Effect of Kenaf Fibre as Reinforcing Fillers in Corn Starch-Based Biocomposite Film
Abstract
1. Introduction
2. Methodology
2.1. Materials
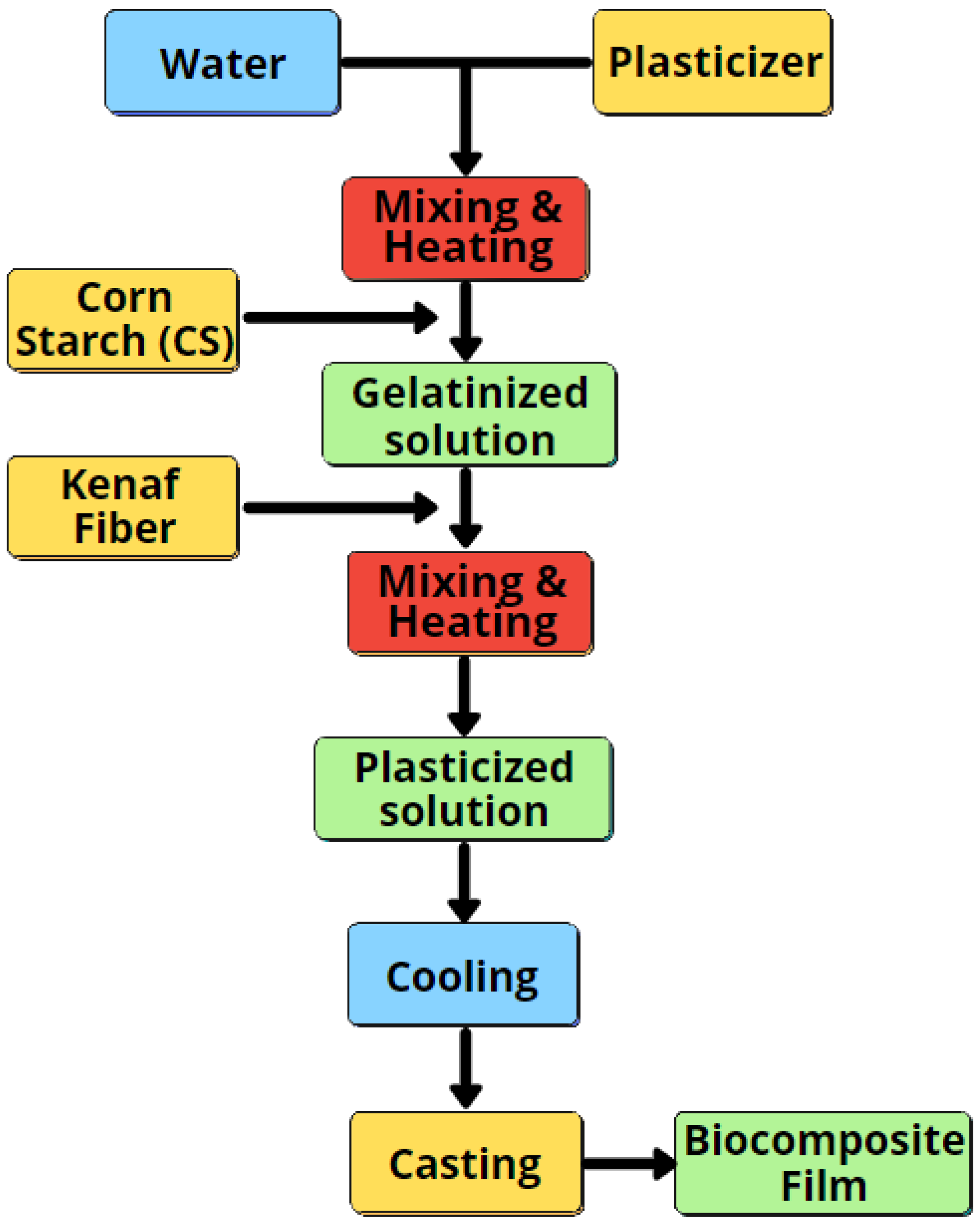
2.2. Preparation of Biocomposite Films
2.3. Film Weight
2.4. Film Thickness
2.5. Film Density
2.6. Film Moisture Content
2.7. Water Absorption (WA)
2.8. Water Solubility (WS)
2.9. Fourier Transform Infrared Spectroscopy (FTIR)
2.10. X-ray Diffraction (XRD)
2.11. Tensile Properties of Films
2.12. Field Emission Scanning Electron Microscopy (FESEM)
3. Results and Discussion
3.1. Physical Appearance of Corn Starch/Kenaf (CS/K) Biocomposite Films
3.2. Physical Properties of CS/Kenaf Biocomposite Film
3.3. Water Solubility (WS)
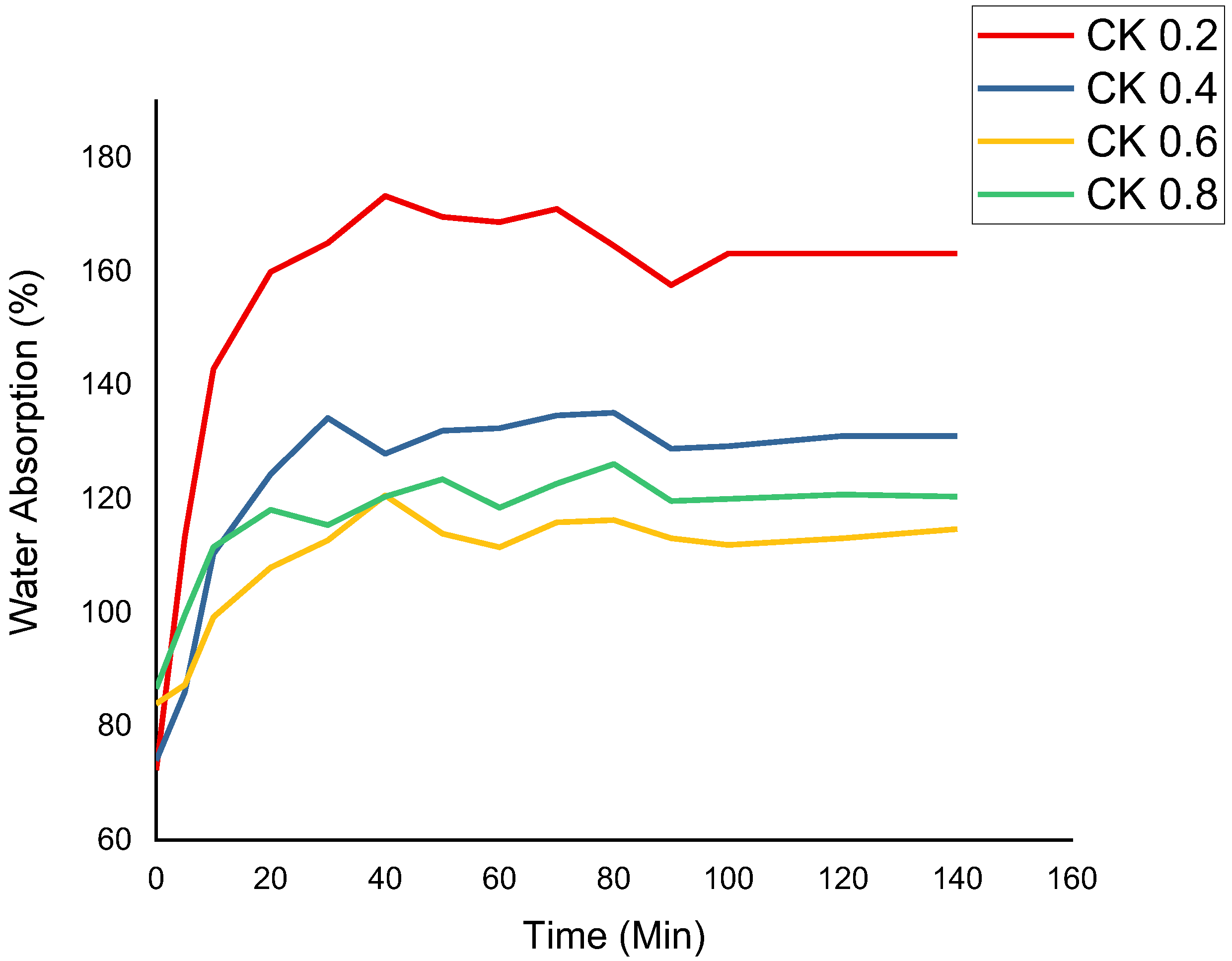
3.4. Water Absorption (WA)
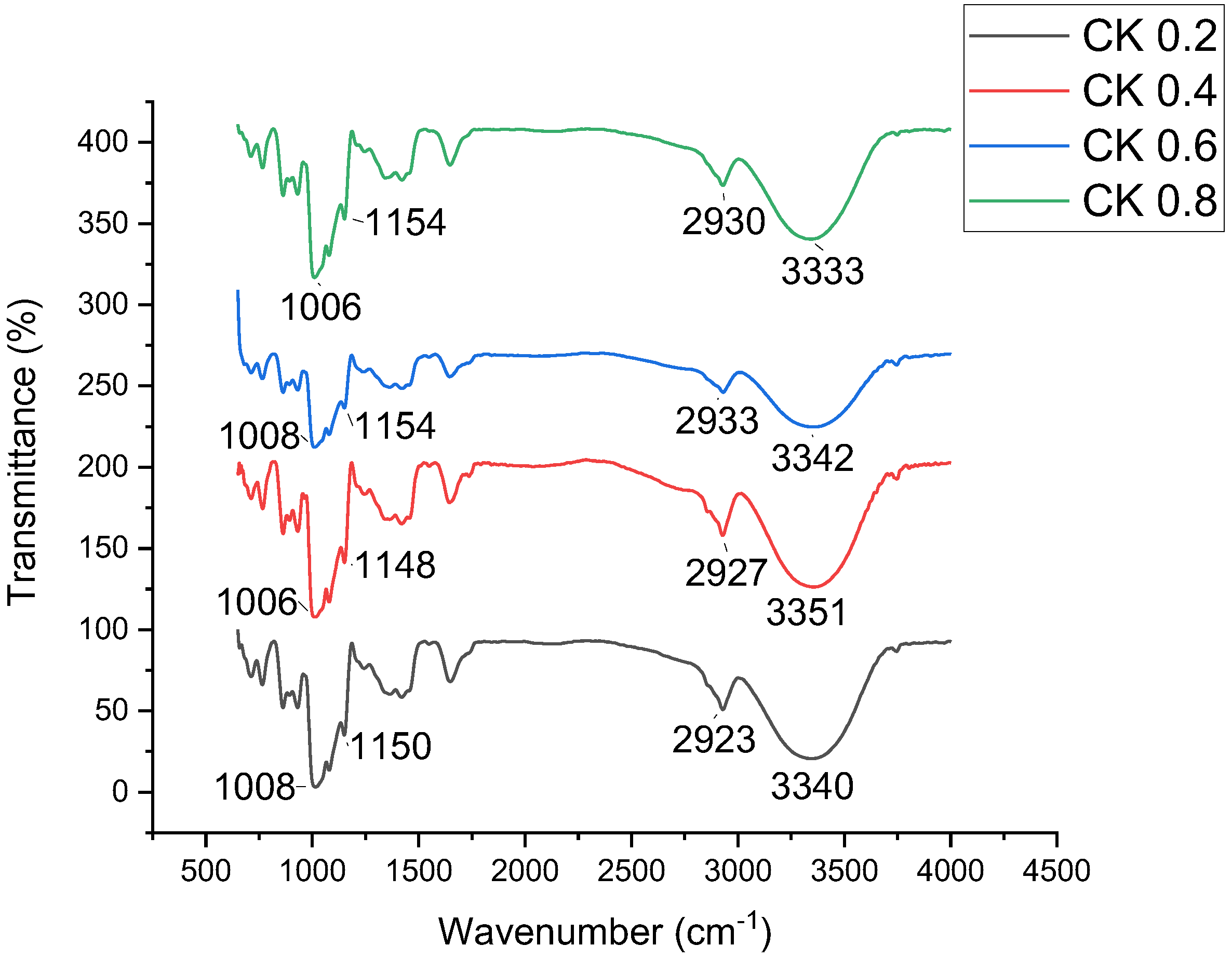
3.5. Fourier Transform Infrared Spectroscopy (FTIR)
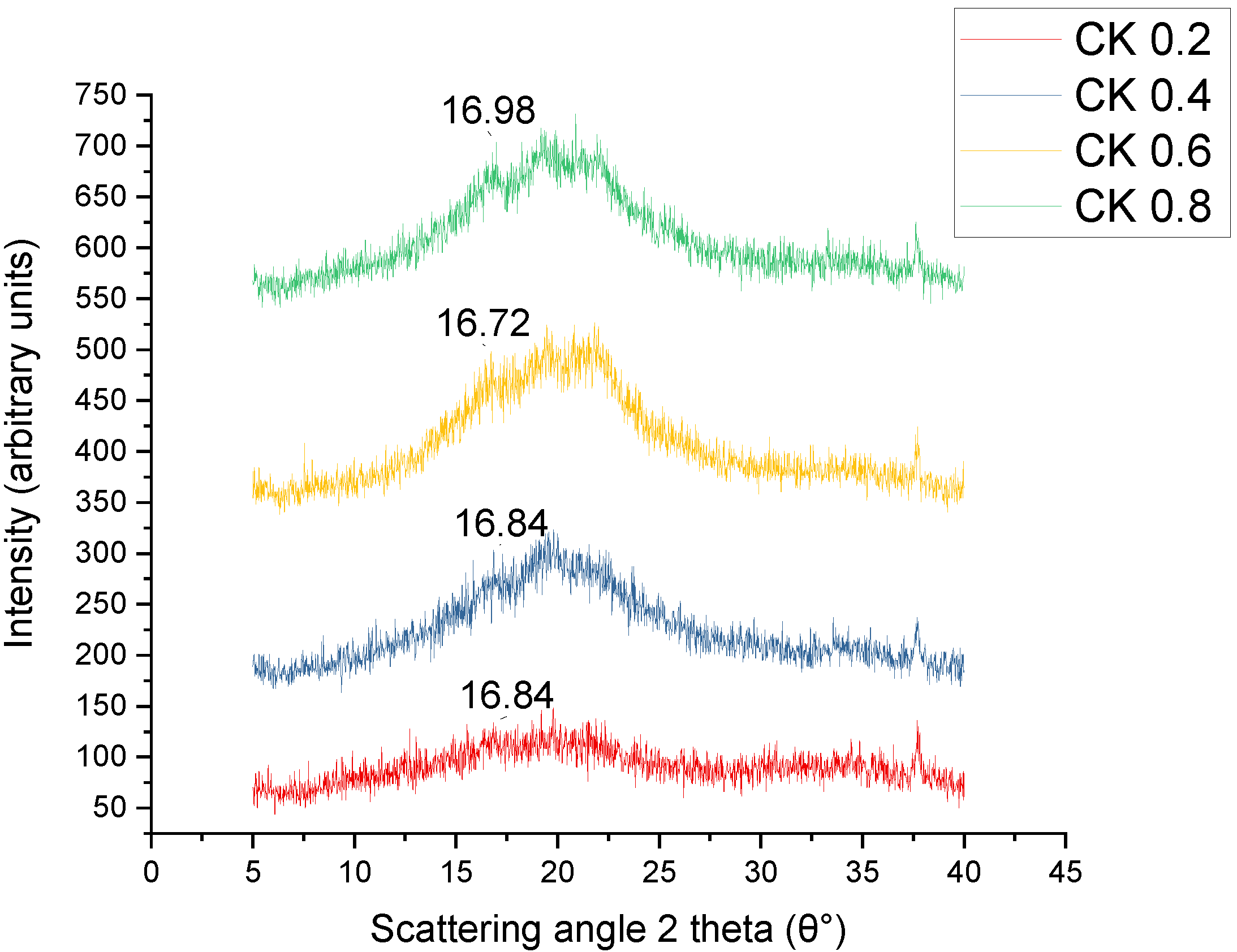
3.6. X-ray Diffraction (XRD)
3.7. Tensile Properties
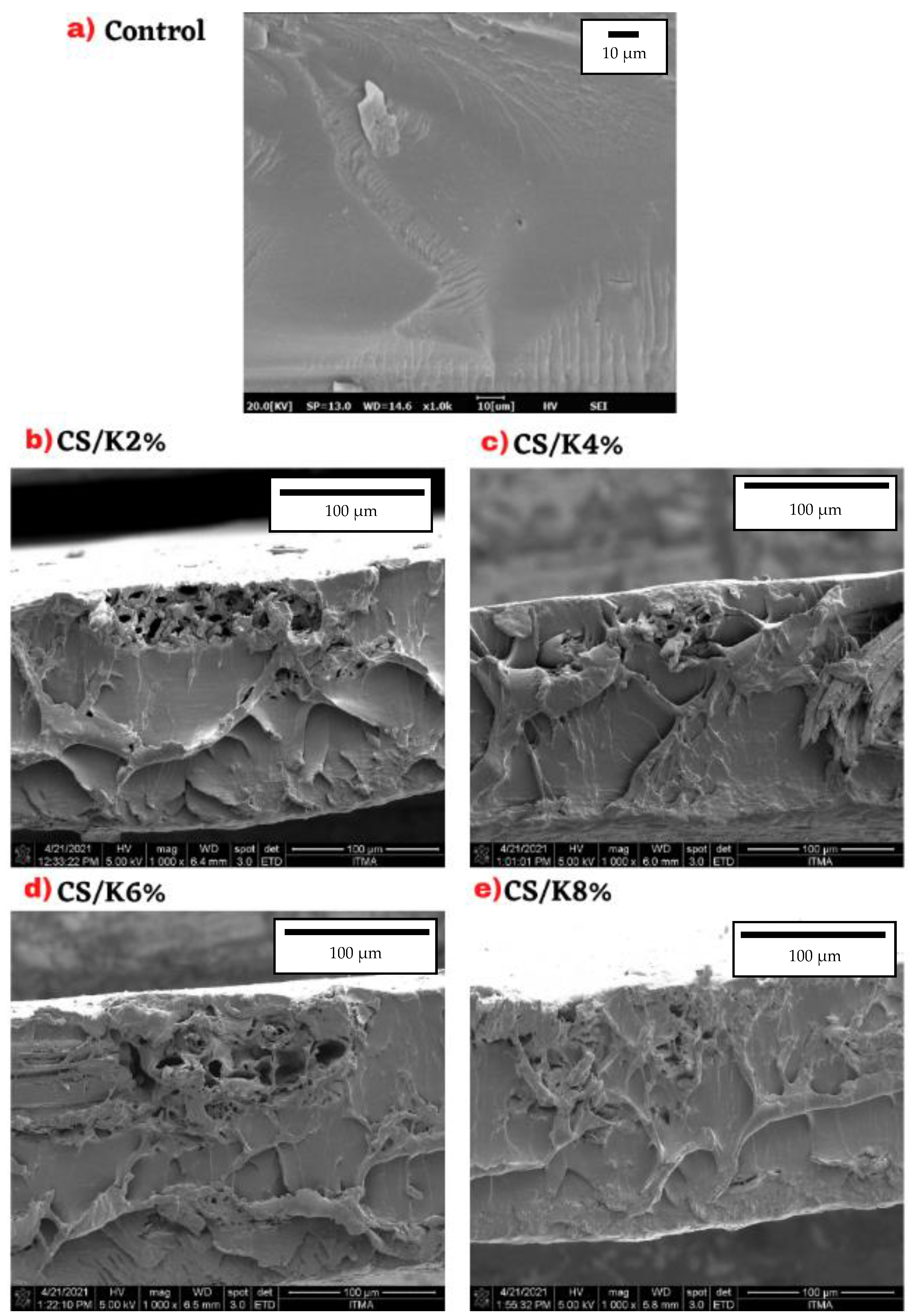
3.8. Field Emission Scanning Electron Microscopy (FESEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacKenzie, D. How bad will it get? New Sci. 2020, 245, 7. [Google Scholar] [CrossRef]
- Rajmohan, K.V.S.; Ramya, C.; Raja Viswanathan, M.; Varjani, S. Plastic pollutants: Effective waste management for pollution control and abatement. Curr. Opin. Environ. Sci. Health 2019, 12, 72–84. [Google Scholar] [CrossRef]
- Klemeš, J.J.; Van Fan, Y.; Tan, R.R.; Jiang, P. Minimising the present and future plastic waste, energy and environmental footprints related to COVID-19. Renew. Sustain. Energy Rev. 2020, 127, 109883. [Google Scholar] [CrossRef] [PubMed]
- Diyana, Z.N.; Jumaidin, R.; Selamat, M.Z.; Ghazali, I.; Julmohammad, N.; Huda, N.; Ilyas, R.A. Physical Properties of Thermoplastic Starch Derived from Natural Resources and Its Blends: A Review. Polymers 2021, 13, 1396. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Sapuan, S.M.; Harussani, M.M.; Hakimi, M.Y.A.Y.; Haziq, M.Z.M.; Atikah, M.S.N.; Asyraf, M.R.M.; Ishak, M.R.; Razman, M.R.; Nurazzi, N.M.; et al. Polylactic Acid (PLA) Biocomposite: Processing, Additive Manufacturing and Advanced Applications. Polymers 2021, 13, 1326. [Google Scholar] [CrossRef]
- Alsubari, S.; Zuhri, M.Y.M.; Sapuan, S.M.; Ishak, M.R.; Ilyas, R.A.; Asyraf, M.R.M. Potential of natural fiber reinforced polymer composites in sandwich structures: A review on its mechanical properties. Polymers 2021, 13, 423. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A.; Sherwani, S.F.K.; Yusuf, J.; Ilyas, R.A. Recent developments in sustainable arrowroot (Maranta arundinacea Linn) starch biopolymers, fibres, biopolymer composites and their potential industrial applications: A review. J. Mater. Res. Technol. 2021, 13, 1191–1219. [Google Scholar] [CrossRef]
- Yu, H.; Sun, X.; Solvang, W.D.; Zhao, X. Reverse Logistics Network Design for Effective Management of Medical Waste in Epidemic Outbreaks: Insights from the Coronavirus Disease 2019 (COVID-19) Outbreak in Wuhan (China). Int. J. Environ. Res. Public Health 2020, 17, 1770. [Google Scholar] [CrossRef]
