Infectious Inflammatory Processes and the Role of Bioactive Agent Released from Imino-Chitosan Derivatives Experimental and Theoretical Aspects
Abstract
1. Introduction
2. Theoretical Design
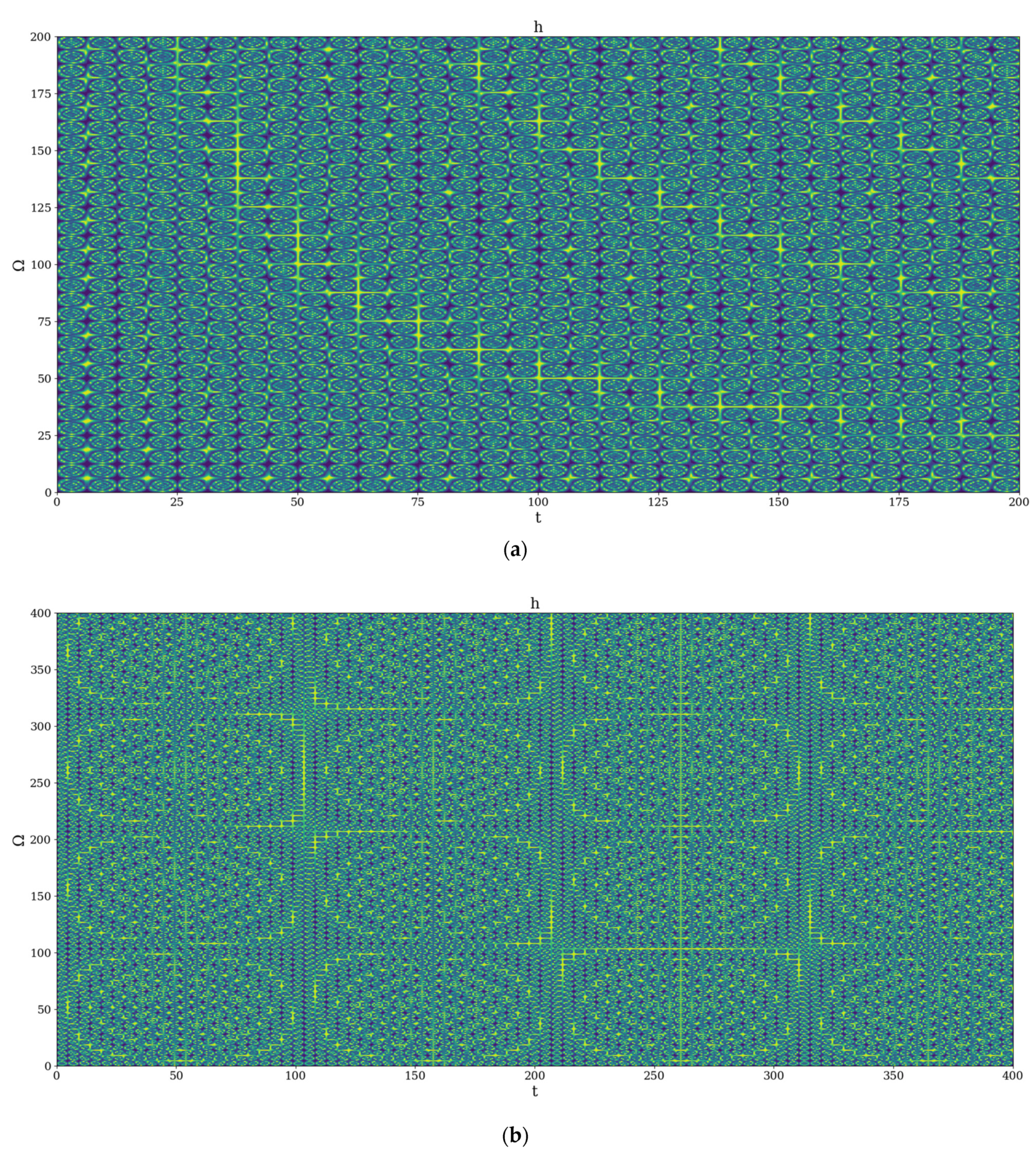
2.1. Scale Space and Drug Release Laws
2.2. Scale Space and Drug Release Mechanisms
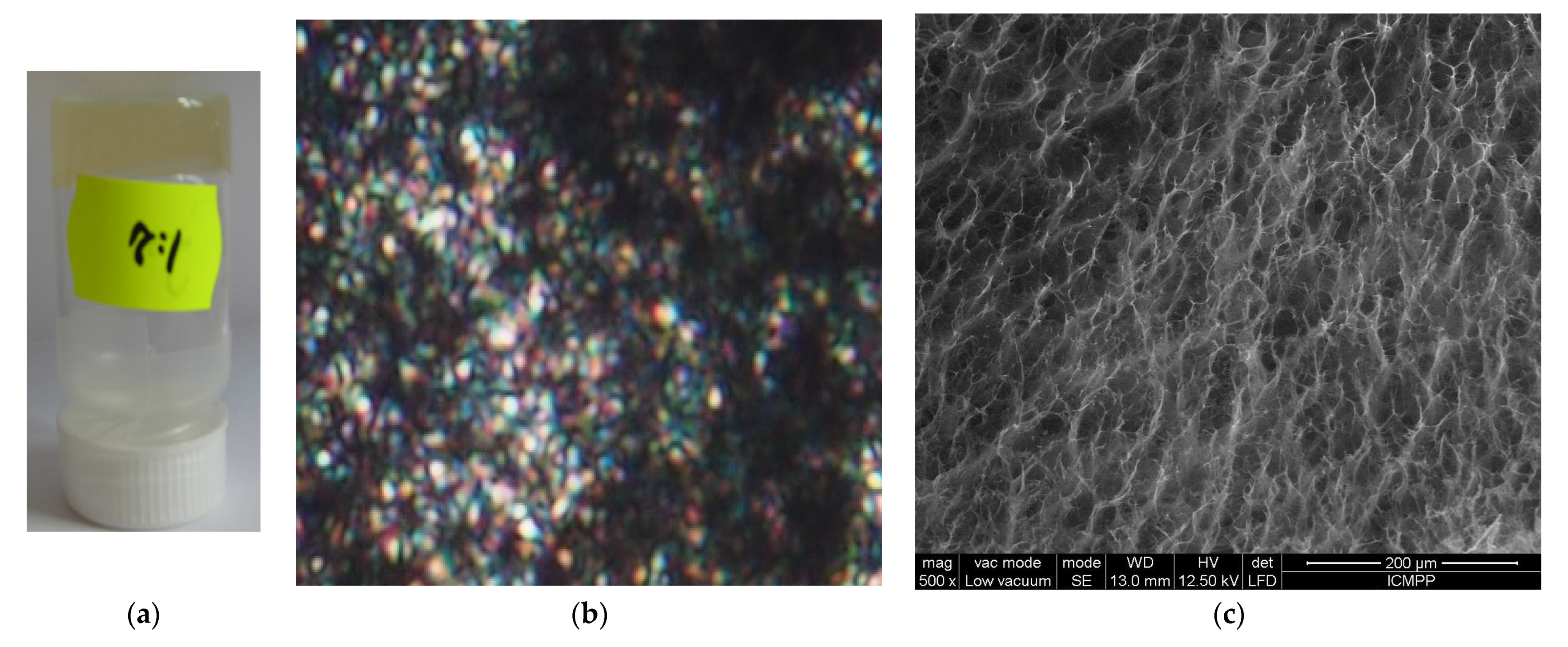
3. Experimental Design
4. Results and Discussion
5. Perspectives in Infectious Inflammatory Processes
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Y.; Rashidpour, A.; Almajano, M.P.; Meton, I. Chitosan-Based Drug Delivery System: Applications in Fish Biotechnology. Polymers 2020, 12, 1177. [Google Scholar] [CrossRef] [PubMed]
- Marin, L.; Ailincai, D.; Mares, M.; Paslaru, E.; Cristea, M.; Nica, V.; Simionescu, B.C. Imino-chitosan biopolymeric films. Obtaining, self-assembling, surface and antimicrobial properties. Carbohydr. Polym. 2015, 117, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, M.; Wang, C.; Yan, S.; Lu, P.; Wang, S. Developed Chitosan/Oregano Essential Oil Biocomposite Packaging Film Enhanced by Cellulose Nanofibril. Polymers 2020, 12, 1780. [Google Scholar] [CrossRef]
- Fauzi, N.I.M.; Fen, Y.W.; Omar, N.A.S.; Saleviter, S.; Daniyal, W.M.E.M.M.; Hashim, H.S.; Nasrullah, M. Nanostructured chitosan/maghemite composites thin film for potential optical detection of mercury ion by surface plasmon resonance investigation. Polymers 2020, 12, 1497. [Google Scholar] [CrossRef] [PubMed]
- Craciun, A.M.; Mititelu-Tartau, L.; Gavril, G.; Marin, L. Chitosan crosslinking with pyridoxal 5-phosphate vitamer toward biocompatible hydrogels for in vivo applications. Int. J. Biol. Macromol. 2021, 193, 1734–1743. [Google Scholar] [CrossRef] [PubMed]
- Kuen, C.Y.; Galen, T.; Fakurazi, S.; Othman, S.S.; Masarudin, M.J. Increased cytotoxic efficacy of protocatechuic acid in A549 human lung cancer delivered via hydrophobically modified-chitosan nanoparticles as an anticancer modality. Polymers 2020, 12, 1951. [Google Scholar] [CrossRef] [PubMed]
