A Facile and Cost-Effective Method to Prepare Biodegradable Poly(ester urethane)s with Ordered Aliphatic Hard-Segments for Promising Medical Application as Long-Term Implants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
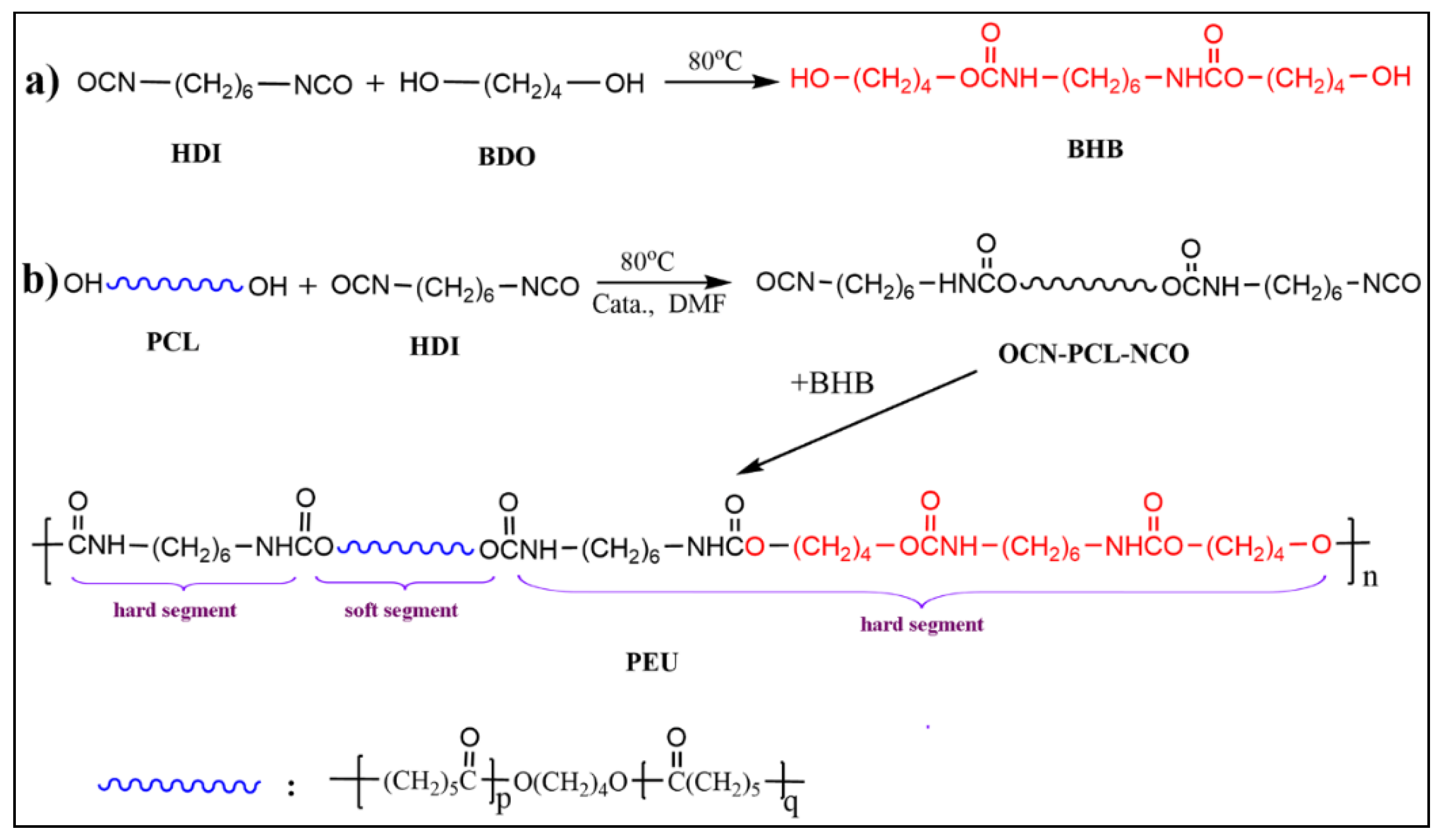
2.2. Synthesis of BHB
2.3. Preparation of PEUs and PEU Films
2.4. Characterization and Instruments
3. Results and Discussion
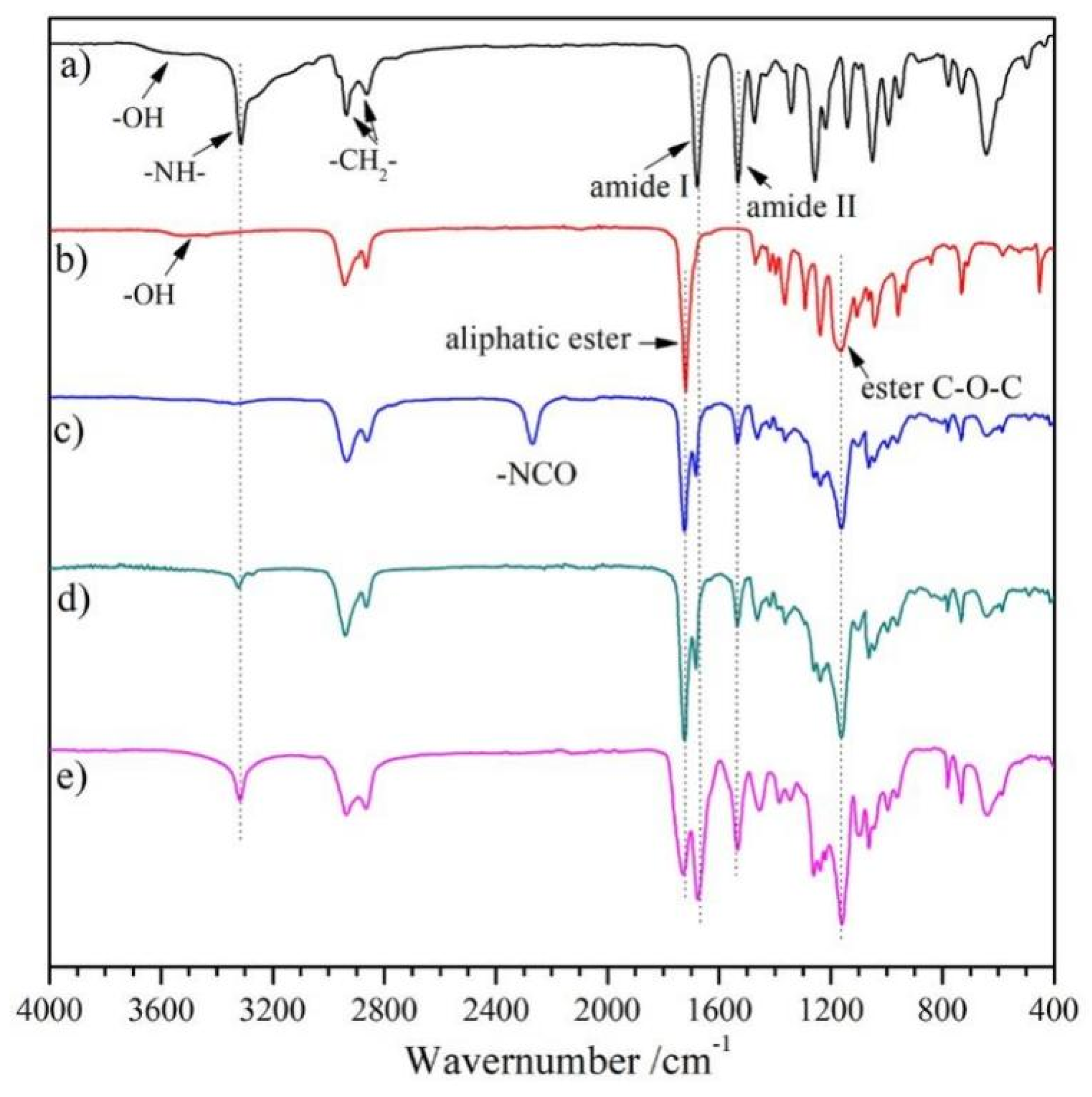
3.1. Synthesis and Characterization
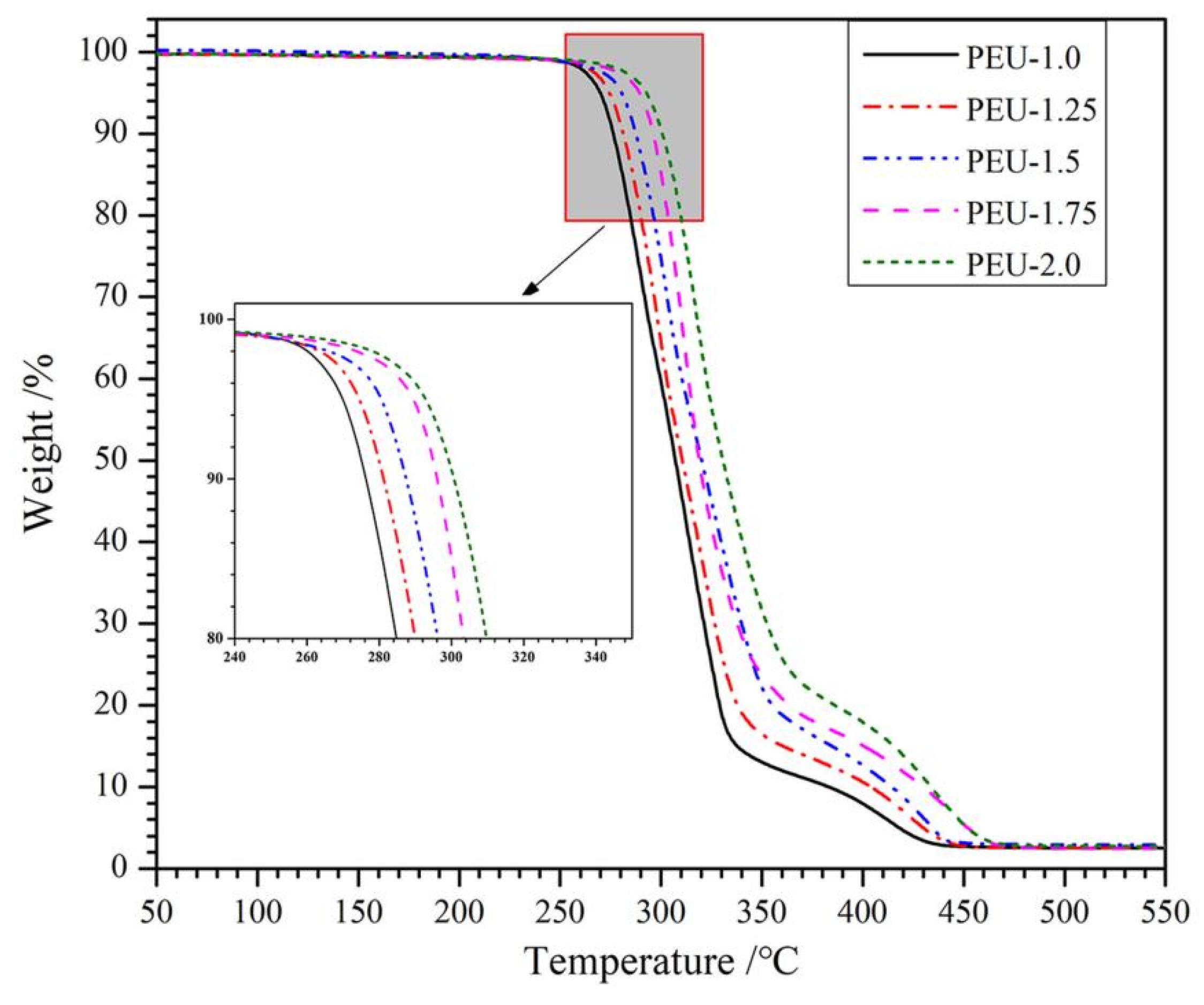
3.2. Thermal Stability
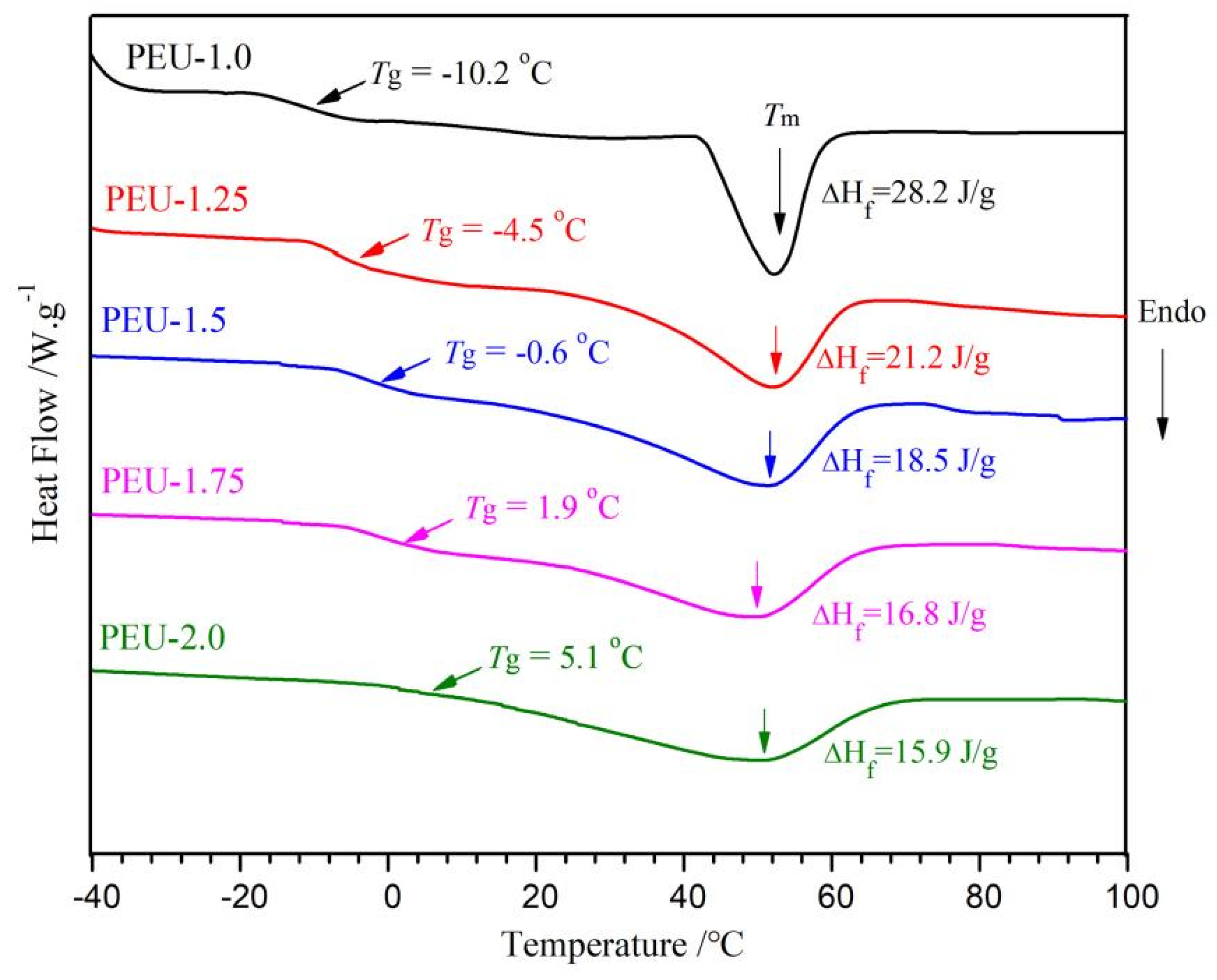
3.3. Thermal Transition
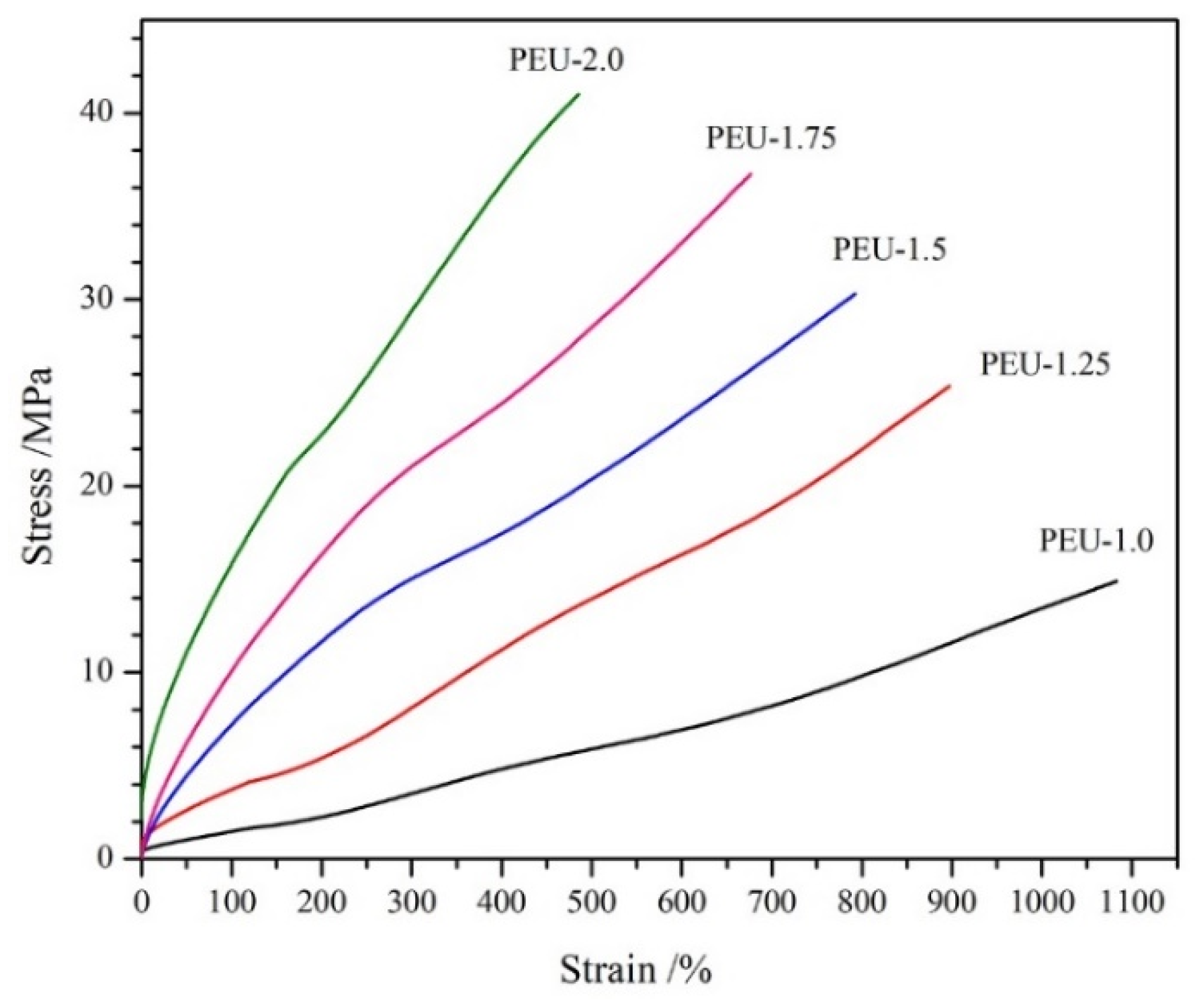
3.4. Tensile Features
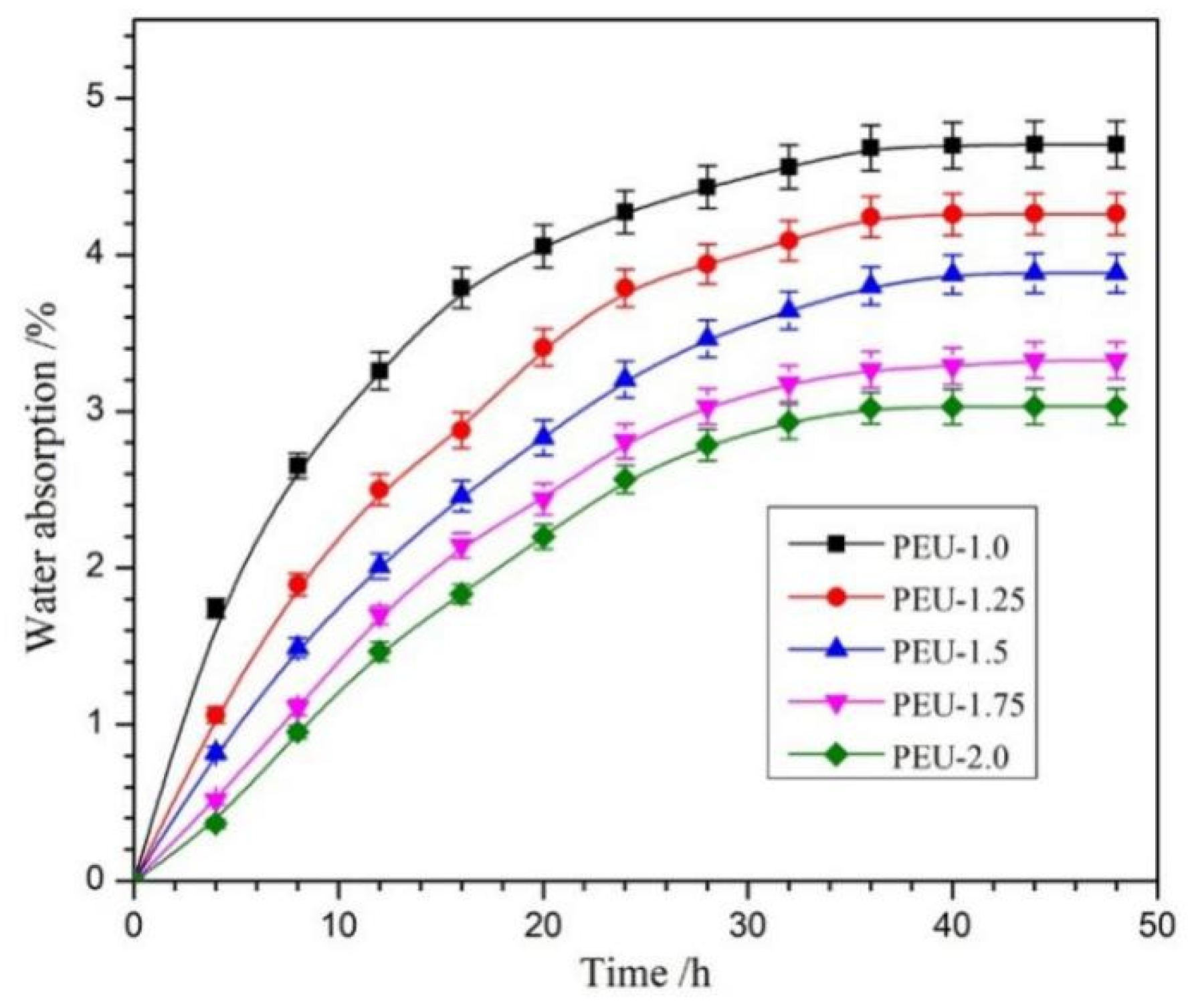
3.5. Water Absorption
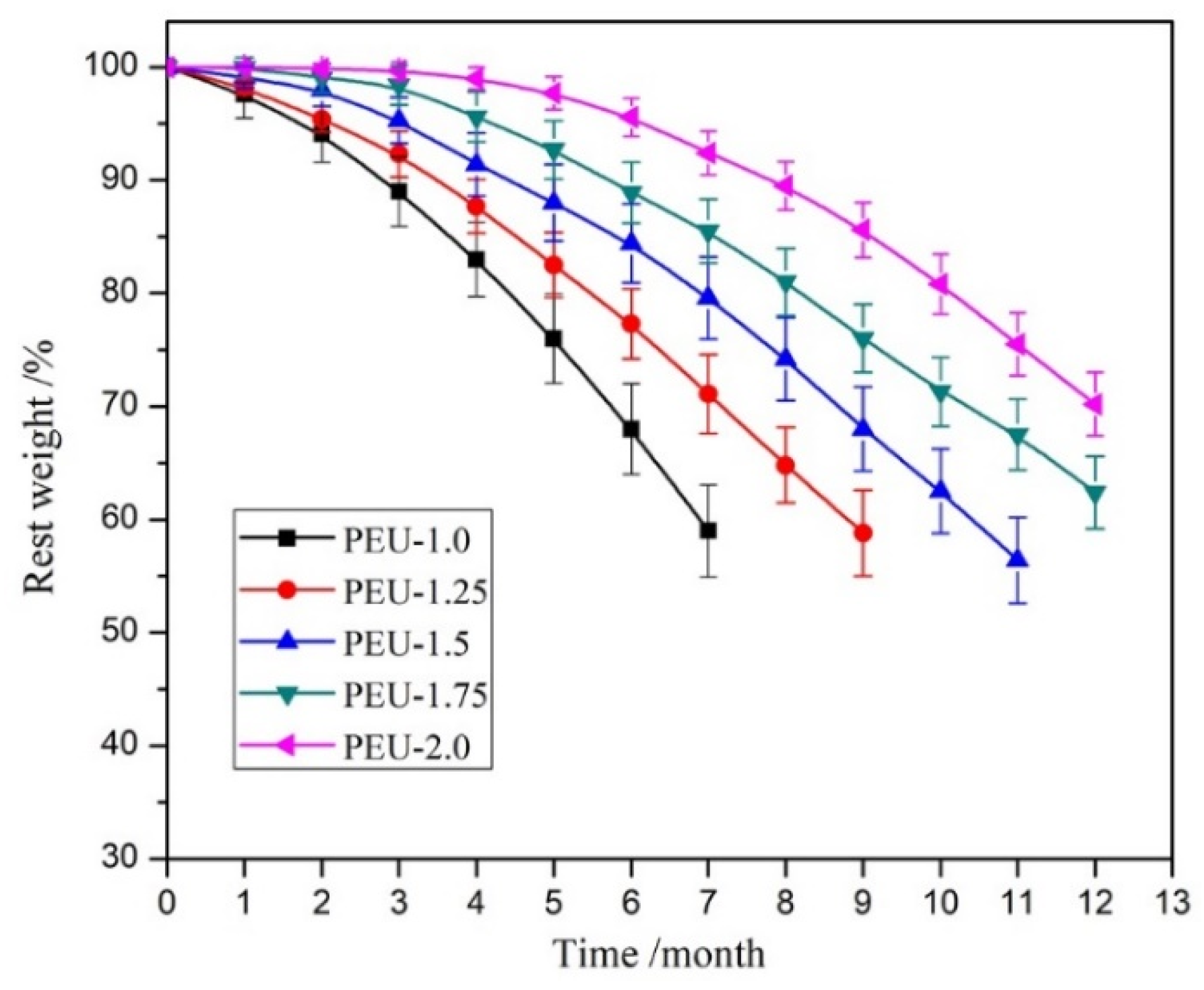
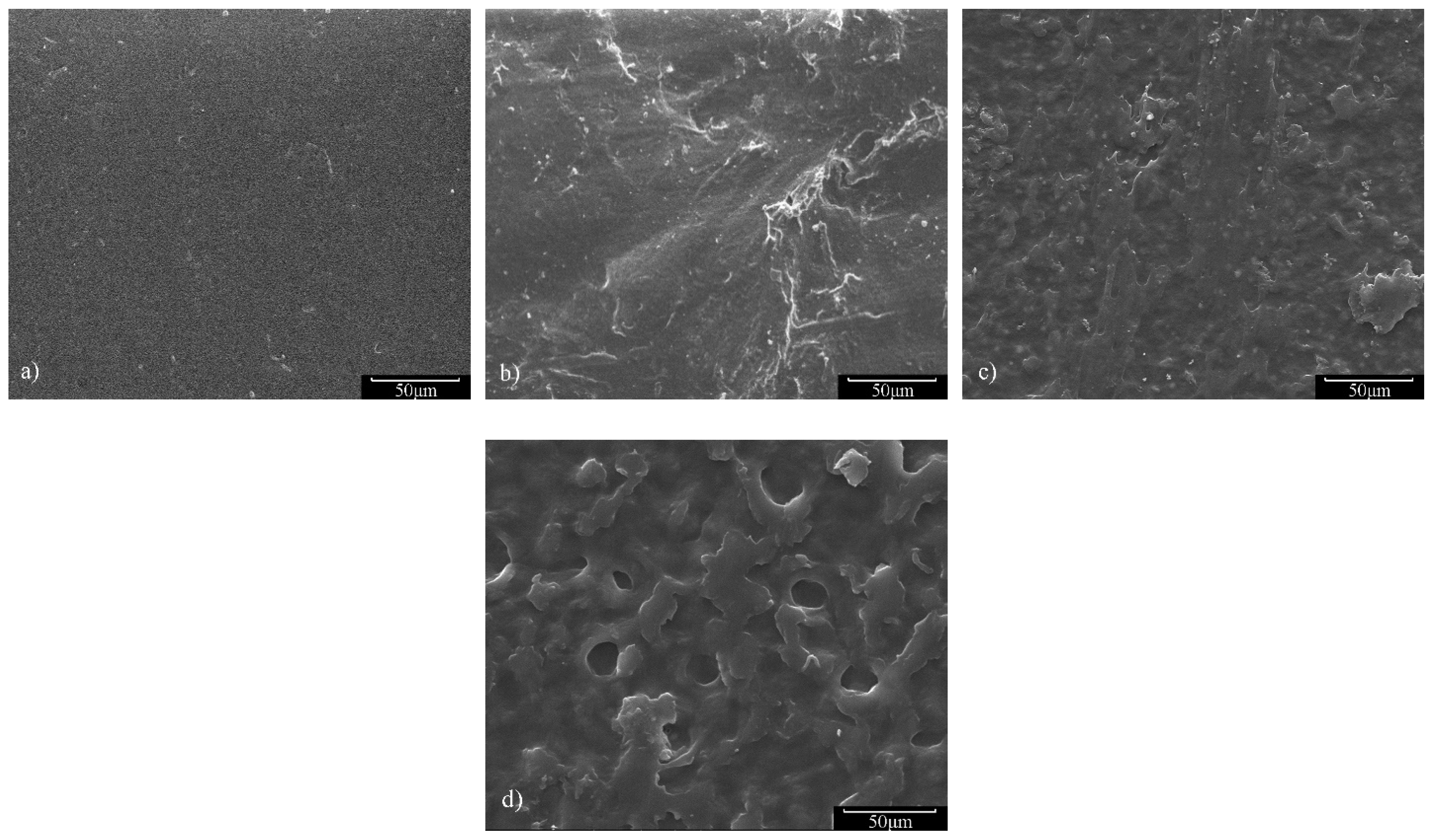
3.6. Hydrolytic Degradability
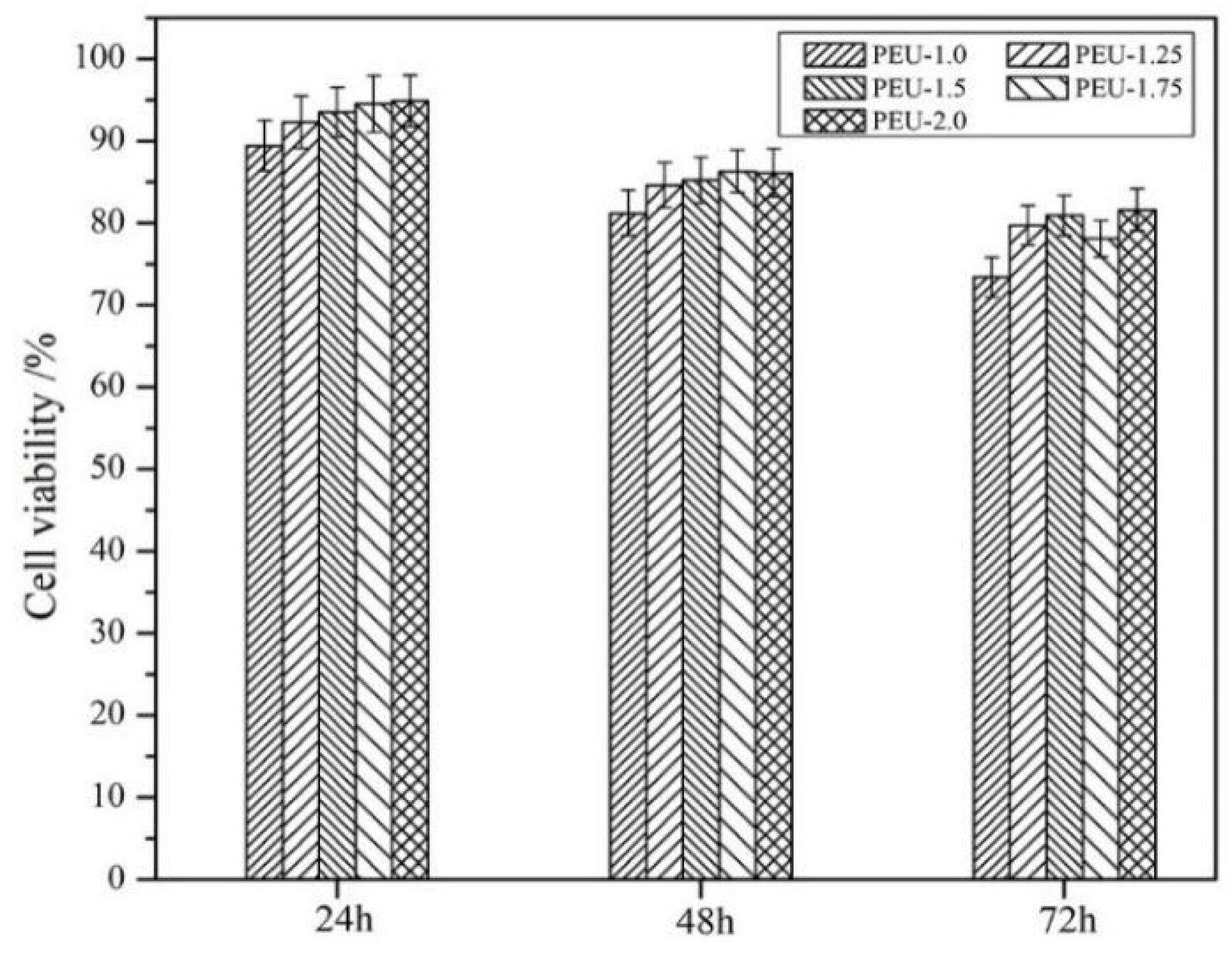
3.7. Cytotoxicity Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atiqah, A.; Mastura, M.T.; Ahmed, A.B.A.; Jawaid, M.; Sapuan, S.M. A review on polyurethane and its polymer composites. Curr. Org. Synth. 2017, 14, 233–248. [Google Scholar] [CrossRef]
- Krol, P. Synthesis methods, chemical structures and phase structures of linear polyurethanes. Properties and applications of linear polyurethanes in polyurethane elastomers, copolymers and ionomers. Prog. Mater. Sci. 2007, 52, 915–1015. [Google Scholar] [CrossRef]
