Ternary Electrical Memory Devices Based on Polycarbazole: SnO2 Nanoparticles Composite Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
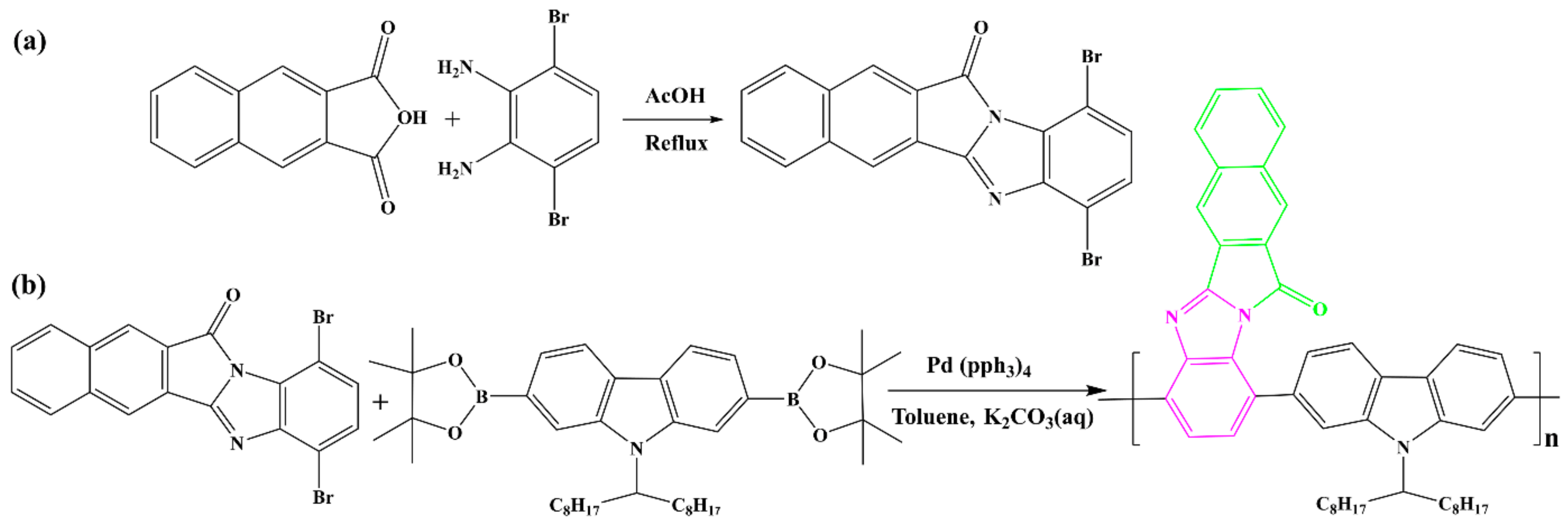
2.2. Synthesis of Monomer
2.3. Synthesis of Polymer
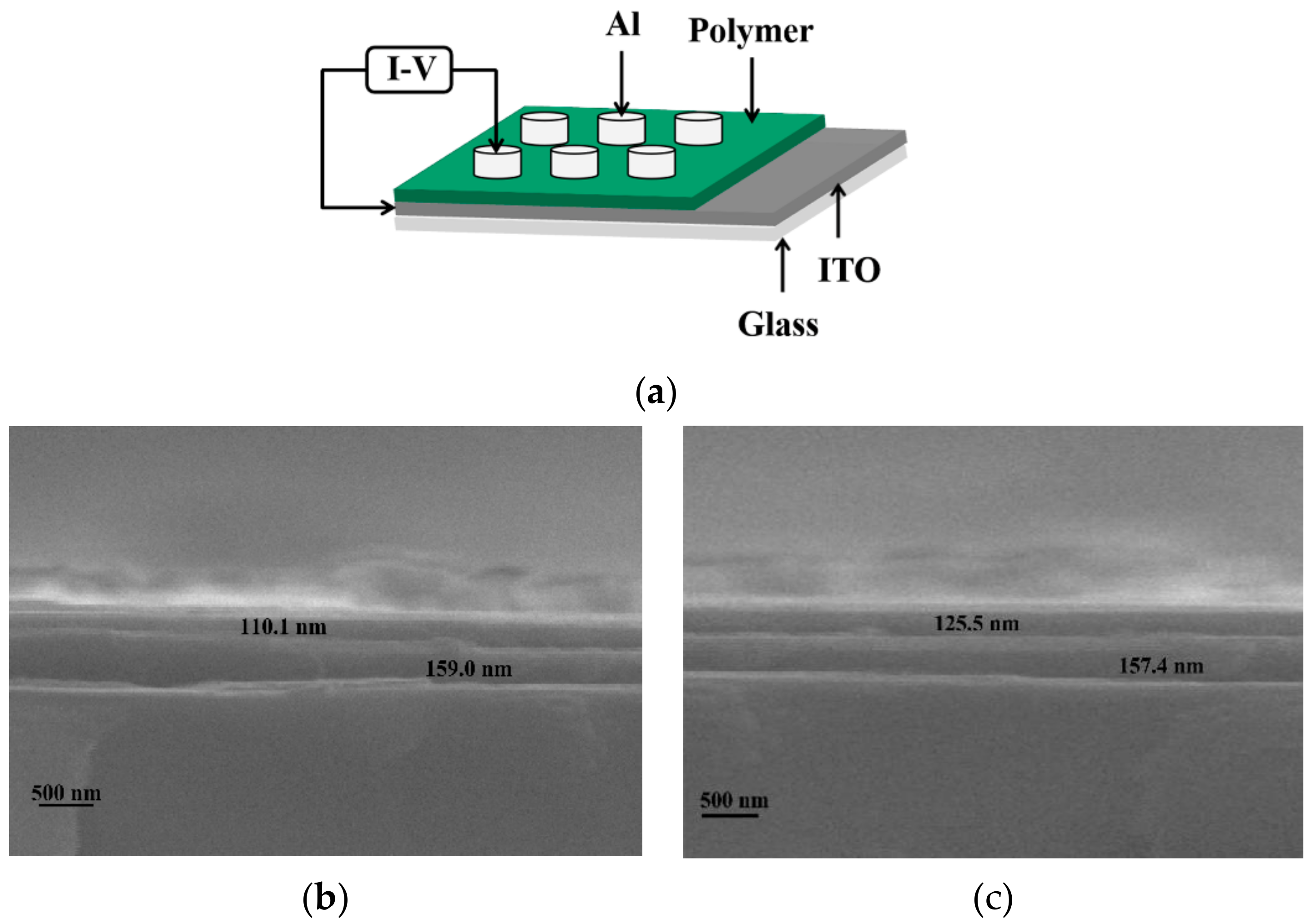
2.4. Memory Device Preparation
3. Results and Discussion
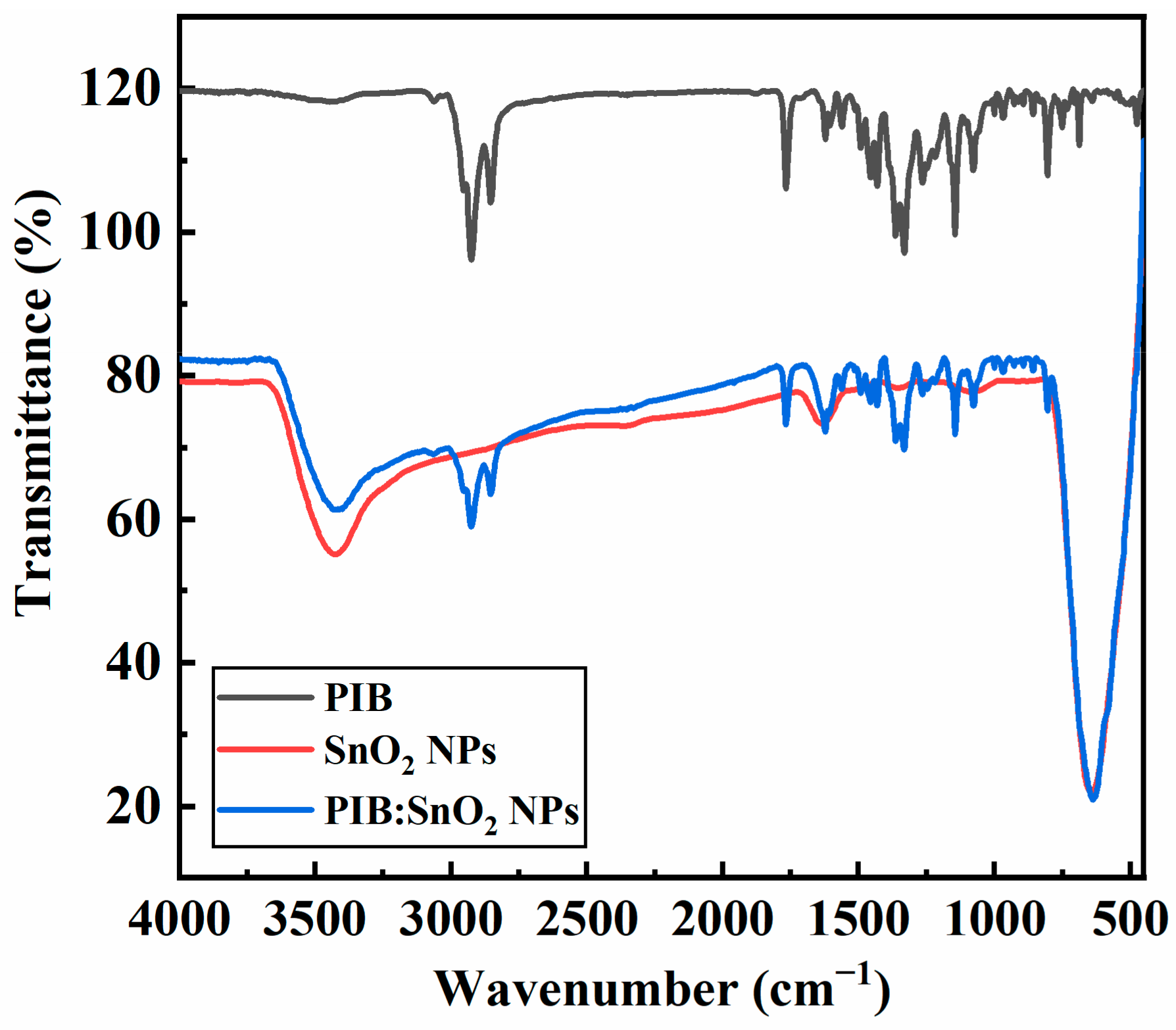
3.1. Polymer Characterization
3.2. Molecular Weight and Thermal Stability of Polymer
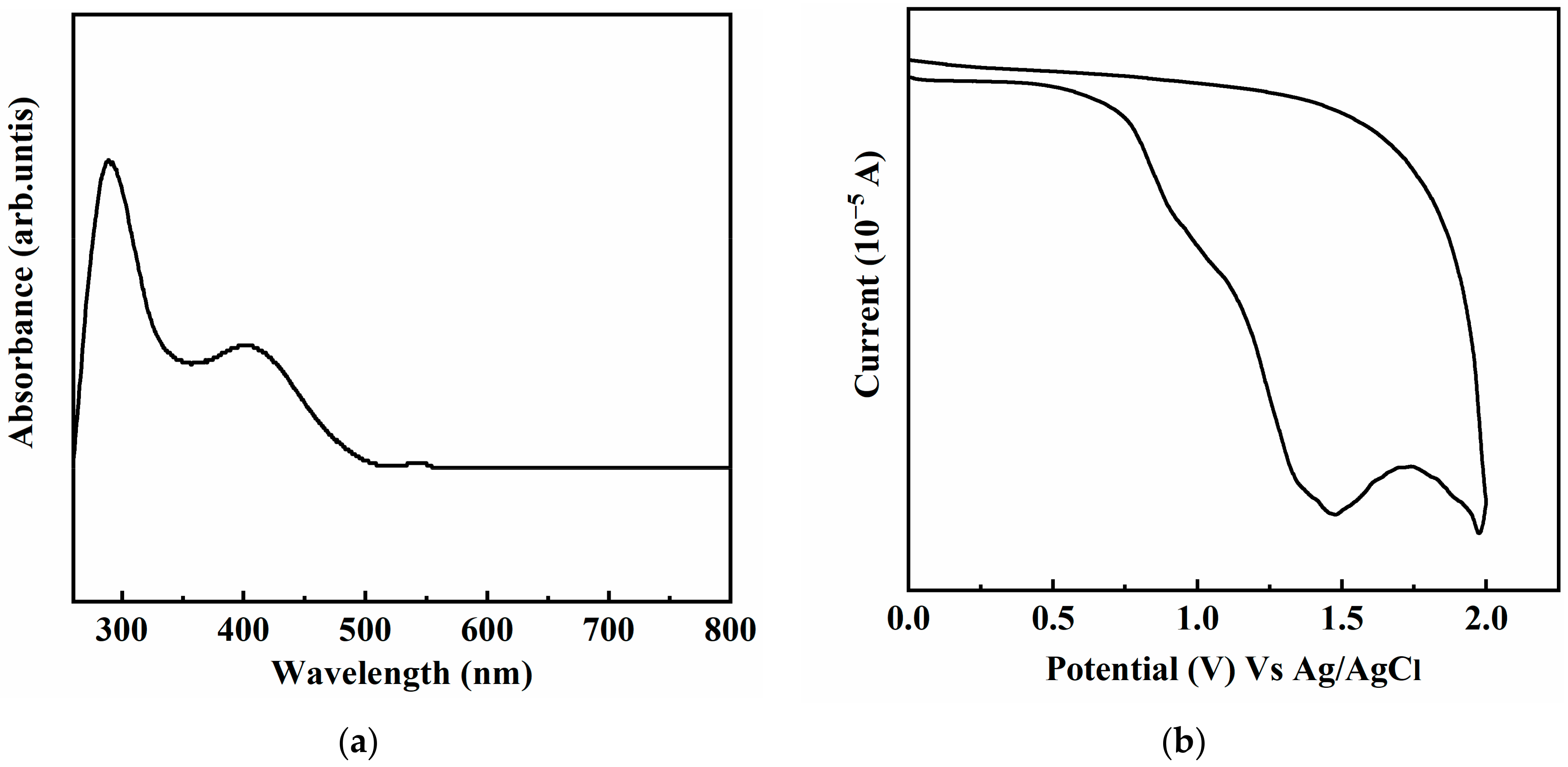
3.3. Optical and Electrochemical Properties
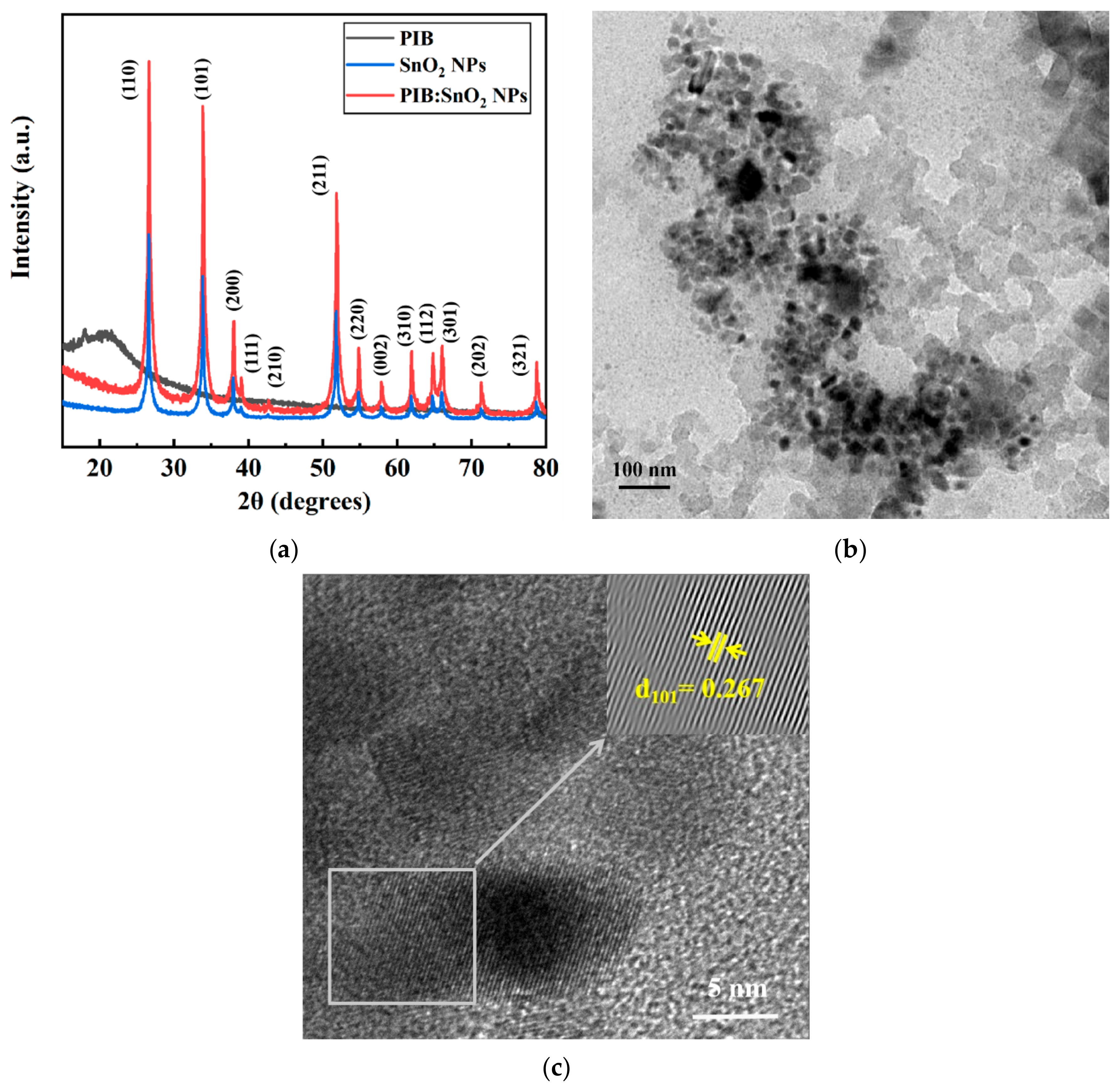
3.4. XRD of SnO2 NPs and Micrograph of the Device
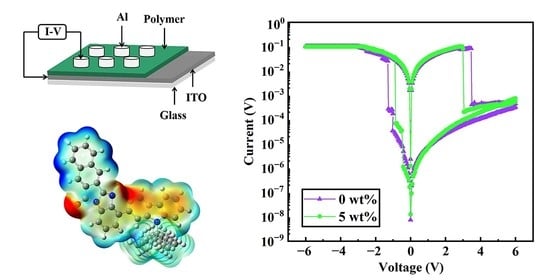
3.5. Structure and Cross-Section Possessed by Memory Devices
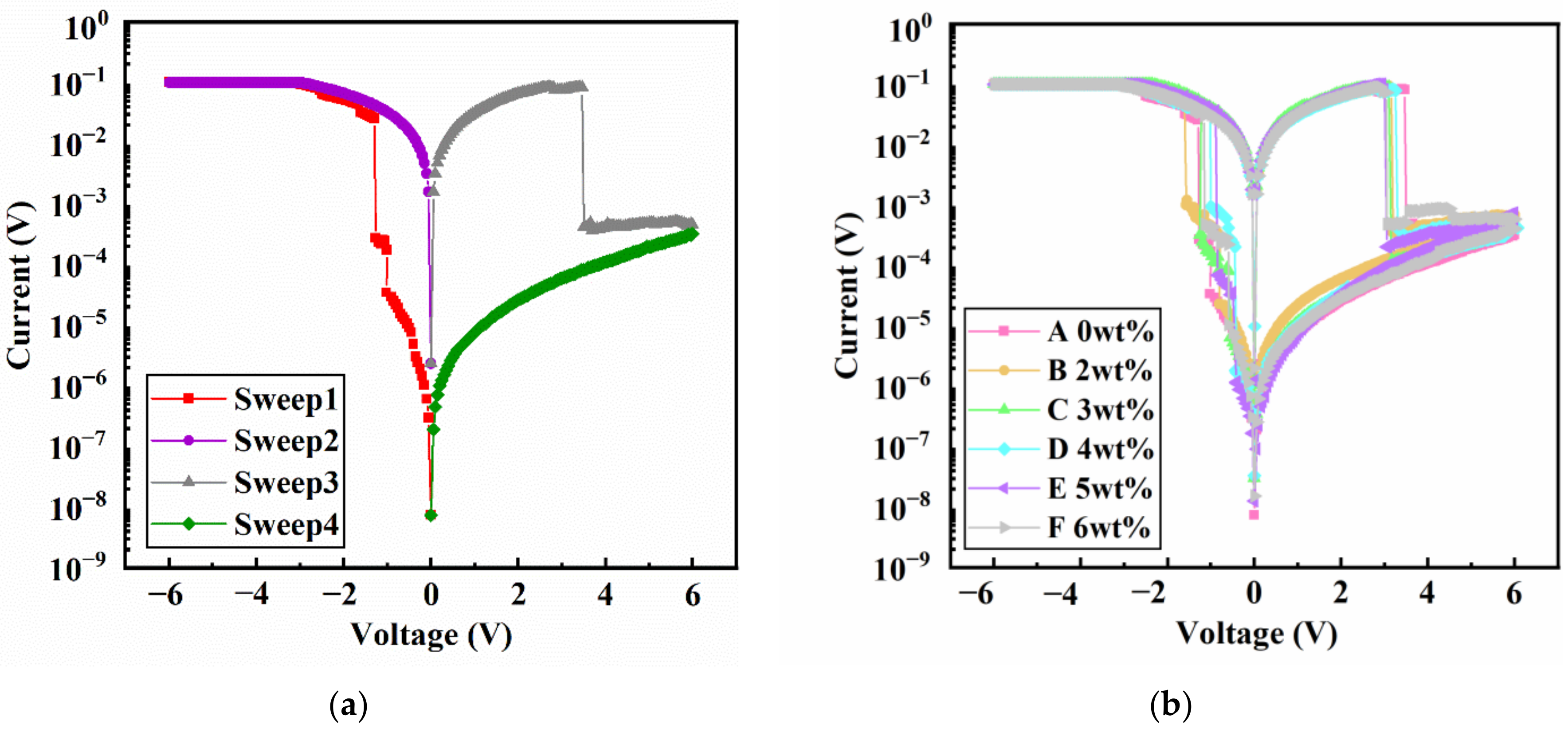
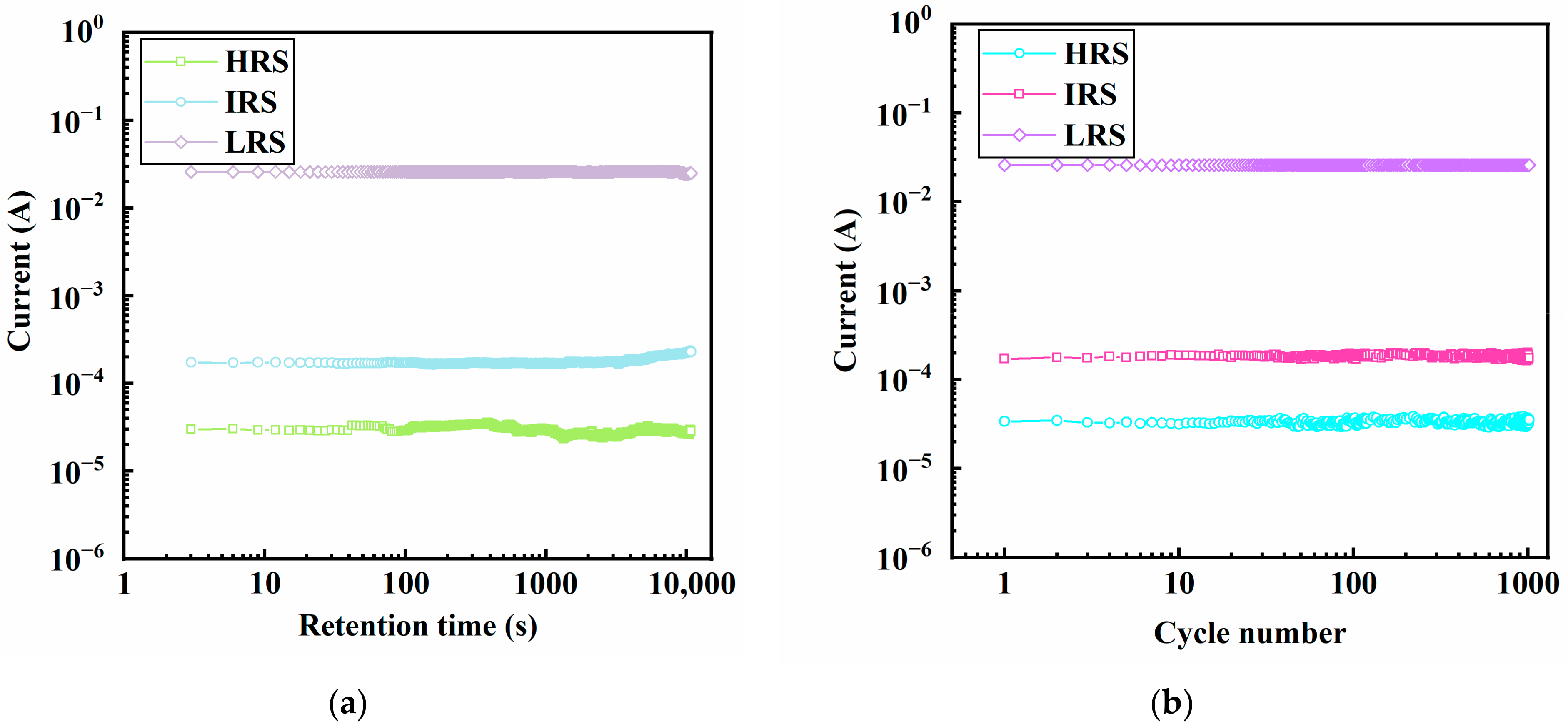
3.6. Characterization of the Memory Devices
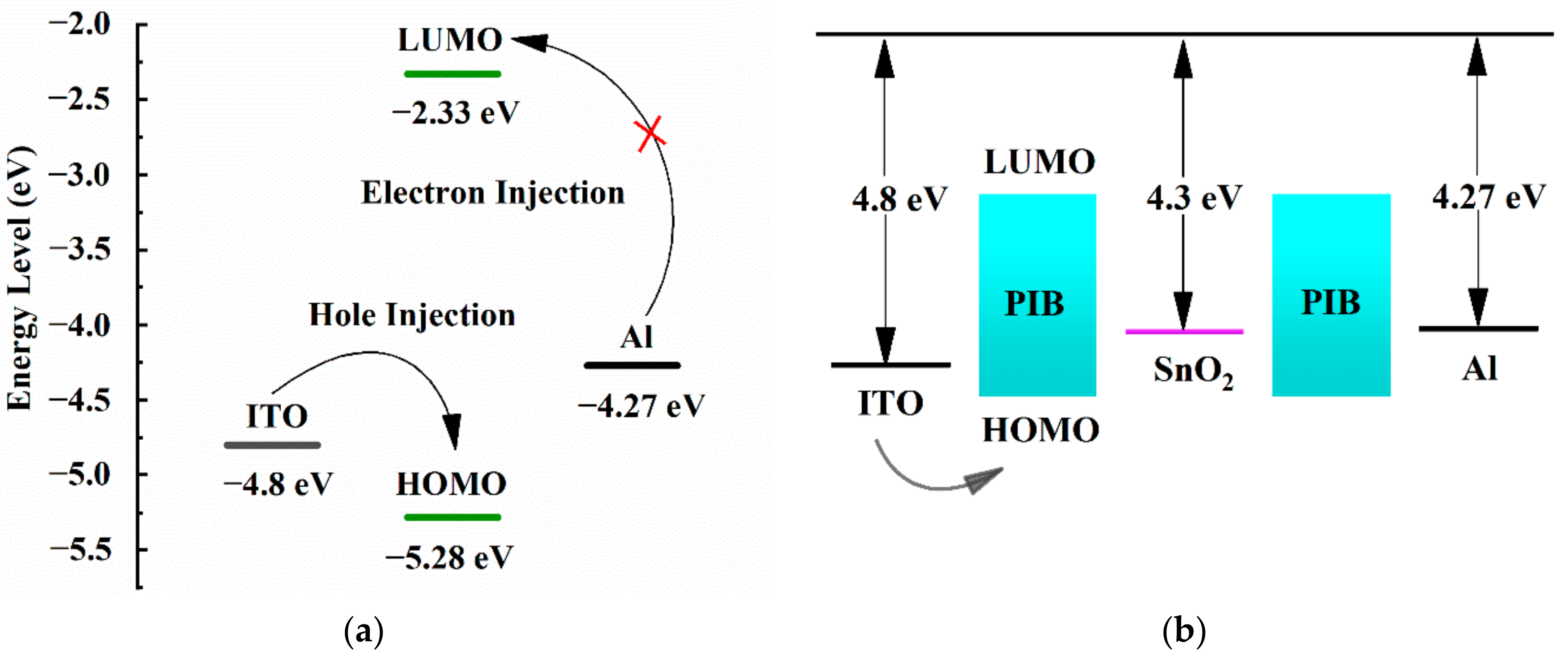
3.7. Switching Mechanism of Devices
3.8. Device Switching Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; He, J.; Zhuang, H.; Li, H.; Li, N.; Xu, Q.; Chen, D.; Lu, J. Rational design of small molecules to implement organic quaternary memory devices. Adv. Funct. Mater. 2016, 26, 146–154. [Google Scholar] [CrossRef]
- You, Y.; Yang, K.; Yuan, S.; Dong, S.; Zhang, H.; Huang, Q.; Gillin, W.P.; Zhan, Y.; Zheng, L. An organic multilevel non-volatile memory device based on multiple independent switching modes. Org. Electron. 2014, 15, 1983–1989. [Google Scholar] [CrossRef]