- Windfeld, E.S.; Brooks, M.S.-L. Medical waste management—A review. J. Environ. Manag. 2015, 163, 98–108. [Google Scholar] [CrossRef]
- Chen, H.L.; Nath, T.K.; Chong, S.; Foo, V.; Gibbins, C.; Lechner, A.M. The plastic waste problem in Malaysia: Management, recycling and disposal of local and global plastic waste. SN Appl. Sci. 2021, 3, 437. [Google Scholar] [CrossRef]
- Lechner, A.M.; Gomes, R.L.; Rodrigues, L.; Ashfold, M.J.; Selvam, S.B.; Wong, E.P.; Raymond, C.M.; Zieritz, A.; Sing, K.W.; Moug, P.; et al. Challenges and considerations of applying nature-based solutions in low- and middle-income countries in Southeast and East Asia. Blue-Green Syst. 2020, 2, 331–351. [Google Scholar] [CrossRef]
- Jumaidin, R.; Diah, N.A.; Ilyas, R.A.; Alamjuri, R.H.; Yusof, F.A.M. Processing and Characterisation of Banana Leaf Fibre Reinforced Thermoplastic Cassava Starch Composites. Polymers 2021, 13, 1420. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Zuhri, M.Y.M.; Norrrahim, M.N.F.; Misenan, M.S.M.; Jenol, M.A.; Samsudin, S.A.; Nurazzi, N.M.; Asyraf, M.R.M.; Supian, A.B.M.; Bangar, S.P.; et al. Natural Fiber-Reinforced Polycaprolactone Green and Hybrid Biocomposites for Various Advanced Applications. Polymers 2022, 14, 182. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Aisyah, H.A.; Nordin, A.H.; Ngadi, N.; Zuhri, M.Y.M.; Asyraf, M.R.M.; Sapuan, S.M.; Zainudin, E.S.; Sharma, S.; Abral, H.; et al. Natural-Fiber-Reinforced Chitosan, Chitosan Blends and Their Nanocomposites for Various Advanced Applications. Polymers 2022, 14, 874. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Zuhri, M.Y.M.; Aisyah, H.A.; Asyraf, M.R.M.; Hassan, S.A.; Zainudin, E.S.; Sapuan, S.M.; Sharma, S.; Bangar, S.P.; Jumaidin, R.; et al. Natural Fiber-Reinforced Polylactic Acid, Polylactic Acid Blends and Their Composites for Advanced Applications. Polymers 2022, 14, 202. [Google Scholar] [CrossRef]
- Syafiq, R.M.O.; Sapuan, S.M.; Zuhri, M.Y.M.; Othman, S.H.; Ilyas, R.A. Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films. Nanotechnol. Rev. 2022, 11, 423–437. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Development and characterization of sugar palm nanocrystalline cellulose reinforced sugar palm starch bionanocomposites Development and characterization of sugar palm nanocrystalline cellulose reinforced sugar palm starch bionanocomposites. Carbohydr. Polym. 2018, 202, 186–202. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ibrahim, R.; Abral, H.; Ishak, M.R.; Zainudin, E.S.; Atikah, M.S.N.; Mohd Nurazzi, N.; Atiqah, A.; Ansari, M.N.M.; et al. Effect of sugar palm nanofibrillated cellulose concentrations on morphological, mechanical and physical properties of biodegradable films based on agro-waste sugar palm (Arenga pinnata (Wurmb.) Merr) starch. J. Mater. Res. Technol. 2019, 8, 4819–4830. [Google Scholar] [CrossRef]
