure sensors assembled from ionic conductive hydrogels with dynamic cross-links. ACS Appl. Mater. Interfaces 2020, 12, 25334–25344. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, H.; Wu, J.; Han, L.; Yang, Z.; Jiang, Z.; Wang, R.; Huang, Z.; Xu, M. Ultrafast self-healing, reusable, and conductive polysaccharide-based hydrogels for sensitive ionic sensors. ACS Sustain. Chem. Eng. 2020, 8, 18506–18518. [Google Scholar] [CrossRef]
- Hussain, I.; Ma, X.; Luo, Y.; Luo, Z. Fabrication and characterization of glycogen-based elastic, self-healable, and conductive hydrogels as a wearable strain-sensor for flexible e-skin. Polymer 2020, 210, 122961. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Wu, Q.; Wan, Z.; Li, D.; Jin, Y. Highly strong and flexible composite hydrogel reinforced by aligned wood cellulose skeleton via alkali treatment for muscle-like sensors. Chem. Eng. J. 2020, 400, 125876. [Google Scholar] [CrossRef]
- Liu, M.; Li, B.; Zhou, H. Extraordinary rate capability achieved by a 3D “skeleton/skin” carbon aerogel–polyaniline hybrid with vertically aligned pores. Chem. Commun. 2017, 53, 2810–2813. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, Y.; Xie, Z.; Tan, X.; Zhang, X.; Liu, R.; Song, Y.-F.; Zhang, S. Ionic liquids achieve the exfoliation of ultrathin two-dimensional VOPO4·2H2O crystalline nanosheets: Implications on energy storage and catalysis. ACS Appl. Nano Mater. 2021, 4, 2503–2514. [Google Scholar] [CrossRef]
- Berton, P.; Tian, H.; Rogers, R. Phase behavior of aqueous biphasic systems with choline alkanoate ionic liquids and phosphate solutions: The influence of pH. Molecules 2021, 26, 1702. [Google Scholar] [CrossRef] [PubMed]
- KhorsandKheirabad, A.; Zhou, X.; Xie, D. Hydrazine-enabled one-step synthesis of metal nanoparticle–functionalized gradient porous poly (ionic liquid) membranes. Macromol. Rapid Commun. 2021, 42, 2000143. [Google Scholar] [CrossRef]
- Liu, Z.T.; Huang, K.L.; Wu, Y.S.; Lyu, Y.P.; Lee, C.L. A comparison of physically and chemically defective graphene nanosheets as catalyst supports for cubic Pd nanoparticles in an alkaline oxygen reduction reaction. Electrochim. Acta 2015, 186, 552–561. [Google Scholar] [CrossRef]
- Park, S.H.; Lim, J.; Song, I.Y.; Lee, J.R.; Park, T. Physically stable polymer-membrane electrolytes for highly efficient solid-state dye-sensitized solar cells with long-term stability. Adv. Energy Mater. 2014, 4, 1300489. [Google Scholar] [CrossRef]
- Nan, M.; Wang, F.; Kim, S.; Li, H.; Jin, Z.; Bang, D.; Kim, C.-S.; Park, J.-O.; Choi, E. Ecofriendly high-performance ionic soft actuators based on graphene-mediated cellulose acetate. Sens. Actuators B Chem. 2019, 301, 127127. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Lu, A. Transparent, antifreezing, ionic conductive cellulose hydrogel with stable sensitivity at subzero temperature. ACS Appl. Mater. Interfaces 2019, 11, 41710–41716. [Google Scholar] [CrossRef]
- Liu, C.; Huang, N.; Xu, F.; Tong, J.; Chen, Z.; Gui, X.; Fu, Y.; Lao, C. 3D printing technologies for flexible tactile sensors toward wearable electronics and electronic skin. Polymers 2018, 10, 629. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhang, Y.; Lu, H.; Wang, Y.; Xu, J.; Zhu, J.; Zhang, C.; Liu, T. Cryopolymerization enables anisotropic polyaniline hybrid hydrogels with superelasticity and highly deformation-tolerant electrochemical energy storage. Nat. Commun. 2020, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Ruan, C.; Li, P.; Xu, J.; Xie, Y. Enhancement of electrochemical performance of cobalt (II) coordinated polyaniline: A combined experimental and theoretical study. Electrochim. Acta 2020, 338, 135881. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, L.; Zhang, D.; Song, W. High performance, flexible and renewable nano-biocomposite artificial muscle based on mesoporous cellulose/ionic liquid electrolyte membrane. Sens. Actuators B Chem. 2019, 283, 579–589. [Google Scholar] [CrossRef]