- Ailincai, D.; Marin, L.; Morariu, S.; Mares, M.; Bostanaru, A.C.; Pinteala, M.; Simionescu, B.C.; Barboiu, M. Dual crosslinkediminoboronate-chitosan hydrogels with strong antifungal activity against Candida planktonic yeasts and biofilms. Carbohydr. Polym. 2016, 152, 306–316. [Google Scholar] [CrossRef]
- Anisiei, A.; Rosca, I.; Sandu, A.-I.; Bele, A.; Cheng, X.; Marin, L. Imination of Microporous Chitosan Fibers—A Route to Biomaterials with “On Demand” Antimicrobial Activity and Biodegradation for Wound Dressings. Pharmaceutics 2022, 14, 117. [Google Scholar] [CrossRef]
- Zhu, Y.; Long, H.; Zhang, W. Imine-Linked Porous Polymer Frameworks with High Small Gas (H2, CO2, CH4, C2H2) Uptake and CO2/N2 Selectivity. Chem. Mater. 2013, 25, 1630–1635. [Google Scholar] [CrossRef]
- Pusca, S.; Paun, M.A.; Toma, C. Viscoelastic behaviour analysis of the technical polymers by bidimensional pulses generation. Mater. Plast. 2007, 44, 39–42. [Google Scholar]
- Xiong, S.; Duan, L.; Cheng, X. Fluorescent chitosan hydrogel for highly and selectively sensing of p-nitrophenol and 2,4,6-trinitrophenol. Carbohydr. Polym. 2019, 225, 115253. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future perspectives. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Fisher, O.Z.; Khademhosseini, A.; Pepas, N.A. Drug delivery: Nanoscale devices. In Encyclopedia of Materials: Science and Technology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1–9. [Google Scholar]
- Pepas, N.A.; Brannon-Peppas, L. Drug Delivery Biomaterials. In Encyclopedia of Materials: Science and Technology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 196–207. [Google Scholar]
- Kosmidis, K.; Ardyrakis, P.; Macheras, P. Fractal kinetics in drug release from finite matrices. J. Chem. Phys. 2003, 119, 6373–6377. [Google Scholar] [CrossRef]
