Improving Properties of an Experimental Universal Adhesive by Adding a Multifunctional Dendrimer (G-IEMA): Bond Strength and Nanoleakage Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Formulation of the Experimental Adhesives
2.2. Microtensile Bond Strength (µTBS) to Dentin and Failure Mode Classification
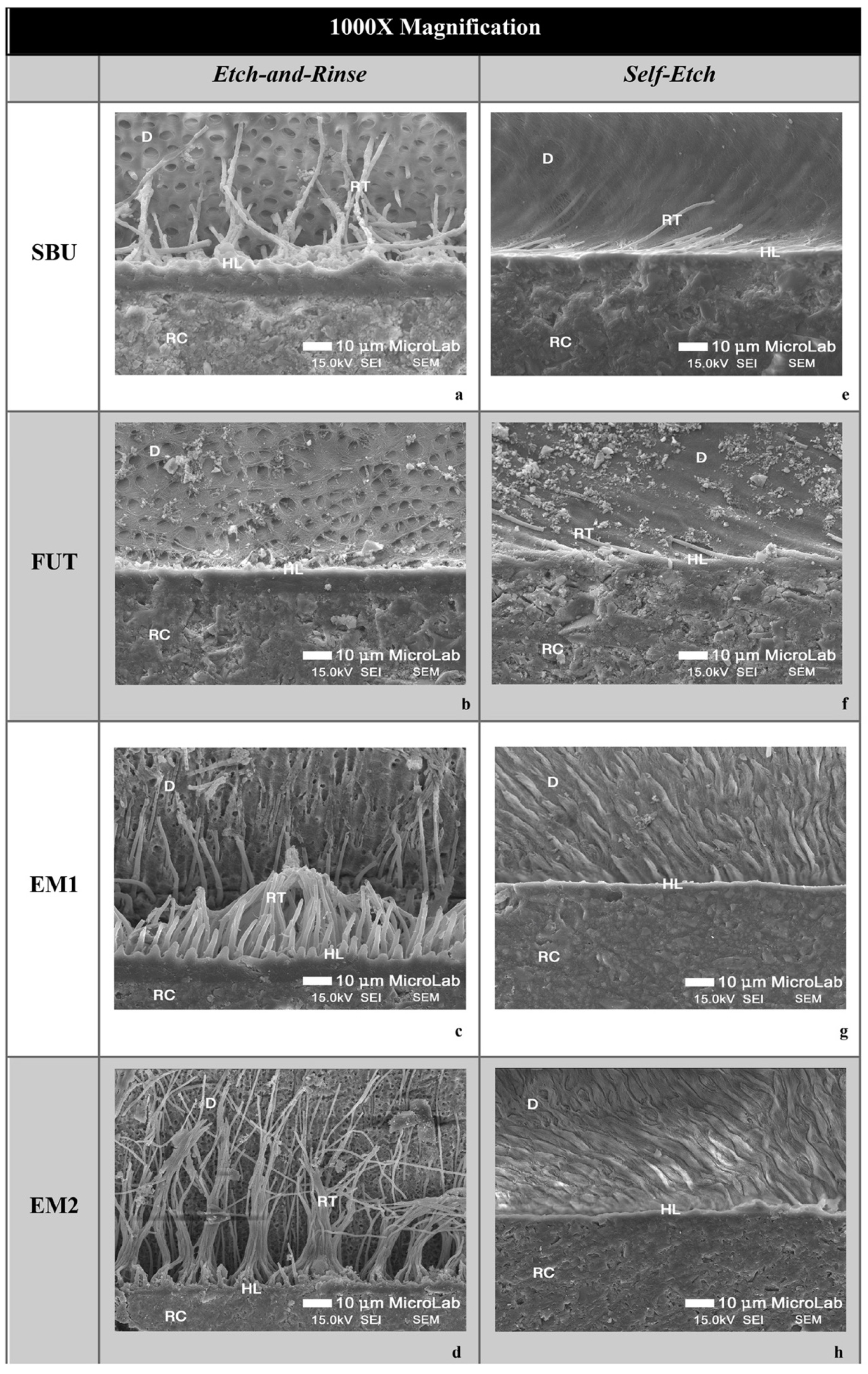
2.3. SEM Analysis of Bonded Interface
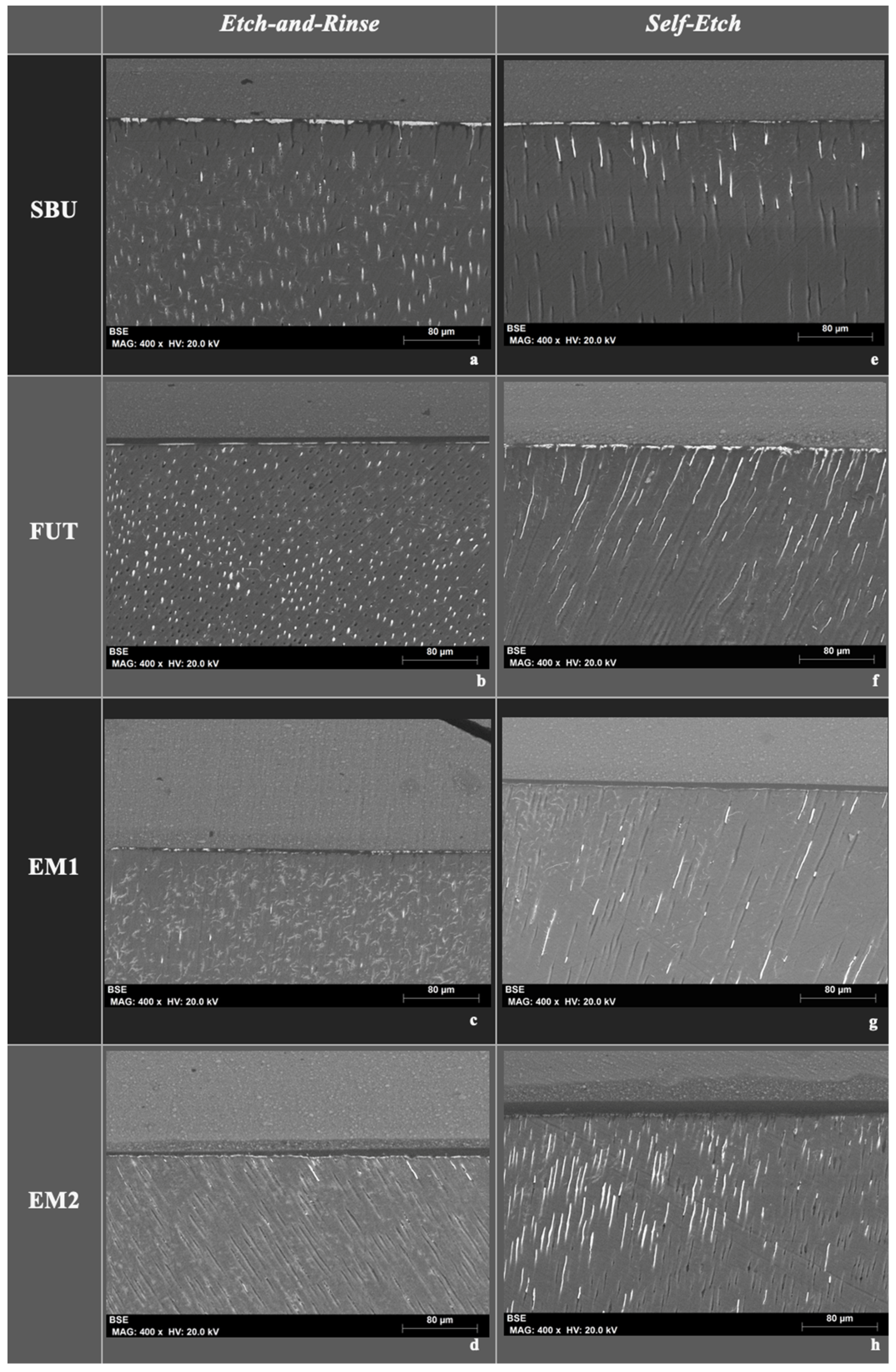
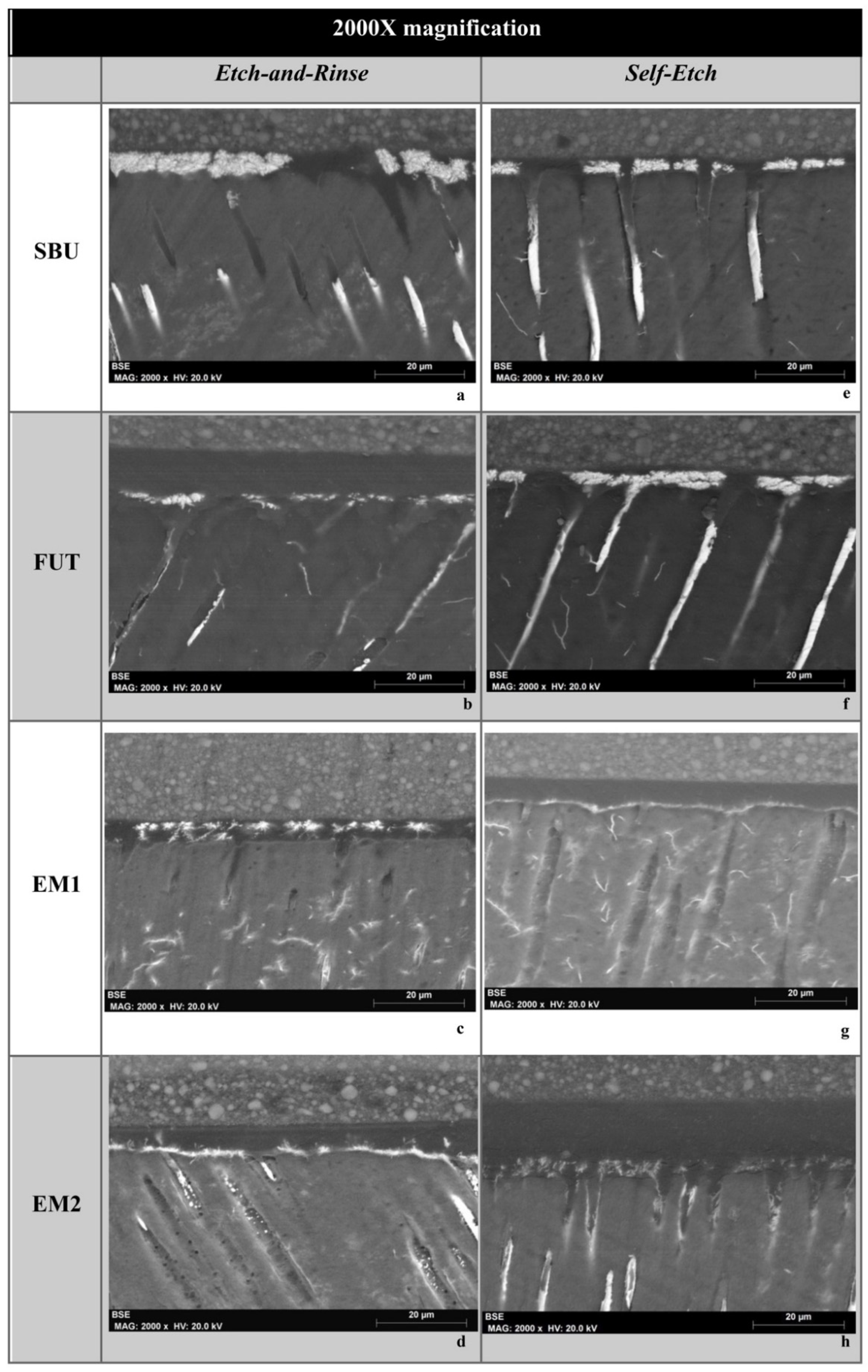
2.4. Nanoleakage
2.5. Statistical Analysis
3. Results
3.1. Microtensile Bond Strength (µTBS) to Dentin and Failure Modes
3.2. SEM Imaging of the Adhesive Interface
3.3. Nanoleakage
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marshall, S.J.; Bayne, S.C.; Baier, R.; Tomsia, A.P.; Marshall, G.W. A review of adhesion science. Dent. Mater. 2010, 26, e11–e16. [Google Scholar] [CrossRef] [PubMed]
- Bertassoni, L.E.; Orgel, J.P.R.; Antipova, O.; Swain, M. V The dentin organic matrix - limitations of restorative dentistry hidden on the nanometer scale. Acta Biomater. 2012, 8, 2419–2433. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; Yoshihara, K.; Van Landuyt, K.; Yoshida, Y.; Peumans, M. From Buonocore’s Pioneering Acid-Etch Technique to Self-Adhering Restoratives. A Status Perspective of Rapidly Advancing Dental Adhesive Technology. J. Adhes. Dent. 2020, 22, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.F.; Alegría-Acevedo, L.F.; Méndez-Bauer, L.; Bermudez, J.; Dávila-Sánchez, A.; Buvinic, S.; Hernández-Moya, N.; Reis, A.; Loguercio, A.D.; Farago, P.V.; et al. Biological, mechanical and adhesive properties of universal adhesives containing zinc and copper nanoparticles. J. Dent. 2019, 82, 45–55. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Mohrbacher, H.; Celis, J.P.; Roos, J.R.; Braem, M.; Lambrechts, P.; Vanherle, G. Chemical Characterization of the Resin-Dentin Interface by Micro-Raman Spectroscopy. J. Dent. Res. 1993, 72, 1423–1428. [Google Scholar] [CrossRef]
- Spencer, P.; Ye, Q.; Park, J.; Topp, E.M.; Misra, A.; Marangos, O.; Wang, Y.; Bohaty, B.S.; Singh, V.; Sene, F.; et al. Adhesive/Dentin interface: the weak link in the composite restoration. Ann. Biomed. Eng. 2010, 38, 1989–2003. [Google Scholar] [CrossRef]
