1. Introduction
In recent years, with the improvement of people’s lives, the impact on the environment is also increasing. The discharge of industrial sewage and domestic sewage causes water pollution. Water pollution sickens 250 million people every year. In the period of rapid development, Japan experienced a number of sanitation incidents due to water pollution, such as Minamata disease (Hg) and Itai-itai disease (Cd). In addition, with the outbreak of the 2011 Great East Japan Earthquake, a large amount of radioactive cesium sewage produced from the Fukushima First Nuclear Power Plant was reported [
1].
Surfactant molecules are composed of hydrophilic and hydrophobic groups, which are widely used in cosmetics, detergents, and other everyday products as emulsifiers, foaming agents, and cleaning agents [
2,
3]. The output value of surfactants in the world reached 47.6 billion US dollars in 2020, an increase of 8.68% compared with the previous year [
4]. The yield increased from 3000 tons in 1950 to 17.8 million tons in 2015 [
5,
6,
7,
8,
9]. Anionic surfactants account for the highest proportion, at about 45%. Although surfactants are important chemical products, when they are discharged into the environment, they produce a large number of bubbles, blocking the exchange of oxygen between water and air, leading to the destruction of the living environment of aquatic organisms, thus affecting biodiversity.
In the past, various adsorbents of surfactants have been widely studied. For example, Yang et al. [
10] determined the most suitable method of using acrylic resin to adsorb surfactants by comparing the adsorption properties of directly purchased resin XAD-7 and an acrylic resin with different pore sizes. In addition, the adsorptive removal of sodium dodecyl sulfate (SDS) using activated coconut shell was discussed by Pravin et al. [
11]. Furthermore, research into improving the adsorption capacity of surfactants by modified activated carbon has also been carried out in our laboratory. With the rapid development of new materials, metal–organic frameworks (MOFs) have received more attention in recent years. MOFs as a new porous material, also possess excellent adsorption properties. Mozhgan et al. [
12] investigated the adsorption of SDS using hierarchical porous zeolitic imidazolate framework-8/carbon fiber (ZIF-8/carbon fiber) nano sorbent. MOFs are also widely used for the adsorption of other substances. For example, selective dye adsorption by UiO-66 MOF [
13], adsorption of heavy metal ion Cr(VI) on UiO-66-NH
2 MOFs [
14], or chemical fixation of CO
2 by amine functionalized MOF [
15] have been reported. Modifying a material with some functional groups can be one of the main methods of achieving efficient surfactant adsorption [
16]. At present, the volume of wastewater including surfactants that need treatment is excessive, and thus we still need to explore adsorption methods that achieve stronger adsorption capacity with cheaper raw materials. The methods for treating environmental pollutants through modified natural materials have been reported [
17,
18,
19]. It can be considered that modified cellulose is also being used to remove dyes and heavy metal ions from the wastewater. However, there have been few studies on the use of cellulose-based adsorbents for the adsorption of surfactants. Thus, in this study, we paid attention to synthesizing a new type of adsorption material based on cellulose as a natural polymer material, which is expected to be environmentally friendly and inexpensive for removing anionic surfactants. Specifically, we tried to synthesize the adsorbent by modifying a quaternary ammonium cation of cellulose.
Cellulose is the main component of plant cell walls and plant fibers and is the most abundant carbohydrate on earth. The annual output of cotton, which has more than 90% cellulose content, is 26 million tons [
20], and the annual recovery of wastepaper, of which the main component of cellulose, is 240 million tons [
21]. Cellulose is a polymer composed of glucose monomers, and the molecules consist of hydrogen bonded into a seal form. The sixth hydroxyl group of D-glucose in cellulose has high activity and reacts easily with other substances [
22]. On the other hand, quaternary ammonium cations are positively charged polyatomic ions that are generally synthesized by tertiary amines. Thus, quaternary ammonium salts with the positive charge can attract the hydrophilic anion of anionic surfactants such as SDS by electrostatic gravitation as well as by van der Waals forces to attract the hydrophobic functional group. For example, N,N-dimethyldodecylamine was selected in our experiments, expecting to enhance the adsorption efficiency through the interaction between the dodecyl group of quaternary ammonium cation and SDS.
The objective of the present research was to investigate the SDS adsorption efficiency of modified cellulose in water, aiming to develop a method for the efficient adsorption of SDS and apply it in practice. We first synthesized an environmentally friendly, low-cost, and effective adsorbent of anionic surfactants by using a natural polymer material based on cotton. In this process, it was important to select an appropriate tertiary amine that reacted with epichlorohydrin for synthesizing the corresponding quaternary ammonium cation. The quaternary ammonium cation in cellulose was modified by the Williamson reaction under alkaline conditions. Subsequently, the characteristics of the modified cellulose were explored, and the SDS adsorption mechanism of the modified cellulose was investigated in this study.
2. Materials and Methods
The cellulose was derived from Osaki cotton balls from Osaki Medical Co., Ltd. (Nagoya, Japan) in this study. The sodium dodecyl sulfate (SDS) and epichlorohydrin were sourced from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan), and the dodecyl dimethyl tertiary amine was from FIJIFILM Wako Chemical Co., Ltd. (Osaka, Japan). All reagents used were of analytical grade. During the whole working process, water (>18.2 MΩ) treated by an ultra-pure water system (RFU 424TA, Advantech Aquarius, Irvine, CA, USA) was used. In addition, a water bath incubator (BT100, Yamato Kagaku Co., Ltd., Tokyo, Japan), a vacuum drying oven (DP33, Yamato Kagaku Co., Ltd., Tokyo, Japan), and a pH meter (HORIBA F-72, Tokyo, Japan) were used in this work.
Furthermore, a scanning electron microscopy and energy dispersive spectrometry (SEM-EDS, JEOL, Akishima, Tokyo, Japan: JCM-6000 with JED-2300), ion sputtering (JFC-1100E, JEOL, Akishima, Tokyo, Japan), Fourier transform infrared spectroscopy (FT-IR4200, Jasco, Hachioji, Tokyo, Japan) nuclear magnetic resonance (NMR, AVANCE III HD400, Bruker, Billerica, MA, USA), Zeta-potential measurement with electrophoretic light scattering (ELS, Otsuka, Tokyo, Japan: ELSZ-2000ZS), and differential refractometry (RID-6A, SHIMADZU, Kyoto, Japan) were employed in this work.
2.1. Synthesis of the Adsorbent
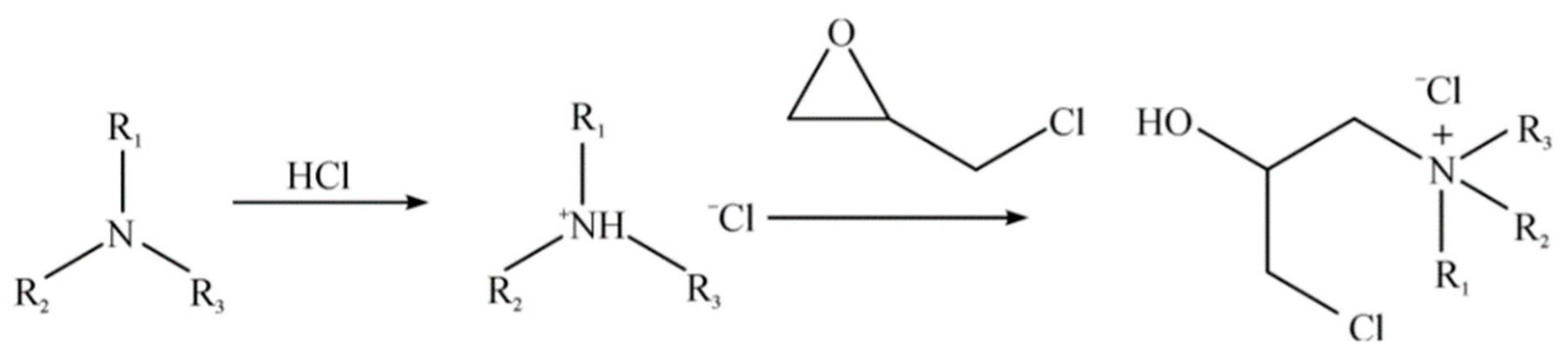
First, epichlorohydrin was grafted onto tertiary amines of dodecyl dimethyl to synthesize quaternary ammonium salts [
23]. Concentrated hydrochloric acid was diluted to 4 M with ultra-pure water, and then dodecyl dimethyl tertiary amine was slowly dropped from a drip funnel in an ice bath until the ratio of hydrochloric acid to tertiary amine was 1:1, and the mixture was left to react for 1 h. Secondly, epichlorohydrin was slowly dropped into a flask at 35 °C for 30 min until the ratio of epichlorohydrin to tertiary amine was 1.5:1, and the mixture was left to react for 8 h. The reaction principle, based on Bao et al. [
22], is shown in
Figure 1. Next, ultra-pure water was added to remove the top layer of oil. The remaining liquid was extracted by ethyl acetate and chloroform successively. After extraction, the liquid was distilled at 100 °C, and the remaining liquid was considered to be a quaternary ammonium salt solution when the volume of the solution did not decrease.
