The Imperative Need of Metal Salt for the Treatment of Industrial Wastewater via the Synergic Coagulation-Flocculation Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Kuwait Tile Wastewater Sample
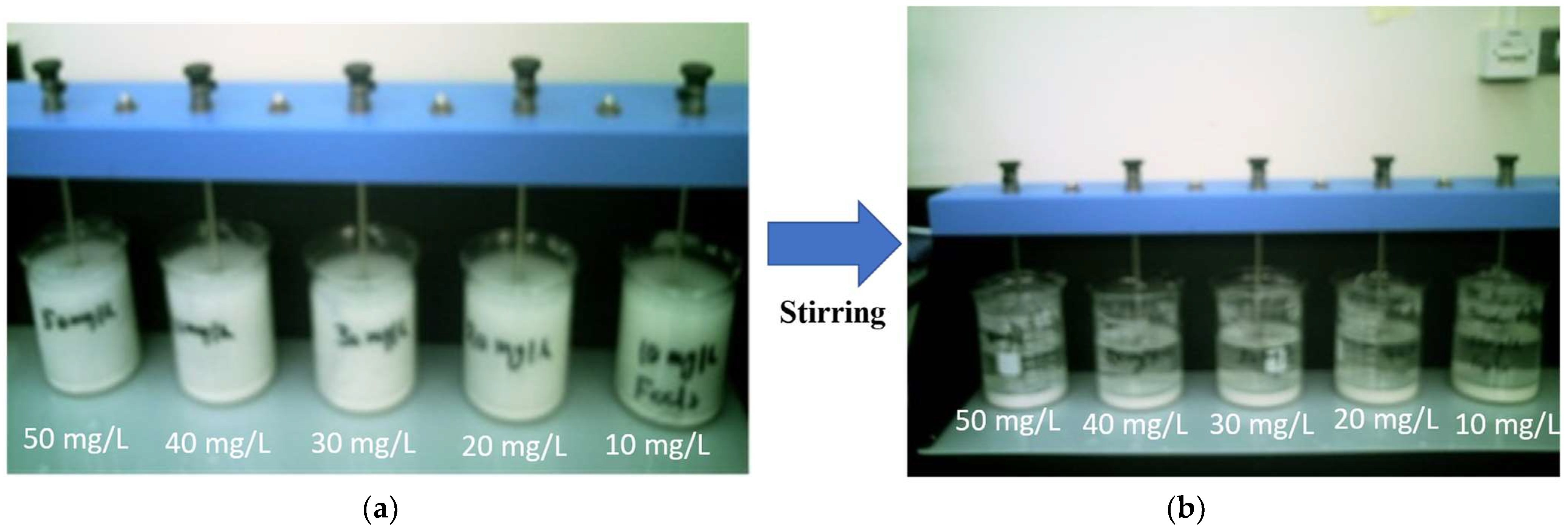
2.2. Batch Experiment
- ❖
- Coagulation precedes flocculation;
- ❖
- Chemicals (coagulants) are added in the coagulation step;
- ❖
- Mixing in coagulation is fast and occurs for a short time (seconds up to a minute);
- ❖
- In flocculation, no chemicals are added, with gentle mixing occurring for a long time (minutes).