- Spencer, P.; Ye, Q.; Song, L.; Parthasarathy, R.; Boone, K.; Misra, A.; Tamerler, C. Threats to adhesive/dentin interfacial integrity and next generation bio-enabled multifunctional adhesives. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2673–2683. [Google Scholar] [CrossRef]
- Pioch, T.; Staehle, H.J.; Duschner, H.; García-Godoy, F. Nanoleakage at the composite-dentin interface: a review. Am. J. Dent. 2001, 14, 252–258. [Google Scholar]
- Perdigão, J. Dentin bonding-Variables related to the clinical situation and the substrate treatment. Dent. Mater. 2010, 26, e24–e37. [Google Scholar] [CrossRef]
- Wagner, A.; Wendler, M.; Petschelt, A.; Belli, R.; Lohbauer, U. Bonding performance of universal adhesives in different etching modes. J. Dent. 2014, 42, 800–807. [Google Scholar] [CrossRef]
- Liu, Y.; Tjäderhane, L.; Breschi, L.; Mazzoni, A.; Li, N.; Mao, J.; Pashley, D.H.; Tay, F.R. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J. Dent. Res. 2011, 90, 953–968. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, D.E.; Baldion, P.A.; Castellanos, J.E. Resin-dentin bonding interface: Mechanisms of degradation and strategies for stabilization of the hybrid layer. Int. J. Biomater. 2019, 2019, 5268342. [Google Scholar] [CrossRef] [PubMed]
- Sano, H.; Shono, T.; Takatsu, T.; Hosoda, H. Microporous dentin zone beneath resin-impregnated layer. Oper. Dent. 1994, 19, 59–64. [Google Scholar] [PubMed]
- Zhou, W.; Liu, S.; Zhou, X.; Hannig, M.; Rupf, S.; Feng, J.; Peng, X.; Cheng, L. Modifying adhesive materials to improve the longevity of resinous restorations. Int. J. Mol. Sci. 2019, 20, 723. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Liu, F.; He, J. Preparation of low shrinkage methacrylate-based resin system without Bisphenol A structure by using a synthesized dendritic macromer (G-IEMA). J. Mech. Behav. Biomed. Mater. 2014, 35, 1–8. [Google Scholar] [CrossRef]
- Vasconcelos e Cruz, J.; Brito, J.; Polido, M.; Gonçalves, L.L. A new experimental adhesive system containing G-IEMA – physicochemical properties. J. Adhes. Sci. Technol. 2018, 33, 418–432. [Google Scholar] [CrossRef]