- Omran, A.A.B.; Mohammed, A.A.B.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Koloor, S.S.R.; Petrů, M. Micro- and Nanocellulose in Polymer Composite Materials: A Review. Polymers 2021, 13, 231. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Souza, B.W.S.; Teixeira, J.A.; Vicente, A.A. Effect of glycerol and corn oil on physicochemical properties of polysaccharide films—A comparative study. Food Hydrocoll. 2012, 27, 175–184. [Google Scholar] [CrossRef]
- Wang, J.-l.; Cheng, F.; Zhu, P.-x. Structure and properties of urea-plasticized starch films with different urea contents. Carbohydr. Polym. 2014, 101, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Isotton, F.S.; Bernardo, G.L.; Baldasso, C.; Rosa, L.M.; Zeni, M. The plasticizer effect on preparation and properties of etherified corn starch films. Ind. Crops Prod. 2015, 76, 717–724. [Google Scholar] [CrossRef]
- Ibrahim, M.I.J.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M. Physical, thermal, morphological, and tensile properties of cornstarch-based films as affected by different plasticizers. Int. J. Food Prop. 2019, 22, 925–941. [Google Scholar] [CrossRef]
- Hazrol, M.D.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M.; Abdul Wahab, N.I. Corn Starch (Zea mays) Biopolymer Plastic Reaction in Combination with Sorbitol and Glycerol. Polymers 2021, 13, 242. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Atikah, M.S.N.; Asyraf, M.R.M.; Rafiqah, S.A.; Aisyah, H.A.; Nurazzi, N.M.; Norrrahim, M.N.F. Effect of hydrolysis time on the morphological, physical, chemical, and thermal behavior of sugar palm nanocrystalline cellulose (Arenga pinnata (Wurmb.) Merr). Text. Res. J. 2021, 91, 152–167. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R. Isolation and characterization of nanocrystalline cellulose from sugar palm fibres (Arenga Pinnata). Carbohydr. Polym. 2018, 181, 1038–1051. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ibrahim, R.; Abral, H.; Ishak, M.R.; Zainudin, E.S.; Asrofi, M.; Atikah, M.S.N.; Huzaifah, M.R.M.; Radzi, A.M.; et al. Sugar palm (Arenga pinnata (Wurmb.) Merr) cellulosic fibre hierarchy: A comprehensive approach from macro to nano scale. J. Mater. Res. Technol. 2019, 8, 2753–2766. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Sugar palm nanofibrillated cellulose (Arenga pinnata (Wurmb.) Merr): Effect of cycles on their yield, physic-chemical, morphological and thermal behavior. Int. J. Biol. Macromol. 2019, 123, 379–388. [Google Scholar] [CrossRef]
- Verma, R.; Shukla, M. Characterization of Mechanical Properties of Short Kenaf Fiber-HDPE Green Composites. Mater. Today Proc. 2018, 5, 3257–3264. [Google Scholar] [CrossRef]
- G, R. Development of Sustainable Textiles from Kenaf-Cotton Blended Yarn. Trends Text. Eng. Fash. Technol. 2018, 1. [Google Scholar] [CrossRef][Green Version]