3. Results and Discussion
3.1. Characteristics of Kuwait Tile Factory-Industrial Wastewater
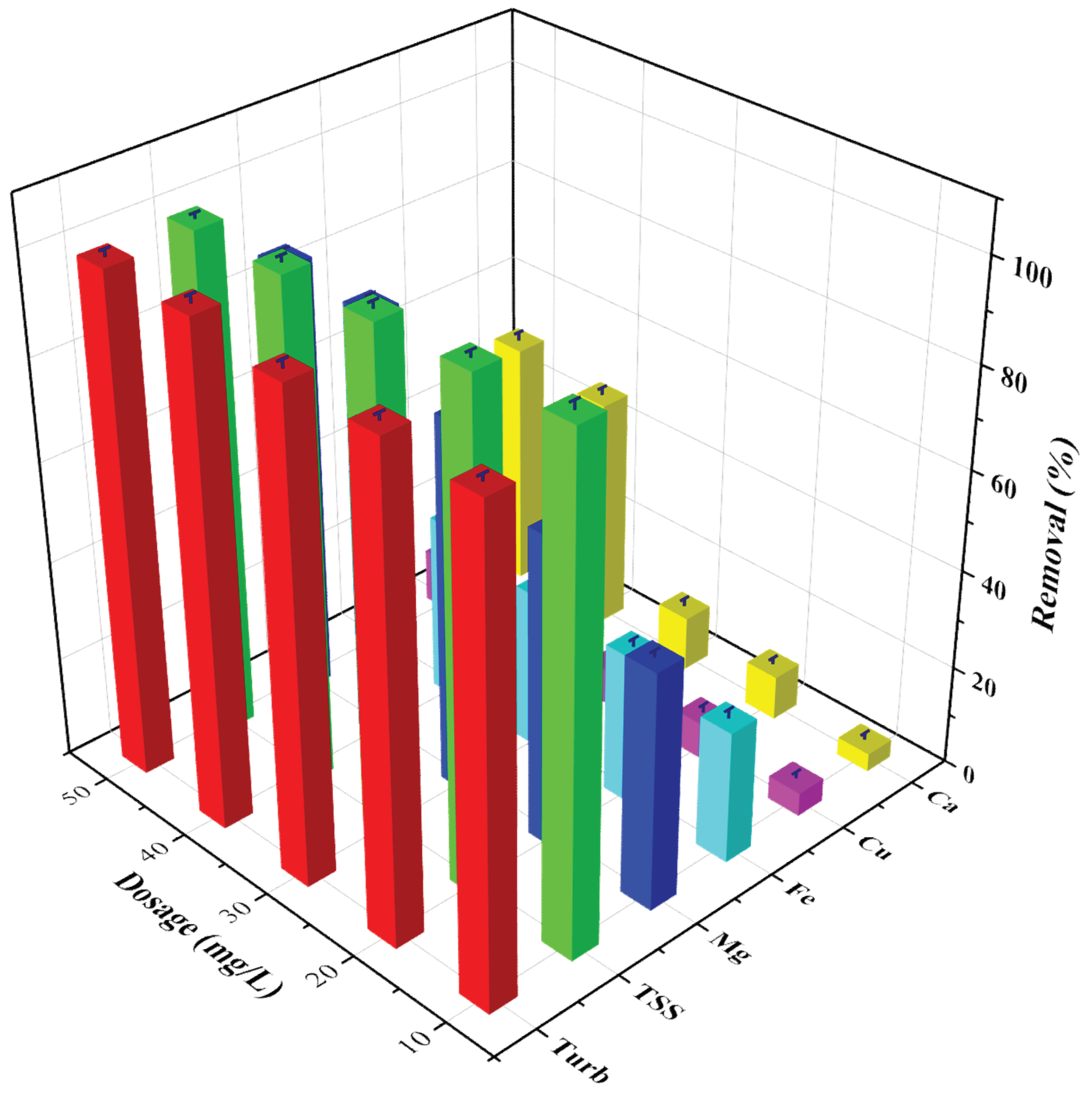
3.2. Effect of Dosage
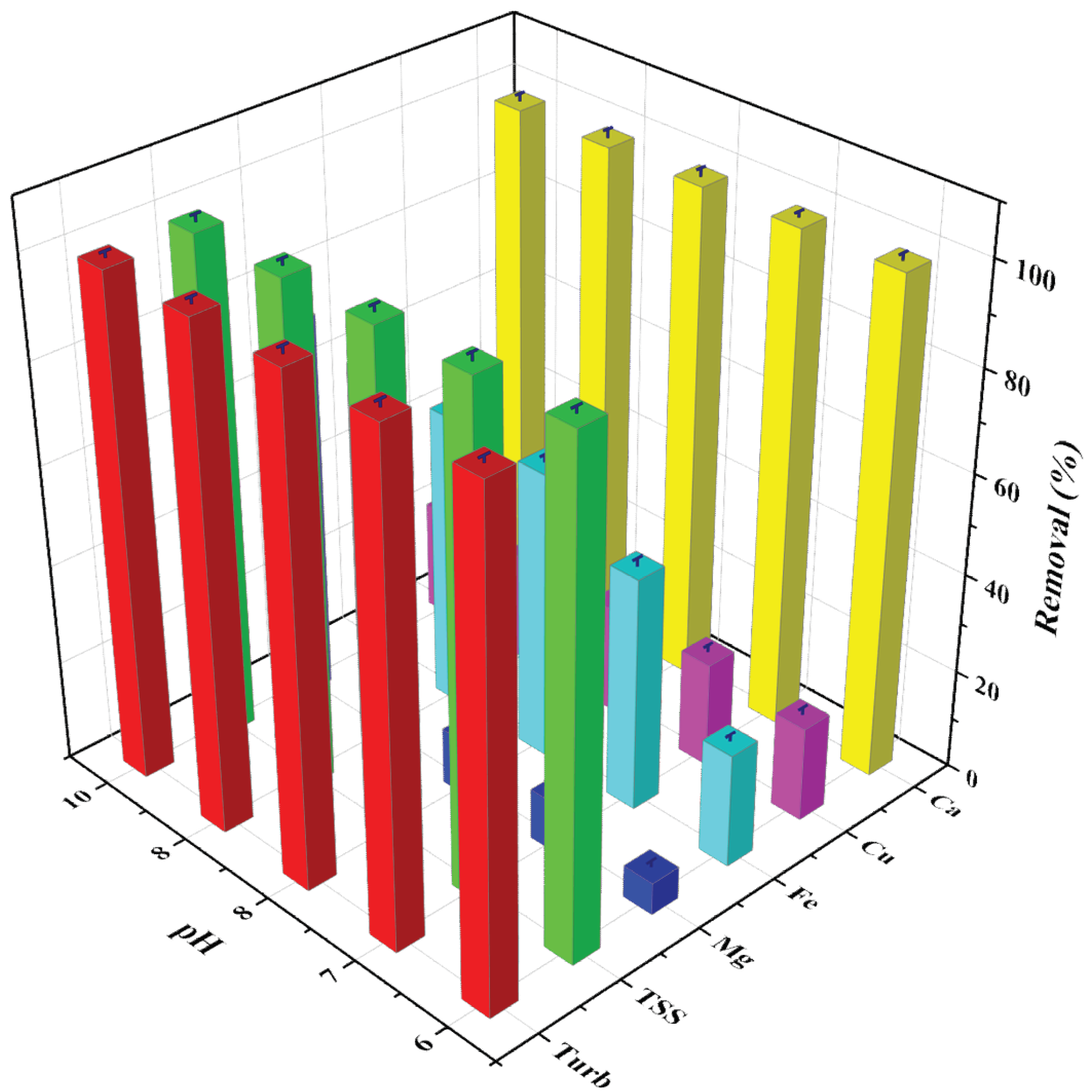
3.3. Effect of pH
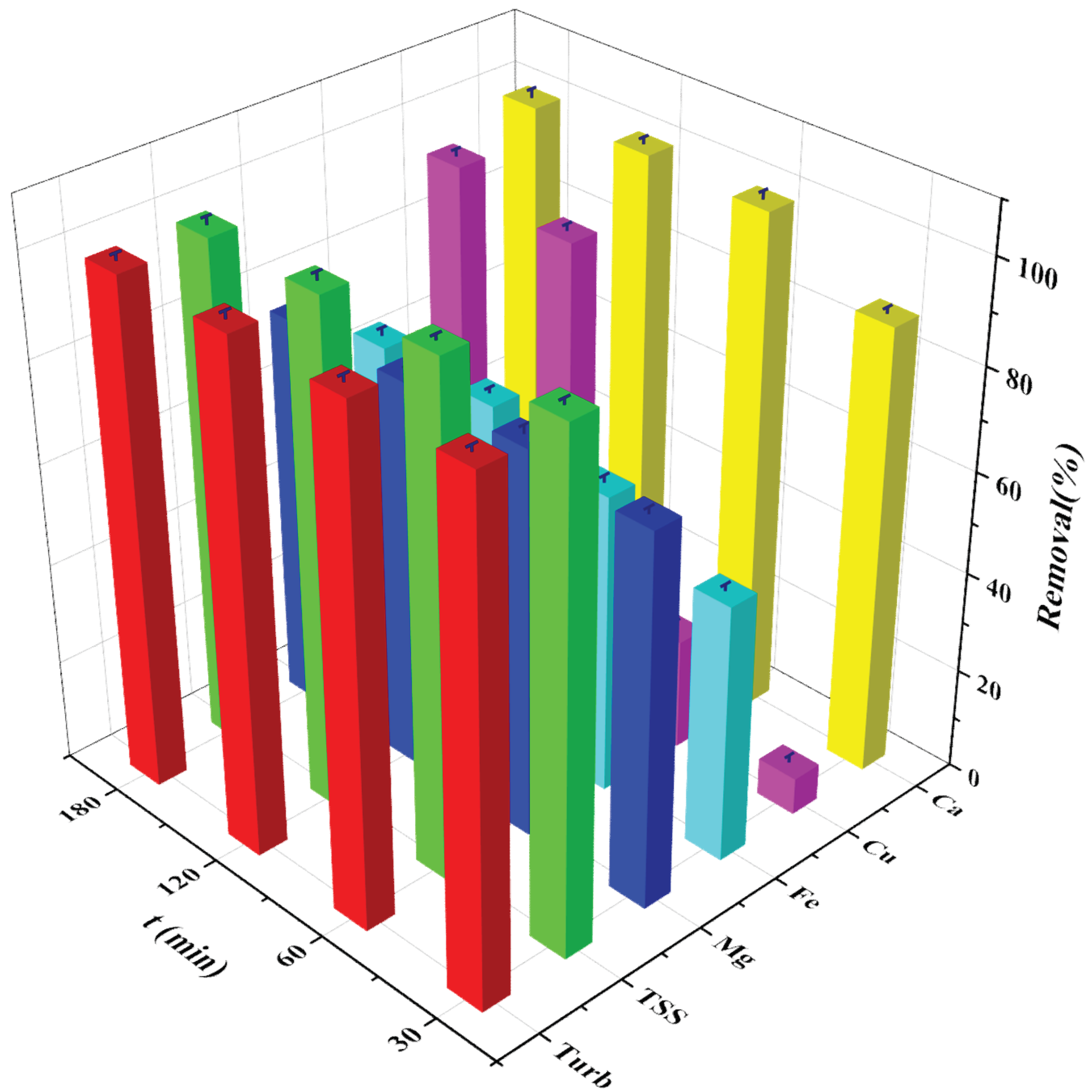
3.4. Effect of Time
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TSS | Total suspended solids |
| FeCl3 | Ferric chloride hexahydrate |
| TDS | Total dissolved solids |
| COD | Chemical oxygen demand |
| BOD | Biological oxygen demand |
| Ca | Calcium |
| Mg | Magnesium |
| Fe | Iron |
| Cu | Copper |
| KEPA | Kuwait Environmental Public Authority |
| SO4 | Sulfate |
| PACl | Polyaluminum chloride |
| NTU | Nephelometric Turbidity Units |
| Fe(OH)3 | Ferric hydroxide |
References
- Ahmad, M.; Manzoor, K.; Venkatachalam, P.; Ikram, S. Kinetic and thermodynamic evaluation of adsorption of Cu(II) by thiosemicarbazide chitosan. Int. J. Biol. Macromol. 2016, 92, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Durán-Sánchez, A.; Álvarez-García, J.; González-Vázquez, E.; del Río-Rama, M.d. Wastewater management: Bibliometric analysis of scientific literature. Water 2020, 12, 2963. [Google Scholar] [CrossRef]
