Fabrication and Characterization of Gelatin/Polyvinyl Alcohol Composite Scaffold
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PVA/Gelatin Composite Materials
2.3. Characterization of PVA/Gelatin Composite Scaffold
2.4. Mechanical Test
2.5. Scanning Electron Microscopy (SEM)
2.6. In Vitro Swelling Characterizations
2.7. In Vitro Degradation of Composite Scaffold
2.8. Statistic Analysis
3. Results
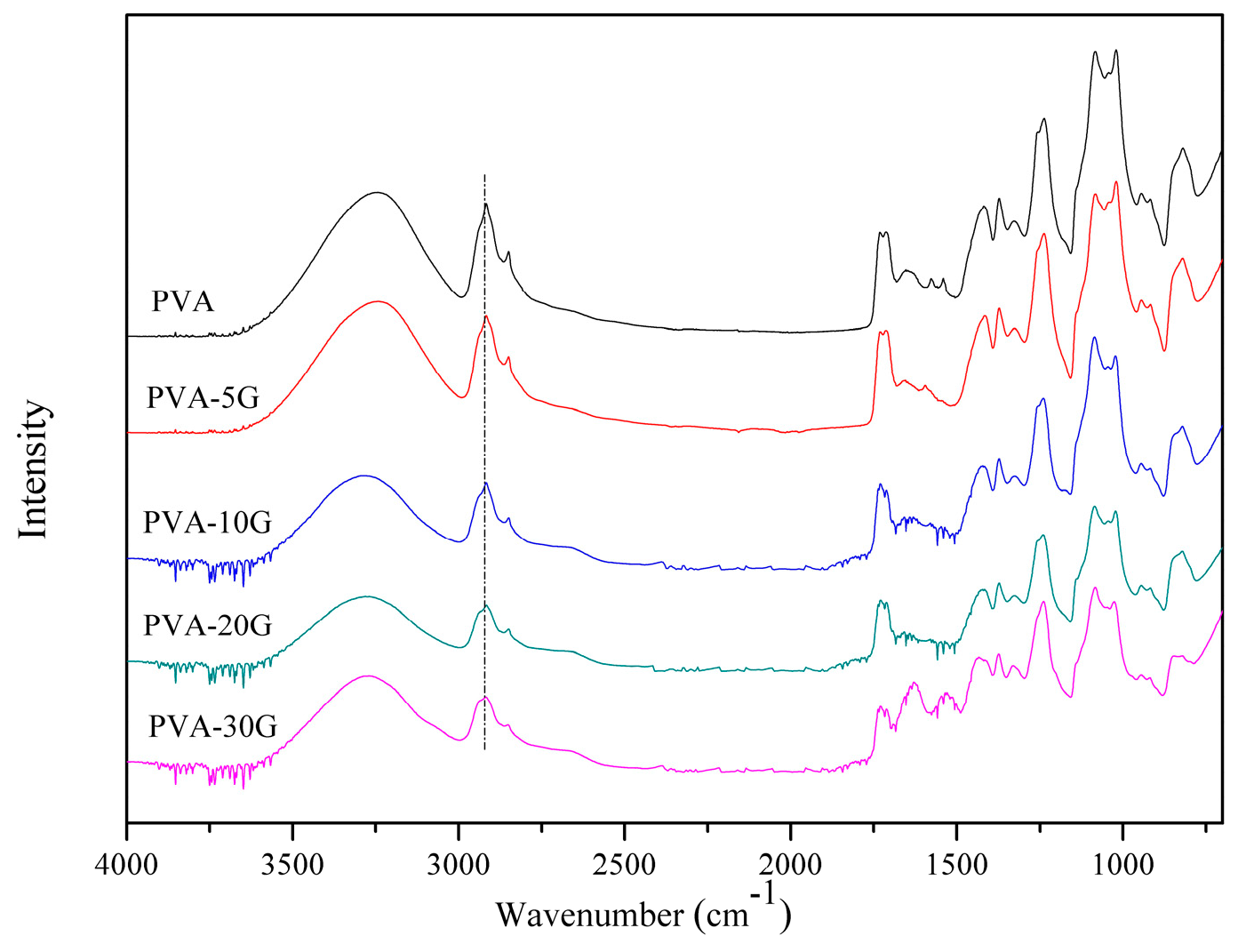
3.1. FTIR Analysis
3.2. XPS Analysis
3.3. XRD Analysis
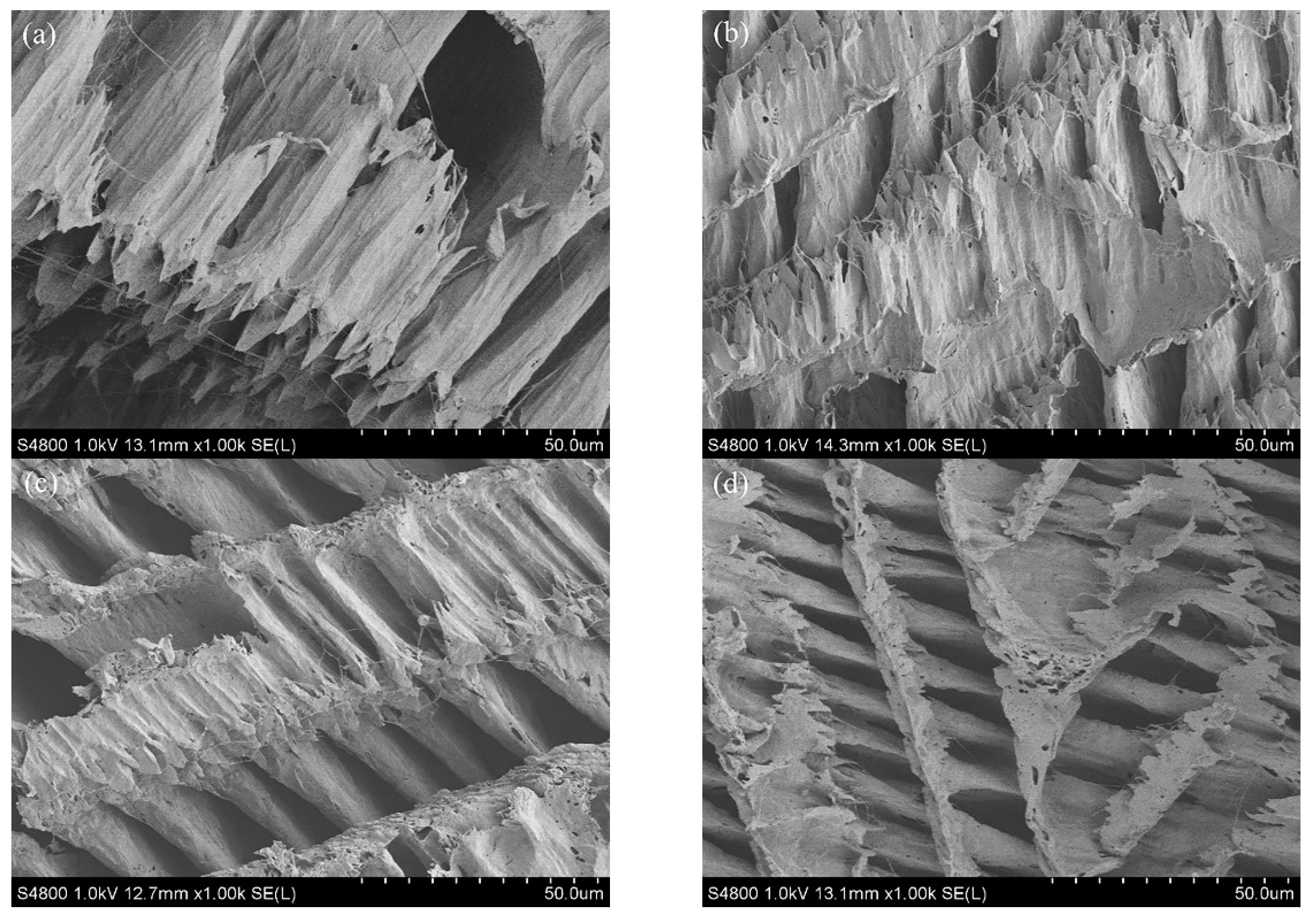
3.4. SEM
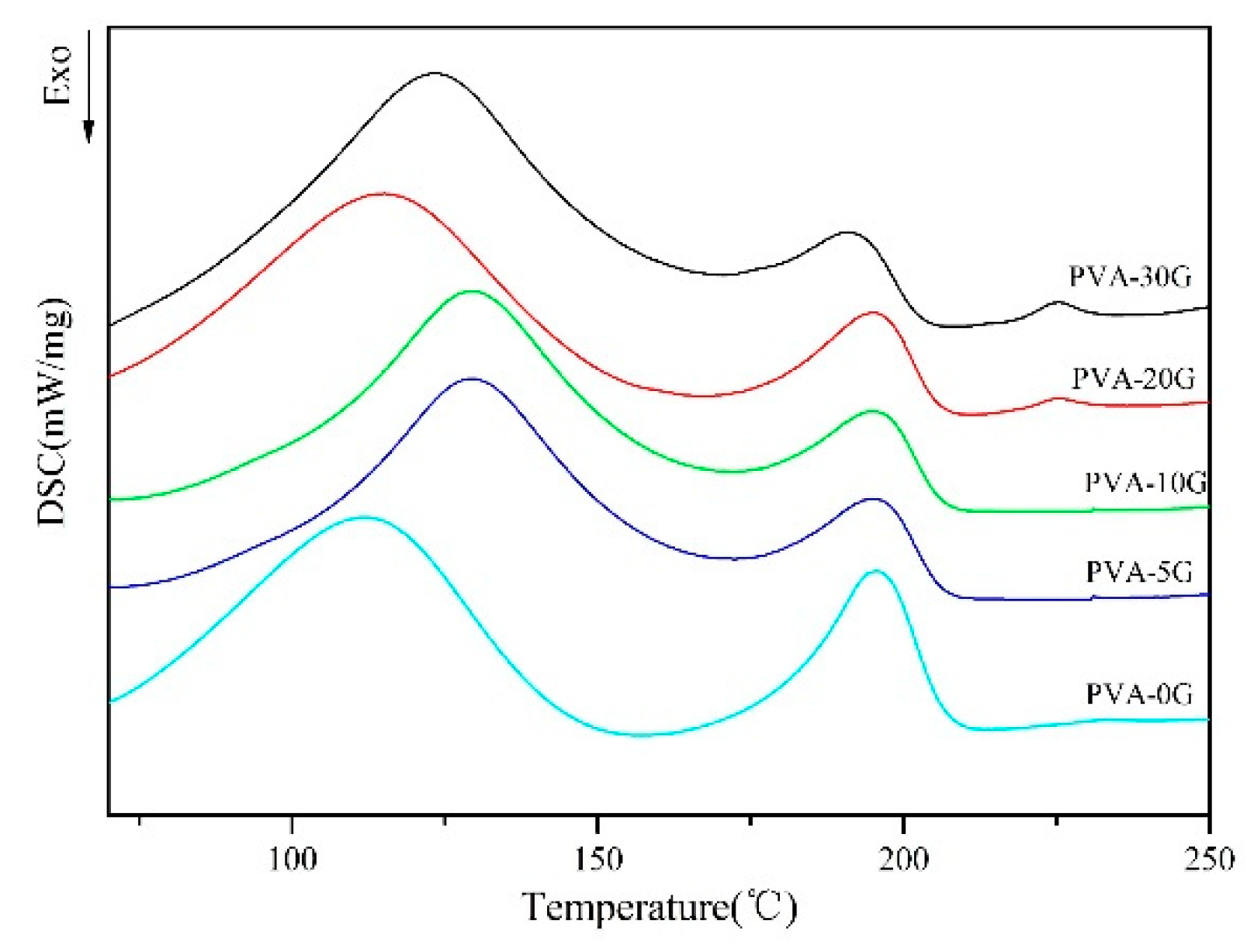
3.5. Thermal Properties of Scaffold Materials
3.6. Swelling Properties
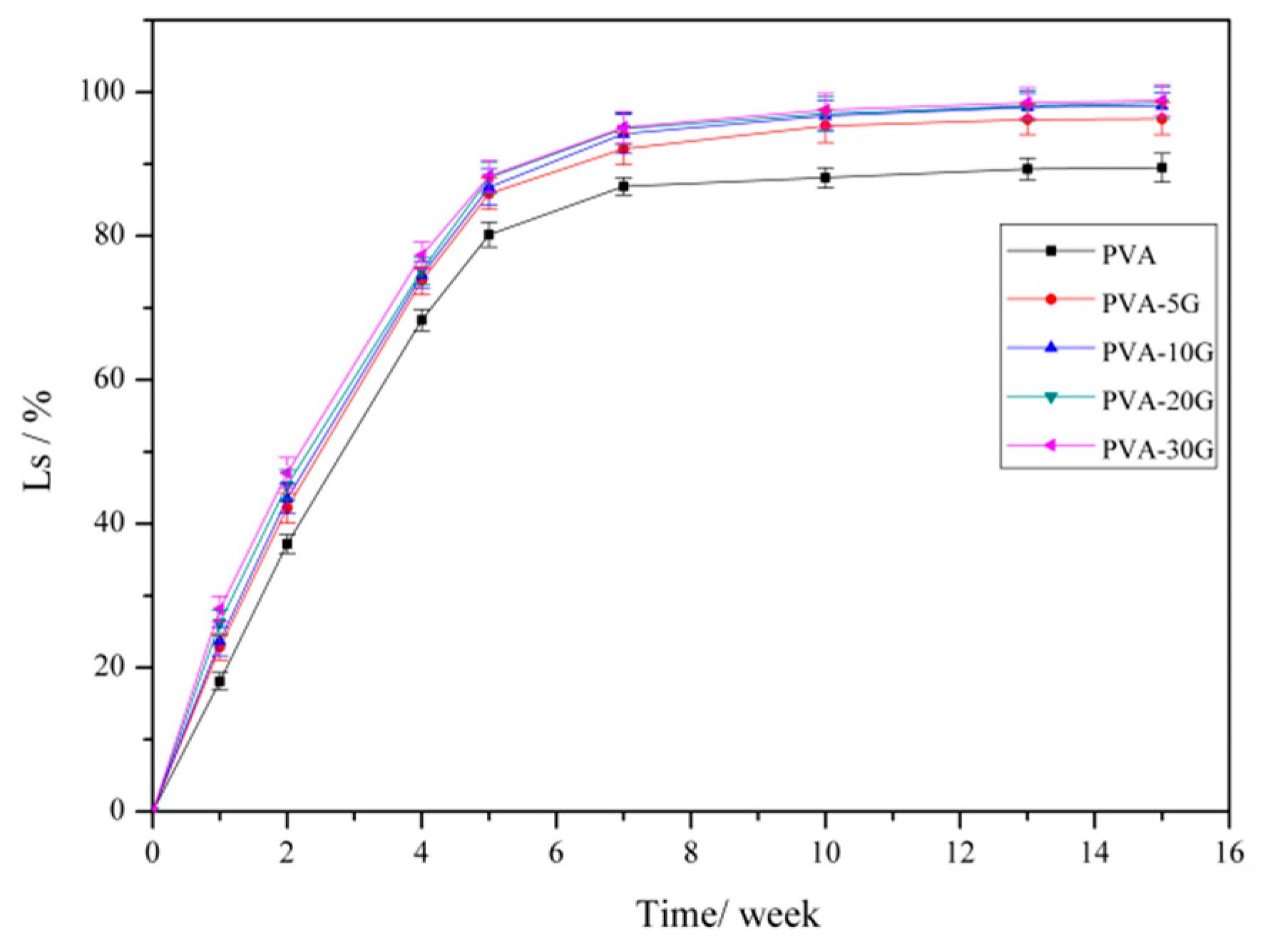
3.7. Degradation Properties
3.8. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parveen, S.; Krishnakumar, K.; Sk, S. New Era in Health Care: Tissue Engineering. J. Stem Cells Regen. Med. 2006, 1, 8–24. [Google Scholar] [PubMed]
- Sharma, C.; Gautam, S.; Dlnda, A.K.; Mlshra, N.C. Cartilage Tissue Engineering: Current Scenario and Challenges. Adv. Mater. Lett. 2011, 2, 90–99. [Google