- Keshk, S.; Suwinarti, W.; Sameshima, K. Physicochemical characterization of different treatment sequences on kenaf bast fiber. Carbohydr. Polym. 2006, 65, 202–206. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Natural Fibers, Biopolymers, and Biocomposites; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Joshi, S.V.; Drzal, L.T.; Mohanty, A.K.; Arora, S. Are natural fiber composites environmentally superior to glass fiber reinforced composites? Compos. Part A Appl. Sci. Manuf. 2004, 35, 371–376. [Google Scholar] [CrossRef]
- Aziz, S.H.; Ansell, M.P. The effect of alkalization and fibre alignment on the mechanical and thermal properties of kenaf and hemp bast fibre composites: Part 1—Polyester resin matrix. Compos. Sci. Technol. 2004, 64, 1219–1230. [Google Scholar] [CrossRef]
- Akil, H.M.; Omar, M.F.; Mazuki, A.A.M.; Safiee, S.; Ishak, Z.A.M.; Abu Bakar, A. Kenaf fiber reinforced composites: A review. Mater. Des. 2011, 32, 4107–4121. [Google Scholar] [CrossRef]
- Hazrol, M.D.; Sapuan, S.M.; Ilyas, R.A.; Othman, M.L.; Sherwani, S.F.K. Electrical properties of sugar palm nanocrystalline cellulose reinforced sugar palm starch nanocomposites. Polimery 2020, 65, 363–370. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Atikah, M.S.N.; Ibrahim, R.; Syafiq, R.; Hazrol, M.D.; Nazrin, A.; Ibrahim, M.I.J. Tensile Properties of Sugar Palm Fiber-Reinforced Polymer Composites: A Comprehensive Review. In Biofiller-Reinforced Biodegradable Polymer Composites; Jumaidin, R., Sapuan, S.M., Ismail, H., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 243–266. [Google Scholar]
- ASTM D882-02 ASTM International. Standard Test Method for Tensile Properties of Thin Plastic Sheeting. In Annual Book of ASTM Standards; ASTM: Philadelphia, PA, USA, 2002. [Google Scholar]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Effect of delignification on the physical, thermal, chemical, and structural properties of sugar palm fibre. BioResources 2017, 12, 8734–8754. [Google Scholar] [CrossRef]
- Suppakul, P.; Chalernsook, B.; Ratisuthawat, B.; Prapasitthi, S.; Munchukangwan, N. Empirical modeling of moisture sorption characteristics and mechanical and barrier properties of cassava flour film and their relation to plasticizing–antiplasticizing effects. J. Food Sci. Technol. 2013, 50, 290–297. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Recent developments in sugar palm (Arenga pinnata) based biocomposites and their potential industrial applications: A review. Renew. Sustain. Energy Rev. 2016, 54, 533–549. [Google Scholar] [CrossRef]
- Norizan, M.N.; Abdan, K.; Ilyas, R.A.; Biofibers, S.P. Effect of fiber orientation and fiber loading on the mechanical and thermal properties of sugar palm yarn fiber reinforced unsaturated polyester resin composites. Polimery 2020, 65, 34–43. [Google Scholar] [CrossRef]
