Preparation of Stable POSS-Based Superhydrophobic Textiles Using Thiol–Ene Click Chemistry
Abstract
:1. Introduction
2. Experimental
2.1. Materials
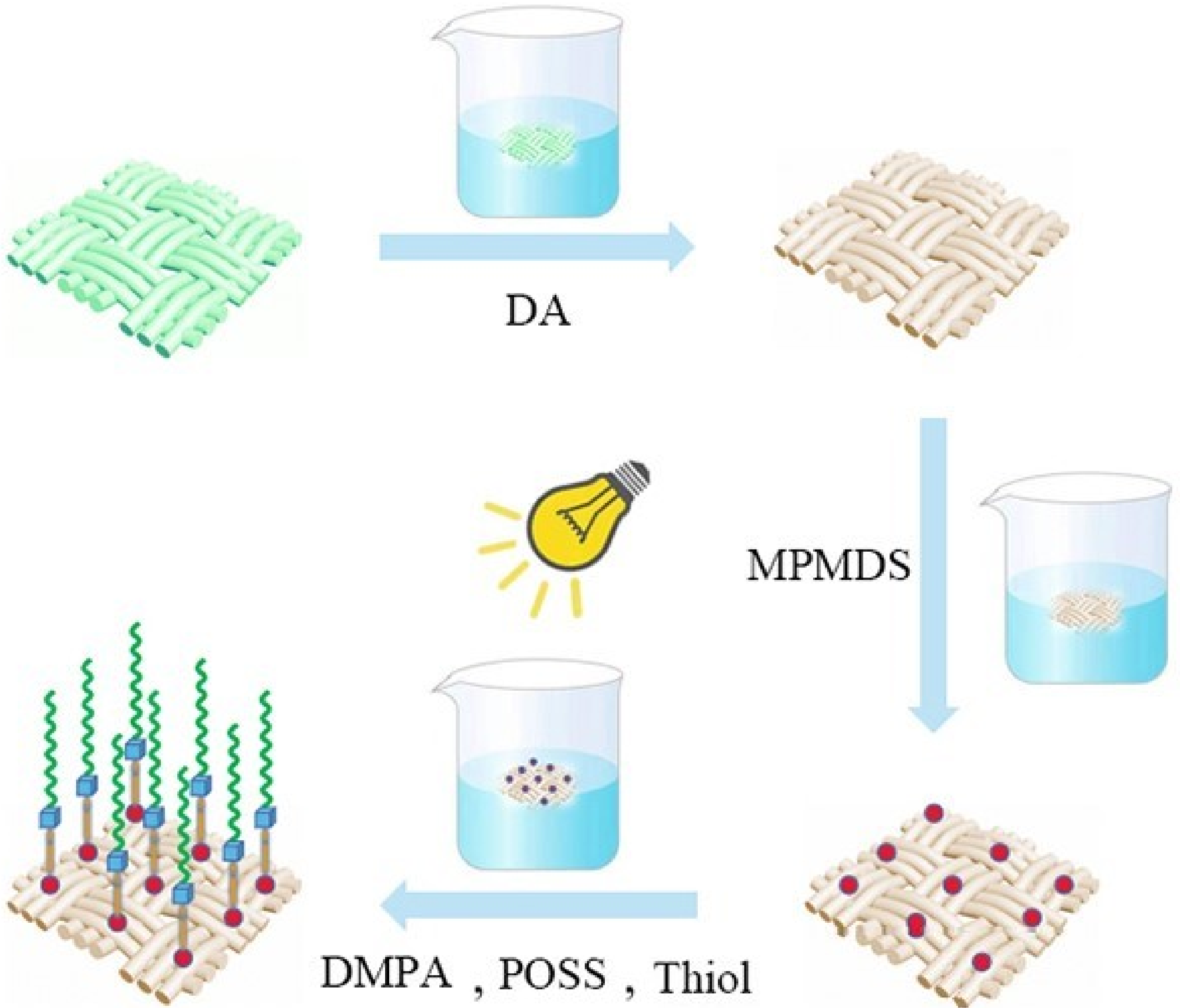
2.2. Preparation of Dopamine-Modified Fabric
2.3. Preparation of Mercapto-Modified Fabric
2.4. Preparation of Superhydrophobic Fabric
2.5. Characterization
2.5.1. Contact Angle and Rolling Angle
2.5.2. Surface Morphology and Elemental Analysis
2.5.3. X-ray Photoelectron Spectroscopy Test
2.5.4. Acid Resistance Test
2.5.5. Friction Fastness Test
2.5.6. Ultraviolet Fastness Test
2.5.7. Testing of Fastness to Organic Solvents
2.5.8. Soap Fastness Test
2.5.9. Self-Cleaning and Oil–Water Separation Test
3. Results and Discussion
3.1. Brief Description of the Formation Mechanism of Superhydrophobic Fabric
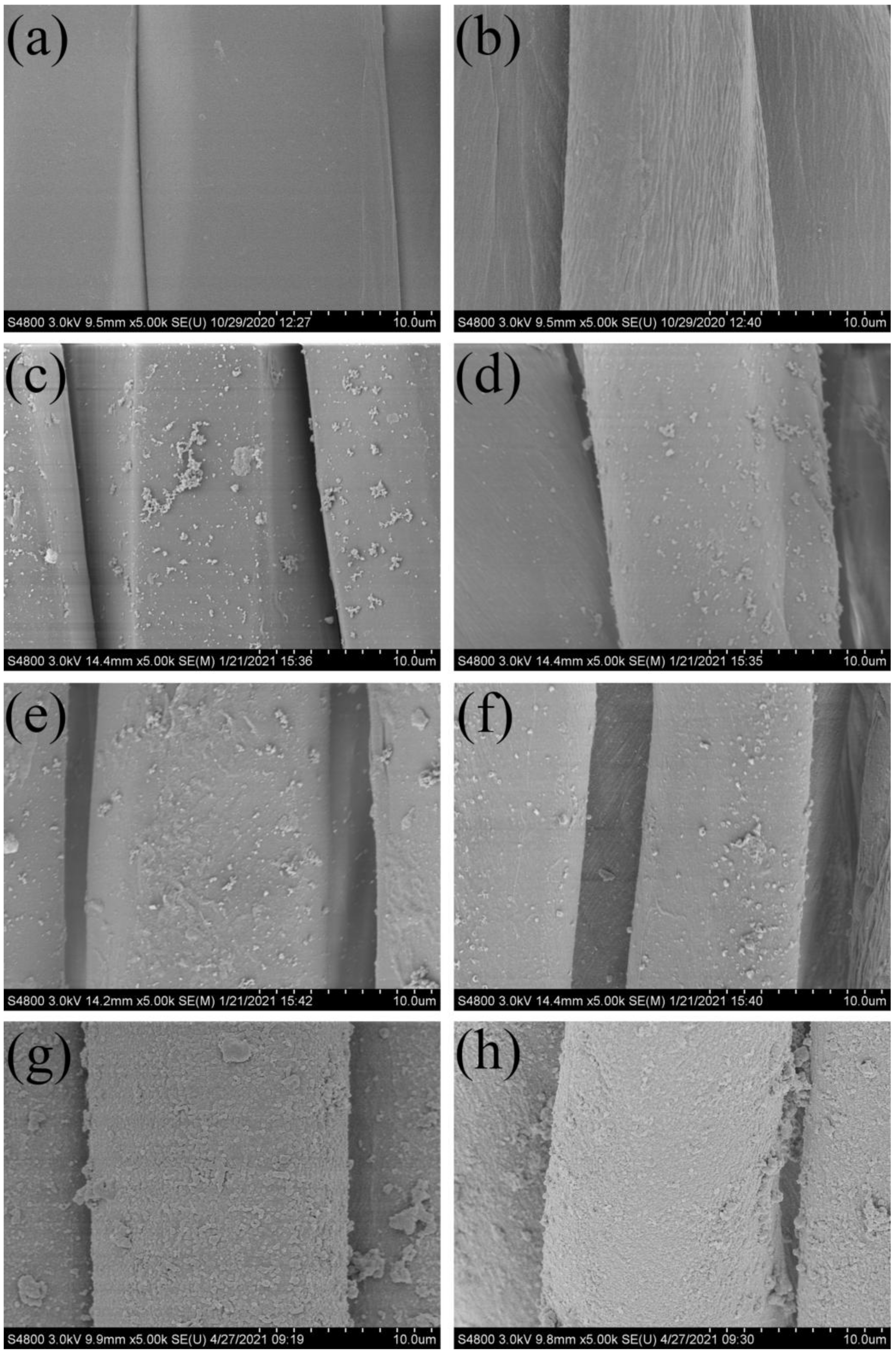
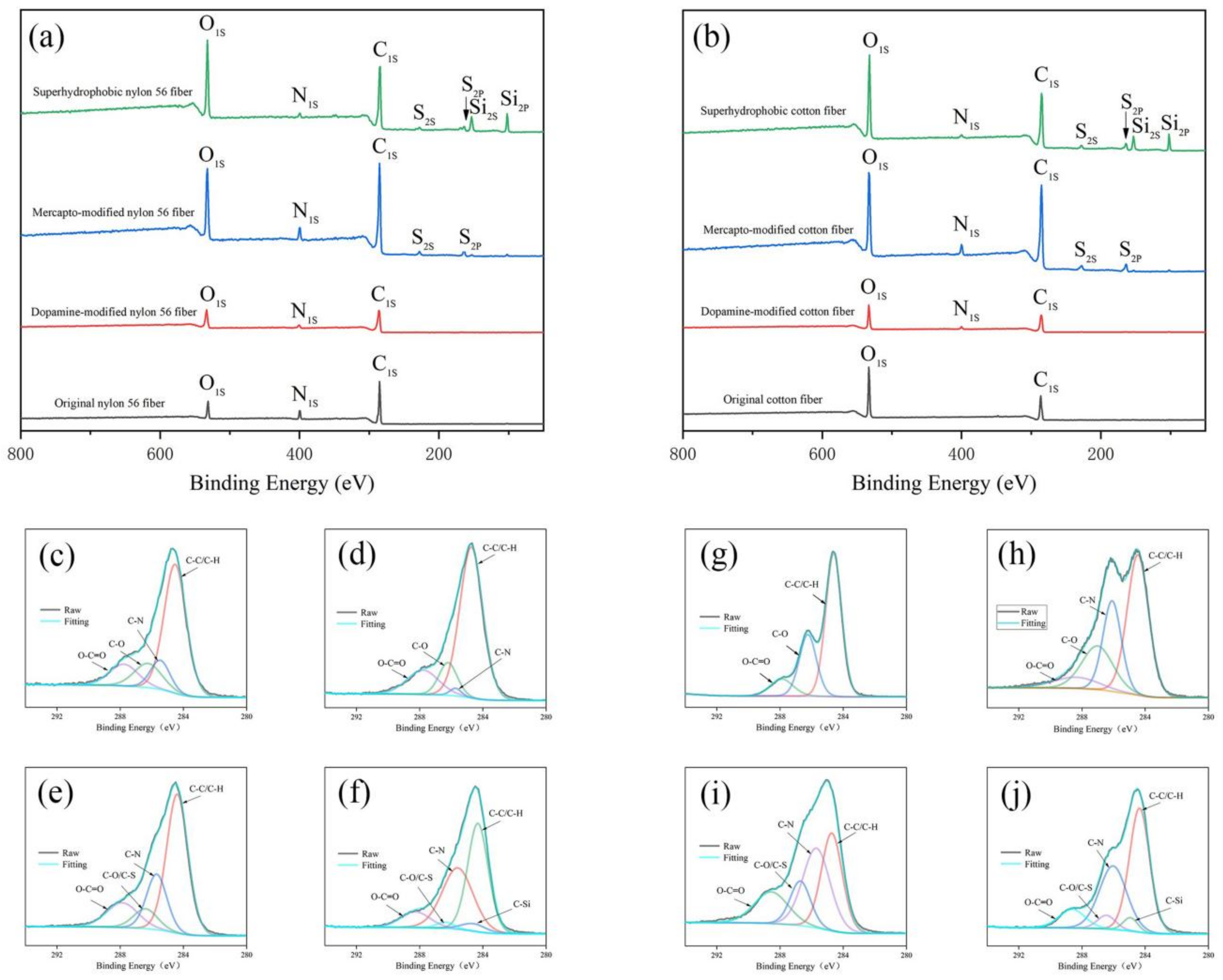
3.2. Surface Morphology and Composition of Superhydrophobic Fabrics
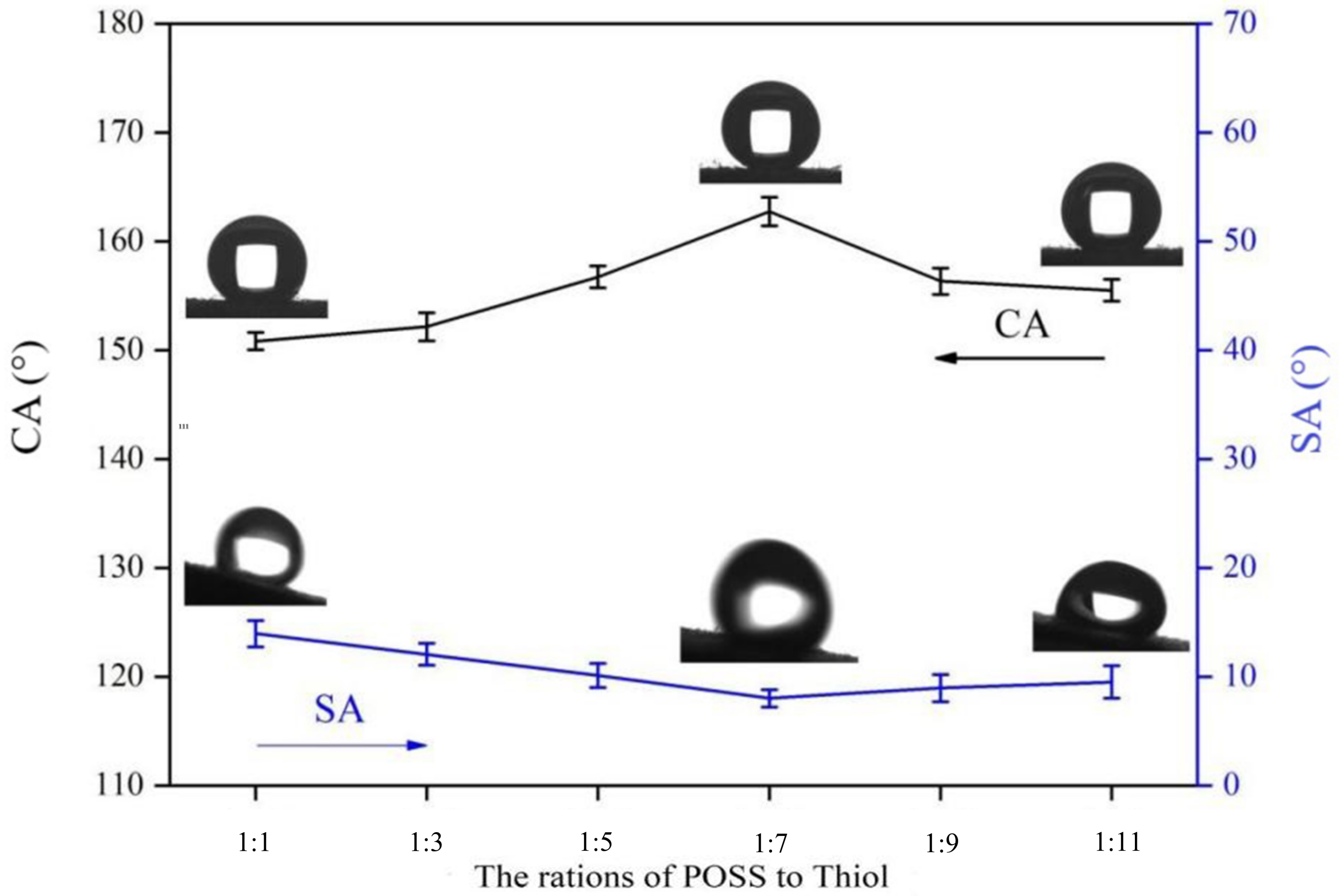
3.3. The Effect of Reactant Ratio on the Superhydrophobic Finishing of Fabrics
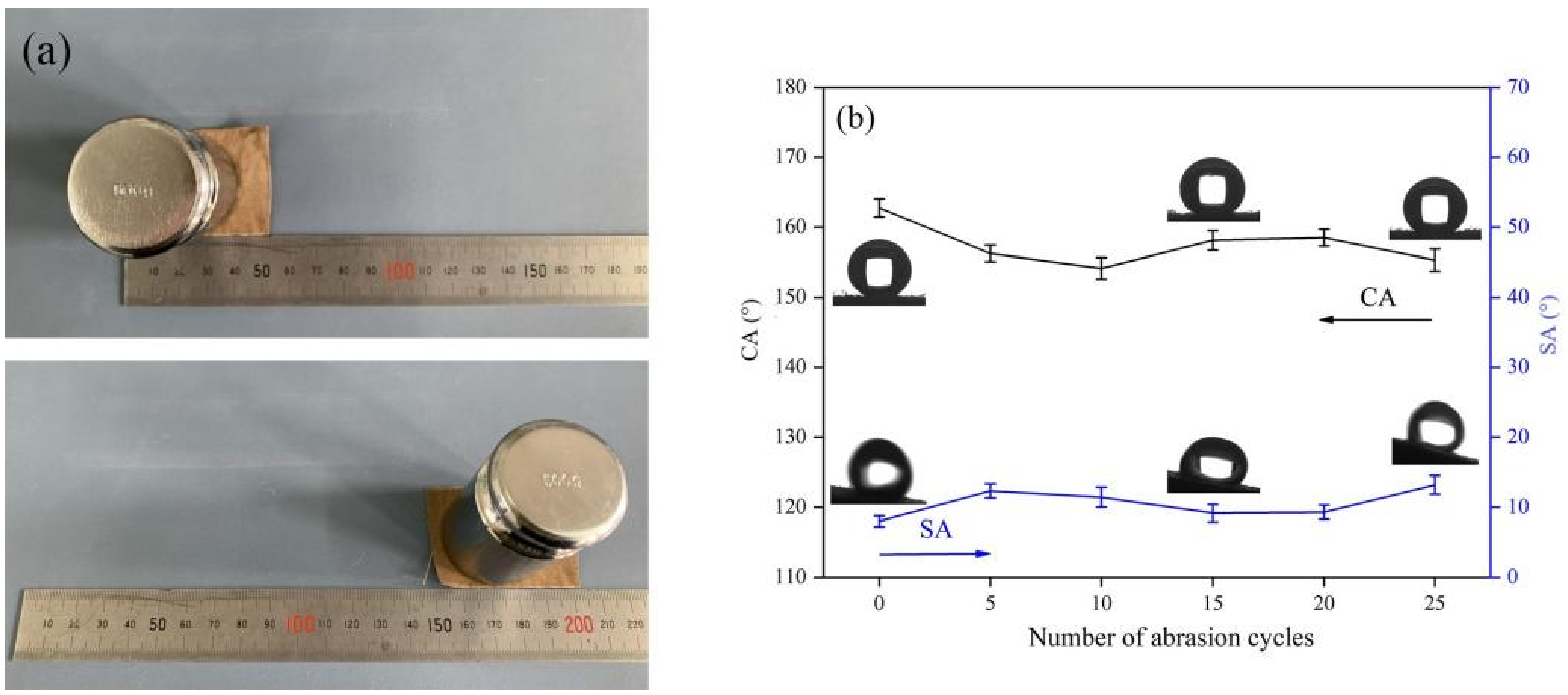
3.4. Mechanical Stability of the Superhydrophobic Fabric
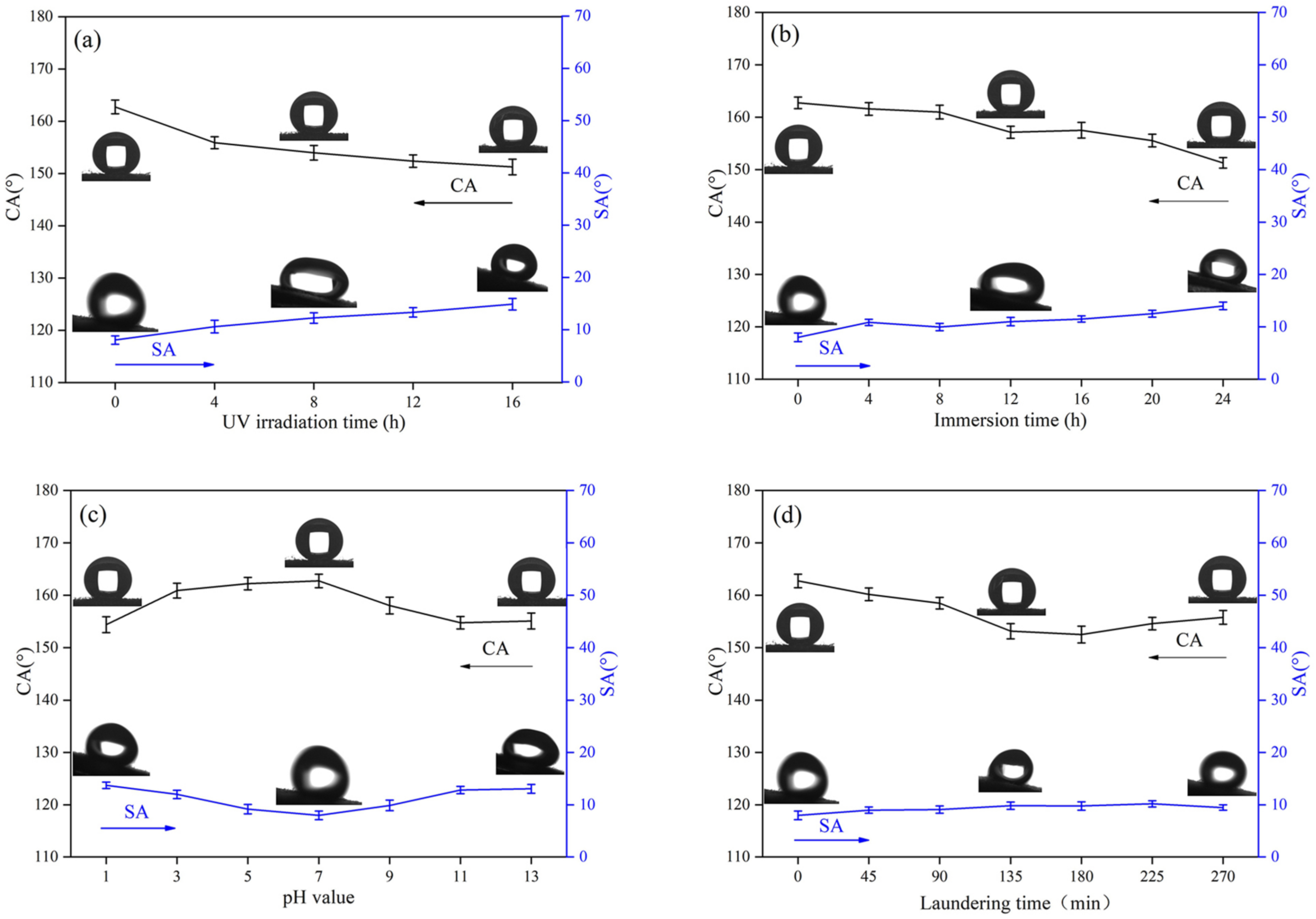
3.5. Chemical Stability and Durability of Superhydrophobic Fabrics
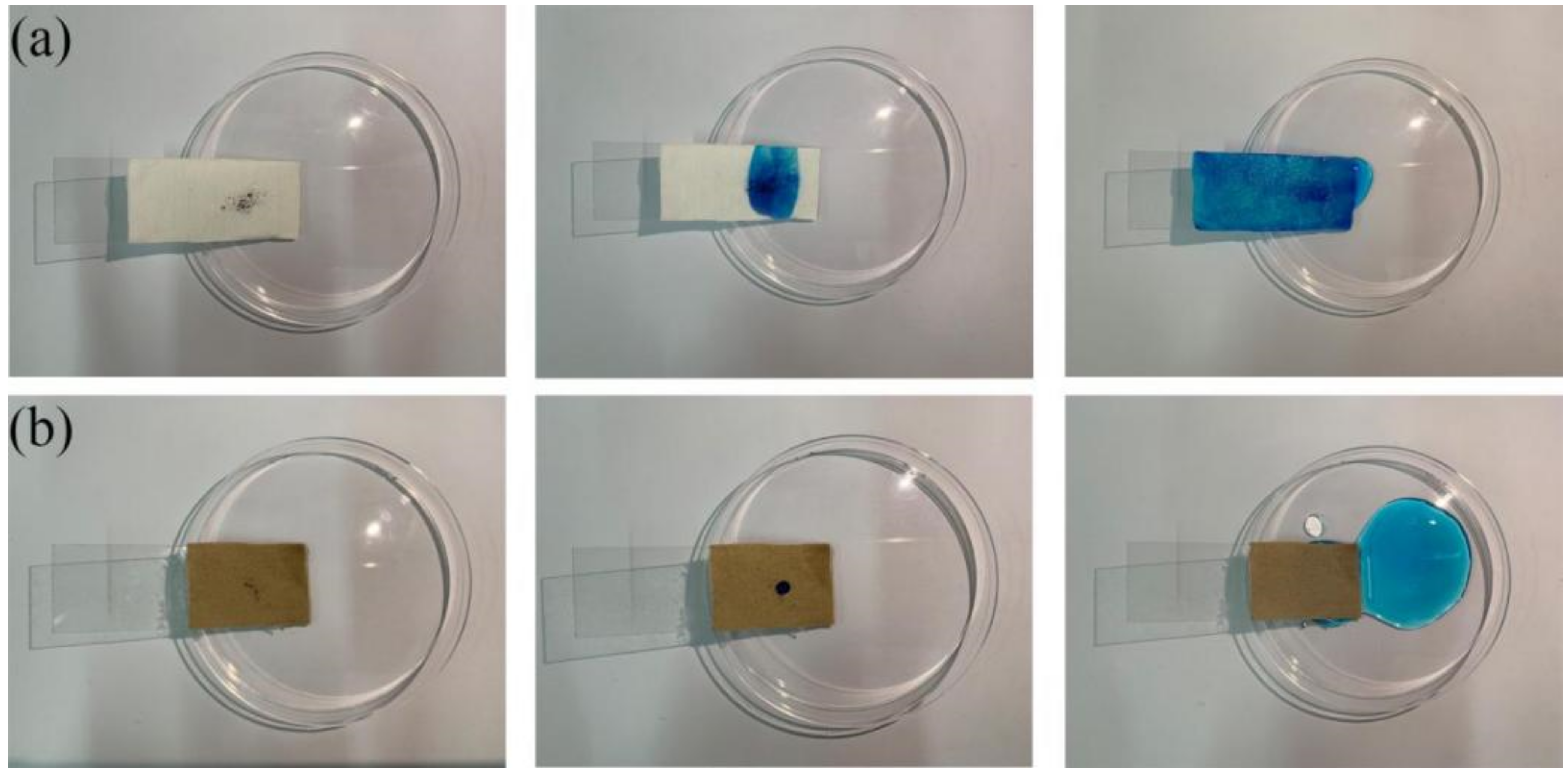
3.6. Self-Cleaning Performance of the Superhydrophobic Fabric
3.7. Oil–Water Separation Performance of the Superhydrophobic Fabric