- Wang, A.; Wang, Y.; Zhang, B.; Wan, K.; Zhu, J.; Xu, J.; Zhang, C.; Liu, T. Hydrogen-bonded network enables semi-interpenetrating ionic conductive hydrogels with high stretchability and excellent fatigue resistance for capacitive/resistive bimodal sensors. Chem. Eng. J. 2021, 411, 128506. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.; Han, H.; Zheng, H.; Xu, W.; Wang, Z. Dopamine-triggered hydrogels with high transparency, self-adhesion, and thermoresponse as skinlike sensors. ACS Nano 2021, 15, 1785–1794. [Google Scholar] [CrossRef]
- Duan, J.; Wen, H.; Zong, S.; Li, T.; Lv, H.; Liu, L. Soft/hard controllable conversion galactomannan ionic conductive hydrogel as a flexible sensor. ACS Appl. Electron. Mater. 2021, 3, 5000–5014. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Zhou, G.; Qin, F.; Jing, M.; Li, L.; Song, W.; Sun, Z. A high-efficiency bioinspired photoelectric-electromechanical integrated nanogenerator. Nat. Commun. 2020, 11, 6158. [Google Scholar] [CrossRef]
- Zhou, Y.; Wan, C.; Yang, Y.; Yang, H.; Wang, S.; Dai, Z.; Ji, K.; Jiang, H.; Chen, X.; Long, Y. Highly Stretchable, Elastic, and Ionic Conductive Hydrogel for Artificial Soft Electronics. Adv. Funct. Mater. 2019, 29, 1806220. [Google Scholar] [CrossRef]
- Del Castillo-Castro, T.; Castillo-Ortega, M.M.; Herrera-Franco, P.J. Electrical, mechanical and piezo-resistive behavior of a polyani-line/poly(n-butyl methacrylate) composite. Compos. Part A Appl. Sci. Manuf. 2009, 40, 1573–1579. [Google Scholar] [CrossRef]
- Vallim, M.R.; Felisberti, M.I.; De Paoli, M.-A. Blends of polyaniline with nitrilic rubber. J. Appl. Polym. Sci. 2000, 75, 677–684. [Google Scholar] [CrossRef]
- Zheng, S.; Wu, X.; Huang, Y.; Xu, Z.; Yang, W.; Liu, Z.; Huang, S.; Xie, B.; Yang, M. Highly sensitive and multifunctional piezoresistive sensor based on polyaniline foam for wearable Human-Activity monitoring. Compos. Part A Appl. Sci. Manuf. 2019, 121, 510–516. [Google Scholar] [CrossRef]
- Xu, M.; Qi, J.; Li, F.; Zhang, Y. Transparent and flexible tactile sensors based on graphene films designed for smart panels. J. Mater. Sci. 2018, 53, 9589–9597. [Google Scholar] [CrossRef]
- Chang, S.; Li, J.; He, Y.; Liu, H.; Cheng, B. A high-sensitivity and low-hysteresis flexible pressure sensor based on carbonized cotton fabric. Sens. Actuator A Phys. 2019, 294, 45–53. [Google Scholar] [CrossRef]
- Arunachalam, S.; Izquierdo, R.; Nabki, F. Low-hysteresis and fast response time humidity sensors using suspended functionalized carbon nanotubes. Sensors 2019, 19, 680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Han, C.; Liang, R.; Xu, J.; Li, B.; Hou, J.; Tang, T.; Zeng, Z.; Li, J. Fabrication and Performance of Graphene Flexible Pressure Sensor with Micro/Nano Structure. Sensors 2021, 21, 7022. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Xu, H.; Jia, N.; Li, Y.; Zhu, L.; Sun, Z. A Highly Sensitive, Ultra-Durable, Eco-Friendly Ionic Skin for Human Motion Monitoring. Polymers 2022, 14, 1902. https://doi.org/10.3390/polym14091902
Li Z, Xu H, Jia N, Li Y, Zhu L, Sun Z. A Highly Sensitive, Ultra-Durable, Eco-Friendly Ionic Skin for Human Motion Monitoring. Polymers. 2022; 14(9):1902. https://doi.org/10.3390/polym14091902
Chicago/Turabian StyleLi, Zhaoxin, Haoyan Xu, Na Jia, Yifei Li, Liangkuan Zhu, and Zhuangzhi Sun. 2022. "A Highly Sensitive, Ultra-Durable, Eco-Friendly Ionic Skin for Human Motion Monitoring" Polymers 14, no. 9: 1902. https://doi.org/10.3390/polym14091902
APA StyleLi, Z., Xu, H., Jia, N., Li, Y., Zhu, L., & Sun, Z. (2022). A Highly Sensitive, Ultra-Durable, Eco-Friendly Ionic Skin for Human Motion Monitoring. Polymers, 14(9), 1902. https://doi.org/10.3390/polym14091902