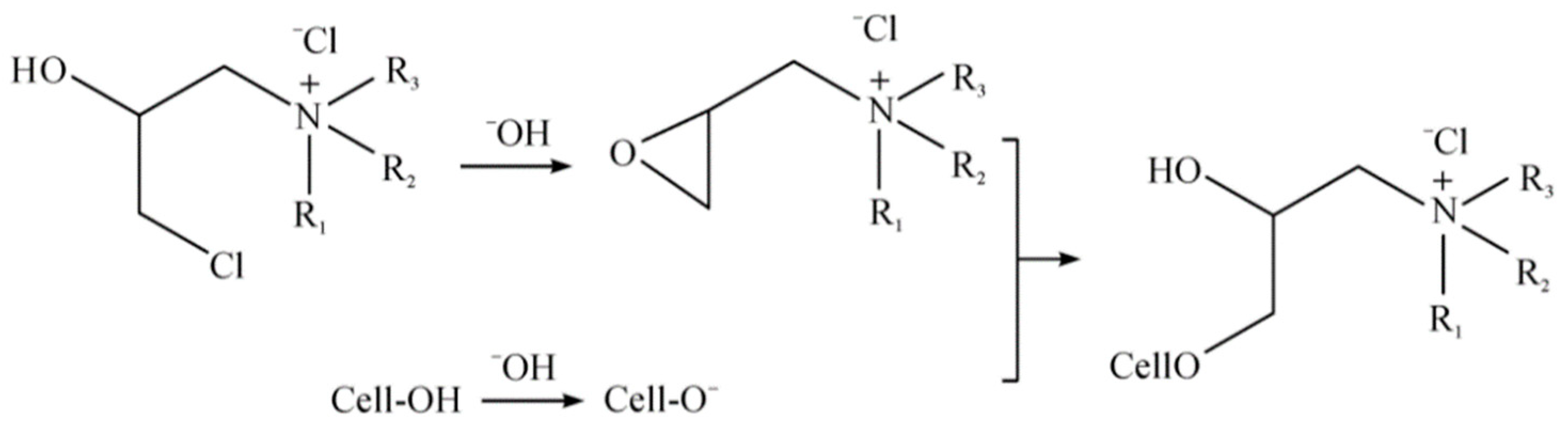
The adsorbent was synthesized with quaternary ammonium cations and cellulose. At a ratio of 1:60, cotton balls were put into a bath of ultra-pure water and the quaternary ammonium salt solution was added at a concentration of 40 g·L
−1. By heating the mixture to 75 °C, the cotton balls came in full contact with the quaternary ammonium salt. The mixture was then cooled to 50 °C, and sodium hydroxide was added at a concentration of 20 g·L
−1 for 30 min [
22,
24]. The reaction principle, based on Bao et al. [
22], is shown in
Figure 2. Finally, the cotton balls were removed, rinsed thoroughly with pure water for 5 min, and dried in a drying oven at 60 °C to obtain cellulose modified with quaternary ammonium salts.
2.2. Characterization of the Adsorbent
In this study, nuclear magnetic resonance (NMR) was first used to analyze the functional groups of quaternary ammonium salts dissolved in heavy water to determine whether the quaternary ammonium reaction was successful. After sputtering gold powder, the surface of the modified cellulose was observed by scanning electron microscopy-energy dispersive spectroscopy (SEM-EDS). The functional groups of cellulose before and after modification were analyzed by Fourier transform infrared spectroscopy (FT-IR) to determine whether the quaternary ammonium cation had modified the cellulose. Then, the zeta potential of cellulose before and after modification in different pH solutions was measured by electrophoretic light scattering (ELS). Finally, after the adsorption experiment, X-ray photoelectron spectroscopy (XPS) was used to analyze the elements of cellulose before and after the adsorption of SDS.
2.3. Adsorption Experiments
In order to study the optimal conditions of SDS adsorption, the effect of pH, adsorption time, and adsorption temperature on the SDS adsorption capacity of the modified cellulose was assessed. The modified cellulose was placed in a 200 mL conical flask containing 30 mL of an aqueous solution with a known amount of SDS, and the suspensions were placed in a constant temperature shaker (TAITEC Plus Shaker EP
−1 with Thermo Minder SX-10R, Saitama, Japan) set at a prescribed temperature. Adsorption experiments were performed in the pH range of 3–11, with a contact time of 1 to 6 h, a temperature of 20–60 °C, and initial SDS concentrations of 100–600 mg·L
−1. The pH of the test solution was adjusted by adding 0.1 mol·L
−1 hydrochloric acid or sodium hydroxide. Finally, the adsorbed cellulose was taken out, and the residual concentration was measured by differential refractometry (RID) to calculate the adsorption capacity. The calculation method is shown in Equations (1) and (2).
where
represents the adsorption capacity at equilibrium (mg·g
−1);
C0 is the concentration of SDS in the standard solution (mg·L
−1);
Ci and
Ct are the initial and equilibrium SDS concentrations (mg·L
−1) in the solution, respectively;
nt and
n0 are the tortuosity ratio of the adsorbed SDS and the SDS in the standard solution, respectively;
V is the volume of the solution (L); and
m is the mass of adsorbent (g) [
25,
26,
27].
3. Results and Discussion
3.1. Characterization
3.1.1. NMR Analysis Results of the Quaternary Ammonium Salt
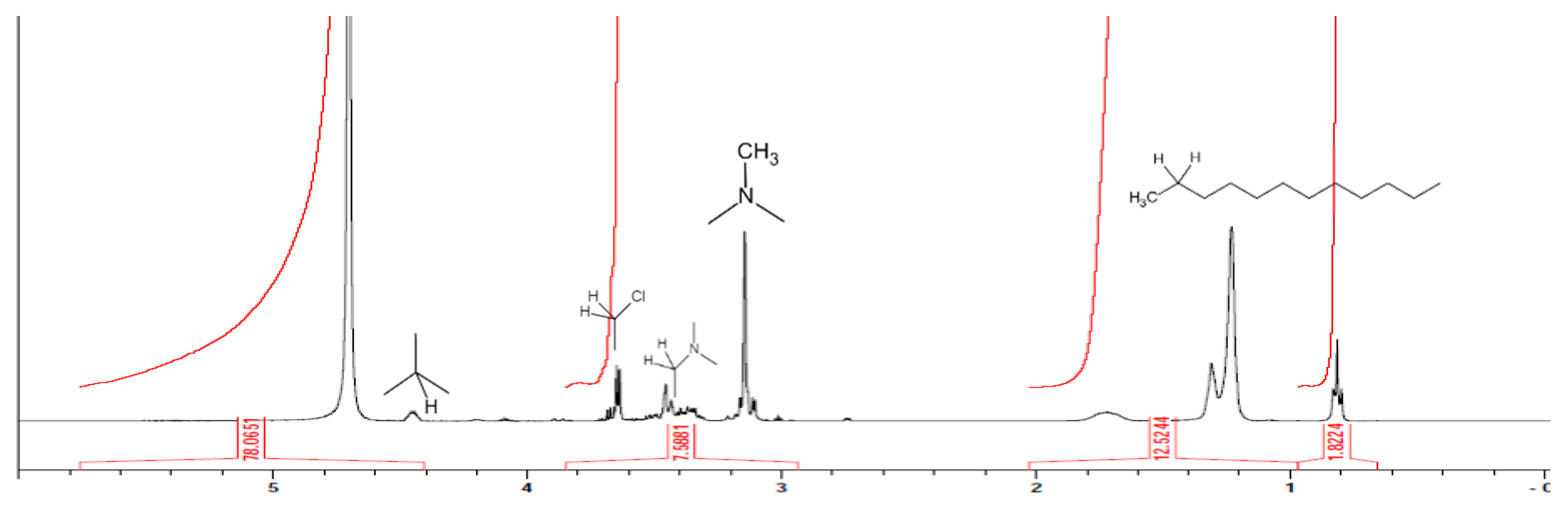
After the quaternary ammonium salt was dissolved in heavy water, nuclear magnetic resonance (NMR) was used to confirm the synthesis of the quaternary ammonium salt, and the results of this analysis are shown in
Figure 3. According to an analysis of the chemical shift of different peaks, we can discover the kinds of functional groups hydrogen belongs to. The dodecyl group appeared at 0.5–2.0 ppm, the methyl group linked to nitrogen appeared at 3.1 ppm, the methylene group linked to nitrogen appeared at 3.3–3.4 ppm, the methylene group linked to chlorine appeared at 3.5–3.6 ppm and the methine group linked to the hydroxyl group appeared at 4.4 ppm [
22]. Therefore, it can be seen from the measurements of the substance that the quaternary ammonium salts were indeed grafted with dodecyl dimethyl.
3.1.2. FT-IR Analysis Results of Cellulose
After making thin sheets of cotton floss with KBr, the structure of cotton floss was analyzed by Fourier transform infrared spectroscopy (FT-IR). The results are shown in
Figure 4. It can be seen that the modified cellulose had a more obvious peak at 1637 cm
−1 and 2900 cm
−1 than pure cellulose. The peak at 1637 cm
−1 was generated by the variable angular vibration of the quaternary ammonium group, and the peak at 2900 cm
−1 was generated by the stretching vibration of methylene in the long-chain dodecyl group [
28,
29]. This confirmed that the quaternary ammonium group had modified the cellulose.