- Jang, J.; Choi, H.H.; Kim, M.; Kim, J.K.; Chung, S.; Park, J.H. Self-erasable titanium oxide resistive memory devices. Ind. Eng. Chem. Res. 2019, 78, 338–343. [Google Scholar] [CrossRef]
- Zhang, B.; Li, D.; Wu, Y.; Fan, F.; Chen, Y. A donor-acceptor structured conjugated copolymer for flexible memory device. Org. Electron. 2017, 49, 269–277. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Li, H.; Sun, X.; Yan, S.; Ren, Z. A recyclable and photocontrollable resistive memory device based on polycoumarinsiloxanes. J. Mater. Chem. C 2020, 8, 7527–7533. [Google Scholar] [CrossRef]
- Tian, Y.; Song, Y.; Yao, H.; Yu, H.; Tan, H.; Song, N.; Shi, K.; Zhang, B.; Zhu, S.; Guan, S. Improving resistive switching characteristics of polyimide-based volatile memory devices by introducing triphenylamine branched structures. Dyes Pigm. 2019, 163, 190–196. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, S.; Di, Y.; Liu, H.; Yao, H.; Zhang, Y.; Guan, S. HOMO-controlled donor-acceptor contained polyimide for nonvolatile resistive memory device. Dyes Pigment 2021, 186, 109020. [Google Scholar] [CrossRef]
- Fan, F.; Zhang, B.; Cao, Y.; Yang, X.; Gu, J.; Chen, Y. Conjugated polymer covalently modified graphene oxide quantum dots for ternary electronic memory devices. Nanoscale 2017, 9, 10610–10618. [Google Scholar] [CrossRef]