- Ghazy, O.A.; Khalil, S.A.; Senna, M.M. Synthesis of montmorillonite/chitosan/ammonium acrylate composite and its potential application in river water flocculation. Int. J. Biol. Macromol. 2020, 163, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, E.K. Chemically enhanced primary treatment of textile industrial effluents. Polish J. Environ. Stud. 2009, 18, 651–655. [Google Scholar]
- Ahmad, M.; Manzoor, K.; Ikram, S. Versatile nature of hetero-chitosan based derivatives as biodegradable adsorbent for heavy metal ions; a review. Int. J. Biol. Macromol. 2017, 105, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Sagar, B.; Neha, P.; Arti, P. Environmental Solution for Effluent of Tile Industry. Int. J. Adv. Res. Innov. Ideas Educ. 2016, 2, 2395–4396. [Google Scholar]
- Amin, S.K.; Sibak, H.A.; El-Sherbiny, S.A.; Abadir, M.F. An overview of ceramic wastes management in construction. Int. J. Appl. Eng. Res. 2016, 11, 2680–2685. [Google Scholar]
- Hu, P.; Ren, J.; Hu, X.; Yang, H. Comparison of two starch-based flocculants with polyacrylamide for the simultaneous removal of phosphorus and turbidity from simulated and actual wastewater samples in combination with FeCl3. Int. J. Biol. Macromol. 2020, 167, 223–232. [Google Scholar] [CrossRef]
- Li, C.; Sun, W.; Lu, Z.; Ao, X.; Li, S. Ceramic nanocomposite membranes and membrane fouling: A review. Water Res. 2020, 175, 115674. [Google Scholar] [CrossRef]
- Chong, M.F.; Lee, K.P.; Chieng, H.J.; Ramli, I.I.S.B. Removal of boron from ceramic industry wastewater by adsorption-flocculation mechanism using palm oil mill boiler (POMB) bottom ash and polymer. Water Res. 2009, 43, 3326–3334. [Google Scholar] [CrossRef]
- Teo, P.T.; Anasyida, A.S.; Basu, P.; Nurulakmal, M.S. Recycling of Malaysia’s electric arc furnace (EAF) slag waste into heavy-duty green ceramic tile. Waste Manag. 2014, 34, 2697–2708. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.H.; Ahn, J.W. A perspective of chemical treatment for cyanobacteria control toward sustainable freshwater development. Environ. Eng. Res. 2017, 22, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Al-Anzi, B.; Abusam, A.; Shahalam, A. Assessment of Wastewater Reuse in Kuwait and Its Impact on Amounts of Pollutants Discharged into the Sea. J. Environ. Prot. 2012, 3, 935–939. [Google Scholar] [CrossRef] [Green Version]
- Al-Qurashi, A.A.H.; Husain, T. Sustainable Water Resources Development plan for the Middle-East countries. Bridging the Gap: Meeting the World’s Water and Environmental Resources Challenges. 2001, p. 2004. Available online: https://doi.org/10.1061/40569(2001)366 (accessed on 26 April 2012).
- Hamoda, M.F. Desalination and water resource management in Kuwait. Desalination 2001, 138, 165. [Google Scholar] [CrossRef]
- Wu, T.Y.; Mohammad, A.W.; Lim, S.L.; Lim, P.N.; Hay, J.X.W. Recent advances in the reuse of wastewaters for promoting sustainable development. In Wastewater Reuse and Management; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Kuhtz, S.; Zhou, C.; Albino, V.; Yazan, D.M. Energy use in two Italian and Chinese tile manufacturers: A comparison using an enterprise input-output model. Energy 2010, 35, 364–374. [Google Scholar] [CrossRef]
- Available online: https://www.google.com/search?q=Tile%20factory%20in%20kuwait (accessed on 1 March 2022).