- Janik, H.; Marzec, M. A review: Fabrication of porous polyurethane scaffolds. Mat. Sci. Eng. C 2015, 48, 586–591. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Hu, Z.; Xu, A. The preparation and performance of a new polyurethane vascular prosthesis. Cell Biochem. Biophys. 2013, 66, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Lamba, N.M.K.; Woodhouse, K.A.; Cooper, S.L. Polyurethanes in Biomedical Applications; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- John, K.R.S. The use of polyurethane materials in the surgery of the spine: A review. Spine J. 2014, 14, 3038–3047. [Google Scholar] [CrossRef]
- Ergene, E.; Yagci, B.S.; Gokyer, S.; Eyidogan, A.; Aksoy, E.A.; Huri, P.Y. A novel polyurethane-based biodegradable elastomer as a promising material for skeletal muscle tissue engineering. Biomed. Mater. 2019, 14, 025014. [Google Scholar] [CrossRef]
- Howard, G.T. Biodegradation of polyurethane: A review. Int. Biodeterior. Biodegrad. 2002, 49, 245–252. [Google Scholar] [CrossRef]
- Lin, H.H.; Hsieh, F.Y.; Tseng, C.S.; Hsu, S.H. Preparation and characterization of a biodegradable polyurethane hydrogel and the hybrid gel with soy protein for 3D cell-laden bioprinting. J. Mater. Chem. B 2016, 41, 6694–6705. [Google Scholar] [CrossRef]
- Xia, J.; Li, J.; Ding, M.; Hong, T.; Ling, Q.; Zhong, Y. Synthesis and degradation of nontoxic biodegradable waterborne polyurethanes elastomer with poly(ε-caprolactone) and poly(ethylene glycol) as soft segment. Eur. Polym. J. 2007, 43, 1838–1846. [Google Scholar]