- Ailincai, D.; Agop, M.; Marinas, I.C.; Zala, A.; Irimiciuc, S.A.; Dobreci, L.; Petrescu, T.-C.; Volovat, C. Theoretical model for the diclofenac release from PEGylated chitosan hydrogels. Drug Deliv. 2021, 28, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Nottale, L. Scale Relativity and Fractal Space-Time: A New Approach to Unifying Relativity and Quantum Mechanics; Imperial College Press: London, UK, 2011. [Google Scholar]
- Merches, I.; Agop, M. Differentiability and Fractality in Dynamics of Physical Systems; World Scientific: Singapore, 2016. [Google Scholar]
- Marin, L.; Popa, M.; Anisiei, A.; Irimiciuc, S.-A.; Agop, M.; Petrescu, T.-C.; Vasincu, D.; Himiniuc, L. A Theoretical Model for Release Dynamics of an Antifungal Agent Covalently Bonded to the Chitosan. Molecules 2021, 26, 2089. [Google Scholar] [CrossRef]
- Marin, L.; Destri, S.; Porzio, W.; Bertini, F. Synthesis and characterization of new azomethine derivatives exhibiting liquid crystalline properties. Liq. Cryst. 2009, 36, 21–32. [Google Scholar] [CrossRef]
- Olaru, A.M.; Marin, L.; Morariu, S.; Pricope, G.; Pinteala, M.; Tartau-Mititelu, L. Biocompatible chitosan-based hydrogels for potential application in local tumour therapy. Carbohydr. Polym. 2018, 179, 59–70. [Google Scholar] [CrossRef]
- Craciun, A.M.; Tartau, L.; Pinteala, M.; Marin, L. Nitrosalicyl-imine-chitosan hydrogels based drug delivery systems for long term sustained release in local therapy. J. Colloid Interface Sci. 2019, 536, 196–207. [Google Scholar] [CrossRef]
- Ailincai, D.; Mititelu-Tartau, L.; Marin, L. Citryl-imine-PEG-ylated chitosan hydrogels—Promising materials for drug delivery applications. Int. J. Biol. Macromol. 2020, 162, 1323–1337. [Google Scholar] [CrossRef]
- Spampinato, C.; Leonardi, D. Candida Infections, Causes, Targets, and Resistance Mechanisms: Traditional and Alternative Antifungal Agents. Biomed Res. Int. 2013, 2013, 204237. [Google Scholar] [CrossRef] [PubMed]
- Paun, M.A.; Avanaki, M.R.N.; Dobre, G.; Hojjat, A.; Podoleanu, A.G. Wavefront aberration correction in single mode fibre systems. J. Optoelectron. Adv. Mater. 2009, 11, 1681–1685. [Google Scholar]