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gong, X.; Zhang, L.G.; He, S.; Jiang, S.H.; Wang, W.F.; Wu, Y.Z. Rewritable superhydrophobic coatings fabricated using water-soluble polyvinyl alcohol. Mater. Des. 2020, 196, 109112. [Google Scholar] [CrossRef]
- Li, Z.H.; Guo, Z.G. Flexible 3D porous superhydrophobic composites for oil-water separation and organic solvent detection. Mater. Des. 2020, 196, 109144. [Google Scholar] [CrossRef]
- Zhao, X.X.; Park, D.S.; Choi, J.; Park, S.; Soper, S.; Murphy, M. Robust, transparent, superhydrophobic coatings using novel hydrophobic/hydrophilic dual-sized silica particles. J. Colloid Interface Sci. 2020, 574, 347–354. [Google Scholar] [CrossRef]
- Zeng, T.C.; Zhang, P.F.; Li, X.X.; Yin, Y.J.; Chen, K.L.; Wang, C.X. Facile fabrication of durable superhydrophobic and oleophobic surface on cellulose substrate via thiol-ene click modification. Appl. Surf. Sci. 2019, 493, 1004–1012. [Google Scholar] [CrossRef]
- Jia, S.S.; Deng, S.L.; Luo, S.; Qing, Y.; Yan, C.N.; Wu, Y.Q. Texturing commercial epoxy with hierarchical and porous structure for robust superhydrophobic coatings. Appl. Surf. Sci. 2019, 466, 84–91. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, C.P.; Song, L.X.; Ma, Y.; Alia, Z.; Gua, J.W.; Zhang, B.L.; Zhang, H.P.; Zhang, Q.Y. A stable 3D sol-gel network with dangling fluoroalkyl chains and rapid selfhealing ability as a long-lived superhydrophobic fabric coating. Chem. Eng. J. 2018, 334, 598–610. [Google Scholar] [CrossRef]