3.1.3. SEM-EDS Analysis Results of Cellulose
After the cellulose was coated with gold powder by ion sputtering, the material was observed by scanning electron microscopy and energy dispersive spectrometry (SEM-EDS) at 15 kV voltage and under high vacuum conditions. The results of these samples [(a) pure cellulose, (b) modified cellulose, (c) adsorbed cellulose] are shown in
Figure 5 (with the magnification of 2000). In the case of modified cellulose, the sample with the magnification of 220 (
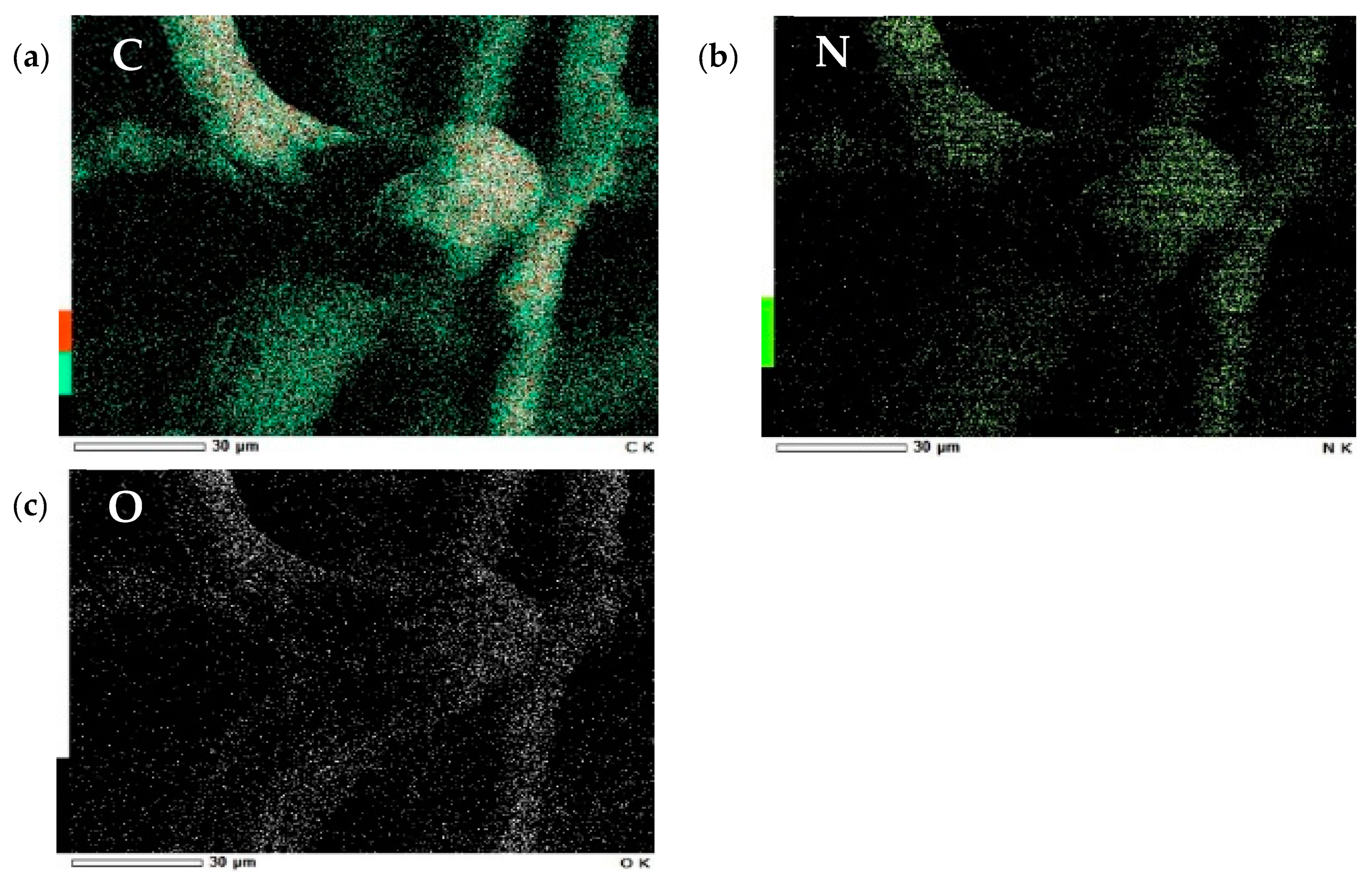
Figure 5b*) was also measured for the observation of the overall look of the sample. It was found that the quaternary ammonium salts adhered to the modified cellulose, making it coarser than pure cellulose. After adsorption, we can see that SDS is evenly wrapped around the fiber surface. The EDS results (
Figure 6) showed the distribution of elements on the modified cellulose’s surface, which also indicated that the quaternary ammonium group was modified in the cellulose.
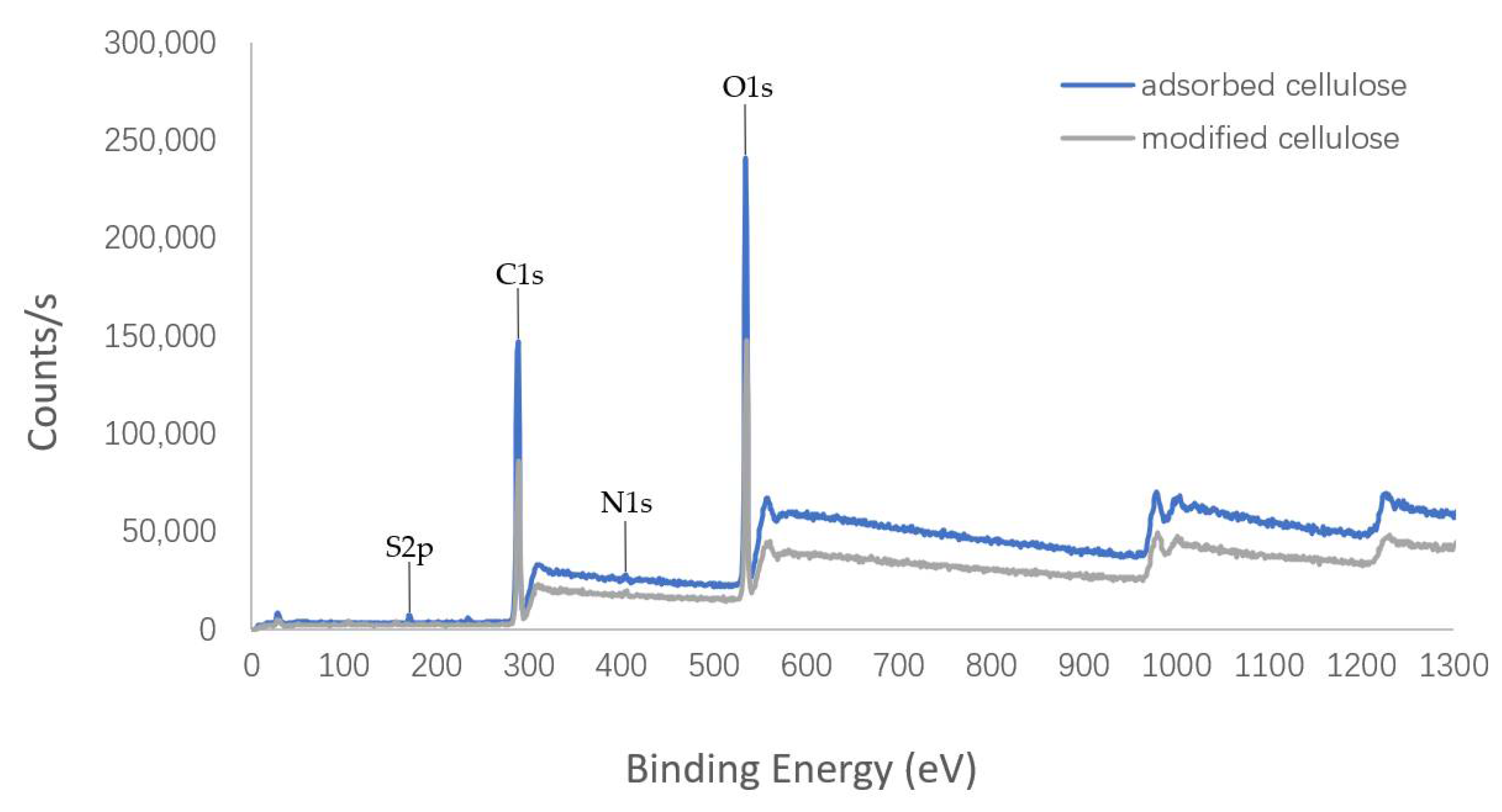
3.1.4. XPS Analysis Results of Cellulose
The surface elemental information of cellulose was investigated by XPS and elemental analysis was carried out. The analysis results are shown in
Figure 7 and
Table 1. Although nitrogen was detected in the modified cellulose, it was low compared with the other elements (C and O). The result may be related to the fact that the proportion of nitrogen in the quaternary ammonium salt in cellulose is very low. The modification rate was estimated to be only 15.7%, which may be attributable to the large volume of dodecyl, and thus it was not easy to modify the cellulose. Moreover, the elemental composition of the adsorbed cellulose, including the detection of sulfur, suggested that the SDS can be truly absorbed by the modified cellulose.
3.2. Adsorption Experiments
3.2.1. The Effect of pH
In order to investigate the effects of the solution’s pH on the uptake of SDS, adsorption experiments were conducted at different pH values (3, 5, 7, 9, and 11). As shown in
Figure 8, the adsorption amount reached the maximum when the pH was around seven. At a lower pH value, the hydrogen ions compete with the quaternary ammonium cations, affecting the interaction between quaternary ammonium cations and SDS. On the other hand, when the pH was higher than seven, the hydroxide ions preferentially combined with quaternary ammonium cations, resulted in a deterioration in the adsorption capacity. Therefore, the pH was adjusted to seven for the removal of SDS by the modified cellulose for further experiments because the pH value of the solution was about nine before the adjustment.
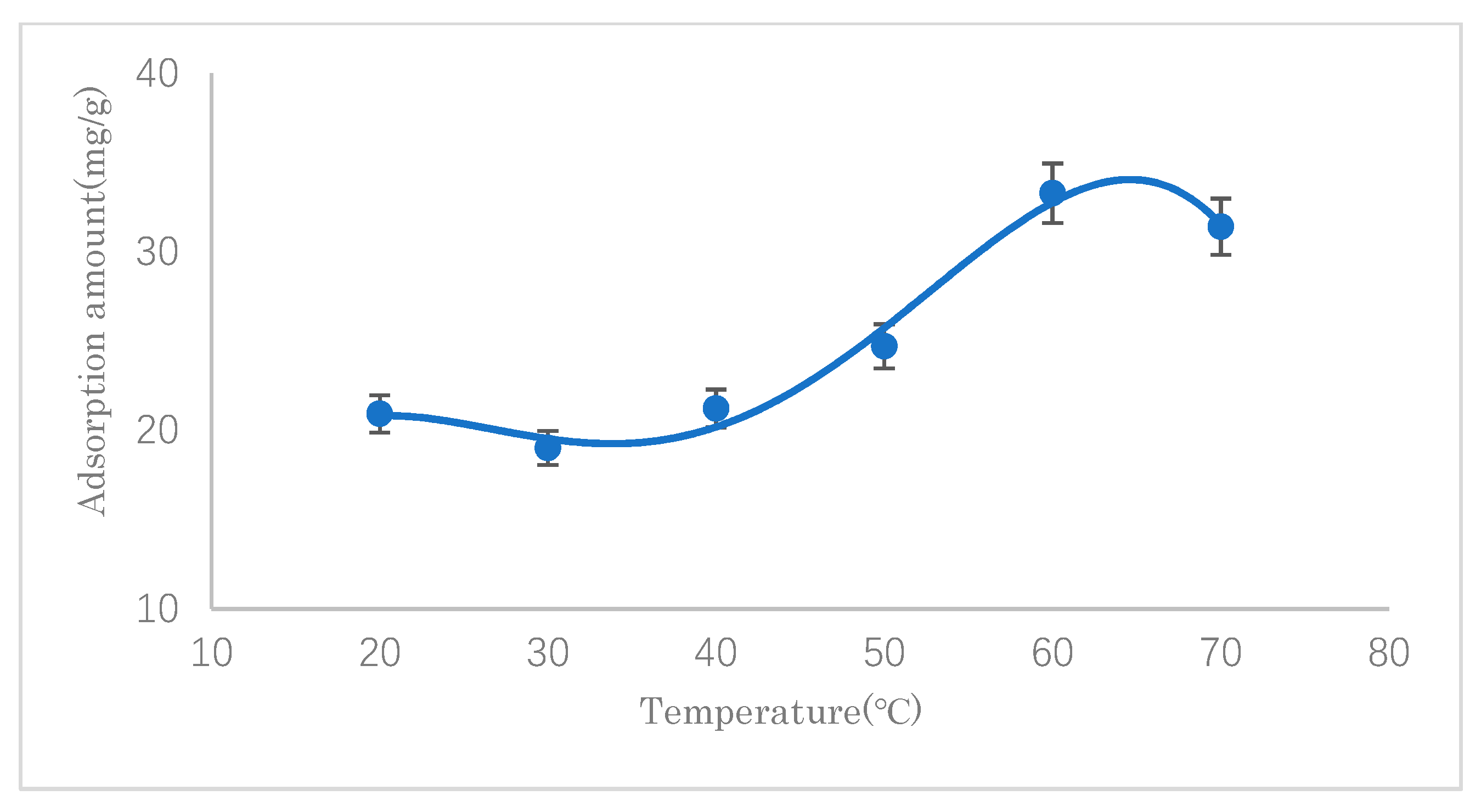
3.2.2. The Effect of Temperature
The effects of the reaction temperature on the SDS adsorption capacity of modified cellulose were investigated at different temperatures (20–70 °C). The results are shown in
Figure 9. It can be seen that the adsorption capacity reached the maximum value when approaching 60 °C. Before 60 °C, with an increase in the reaction temperature, the adsorption amount generally increased, which indicated that the adsorption reaction was endothermic. After 60 °C, the adsorption amount decreased, which may be attributable to the high-temperature desorption of SDS that had been adsorbed through the intermolecular van der Waals force. Therefore, 60 °C was chosen for further experimental work.