- Cruz, J.V.e.; Polido, M.; Brito, J.; Gonçalves, L.L. Dentin Bonding and SEM Analysis of a New Experimental Universal Adhesive System Containing a Dendrimer. Polymers 2020, 12, 461. [Google Scholar] [CrossRef]
- Armstrong, S.; Breschi, L.; Özcan, M.; Pfefferkorn, F.; Ferrari, M.; Van Meerbeek, B. Academy of Dental Materials guidance on in vitro testing of dental composite bonding effectiveness to dentin/enamel using micro-tensile bond strength (μTBS) approach. Dent. Mater. 2017, 33, 133–143. [Google Scholar] [CrossRef]
- Perdigao, J.; Lambrechts, P.; Van Meerbeek, B.; Vanherle, G.; Lopes, A.L.B. Field emission SEM comparison of four postfixation drying techniques for human dentin. J. Biomed. Mater. Res. 1995, 29, 1111–1120. [Google Scholar] [CrossRef]
- Schneider, H.; Fröhlich, M.; Erler, G.; Engelke, C.; Merte, K. Interaction patterns between dentin and adhesive on prepared class V cavities in vitro and in vivo. J. Biomed. Mater. Res. 2000, 53, 86–92. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H.; Yoshiyama, M. Two modes of nanoleakage expression in single-step adhesives. J. Dent. Res. 2002, 81, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Hariri, I.; Shimada, Y.; Sadr, A.; Ichinose, S.; Tagami, J. The effects of aging on shear bond strength and nanoleakage expression of an etch-and-rinse adhesive on human enamel and dentin. J. Adhes. Dent. 2012, 14, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Hanabusa, M.; Mine, A.; Kuboki, T.; Momoi, Y.; Van Ende, A.; Van Meerbeek, B.; De Munck, J. Bonding effectiveness of a new “multi-mode” adhesive to enamel and dentine. J. Dent. 2012, 40, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Perdigão, J.; Swift, E.J. Universal Adhesives. J. Esthet. Restor. Dent. 2015, 27, 331–334. [Google Scholar] [CrossRef]
- Kaczor, K.; Gerula-Szymańska, A.; Smektała, T.; Safranow, K.; Lewusz, K.; Nowicka, A. Effects of different etching modes on the nanoleakage of universal adhesives: A systematic review and meta-analysis. J. Esthet. Restor. Dent. 2018, 30, 287–298. [Google Scholar] [CrossRef]
- Cuevas-Suárez, C.E.; da Rosa, W.L.; de, O.; Lund, R.G.; da Silva, A.F.; Piva, E. Bonding performance of universal adhesives: An updated systematic review and meta-analysis. J. Adhes. Dent. 2019, 21, 7–26. [Google Scholar] [CrossRef]