- Raimondoa, M.; Veronesi, F.; Boveri, G.; Guarini, G.; Motta, A.; Zanoni, R. Superhydrophobic properties induced by sol-gel routes on copper surfaces. Appl. Surf. Sci. 2017, 422, 1022–1029. [Google Scholar] [CrossRef]
- Wang, Y.T.; Peng, H.K.; Li, T.T.; Shiu, B.C.; Zhang, X.F.; Lou, C.W.; Lin, J.H. Lightweight, flexible and superhydrophobic conductive composite films based on layer-by-layer self-assembly for high-performance electromagnetic interference shielding. Compos. Part A Appl. Sci. 2021, 141, 106199. [Google Scholar] [CrossRef]
- Gao, T.T.; Gu, X.X.; Guo, S.T.; Wang, G.Y. Synthesis, self-assembly of perfluoropolyether based ABA-triblock copolymers for superhydrophobic surface applications. Polymer 2020, 205, 122732. [Google Scholar] [CrossRef]
- Pour, F.Z.; Karimi, H.; Avargani, V.M. Preparation of a superhydrophobic and superoleophilic polyester textile by chemical vapor deposition of dichlorodimethylsilane for Water–Oil separation. Polyhedron 2019, 159, 54–63. [Google Scholar] [CrossRef]
- Rezaei, S.; Manoucheri, I.; Moradian, R.; Pourabbas, B. One-step chemical vapor deposition and modification of silica nanoparticles at the lowest possible temperature and superhydrophobic surface fabrication. Chem. Eng. J. 2014, 252, 11–16. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Q.; Zhang, Y.; Zhao, J.; Yan, B.; Tang, S.; Xing, T.; Chen, G. Fabrication of superhydrophobic cotton fabric based on reaction of thiol-ene click chemistry. Colloid Surf. A 2020, 586, 124175. [Google Scholar] [CrossRef]