- Guo, J.; Geng, J.; Tian, G.; Ji, D.; Qi, S.; Wu, D. Zinc ion triggered controllable binary/ternary memory conversion behaviors in polyimides containing pendant porphyrin group. Small 2020, 16, e2005659. [Google Scholar] [CrossRef]
- Zhang, Q.; He, J.; Li, H.; Li, N.; Xu, Q.; Chen, D.; Lu, J. A novel ternary memory property achieved through rational introduction of end-capping naphthalimide acceptors. J. Mater. Chem. C 2017, 5, 7961–7968. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, L.; Wang, L.; Liu, B.; Tian, X.; Chen, Y. Covalent modification of graphene oxide with poly(n-vinylcarbazole) containing pendant azobenzene chromophores for nonvolatile ternary memories. Carbon 2018, 134, 500–506. [Google Scholar] [CrossRef]
- Gu, P.Y.; Zhou, F.; Gao, J.; Li, G.; Wang, C.; Xu, Q.F.; Zhang, Q.; Lu, J.M. Synthesis, characterization, and nonvolatile ternary memory behavior of a larger heteroacene with nine linearly fused rings and two different heteroatoms. J. Am. Chem. Soc. 2013, 135, 14086–14089. [Google Scholar] [CrossRef]
- Singh, S.P.; Sharma, S.K.; Kim, D.Y. Carrier mechanism of ZnO nanoparticles-embedded PMMA nanocomposite organic bistable memory device. Solid State Sci. 2020, 99, 106046. [Google Scholar] [CrossRef]