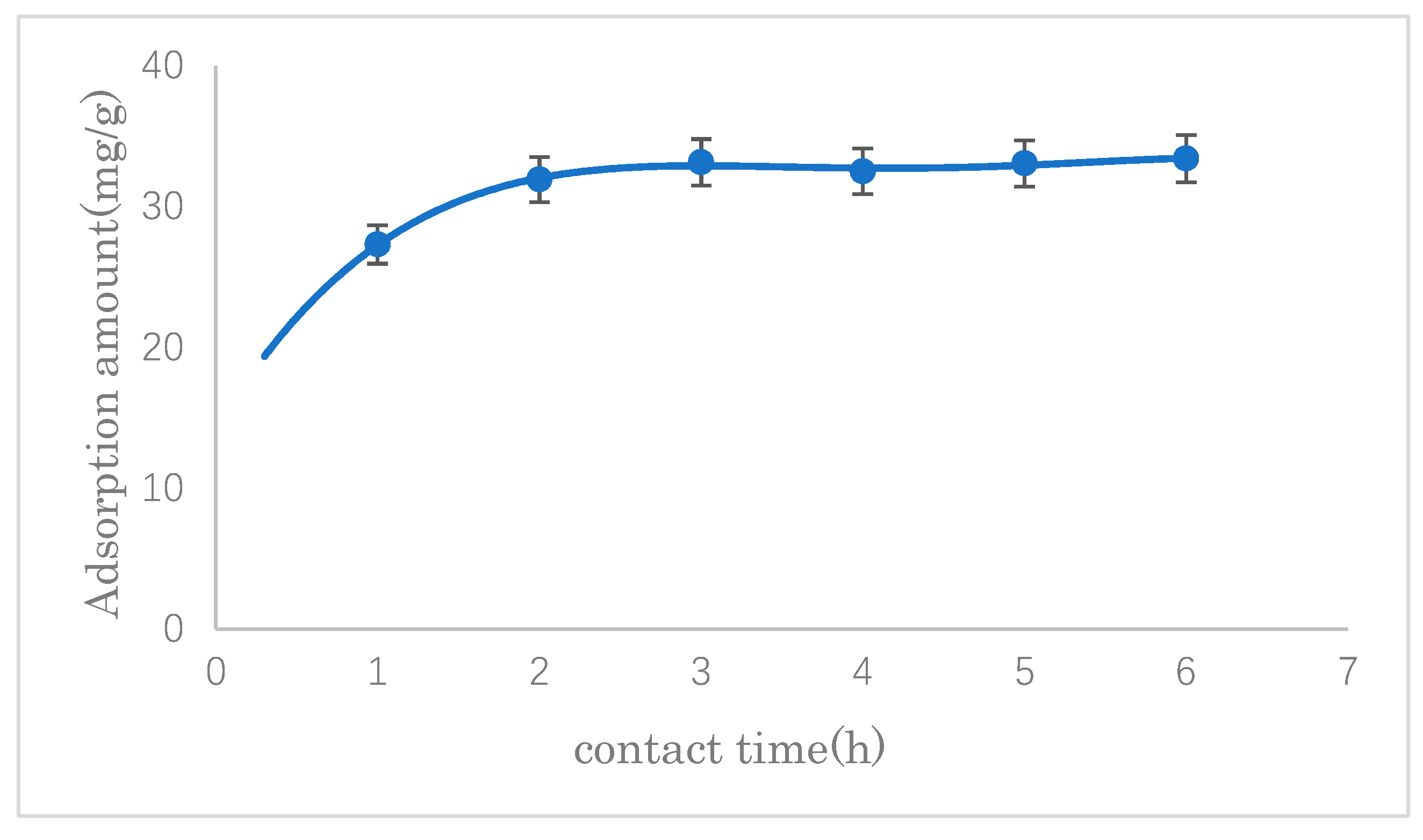
3.2.3. The Effect of Adsorption Time
The effect of contact time on the SDS adsorption capacity of modified cellulose was investigated. The results are shown in
Figure 10. It can be seen that there was a significant increase from the beginning of adsorption up to 2 h, after that, there was no appreciable change. Therefore, the adsorption time was set at 3 h thereafter.
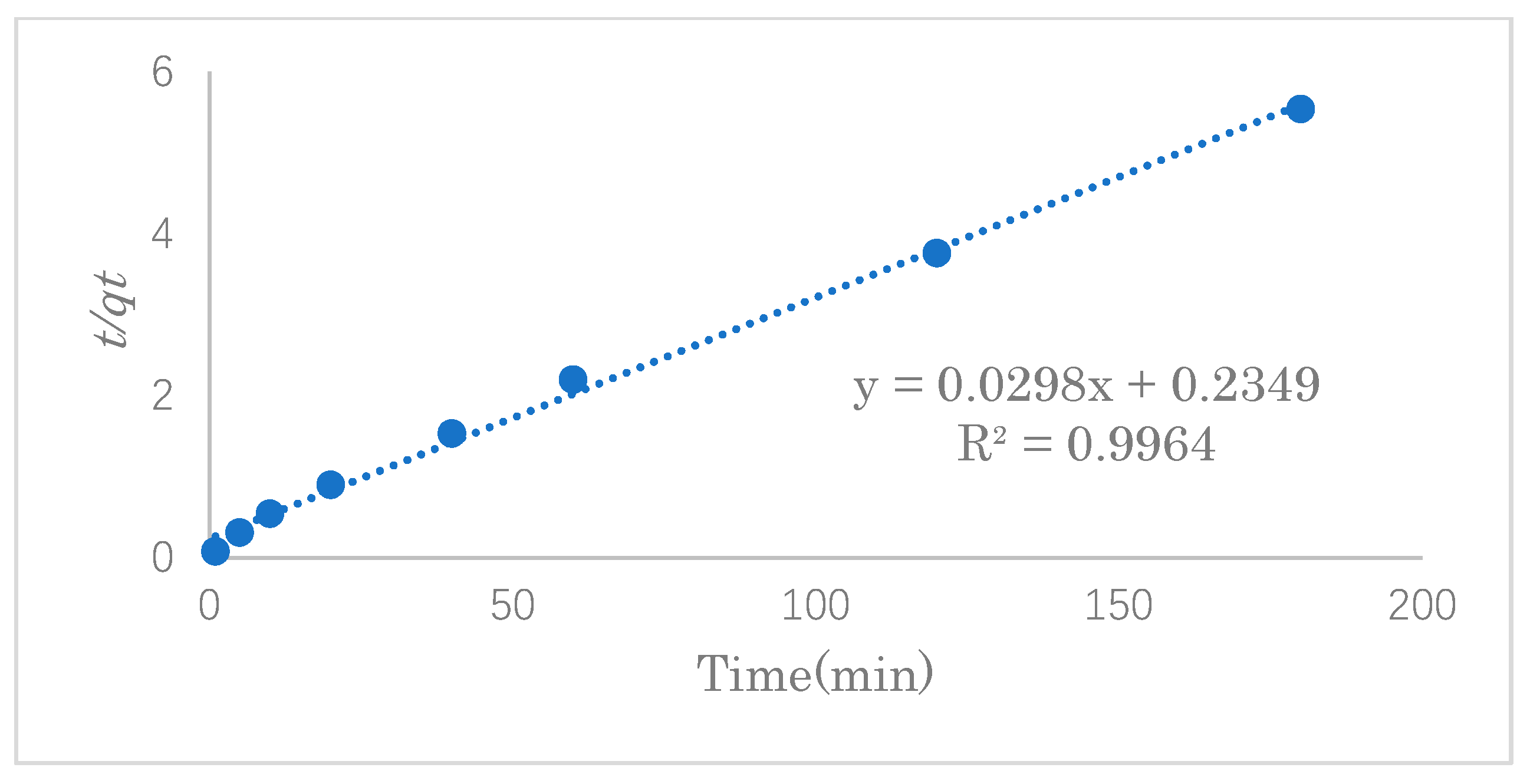
3.3. Adsorption Kinetics Study
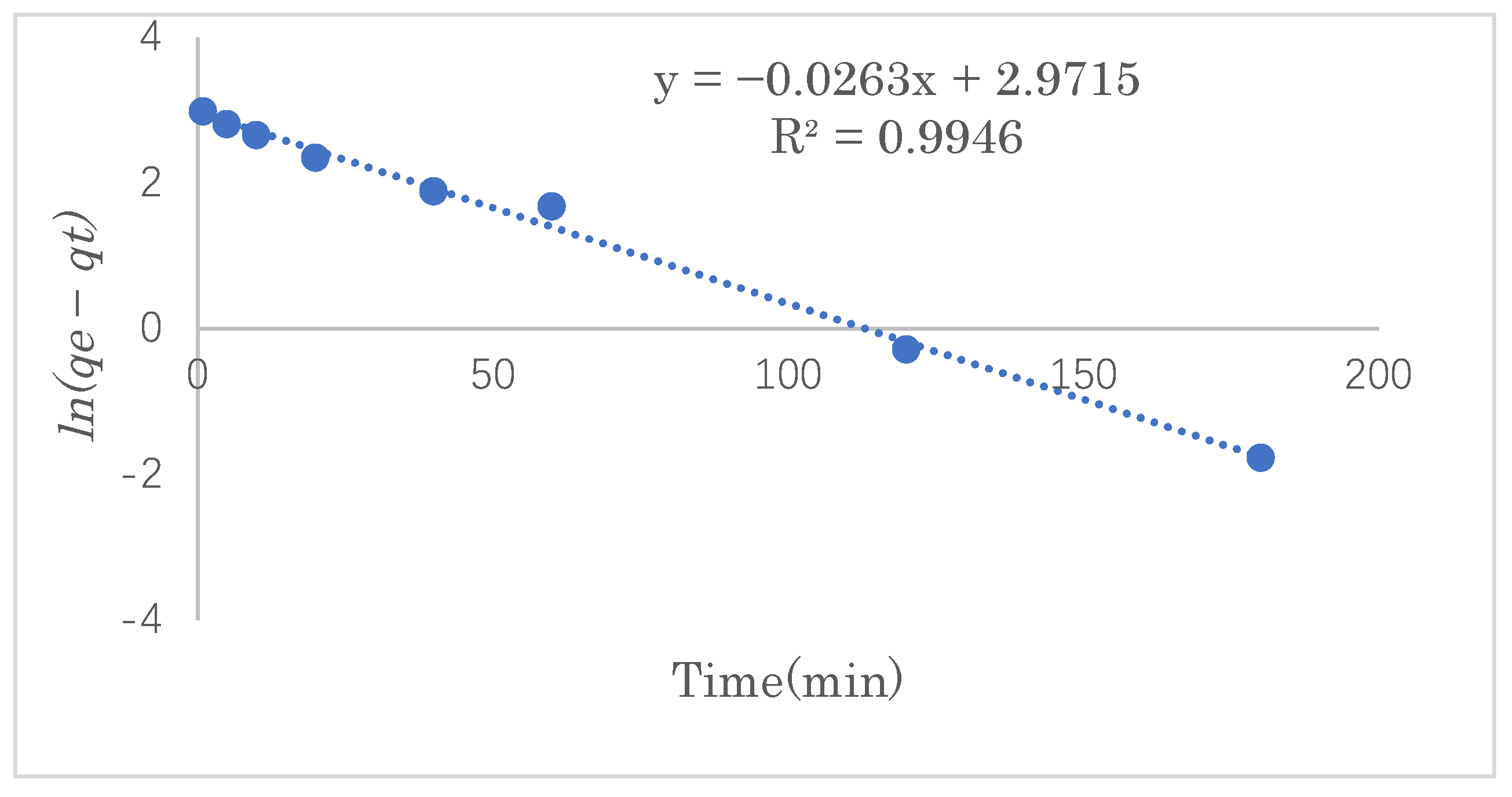
The adsorption kinetics model can be used to determine the mechanism of the adsorption process and provide effective data support for a feasibility study of the process. In this experiment, the SDS adsorption capacity was investigated at 1, 5, 10, 20, 40, and 60 min under the optimal adsorption conditions. The experimental data were fitted into the pseudo-first- and pseudo-second-order reaction equations to study the mechanism of the adsorption process.
The pseudo-first-order rate equation is expressed as follows:
where
qe and
qt are the adsorption capacity (mg·g
−1) of SDS at equilibrium and at time
t, respectively, and
k1 is the rate constant (h
−1) of pseudo-first-order adsorption.
The pseudo-second-order rate equation is expressed as follows:
where
qe and
qt are the adsorption capacity (mg·g
−1) of SDS at equilibrium and at time
t, respectively, and
k2 is the rate constant (g·mg
−1·h
−1) of pseudo-second-order adsorption [
30].
In order to examine the consistency between the kinetics model and the experimental results, pseudo-first-order or pseudo-second-order models were applied to the linear graphs of
ln(qe−qt) vs.
t or those of
t/
qe vs.
t for the SDS adsorption by modified cellulose, as shown in
Figure 11 and
Figure 12.
Table 2 shows the linear constants (
R2) and other parameters of the two kinetic models for the SDS adsorption by modified cellulose calculated according to
Figure 11 and
Figure 12. Judging the two values of