- Ibrahim, M.I.J.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M. Preparation and characterization of cornhusk/sugar palm fiber reinforced Cornstarch-based hybrid composites. J. Mater. Res. Technol. 2020, 9, 200–211. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of plasticizer type and concentration on physical properties of biodegradable films based on sugar palm (arenga pinnata) starch for food packaging. J. Food Sci. Technol. 2016, 53, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.M.A.; Mohammad Amini, A.; Zahedi, Y. Characterisation of a new biodegradable edible film based on sage seed gum: Influence of plasticiser type and concentration. Food Hydrocoll. 2015, 43, 290–298. [Google Scholar] [CrossRef]
- Jouki, M.; Khazaei, N.; Ghasemlou, M.; HadiNezhad, M. Effect of glycerol concentration on edible film production from cress seed carbohydrate gum. Carbohydr. Polym. 2013, 96, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.I.J.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M.; Edhirej, A. Corn (Maize)—Its Fibers, Polymers, Composites, and Applications: A Review. Biodegrad. Compos. 2019, 10, 13–36. [Google Scholar] [CrossRef]
- Edhirej, A.; Sapuan, S.M.; Jawaid, M.; Zahari, N.I. Cassava/sugar palm fiber reinforced cassava starch hybrid composites: Physical, thermal and structural properties. Int. J. Biol. Macromol. 2017, 101, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Saba, N.; Safwan, A.; Sanyang, M.L.; Mohammad, F.; Pervaiz, M.; Jawaid, M.; Alothman, O.Y.; Sain, M. Thermal and dynamic mechanical properties of cellulose nanofibers reinforced epoxy composites. Int. J. Biol. Macromol. 2017, 102, 822–828. [Google Scholar] [CrossRef]
- Kamaruddin, Z.H.; Sapuan, S.M.; Yusoff, M.Z.M.; Jumaidin, R. Rapid Detection and Identification of Dioscorine Compounds in Dioscorea hispida Tuber Plants by LC-ESI-MS. BioResources 2020, 15, 5999–6011. [Google Scholar] [CrossRef]
- AL-Oqla, F.M.; Sapuan, S.M.; Anwer, T.; Jawaid, M.; Hoque, M.E. Natural fiber reinforced conductive polymer composites as functional materials: A review. Synth. Met. 2015, 206, 42–54. [Google Scholar] [CrossRef]
- Lopez, O.; Garcia, M.A.; Villar, M.A.; Gentili, A.; Rodriguez, M.S.; Albertengo, L. Thermo-compression of biodegradable thermoplastic corn starch films containing chitin and chitosan. LWT-Food Sci. Technol. 2014, 57, 106–115. [Google Scholar] [CrossRef]
- Kalia, S.; Avérous, L.; Njuguna, J.; Dufresne, A.; Cherian, B.M. Natural fibers, bio-and nanocomposites. Int. J. Polym. Sci. 2011, 2011, 735932. [Google Scholar] [CrossRef]
- Edhirej, A.; Sapuan, S.M.; Jawaid, M.; Zahari, N.I. Effect of various plasticizers and concentration on the physical, thermal, mechanical, and structural properties of cassava-starch-based films. Starch/Staerke 2017, 69, 1–11. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Labidi, J.; Fernandes, S.C.M. Chitin nanocrystals and nanofibers as nano-sized fillers into thermoplastic starch-based biocomposites processed by melt-mixing. Chem. Eng. J. 2014, 256, 356–364. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Atiqah, A.; Ibrahim, R.; Abral, H.; Ishak, M.R.; Zainudin, E.S.; Nurazzi, N.M.; Atikah, M.S.N.; Ansari, M.N.M.; et al. Sugar palm (Arenga pinnata [Wurmb.] Merr) starch films containing sugar palm nanofibrillated cellulose as reinforcement: Water barrier properties. Polym. Compos. 2019, 41, 1–9. [Google Scholar] [CrossRef]