- Mombrú, D.; Romero, M.; Faccio, R.; Mombrú, A.W. Raman and impedance spectroscopy under applied Dc Bias insights on the electrical transport for donor:acceptor nanocomposites based on poly(vinyl Carbazole) and TiO2 quantum dots. J. Phys. Chem. C 2017, 121, 23383–23391. [Google Scholar] [CrossRef]
- Kashyap, J.; Ashraf, S.M.; Riaz, U. Highly efficient photocatalytic degradation of amido black 10B dye using polycarbazole-decorated TiO2 nanohybrids. ACS Omega 2017, 2, 8354–8365. [Google Scholar] [CrossRef] [Green Version]
- Shakir, M.; Noor-e, I.; Khan, M.S.; Al-Resayes, S.I.; Khan, A.A.; Baig, U. Electrical conductivity, isothermal stability, and ammonia-sensing performance of newly synthesized and characterized organic–inorganic polycarbazole–titanium dioxide nanocomposite. Ind. Eng. Chem. Res. 2014, 53, 8035–8044. [Google Scholar] [CrossRef]
- Sarkar, P.K.; Bhattacharjee, S.; Prajapat, M.; Roy, A. Incorporation of SnO2 nanoparticles in PMMA for performance enhancement of a transparent organic resistive memory device. RSC Adv. 2015, 5, 105661–105667. [Google Scholar] [CrossRef]
- Horti, N.C.; Kamatagi, M.D.; Patil, N.R.; Nataraj, S.K.; Patil, S.A.; Inamdar, S.R. Synthesis and photoluminescence properties of polycarbazole/tin oxide (PCz/SnO2) polymer nanocomposites. Polym. Bull. 2020, 78, 6321–6336. [Google Scholar] [CrossRef]