R2 shows that the adsorption process is highly correlated with both models. However, the maximum adsorption capacity calculated from the pseudo-second-order model was closer to that of the experimental data, so the adsorption of SDS is more consistent with the pseudo-second-order equation. Therefore, it is suggested that the adsorption reaction of SDS in this work was dominated by chemical adsorption as well as the van der Waals force on physical adsorption. The maximum adsorption capacity was estimated to be 33.6 mg·g
−1.
3.4. Adsorption Isotherm Study
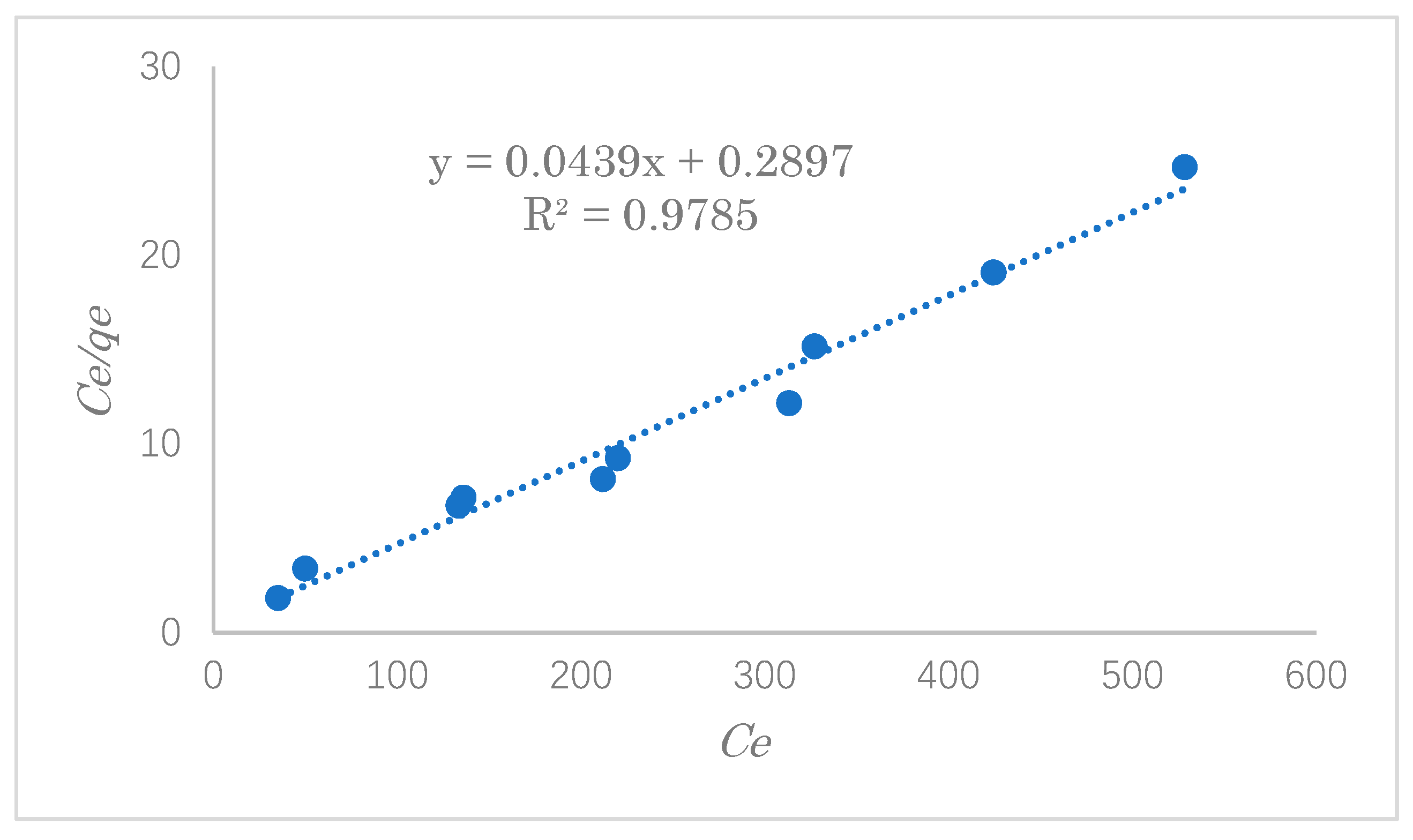
In the process of adsorption, the study of adsorption isotherms is significant and crucial for predicting the adsorption behavior of pollutants onto the adsorbent’s surface. In this experiment, typical adsorption isotherms, such as the Langmuir and Freundlich isotherms, were used to evaluate the experimental data of SDS adsorption. In this experiment, the adsorption capacity was explored with varying initial concentrations of 100, 200, 300, 400, 500, and 600 ppm.
The Langmuir adsorption isothermal model assumes that the surface of the adsorbent is uniform and that the adsorbate is adsorbed on the surface of the adsorbent in the form of a single molecular layer. The Langmuir adsorption isotherm is given by Equation (5):
where
Ce and
qe are the SDS concentration (mg·L
−1) and adsorption capacity (mg·g
−1) when the adsorption reaches equilibrium,
qmax is the maximal adsorption capacity of the adsorbent (mg⋅g
−1) and
KL is the adsorption constant of the Langmuir isotherm (L·mg
−1). The relationship between
Ce/
qe and
Ce gives a straight line with a slope of 1/
qmax and an intercept of 1/(
KL·
qmax).
KL can be applied to the Gibbs adsorption free energy Δ
Gads (J⋅mol
−1) via Equation (6):
where
R is the gas constant (8.314 J⋅K
−1mol
−1),
T is the absolute temperature at equilibrium (K) and
KL is the equilibrium constant at temperature
T.