- Balordia, M.; Cammia, A.; Magistrisa, G.S.D.; Chemelli, C. Role of micrometric roughness on anti-ice properties and durability of hierarchical super-hydrophobic aluminum surfaces. Surf. Coat. Technol. 2019, 374, 549–556. [Google Scholar] [CrossRef]
- Durán, I.R.; Laroche, G. Current trends, challenges, and perspectives of anti-fogging technology: Surface and material design, fabrication strategies, and beyond. Prog. Mater. Sci. 2019, 99, 106–186. [Google Scholar] [CrossRef]
- Liu, E.Y.; Yin, X.L.; Hu, J.H.; Yu, S.R.; Zhao, Y.; Xiong, W. Fabrication of a biomimetic hierarchical superhydrophobic Cu-Ni coating with self-cleaning and anti-corrosion properties. Colloid Surf. A 2020, 586, 124223. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, W.Q.; Yang, M.P.; Liu, C.H.; He, S.; Xie, Y.K.; Wang, Z.F. Robust multifunctional superhydrophobic fabric with UV induced reversible wettability, photocatalytic self-cleaning property, and oil-water separation via thiol-ene click chemistry. Appl. Surf. Sci. 2019, 463, 34–44. [Google Scholar] [CrossRef]
- Kazemi, K.K.; Zarifi, T.; Mohseni, M.; Narang, R.; Golovin, K.; Zarifi, M. Smart Superhydrophobic Textiles Utilizing a Long-Range Antenna Sensor for Hazardous Aqueous Droplet Detection plus Prevention. ACS Appl. Mater. Interfaces 2021, 13, 34877–34888. [Google Scholar] [CrossRef]