- Salah, N.; Habib, S.; Azam, A. Magnetic properties of microwave-synthesized Mn-doped SnO2 nanoparticles. Appl. Phys. A 2016, 122, 986. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, J.; Xi, J.; Du, J.; Tian, J. Multiple-function surface engineering of SnO2 nanoparticles to achieve efficient perovskite solar cells. J. Phys. Chem. Lett. 2021, 12, 9142–9148. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, X.; Bai, J.; Hou, Y.; Wang, S.; Wang, C.; Ma, D. Ternary memory devices based on bipolar copolymers with naphthalene benzimidazole acceptors and fluorene/carbazole donors. Macromolecules 2019, 52, 9364–9735. [Google Scholar] [CrossRef]
- Das, M.; Roy, S. Preparation, characterization and properties of newly synthesized SnO2-polycarbazole nanocomposite via room temperature solution phase synthesis process. Mater. Today 2019, 18, 5438–5446. [Google Scholar] [CrossRef]
- Chen, C.J.; Hu, Y.C.; Liou, G.S. Linkage and acceptor effects on diverse memory behavior of triphenylamine-based aromatic polymers. Polym. Chem. 2013, 4, 4162–4171. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, H.; Liu, J.; Shen, Y. Synthesis and binary/ternary write-once read-many-times electrical memory behaviors of carbazole-based polyimides bearing flexible linkage segment. Eur. Polym. J. 2018, 10, 10–19. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, A.C.; Prakash, R. Electro-oxidation of formic acid using polyindole-SnO2 nanocomposite. Catal. Sci. Technol. 