Scholar]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Sharma, C.; Dinda, A.K.; Mishra, N.C. Fabrication and characterization of natural origin chitosan-gelatin-alginate composite scaffold by foaming method without using surfactant. J. Appl. Polym. Sci. 2012, 127, 3228–3241. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Q.; Chen, X.; Yu, F.; Zhu, Z. Investigation of PVA/ws-chitosan hydrogels prepared by combined γ-irradiation and freeze-thawing. Carbohydr. Polym. 2008, 73, 401–408. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Beckman, E.J.; Piesco, N.P.; Agarwal, S. A new peptide-based urethane polymer: Synthesis, biodegradation, and potential to support cell growth in vitro. Biomaterials 2000, 21, 1247–1258. [Google Scholar] [CrossRef] [Green Version]
- Sivashankari, P.R.; Prabaharan, M. Three-dimensional porous scaffolds based on agarose/chitosan/grapheme oxide composite for tissue engineering. Int. J. Biol. Macromol. 2020, 146, 222–231. [Google Scholar] [CrossRef]
- Ocampo, J.G.; Jaramillo, M.E.; Sierra, D.E.; Orozco, C.O. Suspension Rheology, Porosity and Mechanical Strength of Porous Hydroxyapatite Obtained by Gel-casting and Infiltration. J. Mater. Eng. Perform. 2016, 25, 431–442. [Google Scholar] [CrossRef]