- Mohseni, M.; Far, H.S.; Hasanzadeh, M.; Golovin, K. Non-fluorinated sprayable fabric finish for durable and comfortable superhydrophobic textiles. Prog. Org. Coat. 2021, 157, 106319. [Google Scholar] [CrossRef]
- Luan, V.H.; Bae, D.; Han, J.H.; Lee, W. Mussel-inspired dopamine-mediated graphene hybrid with silver nanoparticles for high performance electrochemical energy storage electrodes. Compos. Part B Eng. 2018, 134, 141e150. [Google Scholar] [CrossRef]
- Zin, G.; Wu, J.; Rezzadoria, K.; Petrus, J.C.C.; Luccio, M.D.; Li, Q.L. Modification of hydrophobic commercial PVDF microfiltration membranes into superhydrophilic membranes by the mussel-inspired method with dopamine and polyethyleneimine. Sep. Purif. Technol. 2019, 212, 641–649. [Google Scholar] [CrossRef]
- Wedel, J.; Pallavi, P.; Stamellou, E.; Yard, B.A. N-acyl dopamine derivates as lead compound for implementation in transplantation medicine. Transplant. Rev. 2015, 29, 109–113. [Google Scholar] [CrossRef]
- Yan, Z.S.; Zhang, Y.H.; Yang, H.Y.; Fan, G.D.; Ding, A.; Liang, H.; Li, G.B.; Ren, N.Q.; Bruggen, B.V.D. Mussel-inspired polydopamine modification of polymeric membranes for the application of water and wastewater treatment: A review. Chem. Eng. Res. Des. 2020, 157, 195–214. [Google Scholar] [CrossRef]
- Marwat, M.A.; Ma, W.G.; Fan, P.Y.; Elahi, H.; Samart, C.; Nan, B.; Tan, H.; Salamon, D.; Ye, B.H.; Zhang, H.B. Ultrahigh energy density and thermal stability in sandwich-structured nanocomposites with dopamine@Ag@BaTiO3. Energy Storage Mater. 2020, 31, 492–504. [Google Scholar] [CrossRef]