- Dinçer, A.R.; Kargı, F. Characterization and biological treatment of ceramic industry wastewater. Bioprocess Biosyst. Eng. 2000, 23, 0209–0212. [Google Scholar] [CrossRef]
- Teh, C.Y.; Wu, T.Y. The potential use of natural coagulants and flocculants in the treatment of urban waters. Chem. Eng. Trans. 2014, 39, 1603–1608. [Google Scholar]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent Advancement of Coagulation-Flocculation and Its Application in Wastewater Treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Subramonian, W.; Wu, T.Y.; Chai, S.P. A comprehensive study on coagulant performance and floc characterization of natural Cassia obtusifolia seed gum in treatment of raw pulp and paper mill effluent. Ind. Crop. Prod. 2014, 61, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Shak, K.P.Y.; Wu, T.Y. Optimized use of alum together with unmodified Cassia obtusifolia seed gum as a coagulant aid in treatment of palm oil mill effluent under natural pH of wastewater. Ind. Crop. Prod. 2015, 76, 1169–1178. [Google Scholar] [CrossRef]
- Abusam, A.; Al-Naser, H.; Shahalam, A. Performance evaluation and modeling of the activated sludge system used in Riqqa, Kuwait. Water Pollut. XI 2012, 164, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Hosseinzadeh, K.; Ghayebzadeh, M.; Shahi, M.; Babrzadeh, M. Polymer Induced Flocculation for Treatment of a Tile Factory Wastewater using Polyacrylamide (PAM): Optimization by Response Surface Methodological Analysis. Medbiotech J. 2019, 3, 70–76. [Google Scholar] [CrossRef]
- Fahiminia, M.; Ardani, R.; Hashemi, S.; Alizadeh, M. Wastewater treatment of stone cutting industries by coagulation process. Am. J. Environ. Sci. 2010, 6, 442–448. [Google Scholar] [CrossRef]
- Baghvand, A.; Zand, A.D.; Mehrdadi, N.; Karbassi, A. Optimizing coagulation process for low to high turbidity waters using aluminum and iron salts. Chem. Eng. J. 2021, 423, 130264. [Google Scholar] [CrossRef]
- Matusiak, J.; Grządka, E.; Bastrzyk, A. Removal of hazardous oxide nanoparticles by the biopolymer flocculation in the presence of divalent salt. Chem. Eng. J. 2021, 423, 130264. [Google Scholar] [CrossRef]
- Godek, E.; Grządka, E.; Maciołek, U.; Bastrzyk, A. Influence of Zwitterionic CAPB on Flocculation of the Aqueous Cationic Guar Gum/Glauconite Suspensions at Various pH. Int. J. Mol. Sci. 2021, 22, 12157. [Google Scholar] [CrossRef]
- Engelhardt, T.L. Coagulation, Flocculation and Clarification of Drinking Water; Hach Company: Loveland, CO, USA, 2014. [Google Scholar]
- Hargreaves, A.J.; Vale, P.; Whelan, J.; Alibardi, L.; Constantino, C.; Dotro, G.; Cartmell, E.; Campo, P. Coagulation-flocculation process with metal salts, synthetic polymers and biopolymers for the removal of trace metals (Cu, Pb, Ni, Zn) from municipal wastewater. Clean Technol. Environ. Policy 2018, 20, 393–402. [Google Scholar] [CrossRef] [Green Version]
- Asrafuzzaman, M.; Fakhruddin, A.N.M.; Hossain, M.A. Reduction of Turbidity of Water Using Locally Available Natural Coagulants. ISRN Microbiol. 2011, 2011, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Subramonian, W.; Wu, T.Y.; Chai, S.P. An application of response surface methodology for optimizing coagulation process of raw industrial effluent using Cassia obtusifolia seed gum together with alum. Ind. Crop. Prod. 2015, 70, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, T.J.; Shakir, E. Effect of settling time, velocity gradient, and camp number on turbidity removal for oilfield produced water. Egypt. J. Pet. 2018, 27, 31–36. [Google Scholar] [CrossRef]