- Wang, H.; Yu, J.; Fang, H.; Wei, H.; Wang, X.; Ding, Y. Largely improved mechanical properties of a biodegradable polyurethane elastomer via polylactide stereocomplexation. Polymer 2018, 137, 1–12. [Google Scholar] [CrossRef]
- Suthapakti, K.; Molloy, R.; Punyodom, W.; Nalampang, K.; Leejarkpai, T.; Topham, P.D. Biodegradable compatibilized poly(l-lactide)/thermoplastic polyurethane blends: Design, preparation and property testing. J. Polym. Environ. 2018, 26, 1818–1830. [Google Scholar] [CrossRef]
- Reed, A.M.; Potter, J.; Szycher, M. A solution grade biostable polyurethane elastomer: ChronoFlex® AR. J. Biomater. Appl. 1994, 8, 210–236. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.; Gordon, B.; Rosenberg, G. Moisture diffusivity of Biomer® versus Biomer®-coated polyisobutylene polyurethane urea (PIB-PUU): A potential blood sac material for the artificial heart. J. Mater. Sci. Lett. 1994, 13, 1390–1391. [Google Scholar] [CrossRef]
- Oertel, G. Polyurethane Handbook; Hanser Gardner: Berlin, Germany, 1994. [Google Scholar]
- Szycher, M. Biostability of polyurethane elastomers: A critical review. J. Biomater. Appl. 1988, 3, 297–402. [Google Scholar] [CrossRef]
- Szycher, M.; Siciliano, A.J. An assessment of 2,4 TDA formation from surgitek polyurethane foam under simulated physiological conditions. J. Biomater. Appl. 1991, 5, 323–336. [Google Scholar] [CrossRef]