- Wang, J.C.; Tiana, J.Y.; Gao, S.S.; Shi, W.X.; Cui, F.Y. Dopamine triggered one step polymerization and codeposition of reactive surfactant on PES membrane surface for antifouling modification. Sep. Purif. Technol. 2020, 249, 117148. [Google Scholar] [CrossRef]
- Lu, J.F.; Liu, X.; Zhang, T.C.; He, H.Q.; Yuan, S.J. Magnetic superhydrophobic polyurethane sponge modified with bioinspired stearic acid@Fe3O4@PDA nanocomposites for oil/water separation. Colloid Surf. A 2021, 624, 126794. [Google Scholar] [CrossRef]
- Ju, J.G.; Fejjaria, K.; Cheng, Y.; Liu, M.Y.; Lia, Z.J.; Kang, W.M.; Liao, Y. Engineering hierarchically structured superhydrophobic PTFE/POSS nanofibrous membranes for membrane distillation. Desalination 2020, 486, 114481. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, Y.; Liu, G.Z.; Jiang, Y.C.; Chen, K.L. Fabrication of self-healing waterbased superhydrophobic coatings from POSS modified silica nanoparticles. Mater. Lett. 2018, 229, 281–285. [Google Scholar] [CrossRef]
- Sabet, S.M.; Mahfuz, H.; Terentis, A.C.; Hashemi, J.; Boesl, B. A facile approach to the synthesis of multi-walled carbon nanotubepolyhedral oligomeric silsesquioxane (POSS) nanohybrids. Mater. Lett. 2016, 168, 9–12. [Google Scholar] [CrossRef]