- Isolan, C.P.; Valente, L.L.; Münchow, E.A.; Basso, G.R.; Pimentel, A.H.; Schwantz, J.K.; da Silva, A.V.; Moraes, R.R. Bond strength of a universal bonding agent and other contemporary dental adhesives applied on enamel, dentin, composite, and porcelain. Appl. Adhes. Sci. 2014, 2, 25. [Google Scholar] [CrossRef]
- Leite, M.L.; de, A.e.S.; Costa, C.A.d.S.; Duarte, R.M.; de Andrade, A.K.M.; Soares, D.G. Bond Strength and Cytotoxicity of a Universal Adhesive According to the Hybridization Strategies to Dentin. Braz. Dent. J. 2018, 29, 68–75. [Google Scholar] [CrossRef]
- Elkaffas, A.A.; Hamama, H.H.H.; Mahmoud, S.H. Do universal adhesives promote bonding to dentin? A systematic review and meta-analysis. Restor. Dent. Endod. 2018, 43, e29. [Google Scholar] [CrossRef]
- Da Rosa, W.L.D.O.; Piva, E.; Da Silva, A.F. Bond strength of universal adhesives: A systematic review and meta-analysis. J. Dent. 2015, 43, 765–776. [Google Scholar] [CrossRef]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the art etch-and-rinse adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sofan, E.; Sofan, A.; Palaia, G.; Tenore, G.; Romeo, U.; Migliau, G. Classification review of dental adhesive systems: from the IV generation to the universal type. Ann. Stomatol. (Roma). 2017, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Perdigão, J.; Ceballos, L.; Giráldez, I.; Baracco, B.; Fuentes, M.V. Effect of a hydrophobic bonding resin on the 36-month performance of a universal adhesive—a randomized clinical trial. Clin. Oral Investig. 2020, 24, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Niu, L.N.; Xie, H.; Zhang, Z.Y.; Zhou, L.Q.; Jiao, K.; Chen, J.H.; Pashley, D.H.; Tay, F.R. Bonding of universal adhesives to dentine--Old wine in new bottles? J. Dent. 2015, 43, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Lohbauer, U.; Nikolaenko, S.A.; Petschelt, A.; Frankenberger, R. Resin tags do not contribute to dentin adhesion in self-etching adhesives. J. Adhes. Dent. 2008, 10, 97–103. [Google Scholar]
- Bedran-Russo, A.; Leme-Kraus, A.A.; Vidal, C.M.P.; Teixeira, E.C. An Overview of Dental Adhesive Systems and the Dynamic Tooth-Adhesive Interface. Dent. Clin. North Am. 2017, 61, 713–731. [Google Scholar] [CrossRef]