- Coury, A. Chemical and biochemical degradation of polymers. In Biomaterials Science: An Introduction to Materials in Medicine; Ratner, B., Hoffman, A., Schoen, F., Lemons, J., Eds.; Elsevier Academic Press: Boston, MA, USA, 2004; pp. 411–430. [Google Scholar]
- Guan, J.; Sacks, M.S.; Beckman, E.J.; Wagner, W.R. Synthesis, characterization, and cytocompatibility of elastomeric, biodegradable poly(ester-urethane)ureas based on poly(caprolactone) and putrescine. J. Biomed. Mater. Res. 2002, 61, 493–503. [Google Scholar] [CrossRef]
- Guan, J.; Sacks, M.S.; Beckman, E.J.; Wagner, W.R. Biodegradable poly(ether ester urethane)urea elastomers based on poly(ether ester) triblock copolymers and putrescine: Synthesis, characterization and cytocompatibility. Biomaterials 2004, 25, 85–96. [Google Scholar] [CrossRef]
- Spaans, C.J.; De Groot, J.H.; Belgraver, V.W. A new biomedical polyurethane with a high modulus based on 1,4-butanediisocyanate and ε-caprolactone. J. Mater. Sci. Mater. Med. 1998, 9, 675–678. [Google Scholar] [CrossRef]
- Spaans, C.J.; Groot, J.H.D.; Dekens, F.G. High molecular weight polyurethanes and a polyurethane urea based on 1,4-butanediisocyanate. Polym. Bull. 1998, 41, 131–138. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Zhang, W.; Zhang, H.; Hou, Z. Synthesis and properties of biodegradable poly(ester-urethane)s based on poly(ε-caprolactone) and aliphatic diurethane diisocyanate for long-term implant application: Effect of uniform-size hard segment content. J. Biomater. Sci. Polym. Ed. 2019, 30, 1212–1226. [Google Scholar] [CrossRef]
- Yin, S.; Xia, Y.; Jia, Q.; Hou, Z.; Zhang, N. Preparation and properties of biomedical segmented polyurethanes based on poly(ether ester) and uniform-size diurethane diisocyanates. J. Biomater. Sci. Polym. Ed. 2017, 28, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Xu, J.; Teng, J.; Jia, Q.; Wang, X. Facile preparation of medical segmented poly(ester-urethane) containing uniformly sized hard segments and phosphorylcholine groups for improved hemocompatibility. Mater. Sci. Eng. C 2020, 109, 110571. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Sun, F.; Du, H. Determination of isocyanate group in polyurethane by “di-n-butylamine-acetone” method. Chin. J. Anal. Lab. 2007, 26, 73–76. [Google Scholar]
- Ji, Q.; Xia, Y.; Yin, S.; Hou, Z.; Wu, R. Influence of well-defined hard segment length on the properties of medical segmented polyesterurethanes based on poly(ε-caprolactone-co-L-lactide) and aliphatic urethane diisocyanates. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 388–397. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, C.; Kessler, M.R. Anionic waterborne polyurethane dispersion from a bio-based ionic segment. RSC Adv. 2014, 4, 35476–35483. [Google Scholar] [CrossRef]