- Venkatesan, J.; Jayakumar, R.; Anil, S.; Chalisserry, E.P.; Pallela, R.; Kim, S.K. Development of Alginate-Chitosan-Collagen Based Hydrogels for Tissue Engineering. J. Biomater. Tiss. Eng. 2015, 5, 458–464. [Google Scholar] [CrossRef]
- Al-Mamun, A.; Haque, P.; Debnath, T.; Rahman, M.F.; Islam, J.M.; Rahman, M.M.; Khan, M.A. γ-Irradiated gelatin and polyvinyl alcohol films as artificial articular cartilage. J. Thermoplast. Compos. Mater. 2020, 33, 614–627. [Google Scholar] [CrossRef]
- Tayser, G.; Abu, S.; Majid, A.; Abdul, K.; Abu, M.; Ahmed, A.-A. Properties and Applications of Polyvinyl Alcohol, Halloysite Nanotubes and Their Nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar]
- Saini, I.; Sharma, A.; Dhiman, R.; Aggarwal, S.; Ram, S.; Sharma, P.K. Grafted SiC nanocrystals: For enhanced optical, electrical and mechanical properties of polyvinyl alcohol. J. Alloy. Compd. 2017, 714, 172–180. [Google Scholar] [CrossRef]
- Anis, A.; Banthia, A.K.; Bandyopadhyay, S. Synthesis & Characterization of PVA/STA Composite Polymer Electrolyte Membranes for Fuel Cell Application. J. Mater. Eng. Perform. 2008, 17, 772–779. [Google Scholar]
- Ye, M.; Mohanty, P.; Ghosh, G. Biomimetic apatite-coated porous PVA scaffolds promote the growth of breast cancer cells. Mater. Sci. Eng. C 2014, 44, 310–316. [Google Scholar] [CrossRef]
- Roy, S.; Das, T.; Kuddannaya, S.; Lee, H.; Lim, J.; Hu, X.M.; Yue, C.Y.; Kim, J.W. A Novel Approach of Fabricating Highly Tunable and Fluffy Bioinspired 3D Poly(vinyl alcohol) (PVA) Fiber Scaffolds. Nanoscale 2017, 9, 7081–7093. [Google Scholar] [CrossRef]
- Cijun, S.; Pei, F.; Chengde, G.; Xiong, S.; Tao, X.; Shuping, P. Graphene oxide reinforced poly(vinyl alcohol): Nanocomposite scaffolds for tissue engineering applications. RSC Adv. 2015, 5, 25416–25423. [Google Scholar]
- Kolodziejska, I.; Sikorski, Z.; Niecikowska, C. Parameters affecting the isolation of collagen from squid skins. Food Chem. 1999, 66, 153–157. [Google Scholar] [CrossRef]
- Collins, R.L.L.; Christiansen, D.; Zazanis, G.A.; Silver, F.H. Use of collagen film as a dural substitute: Preliminary animal studies. J. Biomed. Mater. Res. 1991, 25, 267–276. [Google Scholar] [CrossRef]