- Ayu, R.S.; Khalina, A.; Harmaen, A.S.; Zaman, K.; Isma, T.; Liu, Q.; Ilyas, R.A.; Lee, C.H. Characterization Study of Empty Fruit Bunch (EFB) Fibers Reinforcement in Poly(Butylene) Succinate (PBS)/Starch/Glycerol Composite Sheet. Polymers 2020, 12, 1571. [Google Scholar] [CrossRef]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M.; Tawakkal, I.S.M.A.; Ilyas, R.A. Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites. Nanotechnol. Rev. 2021, 10, 431–442. [Google Scholar] [CrossRef]
- Giwa Ibrahim, S.; Karim, R.; Saari, N.; Wan Abdullah, W.Z.; Zawawi, N.; Ab Razak, A.F.; Hamim, N.A.; Umar, R.A. Kenaf (Hibiscus cannabinus L.) Seed and its Potential Food Applications: A Review. J. Food Sci. 2019, 84, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Paraginski, R.T.; Vanier, N.L.; Moomand, K.; de Oliveira, M.; da Rosa Zavareze, E.; e Silva, R.M.; Ferreira, C.D.; Elias, M.C. Characteristics of starch isolated from maize as a function of grain storage temperature. Carbohydr. Polym. 2014, 102, 88–94. [Google Scholar] [CrossRef]
- Ma, X.; Yu, J.; Kennedy, J.F. Studies on the properties of natural fibers-reinforced thermoplastic starch composites. Carbohydr. Polym. 2005, 62, 19–24. [Google Scholar] [CrossRef]
- Zuo, X.; Zhang, M.; He, E.; Guan, B.; Qin, Y.; Yang, J.; Zhu, X.; Dai, J. Structural, magnetic, and dielectric properties of W/Cr co-substituted Aurivillius Bi5FeTi3O15. J. Alloys Compd. 2017, 726, 1040–1046. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, Y. Effects of glycerol and storage relative humidity on the properties of kudzu starch-based edible films. Starch/Staerke 2014, 66, 524–532. [Google Scholar] [CrossRef]
- Ballesteros-Mártinez, L.; Pérez-Cervera, C.; Andrade-Pizarro, R. Effect of glycerol and sorbitol concentrations on mechanical, optical, and barrier properties of sweet potato starch film. NFS J. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Ibrahim, M.I.J.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M. Potential of using multiscale corn husk fiber as reinforcing filler in cornstarch-based biocomposites. Int. J. Biol. Macromol. 2019, 139, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M. Mechanical, Physical and Thermal Properties of Sugar Palm Nanocellulose Reinforced Thermoplastic Starch (TPS)/Poly (Lactic Acid) (PLA) Blend Bionanocomposites. Polymers 2020, 12, 2216. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Sapuan, S.M.; Ibrahim, R.; Atikah, M.S.N.; Atiqah, A.; Ansari, M.N.M.; Norrrahim, M.N.F. Production, Processes and Modification of Nanocrystalline Cellulose from Agro-Waste: A Review. In Nanocrystalline Materials; IntechOpen: London, UK, 2019; pp. 3–32. [Google Scholar]