- Yeganeh, H.; Jamshidi, H.; Jamshidi, S. Synthesis and properties of novel biodegradable poly(ε-caprolactone)/poly(ethylene glycol)-based polyurethane elastomers. Polym. Int. 2007, 56, 41–49. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.; Bryant, P.; Kurusingal, V.; Colwell, J.M.; Laycock, B. Degradation and stabilization of polyurethane elastomers. Prog. Polym. Sci. 2019, 90, 211–268. [Google Scholar] [CrossRef]
- Jeong, J.H.; Han, Y.C.; Yang, J.H.; Kwak, D.S.; Jeong, H.M. Waterborne polyurethane modified with poly(ethylene glycol) macromer for waterproof breathable coating. Prog. Org. Coat. 2017, 103, 69–75. [Google Scholar] [CrossRef]
- Haut, R.C. Biomechanics of soft tissue. In Accidental Injury; Nahum, A.M., Melvin, J.W., Eds.; Springer: New York, NY, USA, 2002; pp. 228–253. [Google Scholar]
- Xu, J.; Hao, T.; Liu, C.; Bi, J.; Sun, J.; Wen, Z.; Hou, Z.; Wei, J. pH-Responsive and degradable polyurethane film with good tensile properties for drug delivery in vitro. Mater. Today Commun. 2021, 29, 102969. [Google Scholar] [CrossRef]
- Abraham, G.A.; Frontini, P.M.; Cuadrado, T.R. Molding of biomedical segmented polyurethane delamination events and stretching behavior. J. Appl. Polym. Sci. 1998, 69, 2159–2167. [Google Scholar] [CrossRef]