- Irimiciuc, S.A.; Hodoroaba, B.C.; Bulai, G.; Gurlui, S.; Craciun, V. Multiple structure formation and molecule dynamics in transient plasmas generated by laser ablation of graphite. Spectrochim. Part B At. Spectrosc. 2020, 165, 105774. [Google Scholar] [CrossRef]
- Liu, L.; Yang, H.; Guo, Y.; Yang, G.; Chen, Y. The impact of chronic endometritis on endometrial fibrosis and reproductive prognosis in patients with moderate and severe intrauterine adhesions: A prospective cohort study. Fertil. Steril. 2019, 111, 1002–1010.e2. [Google Scholar] [CrossRef] [PubMed]
- Polishuk, W.Z.; Anteby, S.O.; Weinstein, D. Puerperal endometritis and intrauterine adhesions. Int. Surg. 1975, 60, 418–420. [Google Scholar] [PubMed]
- Xue, X.; Chen, Q.; Zhao, G.; Zhao, J.Y.; Duan, Z.; Zheng, P.S. The overexpression of TGF-B and CCN2 in intrauterine adhesion involves the NF-kB signaling pathway. Public Libr. Sci. One 2015, 10, e0146159. [Google Scholar]
- Santamaria, X.; Isaacson, K.; Simon, C. Asherman`s Syndrome: It may not be all our fault. Hum. Reprod. 2018, 33, 1374–1380. [Google Scholar] [CrossRef]
- Li, T.; Liu, B.; Mao, W.; Gao, R.; Wu, J.; Deng, Y.; Shen, Y.; Liu, K.; Cao, J. Prostaglandin E2 promotes nitric oxide synthase 2, platelet-activating factor receptor, and matrix metalloproteinase-2 expression in Esherichia coli- challenged ex vivo endometrial explants via the prostaglandin E2 receptor 4/protein kinase a signaling pathway. Theriogenology 2019, 134, 65–73. [Google Scholar]
- Galvão, K.N.; de Oliveira, E.B.; Cunha, F.; Daetz, R.; Jones, K.; Ma, Z.; Jeong, K.C.; Bicalho, R.C.; Higgins, C.H.; Rodrigues, M.X.; et al. Effect of chitosan microparticles on the uterine microbiome of dairy cows with metritis. Appl. Environ. Microbiol. 2020, 86, e01066-20. [Google Scholar] [CrossRef]
- Wenbo, Q.; Lijian, X.; Shuangdan, Z.; Jiahua, Z.; Yanpeng, T.; Xuejun, Q.; Xianghua, H.; Jingkun, Z. Controlled releasing of SDF-1 α in chitosan-heparin hydrogel for endometrium injury healing in rat model. Int. J. Biol. Macromol. 2020, 143, 163–172. [Google Scholar] [CrossRef]