- Ilyas, R.A.; Sapuan, S.M.; Kadier, A.; Krishnan, S.; Atikah, M.S.N.; Ibrahim, R.; Nazrin, A.; Syafiq, R.; Misri, S.; Huzaifah, M.R.M.; et al. Mechanical Testing of Sugar Palm Fiber Reinforced Sugar Palm Biopolymer Composites. In Advanced Processing, Properties, and Applications of Starch and Other Bio-Based Polymers; Al-Oqla, F., Sapuan, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 89–110. [Google Scholar]
- Hazrati, K.Z.; Sapuan, S.M.; Zuhri, M.Y.M.; Jumaidin, R. Extraction and Characterization of Potential Biodegradable Materials Based on Dioscorea hispida Tubers. Polymers 2021, 13, 584. [Google Scholar] [CrossRef]
- Sanyang, M.; Sapuan, S.; Jawaid, M.; Ishak, M.; Sahari, J. Effect of Plasticizer Type and Concentration on Tensile, Thermal and Barrier Properties of Biodegradable Films Based on Sugar Palm (Arenga pinnata) Starch. Polymers 2015, 7, 1106–1124. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, L.; Li, D.; Wei, Q.; Adhikari, B. Effects of high-pressure homogenization on the properties of starch-plasticizer dispersions and their films. Carbohydr. Polym. 2011, 86, 202–207. [Google Scholar] [CrossRef]
- Hazrol, M.D.; Sapuan, S.M.; Zuhri, M.Y.M.; Zainudin, E.S.; Wahab, N.I.A.; Ilyas, R.A. Recent development in kenaf (Hibiscus cannabinus)-based biocomposites and their potential industrial applications: A review. In Design for Sustainability; Elsevier: Amsterdam, The Netherlands, 2021; pp. 329–368. [Google Scholar]
- Aitboulahsen, M.; El Galiou, O.; Laglaoui, A.; Bakkali, M.; Hassani Zerrouk, M. Effect of plasticizer type and essential oils on mechanical, physicochemical, and antimicrobial characteristics of gelatin, starch, and pectin-based films. J. Food Process. Preserv. 2020, 44, 1–10. [Google Scholar] [CrossRef]
- Xu, J.; Andrews, T.D.; Shi, Y. Recent Advances in the Preparation and Characterization of Intermediately to Highly Esterified and Etherified Starches: A Review. Starch-Stärke 2020, 72, 1900238. [Google Scholar] [CrossRef]
- Sherwani, S.F.K.; Sapuan, S.M.; Leman, Z.; Zainuddin, E.S.; Ilyas, R.A. Application of polymer composite materials in motorcycles: A comprehensive review. In Biocomposite and Synthetic Composites for Automotive Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 401–426. [Google Scholar]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M.; Ilyas, R.A.; Syafiq, R.; Sherwani, S.F.K. Nanocellulose Reinforced Thermoplastic Starch (TPS), Polylactic Acid (PLA), and Polybutylene Succinate (PBS) for Food Packaging Applications. Front. Chem. 2020, 8, 1–12. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Asyraf, M.R.M.; Fatimah Athiyah, S.; Shazleen, S.S.; Rafiqah, S.A.; Harussani, M.M.; Kamarudin, S.H.; Razman, M.R.; Rahmah, M.; Zainudin, E.S.; et al. A Review on Mechanical Performance of Hybrid Natural Fiber Polymer Composites for Structural Applications. Polymers 2021, 13, 2170. [Google Scholar] [CrossRef]