2012, 2, 2533–2538. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, X.; Li, Y.; Zhang, M.; Ma, C.; Li, H.; Lu, J.; Zhang, Q. Highly robust organometallic small-molecule-based nonvolatile resistive memory controlled by a redox-gated switching mechanism. ACS Appl. Mater. Interfaces 2019, 11, 40332–40338. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Fang, Y.; Li, H.; He, J.; Ji, Y.; Xu, Q.; Zheng, J.; Lu, J. Thermoresponsive memory behavior in metallo-supramolecular polymer-based ternary memory devices. ACS Appl. Mater. Interfaces 2017, 9, 32930–32938. [Google Scholar] [CrossRef]
- Sun, W.J.; Zhao, Y.Y.; Zhou, J.; Cheng, X.F.; He, J.H.; Lu, J. One-step fabrication of bio-compatible coordination complex film on diverse substrates for ternary flexible memory. Chem. Eur. J. 2019, 25, 4808–4813. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Gu, P.; Wang, Z.; Li, Z.; Li, H.; Lu, J.; Zhang, Q. Nonvolatile tri-state resistive memory behavior of a stable pyrene-fused n-heteroacene with ten linearly-annulated rings. Chem. Eur. J. 2018, 24, 7845–7851. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Li, H.; Xu, Q.; He, J.; Lu, J. Fluorine-induced highly reproducible resistive switching performance:facile morphology control through the transition between J- and H-aggregation. ACS Appl. Mater. Interfaces 2017, 9, 9926–9934. [Google Scholar] [CrossRef]