- Wang, M.; Yue, Y.; Dong, C.; Li, X.; Xu, W.; Xiong, S. Mucosal immunization with high-mobility group box 1 in chitosan enhances DNA vaccine-induced protection against Coxsackievirus B3-induced myocarditis. Clin. Vaccine Immunol. 2013, 20, 1743–1751. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chai, D.; Yue, Y.; Xu, W.; Dong, C.; Xiong, S. Mucosal co-immunization with AIM2 enhances protective SIgA response and increases prophylactic efficacy of chitosan-DNA vaccine against Coxsackievirus B3-induced myocarditis. Hum. Vaccines Immunother. 2014, 10, 1284–1294. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Himiniuc, L.; Socolov, R.; Ghizdovat, V.; Agop, M.; Anton, E.; Toma, B.; Ochiuz, L.; Vasincu, D.; Popa, O.; Onofrei, V. Infectious Inflammatory Processes and the Role of Bioactive Agent Released from Imino-Chitosan Derivatives Experimental and Theoretical Aspects. Polymers 2022, 14, 1848. https://doi.org/10.3390/polym14091848
Himiniuc L, Socolov R, Ghizdovat V, Agop M, Anton E, Toma B, Ochiuz L, Vasincu D, Popa O, Onofrei V. Infectious Inflammatory Processes and the Role of Bioactive Agent Released from Imino-Chitosan Derivatives Experimental and Theoretical Aspects. Polymers. 2022; 14(9):1848. https://doi.org/10.3390/polym14091848
Chicago/Turabian StyleHiminiuc, Loredana, Razvan Socolov, Vlad Ghizdovat, Maricel Agop, Emil Anton, Bogdan Toma, Lacramioara Ochiuz, Decebal Vasincu, Ovidiu Popa, and Viviana Onofrei. 2022. "Infectious Inflammatory Processes and the Role of Bioactive Agent Released from Imino-Chitosan Derivatives Experimental and Theoretical Aspects" Polymers 14, no. 9: 1848. https://doi.org/10.3390/polym14091848
APA StyleHiminiuc, L., Socolov, R., Ghizdovat, V., Agop, M., Anton, E., Toma, B., Ochiuz, L., Vasincu, D., Popa, O., & Onofrei, V. (2022). Infectious Inflammatory Processes and the Role of Bioactive Agent Released from Imino-Chitosan Derivatives Experimental and Theoretical Aspects. Polymers, 14(9), 1848. https://doi.org/10.3390/polym14091848