- Ojogbo, E.; Ogunsona, E.O.; Mekonnen, T.H. Chemical and physical modifications of starch for renewable polymeric materials. Mater. Today Sustain. 2020, 7–8, 100028. [Google Scholar] [CrossRef]
- Hazrol, M.D.; Sapuan, S.M.; Zuhri, M.Y.M.; Ilyas, R.A. Electrical properties of sugar palm nanocellulose fibre reinforced sugar palm starch biopolymer composite. In Proceedings of the Prosiding Seminar Enau Kebangsaan 2019; Institute of Tropical Forest and Forest Products (INTROP), Universiti Putra Malaysia: Bahau, Negeri Sembilan, Malaysia, 2019; pp. 57–62. [Google Scholar]



| Label | Plasticiser | Kenaf Fibre Loading (%) | Appearance of Films |
|---|---|---|---|
| Control | Sorbitol | 0 | Crystal clear, rigid, not brittle nor fragile, non-sticky, flexible, peelable. |
| CS/K2% | Sorbitol | 2 | Brown colour, large fibre gap present, non-sticky, rigid, not brittle nor fragile, peelable, flexible. |
| CS/K4% | Sorbitol | 4 | Brown colour, moderate fibre gap present, rigid, not brittle nor fragile, non-sticky, more flexible than CS/K2%, peelable. |
| CS/K6% | Sorbitol | 6 | Brown colour, less fibre gap present, non-sticky, rigid, not brittle nor fragile, most flexible among all, peelable. |
| CS/K8% | Sorbitol | 8 | Dark brown colour, less fibre gap present, not brittle nor fragile, non-sticky, more flexible than CS/K2% and CS/K4%, easy to peel. |
| Fibre Loading (%) | Thickness (mm) | Weight (mg) | Density (g/cm3) | Moisture Content (%) | Water Solubility (%) |
|---|---|---|---|---|---|
| Control | 0.16 ± 0.02 | 0.08 ± 0.02 | 1.45 ± 0.05 | 9.25 ± 2 | 25.17 |
| Kenaf 2% | 0.16 ± 0.02 | 0.07 ± 0.02 | 1.42 ± 0.05 | 9.86 ± 2 | 38.96 |
| Kenaf 4% | 0.16 ± 0.02 | 0.07 ± 0.02 | 1.45 ± 0.05 | 7.60 ± 2 | 37.28 |
| Kenaf 6% | 0.16 ± 0.02 | 0.08 ± 0.02 | 1.45 ± 0.04 | 5.99 ± 2 | 33.67 |
| Kenaf 8% | 0.17 ± 0.02 | 0.08 ± 0.02 | 1.45 ± 0.03 | 5.88 ± 2 | 25.17 |
| Film Sample | Crystallinity Index (%) |
|---|---|
| Control | 39.86 |
| CS/K2% | 40.33 |
| CS/K4% | 43.71 |
| CS/K6% | 45.06 |
| CS/K8% | 48.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazrol, M.D.; Sapuan, S.M.; Zainudin, E.S.; Wahab, N.I.A.; Ilyas, R.A. Effect of Kenaf Fibre as Reinforcing Fillers in Corn Starch-Based Biocomposite Film. Polymers 2022, 14, 1590. https://doi.org/10.3390/polym14081590
Hazrol MD, Sapuan SM, Zainudin ES, Wahab NIA, Ilyas RA. Effect of Kenaf Fibre as Reinforcing Fillers in Corn Starch-Based Biocomposite Film. Polymers. 2022; 14(8):1590. https://doi.org/10.3390/polym14081590
Chicago/Turabian StyleHazrol, M. D., S. M. Sapuan, E. S. Zainudin, N. I. A. Wahab, and R. A. Ilyas. 2022. "Effect of Kenaf Fibre as Reinforcing Fillers in Corn Starch-Based Biocomposite Film" Polymers 14, no. 8: 1590. https://doi.org/10.3390/polym14081590
APA StyleHazrol, M. D., Sapuan, S. M., Zainudin, E. S., Wahab, N. I. A., & Ilyas, R. A. (2022). Effect of Kenaf Fibre as Reinforcing Fillers in Corn Starch-Based Biocomposite Film. Polymers, 14(8), 1590. https://doi.org/10.3390/polym14081590