The equilibrium constant [
31,
32]
KL can be calculated according to Equation (7):
where
Ce and
qe are the same as in Equation (4).
The Langmuir constant (
KL) can be used to determine the suitability of the adsorbent for the adsorbate by using the Hall separation coefficient (
RL), a dimensionless parameter which is given by Equation (8):
where
C0 (mg⋅L
−1) is the initial concentration. When 0 <
RL < 1, this means that the adsorbent is suitable for the adsorbate [
33,
34].
The Freundlich adsorption isothermal model is a multilayer adsorption process that does not consider adsorption saturation, which occurs on a multi-layered heterogeneous surface. The linear Freundlich isotherm model is represented by Equation (9):
where
KF is the adsorption capacity((mg·g
−1) and 1/
n is the adsorption strength. The relationship between
lnqe and
lnCe can determine the values of 1/
n and
KF. The value of 1/
n can be used to judge the difficulty of the adsorption process as follows: irreversible adsorption,
; favorable adsorption,
; unfavorable adsorption,
[
33].
The results of the adsorption data investigated by the Langmuir and Freundlich equations are shown in
Figure 13 and
Figure 14, respectively. The value of
KL can be calculated from the intercept of the Langmuir isotherm in Equation (4).
KL represents the affinity strength of the adsorbent to the target substance [
35].
Table 3 shows the linear constants (
R2) and other parameters of the two isotherm models for the modified cellulose adsorption of SDS calculated according to
Figure 13 and
Figure 14. By comparing the two values of
R2, we can see that the adsorption isotherms can generally be described more satisfactorily by the Langmuir isotherm. In addition, the value of
RL is between 0 and 1 when the initial concentration is 100 mg·L
−1. This result suggests that the adsorption of SDS on the modified cellulose mainly occurred by a monolayer reaction with the strong adsorption force. The maximum adsorption capacity was estimated to be 22.8 mg·g
−1.
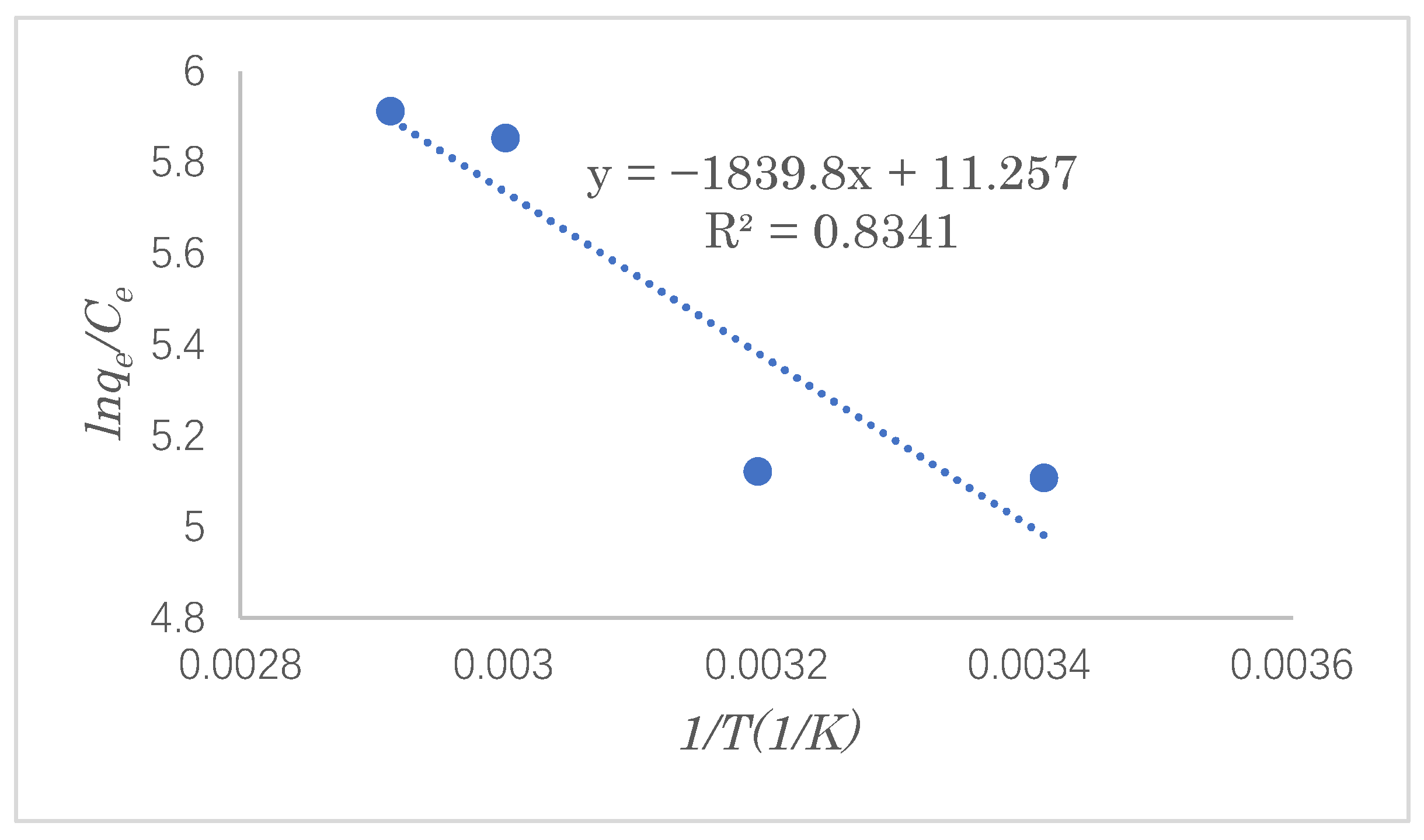
3.5. Adsorption Thermodynamics
In the adsorption process, the direction of heat flow and the reaction in the adsorption process can be guided by a study of the adsorption thermodynamics, which provides theoretical support for improving the reaction’s efficiency. In this experiment, under the optimal adsorption conditions, the SDS adsorption capacity was investigated at adsorption temperatures of 293, 313, 333, and 343 K. The Gibbs free energy, enthalpy change, and entropy change in the SDS adsorption reaction were calculated by the Van’t Hoff equation. The Van’t Hoff equation is as follows (Equation (10)):
The simultaneous equations (Equations (5) and (6)) lead to the following formula (11):
where
qe,
Ce,
R, and
T are the same as in Equations (5) and (6), whereas ∆
S0 (J·mol
−1), ∆
H0 (kJ·mol
−1), and ∆
G0 (kJ·mol
−1) represent the changes in the entropy, enthalpy, and Gibbs free energy during the reaction, respectively. The relationship between
ln(qe/
Ce) and 1/
T shown in
Figure 15 gives a straight line with a slope of ∆
H0/
R and an intercept of ∆
S0/
R [
30]. The thermodynamic parameters calculated via the Van’t Hoff equation are shown in
Table 4. It can be seen that the change in the Gibbs free energy has a negative value, but it tends to decrease with an increase in the temperature, indicating that the adsorption reaction of SDS is spontaneous. From the positive value of the change in enthalpy, it can be concluded that the adsorption reaction of SDS is endothermic. The change in entropy has a positive value, indicating that the degree of randomness of the interface between the adsorbent and the solution increases as the reaction progresses.
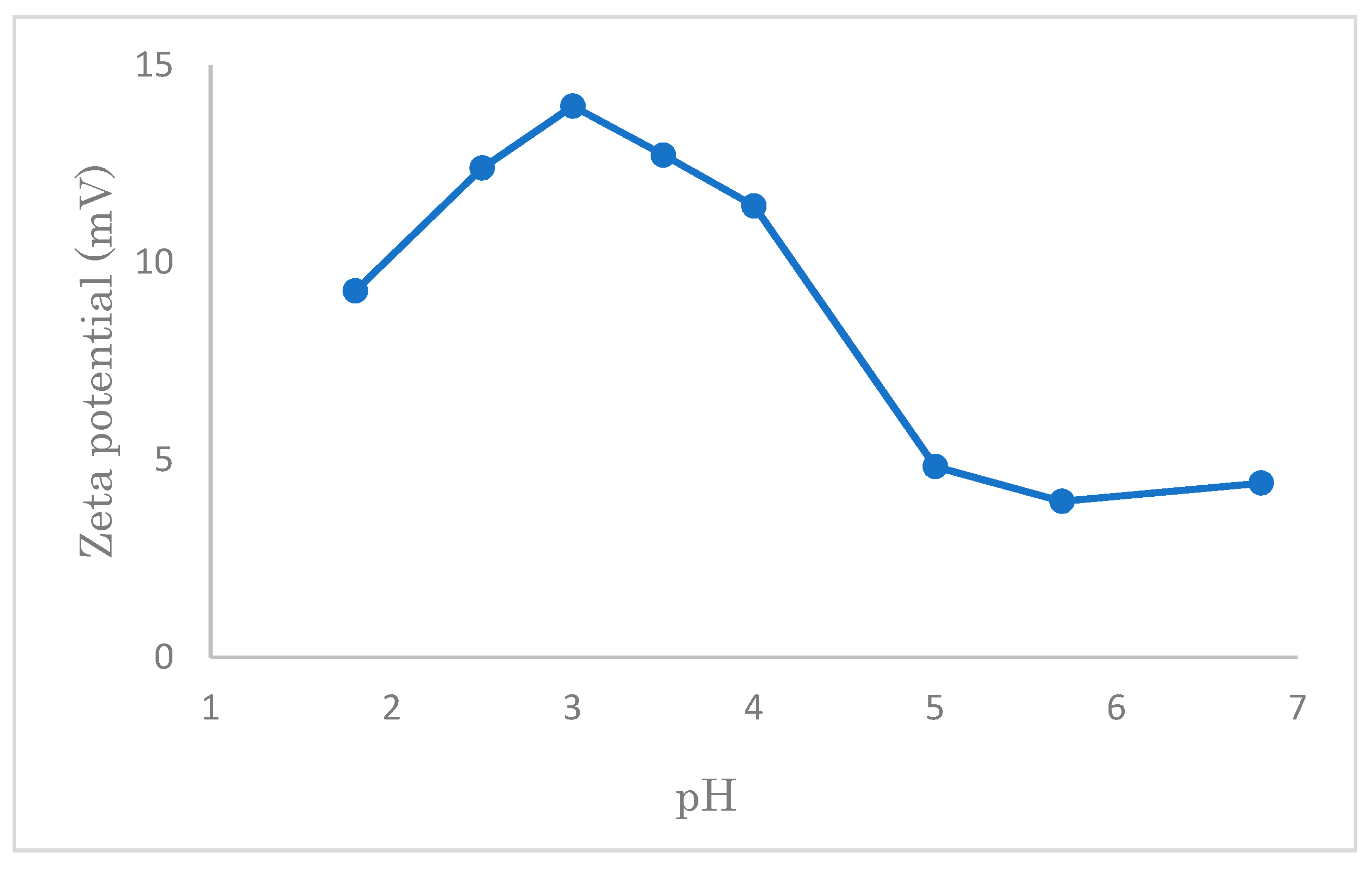
3.6. The Zeta-Potentials of Modified Cellulose Samples
Zeta potential vs. pH data of modified cellulose at different pH is shown in
Figure 16. In our work, the concentration of NaCl was installed 0.01 mol/L based on Li’s work [
36], and the solid–liquid ratio was 1:2000 (i.e., 25 mg for modified cotton). After the solution pH was adjusted to 1.8–6.8 using HCl and NaOH, the zeta potential of each sample was measured by electrophoretic light scattering [
37].