- Liu, X.; Yang, B.; Hou, Z.S.; Zhang, N.; Gao, Y. A mild method for surface-grafting MPC onto poly(ester-urethane) based on aliphatic diurethane diisocyanate with high grafting efficiency. Mater. Sci. Eng. C 2019, 104, 109952. [Google Scholar] [CrossRef] [PubMed]
- Nosbi, N.; Akil, H.M.; Ishak, Z.A.M.; Bakar, A.A. Degradation of compressive properties of pultruded kenaf fiber reinforced composites after immersion in various solutions. Mater. Des. 2010, 31, 4960–4964. [Google Scholar] [CrossRef]
- Zuraida, A.; Humairah, A.R.N.; Suraya, M.R.N.; Nazariah, M. Evaluation of kenaf fibres reinforced starch based biocomposite film through water absorption and biodegradation properties. J. Eng. Sci. 2014, 10, 31–39. [Google Scholar]
- Shi, R.; Zhu, A.; Chen, D. In vitro degradation of starch/PVA films and biocompatibility evaluation. J. Appl. Polym. Sci. 2010, 115, 346–357. [Google Scholar] [CrossRef]
- Zhu, W.; Guo, D.; Chen, Y.; Xiu, W.; Wang, D.; Huang, J.; Lu, W.; Peng, L.; Chen, K.; Zeng, Y. Cytocompatibility of PLA/nano-HA composites for interface fixation. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1122–1126. [Google Scholar] [CrossRef]

| Samples | PCL /mmol | HDI /mmol | BHB | BHB Content /wt % | Mn/KDa | Mw/Mn |
|---|---|---|---|---|---|---|
| PEU-1.0 | 5.0 | 5.0 | 0 | 0 | 89 | 1.49 |
| PEU-1.25 | 5.0 | 6.25 | 1.25 | 6.7 | 85 | 1.51 |
| PEU-1.5 | 5.0 | 7.5 | 2.5 | 12.2 | 78 | 1.62 |
| PEU-1.75 | 5.0 | 8.75 | 3.75 | 16.8 | 76 | 1.58 |
| PEU-2.0 | 5.0 | 10.0 | 5 | 20.7 | 75 | 1.53 |