- Roussy, J.; Van Vooren, M.; Guibal, E. Chitosan for the coagulation and flocculation of mineral colloids. J. Dispers. Sci. Technol. 2005, 25, 663–677. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Role of pH in metal adsorption from aqueous solutions containing chelating agents on chitosan. Ind. Eng. Chem. Res. 1999, 38, 270–275. [Google Scholar] [CrossRef]
- Assaad, E.; Azzouz, A.; Nistor, D.; Ursu, A.V.; Sajin, T.; Miron, D.N.; Monette, F.; Niquette, P.; Hausler, R. Metal removal through synergic coagulation-flocculation using an optimized chitosan–montmorillonite system. Appl. Clay Sci. 2007, 37, 258–274. [Google Scholar] [CrossRef]
- Amokrane, A.; Comel, C.; Veron, J. Landfill leachates pretreatment by coagulation-flocculation. Water Res. 1997, 31, 2775–2782. [Google Scholar] [CrossRef]
- Connolly, R.; Zhao, Y.; Sun, G.; Allen, S. Removal of ammoniacal-nitrogen from an artificial landfill leachate in downflow reed beds. Process Biochem. 2004, 39, 1971–1976. [Google Scholar] [CrossRef]
- Koohestanian, A.; Hosseini, M.; Abbasian, Z. The Separation Method for Removing of Colloidal Particles from Raw Water. Euras. J. Agric. Environ. Sci. 2008, 4, 266–273. [Google Scholar]
- Butt, M.T.; Khokar, A.; Iqbal, K.; Khan, R.A. Coagulation-flocculation studies of laboratory wastewater using different combinations. J. Chem. Soc.Pak. 2013, 35, 637–641. [Google Scholar]
| Tile Industry Wastewater | KEPA Standards for Industrial Discharge to Sewer (Parson 2002) | ||||||
|---|---|---|---|---|---|---|---|
| Parameters | pH | (mg/L) | Turbidity (NTU) | Conductivity (µS/cm) | pH | Concentration (mg/L) | Turbidity (NTU) |
| TSS | 12.8 | 26,350 | 13,610 | 7975 | 6-8 | 300 | 50 |
| TDS | 4800 | 1500 | |||||
| COD | 22.9 | 750 | |||||
| BOD | 0.27 | 500 | |||||
| Alkalinity | 3600 | -- | |||||
| Chloride | 1203 | -- | |||||
| Hardness | 119.4 | -- | |||||
| Ca | 44.9 | -- | |||||
| Mg | 1.77 | -- | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Anzi, B.S.; Naik, M.-u.-d.; Ahmad, M. The Imperative Need of Metal Salt for the Treatment of Industrial Wastewater via the Synergic Coagulation-Flocculation Method. Polymers 2022, 14, 1651. https://doi.org/10.3390/polym14091651
Al-Anzi BS, Naik M-u-d, Ahmad M. The Imperative Need of Metal Salt for the Treatment of Industrial Wastewater via the Synergic Coagulation-Flocculation Method. Polymers. 2022; 14(9):1651. https://doi.org/10.3390/polym14091651
Chicago/Turabian StyleAl-Anzi, Bader S., Mehraj-ud-din Naik, and Mudasir Ahmad. 2022. "The Imperative Need of Metal Salt for the Treatment of Industrial Wastewater via the Synergic Coagulation-Flocculation Method" Polymers 14, no. 9: 1651. https://doi.org/10.3390/polym14091651
APA StyleAl-Anzi, B. S., Naik, M.-u.-d., & Ahmad, M. (2022). The Imperative Need of Metal Salt for the Treatment of Industrial Wastewater via the Synergic Coagulation-Flocculation Method. Polymers, 14(9), 1651. https://doi.org/10.3390/polym14091651