- Braga, R.R.; Fronza, B.M. The use of bioactive particles and biomimetic analogues for increasing the longevity of resin-dentin interfaces: A literature review. Dent. Mater. J. 2020, 39, 62–68. [Google Scholar] [CrossRef]
- Chimeli, T.B.C.; D’Alpino, P.H.P.; Pereira, P.N.; Hilgert, L.A.; Di Hipólito, V.; Garcia, F.C.P. Effects of solvent evaporation on water sorption/solubility and nanoleakage of adhesive systems. J. Appl. Oral Sci. 2014, 22, 294–301. [Google Scholar] [CrossRef][Green Version]
- Van Landuyt, K.L.; De Munck, J.; Snauwaert, J.; Coutinho, E.; Poitevin, A.; Yoshida, Y.; Inoue, S.; Peumans, M.; Suzuki, K.; Lambrechts, P.; et al. Monomer-solvent phase separation in one-step self-etch adhesives. J. Dent. Res. 2005, 84, 183–188. [Google Scholar] [CrossRef]
- Cadenaro, M.; Maravic, T.; Comba, A.; Mazzoni, A.; Fanfoni, L.; Hilton, T.; Ferracane, J.; Breschi, L. The role of polymerization in adhesive dentistry. Dent. Mater. 2019, 35, e1–e22. [Google Scholar] [CrossRef]
- Toledano, M.; Osorio, R. New Advanced Materials for High Performance at the Resin-Dentine Interface. Front. Oral Biol. 2015, 17, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Montagner, A.F.; Sarkis-Onofre, R.; Pereira-Cenci, T.; Cenci, M.S. MMP inhibitors on dentin stability: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Breschi, L.; Mazzoni, A.; Ruggeri, A.; Cadenaro, M.; Di Lenarda, R.; De Stefano Dorigo, E. Dental adhesion review: Aging and stability of the bonded interface. Dent. Mater. 2008, 24, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.C.; Kern, M. The role of host-derived dentinal matrix metalloproteinases in reducing dentin bonding of resin adhesives. Int. J. Oral Sci. 2009, 1, 163–176. [Google Scholar] [CrossRef]
- Yoshida, Y.; Nagakane, K.; Fukuda, R.; Nakayama, Y.; Okazaki, M.; Shintani, H.; Inoue, S.; Tagawa, Y.; Suzuki, K.; De Munck, J.; et al. Comparative study on adhesive performance of functional monomers. J. Dent. Res. 2004, 83, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Proença, J.P.; Polido, M.; Osorio, E.; Erhardt, M.C.G.; Aguilera, F.S.; García-Godoy, F.; Osorio, R.; Toledano, M. Dentin regional bond strength of self-etch and total-etch adhesive systems. Dent. Mater. 2007, 23, 1542–1548. [Google Scholar] [CrossRef]