| Films | PEU-1.0 | PEU-1.25 | PEU-1.5 | PEU-1.75 | PEU-2.0 |
|---|---|---|---|---|---|
| Tensile strength/MPa | 15.1 ± 0.35 | 25.4 ± 0.62 | 30.6 ± 1.58 | 36.8 ± 2.42 | 40.6 ± 2.94 |
| Elongation at break/% | 1085 ± 78 | 903 ± 64 | 797 ± 56 | 678 ± 50 | 477 ± 41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, J.; Liu, Y.; Liu, J. A Facile and Cost-Effective Method to Prepare Biodegradable Poly(ester urethane)s with Ordered Aliphatic Hard-Segments for Promising Medical Application as Long-Term Implants. Polymers 2022, 14, 1674. https://doi.org/10.3390/polym14091674
Bi J, Liu Y, Liu J. A Facile and Cost-Effective Method to Prepare Biodegradable Poly(ester urethane)s with Ordered Aliphatic Hard-Segments for Promising Medical Application as Long-Term Implants. Polymers. 2022; 14(9):1674. https://doi.org/10.3390/polym14091674
Chicago/Turabian StyleBi, Jingjing, Yifan Liu, and Jiaxu Liu. 2022. "A Facile and Cost-Effective Method to Prepare Biodegradable Poly(ester urethane)s with Ordered Aliphatic Hard-Segments for Promising Medical Application as Long-Term Implants" Polymers 14, no. 9: 1674. https://doi.org/10.3390/polym14091674
APA StyleBi, J., Liu, Y., & Liu, J. (2022). A Facile and Cost-Effective Method to Prepare Biodegradable Poly(ester urethane)s with Ordered Aliphatic Hard-Segments for Promising Medical Application as Long-Term Implants. Polymers, 14(9), 1674. https://doi.org/10.3390/polym14091674