- Niamien, P.M.; Essy, F.K.; Trokourey, A.; Yapi, A.; Aka, H.K.; Diabate, D. Correlation between the molecular structure and the inhibiting effect of some benzimidazole derivatives. Mater. Chem. Phys. 2012, 136, 59–65. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, Y.; Ren, Z.; Li, H.; Sun, X.; Yan, S. Flexible and fatigue-resistant ternary electrical memory based on alternative copolysiloxane with carbazole donors and imidazole-modified naphthalimide acceptors. Adv. Mater. Technol. 2019, 4, 1900084. [Google Scholar] [CrossRef]
- Zou, Y.; Gendron, D.; Neagu-Plesu, R.; Leclerc, M. Synthesis and characterization of new low-bandgap diketopyrrolopyrrole-based copolymers. Macromolecules 2009, 42, 6361–6365. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Zhang, C.; Gu, P.; Chen, W.; Li, H.; Lu, J.; Zhang, Q. Thiadizoloquinoxaline-based n-heteroacenes as active elements for high-density data-storage device. ACS Appl. Mater. Interfaces 2018, 10, 15971–15979. [Google Scholar] [CrossRef]


| Sample | SnO2 (mg) | NMP (mL) | PIB (mg) | Toluene (mL) | SnO2 Mass Fraction (wt%) |
|---|---|---|---|---|---|
| A | 0 | 0 | 3 | 1 | 0 |
| B | 0.061 | 1 | 3 | 1 | 2 |
| C | 0.125 | 1 | 3 | 1 | 3 |
| D | 0.187 | 1 | 3 | 1 | 4 |
| E | 0.261 | 1 | 3 | 1 | 5 |
| F | 0.333 | 1 | 3 | 1 | 6 |