- Marchesi, G.; Frassetto, A.; Mazzoni, A.; Apolonio, F.; Diolosà, M.; Cadenaro, M.; Di Lenarda, R.; Pashley, D.H.; Tay, F.; Breschi, L. Adhesive performance of a multi-mode adhesive system: 1-year in vitro study. J. Dent. 2014, 42, 603–612. [Google Scholar] [CrossRef]
- Luque-Martinez, I.V.; Perdigão, J.; M͡noz, M.A.; Sezinando, A.; Reis, A.; Loguercio, A.D. Effects of solvent evaporation time on immediate adhesive properties of universal adhesives to dentin. Dent. Mater. 2014, 30, 1126–1135. [Google Scholar] [CrossRef]
- Yoshida, Y.; Yoshihara, K.; Hayakawa, S.; Nagaoka, N.; Okihara, T.; Matsumoto, T.; Minagi, S.; Osaka, A.; Van Landuyt, K.; Van Meerbeek, B. HEMA inhibits interfacial nano-layering of the functional monomer MDP. J. Dent. Res. 2012, 91, 1060–1065. [Google Scholar] [CrossRef]
- Zanchi, C.H.; Münchow, E.A.; Ogliari, F.A.; Chersoni, S.; Prati, C.; Demarco, F.F.; Piva, E. Development of experimental HEMA-free three-step adhesive system. J. Dent. 2010, 38, 503–508. [Google Scholar] [CrossRef]
- Delgado, A.H.; Young, A.M. Modelling ATR-FTIR Spectra of Dental Bonding Systems to Investigate Composition and Polymerisation Kinetics. Materials 2021, 14, 760. [Google Scholar] [CrossRef] [PubMed]
- Cadenaro, M.; Breschi, L.; Rueggeberg, F.A.; Suchko, M.; Grodin, E.; Agee, K.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H. Effects of residual ethanol on the rate and degree of conversion of five experimental resins. Dent. Mater. 2009, 25, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; Pashley, D.H. Aggressiveness of contemporary self-etching systems: I: Depth of penetration beyond dentin smear layers. Dent. Mater. 2001, 17, 296–308. [Google Scholar] [CrossRef]
- Hardan, L.; Bourgi, R.; Kharouf, N.; Mancino, D.; Zarow, M.; Jakubowicz, N.; Haikel, Y.; Cuevas-Suárez, C.E. Bond strength of universal adhesives to dentin: A systematic review and meta-analysis. Polymers 2021, 13, 814. [Google Scholar] [CrossRef] [PubMed]


| A | Monomers (wt%) | ||||||
| Adhesive | pH | Bis-GMA | UDMA | HEMA | G-IEMA | TEGDMA | 10-MDP |