From
Figure 16, firstly, it can be seen that the zeta potential of the cotton, which was modified by quaternary ammonium cations is positive in the interval of pH 1.8–6.8, while the pure cotton shows negative values higher than pH 2.5 [
38]. This is the reason why the modified cotton has a stronger attraction to the anionic group compared to the pure cotton. After reaching a peak at pH 3, the zeta potential decreases with an increase in pH value.
As shown in
Figure 11 (in
Section 3.2.1), the adsorption of SDS, which is largely influenced by the concentration of hydrogen ions, is maximum at pH 7. At a low value of pH, it is considered that too many hydrogen ions may compete with quaternary ammonium cations, leading to a decrease in the adsorption of SDS, although the zeta potential is high.
3.7. Comparison with Other Adsorbents
A comparison of the maximum SDS adsorption capacity in the present study against those of other adsorbents in the literature is presented in
Table 5. As can be seen from
Table 5, the SDS adsorption capacity of the modified cellulose in this work (32.5 mg·g
−1) was not necessarily high compared with other adsorbents. However, it could be regarded as a potential adsorbent for removing SDS from wastewater for practical use because the method of synthesizing the adsorbent is relatively simple, and cellulose as a raw material is readily available for the removal of SDS.
Moreover, the modified cellulose can be expected to have better adsorption performance if the modification conditions are optimized to improve the modification rate, although the modification rate of quaternary ammonium cations was only 15.7% in this work.
4. Conclusions
In this work, the synthesis of long-chain dodecyl in a quaternary ammonium salt could be confirmed at the laboratory level through NMR analysis. The results of SEM-EDS and FT-IR showed that the quaternary ammonium cation was successfully modified in cellulose. The optimal conditions of SDS adsorption by quaternary ammonium cation-modified cellulose were pH 7, an adsorption time of 3 h, and a reaction temperature of 60 °C. The analysis of the adsorption kinetics showed that the adsorption process of SDS was more consistent with the pseudo-second-order model, indicating that the adsorption was mainly dominated by chemical adsorption. The adsorption process of SDS conformed more to the Langmuir isotherm, suggesting that SDS was mainly adsorbed on the surface of the cellulose in the form of a monolayer. According to the analysis of the adsorption thermodynamics, the adsorption reaction of SDS is a spontaneous and endothermic reaction. From the results of zeta potential, it is clear that the modification of quaternary ammonium salt makes the cellulose surface have a more positive charge, leading to a stronger attraction to SDS. Finally, it can be concluded that the modified cellulose with quaternary ammonium cations synthesized in this study can be an effective adsorbent for SDS from an aqueous solution.
Author Contributions
Experiments, data evaluation, instruments of measurement and writing, M.Z.; experiments and data evaluation, H.Z.; instruments of measurement and data evaluation, N.M.; supervision and writing, N.K. data curation, H.O. All authors have read and agreed to the published version of the manuscript.
Funding
The present work was partially supported by a Grant-in-Aid for Scientific Research from the Japanese Society for the Promotion of Science (Research Program (C), No. 21K12290).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to K. Iwafune of the Common Facility Infrastructure Center and to M. Teraguchi, T. Nomoto and T. Tanaka, T. of the Facility of Engineering at Niigata University for allowing us use the NMR, FT-IR and SEM-EDS equipment. The authors are also grateful to H. Morohashi of the Industrial Research Institute of Niigata Prefecture for the XPS measurements and useful advice.
Conflicts of Interest
The authors declare no conflict of interest.
References
- The Countermeasure of Pollution and Improvement of Water Quality. Apiste Co., Ltd. n.d. Web. Available online: https://www.apiste.co.jp/column/detail/id=4548 (accessed on 31 January 2022).
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Takeuchi, F. Adsorption Chemistry—Key Technology of Surface and Interface Inhibition; Sangyo Tosho Publishing Co., Ltd.: Tokyo, Japan, 1995. [Google Scholar]
- Zhang, H. Development Status and Trend Analysis of Global Surfactant Industry. Hua Jing Res. 2021. Web. Available online: https://m.huaon.com/detail/761468.html (accessed on 31 January 2022).
- Michael, L. River-water biodegradation of surfactants in liquid detergents and shampoos. Water Res. 1991, 25, 1425–1429. [Google Scholar]
- Huang, S.Z.; Chen, G.G.; Wang, D.R.; Zhao, J.; Cui, S.Z. Study on the effects of synthetic detergent (LAS) on crops. Agric. Environ. Prot. 1994, 13, 58–62. [Google Scholar]
- Wakabayashi, A.; Kiikuchi, M.; Lnoue, W. Bioaccumulation profile of sodium linear alkylbenzene sulfonate and sodium alkyl sulfonate in carp. Chemosphere 1978, 7, 917–924. [Google Scholar] [CrossRef]
- Wang, B.H.; Zhang, X.J.; Ji, W.; Kuang, L.; Han, H.J. Environmental hazard analysis of surfactant. Chem. Prog. 2007, 26, 917–924. [Google Scholar]
- Chen, L.; Zhou, X.Q.; Wu, J.D. Hazards and treatment of surfactant wastewater. Ind. Water Treat. 2003, 23, 12–16. [Google Scholar]
- Yang, W.B.; Li, A.M.; Cai, J.G.; Meng, G.H.; Zhang, Q.X. Adsorption of surfactant by acrylic resin with different pore distribution. Sci. China Ser. B-Chem. 2006, 36, 249–255. [Google Scholar]
- Pravin, S.B.; Parag, R.G. Adsorptive removal of sodium dodecyl sulfate using activated coconut shell based adsorbent: Kinetic and thermodynamic study. Desalination Water Treat. 2019, 165, 111–123. [Google Scholar] [CrossRef]
- Mozhgan, S.; Abdolhossein, H.S.; Maen, M.H.; Mahin, S.; Mohammad, R. ZIF-8/carbon fiber for continuous adsorption of sodium dodecyl sulfate (SDS) from aqueous solutions: Kinetics and equilibrium studies. Water Process Eng. 2021, 44, 102437. [Google Scholar]
- Farhad, A.; Rahman, M.; Salman, A.; Atefeh, S.; Mashallah, R.; Hossein, M.; Tejraj, M.A.; Mohammad, A. Superior chemical stability of UiO-66 metal-organic frameworks (MOFs) for selective dye adsorption. Chem. Eng. 2020, 399, 125346. [Google Scholar]
- Wu, S.B.; Ge, Y.J.; Wang, Y.Q.; Chen, X.; Li, F.F.; Xuan, H.; Li, X. Adsorption of Cr(VI) on nano Uio-66-NH2 MOFs in water. Environ. Technol. 2018, 39, 1937–1948. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.X.; Huang, H.L.; Vardhan, H.; Aguila, B.; Zhong, C.L.; Perman, J.A.; Abdullah, M.A.; Ayman, N.; Ma, S.Q. Facile Approach to Graft Ionic Liquid into MOF for Improving the Efficiency of CO2 Chemical Fixation. ACS Appl. 2018, 10, 27124–27130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, L.; Xiao, J.; Tao, M.L.; Zhang, W.Q. Removal of anionic azo dyes from aqueous solutions by quaternary ammonium salt-functionalized fibers with adjustable surface microenvironments. React. Funct. Polym. 2020, 154, 104684. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Elaraby, N.M.; Abdel-Aty, A.M.; Shaban, E.; Abu-Saied, M.A.; Kenawy, E.; El-Naggar, M.E. Improvement of enzymatic properties and decolorization of azo dye: Immobilization of horseradish peroxidase on cationic maize starch. Biocatal. Agric. Biotechnol. 2021, 38, 102208. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Radwan, E.K.; El-Wakeel, S.T.; Kafafy, H.; Gad-Allah, T.A.; El-Kalliny, A.S.; Shaheen, T.I. Synthesis, characterization and adsorption properties of microcrystalline cellulose based nanogel for dyes and heavy metals removal. Biol. Macromol. 2018, 113, 248–258. [Google Scholar] [CrossRef]
- Radwan, E.K.; Kafafy, H.; El-Wakeel, S.T.; Shaheen, T.I.; Gad-Allah, T.A.; El-Kalliny, A.S.; El-Naggar, M.E. Remediation of Cd(II) and reactive red 195 dye in wastewater by nanosized gels of grafted carboxymethyl cellulose. Cellulose 2018, 25, 6645–6660. [Google Scholar] [CrossRef]
- Global Timber Production is at Its Highest Level in 70 Years. UN News. 2019. Available online: https://news.un.org/zh/story/2019/12/1047731 (accessed on 31 January 2022).