| Peak (2θ) | FWHM | Crystallite Size (nm) | Average Size (nm) |
|---|---|---|---|
| 26.57 | 0.176 | 45.9 | |
| 33.87 | 0.208 | 39.6 | 40 |
| 37.93 | 0.201 | 41.4 | |
| 51.77 | 0.260 | 33.6 |
| ESP | HOMO | LUMO | |
|---|---|---|---|
| PIB | 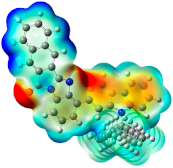 | 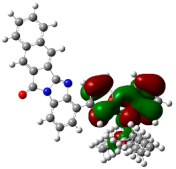 | 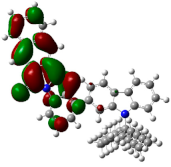 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Dou, F.; Zhou, Y.; Zhao, X.; Chen, J.; Wang, C.; Wang, S. Ternary Electrical Memory Devices Based on Polycarbazole: SnO2 Nanoparticles Composite Material. Polymers 2022, 14, 1494. https://doi.org/10.3390/polym14071494
Zhang Y, Dou F, Zhou Y, Zhao X, Chen J, Wang C, Wang S. Ternary Electrical Memory Devices Based on Polycarbazole: SnO2 Nanoparticles Composite Material. Polymers. 2022; 14(7):1494. https://doi.org/10.3390/polym14071494
Chicago/Turabian StyleZhang, Yingna, Feng Dou, Yijia Zhou, Xiaofeng Zhao, Jiangshan Chen, Cheng Wang, and Shuhong Wang. 2022. "Ternary Electrical Memory Devices Based on Polycarbazole: SnO2 Nanoparticles Composite Material" Polymers 14, no. 7: 1494. https://doi.org/10.3390/polym14071494
APA StyleZhang, Y., Dou, F., Zhou, Y., Zhao, X., Chen, J., Wang, C., & Wang, S. (2022). Ternary Electrical Memory Devices Based on Polycarbazole: SnO2 Nanoparticles Composite Material. Polymers, 14(7), 1494. https://doi.org/10.3390/polym14071494