- Zhang, C.X.; Wu, G.S.; Jiang, H. Tuning interfacial strength of silicone resin composites by varying the grafting density of octamaleamic acid-POSS modified onto carbon fiber. Compos. Part A Appl. Sci. 2018, 109, 555–563. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Yan, H.X.; Xu, P.L.; Guo, L.L.; Yang, K.M.; Liu, R.; Feng, W.X. A novel POSS-containing polyimide: Synthesis and its composite coating with graphene-like MoS2 for outstanding tribological performance. Prog. Org. Coat. 2021, 151, 106013. [Google Scholar] [CrossRef]
- Bassam, A.; Noorullah, B.; Suchetha, S.; Saleh, A.M.; Fakhreia, A.S. Triptycene-containing Poly (vinylene sulfone) derivatives from a metal-free thiol-yne click polymerization followed by a mild oxidation reaction. Polymer 2018, 154, 233–240. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Z.; You, Y.Z.; Hong, C.Y. Synthesis of sequence-controlled polymers via sequential thiol-ene and amino-yne click reactions in one pot. Eur. Polym. J. 2018, 103, 80–87. [Google Scholar] [CrossRef]
- Zhou, J.X.; Liu, Y.M.; Jiao, T.F.; Xing, R.R.; Yang, Z.Q.; Fan, J.Z.; Liu, J.Z.; Li, B.B.; Peng, Q.M. Preparation and enhanced structural integrity of electrospun poly (ε-caprolactone) -based fibers by freezing amorphous chains through thiol-ene click reaction. Colloid Surf. A 2018, 538, 7–13. [Google Scholar] [CrossRef]
- Zhou, L.; He, H.; Li, M.C.; Huang, S.W.; Mei, C.T.; Wu, Q.L. Grafting polycaprolactone diol onto cellulose nanocrystals via click chemistry: Enhancing thermal stability and hydrophobic property. Carbohydr. Polym. 2018, 189, 331–341. [Google Scholar] [CrossRef]