| SBU | 2.7 | 15–25 | - | 15–25 | - | - | N/A |
| FUT | 1.7 | 10–25 | 2.5–5 | 10–25 | - | - | N/A |
| EM1 | 2.0 | 10–15 | 5–10 | 10–25 | 0 | 5–10 | 5–10 |
| EM2 | 2.2 | - | 5–10 | 10–25 | 10–15 | 5–10 | 5–10 |
| B | Photoinitiator system and solvents (wt%) | ||||||
| Adhesive | pH | CQ | BDA | HPDPI | Ethanol | Water | |
| SBU | 2.7 | <2 | <2 | - | 15–25 | 10–15 | |
| FUT | 1.7 | <2.5 | 0 | - | 10–25 | - | |
| EM1 | 2.0 | <2 | <2 | 1.5–2 | 18–28 | 10–15 | |
| EM2 | 2.2 | <2 | <2 | 1.5–2 | 18.28 | 10–15 | |
| Group | Protocol | Application Mode |
|---|---|---|
| SBU FUT | ER |
|
| SE |
| |
| EM1 (with Bis-GMA) EM 2 (with G-IEMA) | ER |
|
| SE |
|
| AR1 | ARMA11 | CS | CSCORREL | ID | TOEPLITZ | |
|---|---|---|---|---|---|---|
| −2 Restricted Log Likelihood | 4814.08 | 4801.72 | 4801.71 | 4801.72 | 4814.15 | NC |
| Akaike’s Information Criterion (AIC) | 4818.08 | 4807.72 | 4805.72 | 4805.72 | 4816.15 | NC |
| Hurvich and Tsai’s Criterion (AICC) | 4818.09 | 4807.76 | 4805.74 | 4805.74 | 4816.16 | NC |
| Bozdogan’s Criterion (CAIC) | 4828.84 | 4823.86 | 4816.48 | 4816.48 | 4821.53 | NC |
| Schwarz’s Bayesian Criterion (BIC) | 4826.84 | 4820.86 | 4814.48 | 4814.48 | 4820.53 | NC |
| Source | Num. Df | Den. Df | F | Sig. |
|---|---|---|---|---|
| Intercept | 1 | 28.30 | 1049.62 | <0.001 |
| Adhesive | 3 | 28.05 | 2.17 | 0.114 |
| Strategy | 1 | 28.31 | 5.51 | 0.026 |
| Adhesive * Protocol | 3 | 28.05 | 1.43 | 0.255 |
| Adhesives | Strategy | µTBS (MPa) | |||
|---|---|---|---|---|---|
| Min | Max | Mean | S.D. | ||
| SBU | ER | 17.6 | 35.5 | 26.2 | 6.5 |
| FUT | 30.2 | 35.9 | 33.9 | 3.2 | |
| EM1 | 26.3 | 42.6 | 32.4 | 6.4 | |
| EM2 | 21.3 | 33.9 | 29.3 | 5.3 | |
| SBU | SE | 19.5 | 28.1 | 23.7 | 3.1 |
| FUT | 21.2 | 26.2 | 24.1 | 2.6 | |
| EM1 | 18.8 | 34.4 | 26.8 | 6.5 | |
| EM2 | 26.0 | 33.7 | 30.2 | 3.4 | |
| Failures (%) | Adhesive | Cohesive Dentin | Cohesive Composite | Mixed |
|---|---|---|---|---|
| SBU | 72.3 | 23.2 | 4.5 | 0 |
| FUT | 84.5 | 3.6 | 10.0 | 1.8 |
| EM1 | 72.5 | 10.4 | 13.0 | 4.1 |
| EM2 | 77.5 | 6.9 | 12.4 | 3.2 |
| Source | df | Mean Square | F | Significance |
|---|---|---|---|---|
| Intercept | 1 | 35463.04 | 788.36 | 0.000 |