- Guo, C.Y. Overview of the World Paper Industry in 2019. China Pulp Pap. 2021. [Google Scholar]
- Bao, J.L.; Guo, Y.Z.; Mao, W.Y.; Zhao, X.; Sun, J.; Zhao, Q.Q.; Shen, Y.F. Research on the synthesis and application of 3-chloro-2-hydroxypropyltrialkylammonium chloride as a quaternary ammonium cation etherifying agents. Zhejiang Sci.-Tech. Univ. 2018, 39, 1673–3851. [Google Scholar]
- Zhou, C.; Xu, J.X. Regionally selective nucleophilic ring-opening reactions of asymmetric ethylene oxide. Prog. Chem. 2011, 23, 165–180. [Google Scholar]
- Li, B.M.; Zhao, Y.Q. Study on cationic modification of cotton fiber. Dye. Finish. Technol. 2011, 33, 18–31. [Google Scholar]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Sarin, V.; Pant, K.K. Removal of chromium from industrial waste by using eucalyptus bark. Bioresour. Technol. 2006, 97, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Duranoğlu, D.; Trochimczuk, A.W.; Beker, U. Kinetics and thermodynamics of hexavalent chromium adsorption onto activated carbon derived from acrylonitrile–divinylbenzene copolymer. Chem. Eng. J. 2012, 187, 193–202. [Google Scholar] [CrossRef]
- Yao, S.Q.; Shi, W.J.; Chen, X.Y.; Yu, Y.; Yang, Q.L. IR Spectra of Quarternary Ammonium Cellulose and Its Adsorption Properties to POPs. Spectrosc. Spectr. Anal. 2009, 29, 2370–2374. [Google Scholar]
- Liu, Y.L.; Kim, H.J. Fourier Transform Infrared Spectroscopy (FT-IR) and Simple Algorithm Analysis for Rapid and Non-Destructive Assessment of Developmental Cotton Fibers. Sensors 2017, 17, 1469. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, H.J.; Liu, P.P.; Fang, W.; Geng, J.J. A novel modified graphene oxide/chitosan composite used as an adsorbent for Cr (VI) in aqueous solutions. Int. J. Biol. Macromol. 2016, 87, 586–596. [Google Scholar] [CrossRef]
- Ayawei, N.; Abasi, C.Y.; Wankasi, D.; Dikio, E.D. Layered Double Hydroxide Adsorption of Lead: Equilibrium, Thermodynamic and Kinetic Studies. Int. J. Adv. Res. Chem. Sci. 2015, 2, 22–32. [Google Scholar]
- Ayawei, N.; Ekubo, A.T.; Wankasi, D.; Dikio, E.D. Synthesis and Application of Layered Double Hydroxide for the removal of Copper in Wastewater. Int. J. Chem. 2015, 7, 122–132. [Google Scholar]
- Mahmoodi, N.M.; Hayati, B.; Bahrami, H.; Arami, M. Dye adsorption and desorption properties of Mentha pulegium in single and binary systems. J. Appl. Polym. Sci. 2011, 122, 1489–1499. [Google Scholar] [CrossRef]
- Rahmati, M.M.; Rabbani, P.; Abdolali, A.; Keshtkar, A.R. Kinetics and equilibrium studies on biosorption of cadmium, lead, and nickel ions from aqueous solutions by intact and chemically modified brown algae. J. Hazard. Mater. 2011, 185, 401–407. [Google Scholar] [CrossRef]
- Zuo, P. Adsorption Behavior of Anionic Surfactant by Ion Exchange Resins; Southeast University: Nanjing, China, 2016. [Google Scholar]
- Li, P.X.; Ishiguro, M. Adsorption of anionic surfactant (sodium dodecyl sulfate) on silica. Soil Sci. Plant Nutr. 2016, 62, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Chai, L.Q.; Shao, J.Z.; Zhou, L.; Xu, M. Study on the Cationic Modification on Cotton and Its Application in the Dyeing with Natural Dyes. Zhejiang Sci.-Tech. Univ. 2010, 27, 1673–3851. [Google Scholar]
- Zeta Potential of Fiber Surface. Otsuka Co., Ltd. n.d. Web. Available online: https://www.otsukael.jp/weblearn/chapter/learnid/71/page/1 (accessed on 15 March 2022).
- Pal, A.; Pan, S.; Saha, S. Synergistically improved adsorption of anionic surfactant and crystal violet on chitosan hydrogel beads. Chem. Eng. 2013, 217, 426–434. [Google Scholar] [CrossRef]
- Gupta, S.; Pal, A.; Ghosh, P.K.; Bandyopadhyay, M. Performance of waste activated carbon as a low-cost adsorbent for the removal of anionic surfactant from aquatic environment. J. Environ. Sci. Health Part A 2003, 38, 81–397. [Google Scholar] [CrossRef]
- Purakayastha, P.D.; Pal, A.; Bandyopadhyay, M. Adsorbent selection for anionic surfactant removal from water, Indian. Chem. Technol. 2005, 12, 281–284. [Google Scholar]
- Sen, T.K.; Thi, M.T.; Afroze, S.; Phan, C.; Ang, M. Removal of anionic surfactant sodium dodecyl sulphate from aqueous solution by adsorption onto pine cone biomass of Pinus Radiate: Equilibrium, thermodynamic, kinetics, mechanism and process design Removal of anionics. Desalination Water Treat. 2012, 45, 263–275. [Google Scholar] [CrossRef]
- Mohd, T.A.T.; Jaafar, M.Z. Adsorption of Anionic Sodium Dodecyl Sulfate Surfactant on Local Sand and Kaolinite Surfaces: The Prospect of Alkaline and Salinity. Recent Technol. Eng. 2019, 7, 2277–3878. [Google Scholar]
Figure 1.
The reaction process for synthesizing the quaternary ammonium salt.
Figure 1.
The reaction process for synthesizing the quaternary ammonium salt.
Figure 2.
The reaction process of modifying the cellulose.
Figure 2.
The reaction process of modifying the cellulose.
Figure 3.
NMR spectra of (3-chloro-2-hydroxypropyl) chloride.
Figure 3.
NMR spectra of (3-chloro-2-hydroxypropyl) chloride.
Figure 4.
FT-IR spectra of cellulose and modified cellulose.
Figure 4.
FT-IR spectra of cellulose and modified cellulose.
Figure 5.
SEM image of (a) the pure cellulose (×2000), (b) the modified cellulose (×2000) [(b*) (×220)], (c) the adsorbed cellulose (×2000).
Figure 5.
SEM image of (a) the pure cellulose (×2000), (b) the modified cellulose (×2000) [(b*) (×220)], (c) the adsorbed cellulose (×2000).
Figure 6.
Element mapping images of modified cellulose [(a): C, (b): N, (c): O].
Figure 6.
Element mapping images of modified cellulose [(a): C, (b): N, (c): O].
Figure 7.
XPS image of cellulose.
Figure 7.
XPS image of cellulose.
Figure 8.
Effect of pH on SDS adsorption.
Figure 8.
Effect of pH on SDS adsorption.
Figure 9.
Effect of temperature on SDS adsorption.
Figure 9.
Effect of temperature on SDS adsorption.
Figure 10.
Effect of contact time on SDS adsorption.
Figure 10.
Effect of contact time on SDS adsorption.
Figure 11.
Pseudo-first-order plot of SDS adsorption by modified cellulose.
Figure 11.
Pseudo-first-order plot of SDS adsorption by modified cellulose.
Figure 12.
Pseudo-second-order plot of SDS adsorption by modified cellulose.
Figure 12.
Pseudo-second-order plot of SDS adsorption by modified cellulose.
Figure 13.
The Langmuir isotherm for the adsorption of SDS by modified cellulose.
Figure 13.
The Langmuir isotherm for the adsorption of SDS by modified cellulose.
Figure 14.
The Freundlich isotherm for the adsorption of SDS by modified cellulose.
Figure 14.
The Freundlich isotherm for the adsorption of SDS by modified cellulose.
Figure 15.
Plot of and 1/T for estimation of the thermodynamic parameters obtained for the adsorption of SDS by modified cellulose.
Figure 15.
Plot of and 1/T for estimation of the thermodynamic parameters obtained for the adsorption of SDS by modified cellulose.
Figure 16.
Zeta potential of modified cellulose (in 0.01 mol/L NaCl) at different pH.
Figure 16.
Zeta potential of modified cellulose (in 0.01 mol/L NaCl) at different pH.
Table 1.
The proportional of element in the cellulose.
Table 1.
The proportional of element in the cellulose.
| Cellulose | C% | O% | N% | S% |
|---|
| Modified | 63.9 | 33.8 | 0.93 | - |
| Adsorbed | 66.1 | 32.2 | 0.62 | 1.04 |
Table 2.
Isotherm parameters of SDS adsorption by the modified cellulose.
Table 2.
Isotherm parameters of SDS adsorption by the modified cellulose.
| | | Pseudo-First-Order Model | Pseudo-Second-Order Model |
|---|
| Target | qeep (mg/g) | qe (mg/g) | k1 (min−1) | R2 | qe (mg/g) | k2 (g/mg min−1) | R2 |
| SDS | 32.5 | 19.5 | 0.0263 | 0.995 | 33.6 | 0.00378 | 0.996 |
Table 3.
Isotherm parameters for SDS adsorption by modified cellulose.
Table 3.
Isotherm parameters for SDS adsorption by modified cellulose.
| Target | T (°C) | Langmuir Isotherm | Freundlich Isotherm |
|---|
| | | qmax (mg/g) | RL | KL | R2 | KF (mg/g) | 1/n | R2 |
| SDS | 60 | 22.8 | 0.0619 | 0.354 | 0.979 | 4.89 | 0.0285 | 0.690 |
Table 4.
Thermodynamic parameters for the adsorption of SDS by modified cellulose.
Table 4.
Thermodynamic parameters for the adsorption of SDS by modified cellulose.
| T (K) | ΔH (kJ/mol) | ΔS (J/mol) | ΔG (kJ/mol) |
|---|
| 293 | 15.29 | 93.59 | −12.14 |
| 313 | - | - | −14.01 |
| 333 | - | - | −15.88 |
| 343 | - | - | −16.82 |
Table 5.
Comparison of the adsorption capacity of different adsorbents.
Table 5.
Comparison of the adsorption capacity of different adsorbents.
| Adsorbent | qmax (mg·g−1) | Reference |
|---|
| Activated coconut shell | 111 | [11] |
| Silica | 0.202 | [36] |
| Chitosan hydrogel beads | 76.9 | [39] |
| Aquaguard waste activated carbon | 61.5 | [40] |
| Granular activated charcoal | 3.75 | [41] |
| Pine cone bio mass | 95.8 | [42] |
| Kaolinite | 1.50 | [43] |
| Modified cellulose | 32.5 | This study |
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).