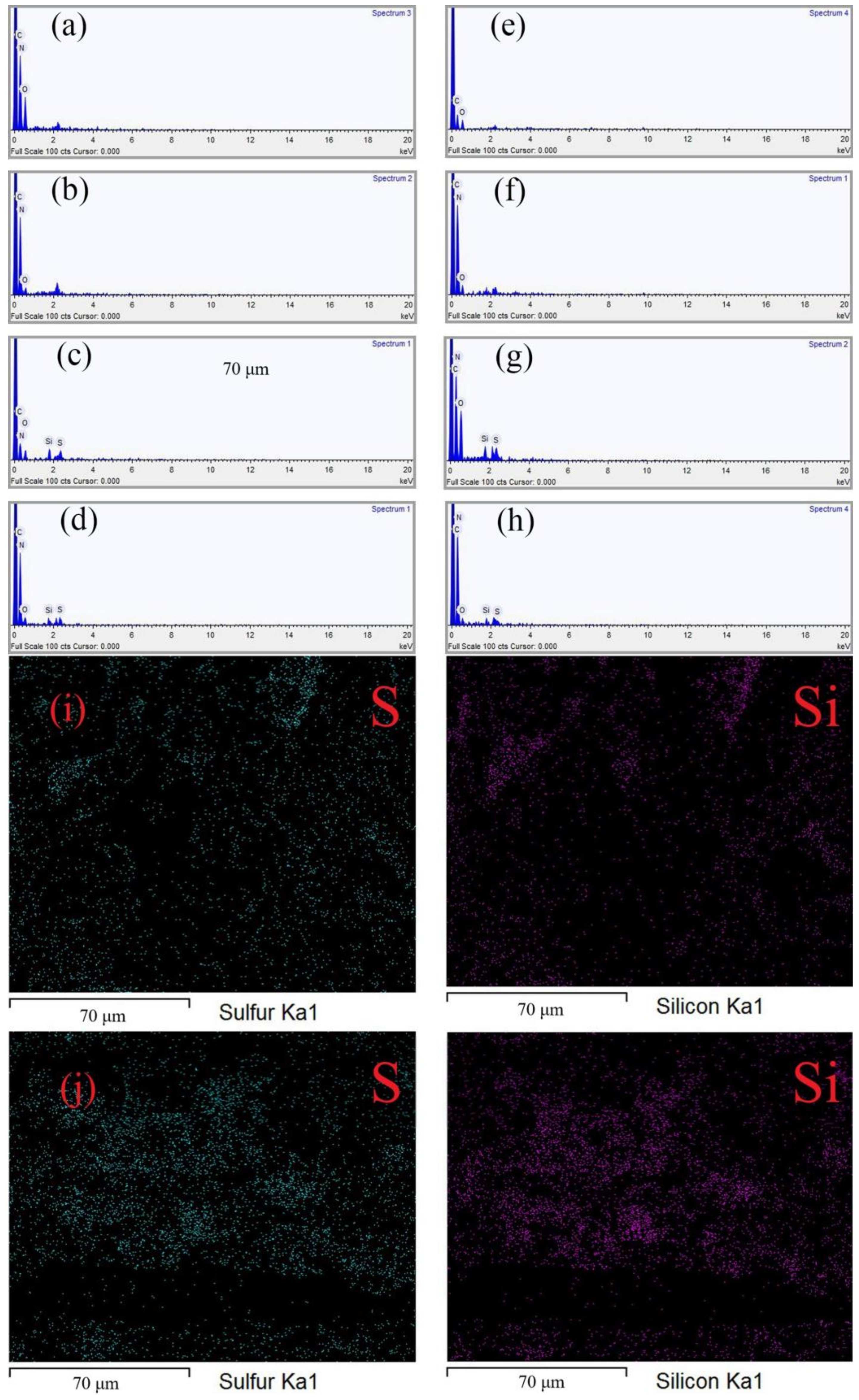


| Element | C | O | N | S | Si | Total |
|---|---|---|---|---|---|---|
| 44.732 | 40.314 | 14.954 | 0 | 0 | 100 |
| 56.618 | 15.423 | 27.959 | 0 | 0 | 100 |
| 47.540 | 29.679 | 15.605 | 2.738 | 4.439 | 100 |
| 63.194 | 14.507 | 18.899 | 2.293 | 1.108 | 100 |
| 50.925 | 49.075 | 0 | 0 | 0 | 100 |
| 71.872 | 21.448 | 6.680 | 0 | 0 | 100 |
| 46.028 | 41.042 | 9.946 | 1.305 | 1.679 | 100 |
| 63.325 | 12.012 | 23.366 | 0.487 | 0.810 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Xing, L.; Xing, T.; Chen, G. Preparation of Stable POSS-Based Superhydrophobic Textiles Using Thiol–Ene Click Chemistry. Polymers 2022, 14, 1426. https://doi.org/10.3390/polym14071426
Wang B, Xing L, Xing T, Chen G. Preparation of Stable POSS-Based Superhydrophobic Textiles Using Thiol–Ene Click Chemistry. Polymers. 2022; 14(7):1426. https://doi.org/10.3390/polym14071426
Chicago/Turabian StyleWang, Baoliang, Lili Xing, Tieling Xing, and Guoqiang Chen. 2022. "Preparation of Stable POSS-Based Superhydrophobic Textiles Using Thiol–Ene Click Chemistry" Polymers 14, no. 7: 1426. https://doi.org/10.3390/polym14071426
APA StyleWang, B., Xing, L., Xing, T., & Chen, G. (2022). Preparation of Stable POSS-Based Superhydrophobic Textiles Using Thiol–Ene Click Chemistry. Polymers, 14(7), 1426. https://doi.org/10.3390/polym14071426