| Strategy | 1 | 393.98 | 8.76 | 0.003 |
| Adhesive | 3 | 1113.31 | 24.75 | 0.000 |
| Strategy * Adhesive | 3 | 496.53 | 11.04 | 0.000 |
| Adhesive | Strategy | Mean | S.D. | 95% CI | |
|---|---|---|---|---|---|
| Min. | Max. | ||||
| SBU | ER | 15.6 | 1.2 | 13.3 | 17.9 |
| SE | 9.2 | 0.8 | 7.5 | 10.9 | |
| FUT | ER | 15.0 | 1.2 | 12.6 | 17.4 |
| SE | 9.5 | 0.9 | 7.7 | 11.2 | |
| EM1 | ER | 5.4 | 0.9 | 3.6 | 7.2 |
| SE | 5.5 | 0.8 | 3,9 | 7.0 | |
| EM2 | ER | 7.5 | 0.8 | 5.9 | 9.1 |
| SE | 11.1 | 1.2 | 8.9 | 13.4 | |
| Adhesive | Protocol | p * |
|---|---|---|
| FUT vs. SBU | ER | n.s. |
| FUT vs. SBU | SE | n.s. |
| FUT vs. EM1 | ER | <0.001 |
| FUT vs. EM1 | SE | 0.001 |
| FUT vs. EM2 | ER | <0.001 |
| FUT vs. EM2 | SE | n.s. |
| SBU vs. EM1 | ER | <0.001 |
| SBU vs. EM1 | SE | 0.002 |
| SBU vs. EM2 | ER | <0.001 |
| SBU vs. EM2 | SE | n.s. |
| EM1 vs. EM2 | ER | n.s. |
| EM1 vs. EM2 | SE | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasconcelos e Cruz, J.; Delgado, A.H.S.; Félix, S.; Brito, J.; Gonçalves, L.; Polido, M. Improving Properties of an Experimental Universal Adhesive by Adding a Multifunctional Dendrimer (G-IEMA): Bond Strength and Nanoleakage Evaluation. Polymers 2022, 14, 1462. https://doi.org/10.3390/polym14071462
Vasconcelos e Cruz J, Delgado AHS, Félix S, Brito J, Gonçalves L, Polido M. Improving Properties of an Experimental Universal Adhesive by Adding a Multifunctional Dendrimer (G-IEMA): Bond Strength and Nanoleakage Evaluation. Polymers. 2022; 14(7):1462. https://doi.org/10.3390/polym14071462
Chicago/Turabian StyleVasconcelos e Cruz, Joana, António H. S. Delgado, Samuel Félix, José Brito, Luísa Gonçalves, and Mário Polido. 2022. "Improving Properties of an Experimental Universal Adhesive by Adding a Multifunctional Dendrimer (G-IEMA): Bond Strength and Nanoleakage Evaluation" Polymers 14, no. 7: 1462. https://doi.org/10.3390/polym14071462
APA StyleVasconcelos e Cruz, J., Delgado, A. H. S., Félix, S., Brito, J., Gonçalves, L., & Polido, M. (2022). Improving Properties of an Experimental Universal Adhesive by Adding a Multifunctional Dendrimer (G-IEMA): Bond Strength and Nanoleakage Evaluation. Polymers, 14(7), 1462. https://doi.org/10.3390/polym14071462