- Maeda, M.; Kadota, K.; Kajihara, M.; Sano, A.; Fujioka, K. Sustained release of human growth hormone (hGH) from collagen film and evaluation of effect on wound healing in db/db mice. J. Control. Release 2001, 77, 261–272. [Google Scholar] [CrossRef]
- Tharanathan, R.N. Biodegradable films and composite coatings: Past, present and future. Trends Food Sci. Technol. 2003, 14, 71–78. [Google Scholar] [CrossRef]
- Elzoghby Ahmed, O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control. Release 2013, 172, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, D.; Liu, Y.; Bo, Y.; Zhan, H.; Qin, L.; Lu, B.; Yi, L. Preparation of chitosan–gelatin hybrid scaffolds with well-organized microstructures for hepatic tissue engineering. Acta Biomater. 2009, 5, 453–461. [Google Scholar]
- Kim, H.; Yang, G.H.; Choi, C.H.; Cho, Y.S.; Kim, G.H. Gelatin/PVA scaffolds fabricated using a 3D-printing process employed with a low-temperature plate for hard tissue regeneration: Fabrication and characterizations. Int. J. Biol. Macromol. 2018, 120, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Yajuan, W.; Xin, Z.; Dan, Q.; Ya, L.; Lihui, Y.; Jingkuan, D. Ultrasonic Assisted Microwave Synthesis of Poly (Chitosan- co- gelatin)/Polyvinyl Pyrrolidone IPN Hydrogel. Ultrason. Sonochem. 2018, 40, 714–719. [Google Scholar]
- Shuaiyang, W.; Junli, R.; Weiying, L.; Runcang, S.; Shijie, L. Properties of polyvinyl alcohol/xylan composite films with citric acid. Carbohydr. Polym. 2014, 103, 94–99. [Google Scholar]
- Fan, L.; Xi’an, F.; Zigui, L.; Wentao, H.; Jian, W.; Zhaoyang, W.; Guangqiang, L.; Yawei, L.; Xin, L. Microstructure, formation mechanism and magnetic properties of Fe1.82Si0.18@Al2O3 soft magnetic composites. J. Magn. Magn. Mater. 2020, 493, 165744. [Google Scholar]
- Hodge, R.M.; Edward, G.H.; Simon, G.P. Water absorption and states of water in semicrystalline poly(vinyl alcohol) films. Polymer 1996, 37, 1371–1376. [Google Scholar] [CrossRef]
- Sarkar, S.; Chorasia, A.; Maji, S.; Sadhukhan, S.; Adhikari, B. Synthesis and Characterization of Gelatin based Polyester Urethane Scaffold. Bull. Mater. Sci. 2006, 29, 475–484. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Carcía-carvajal, Z.Y.; Jobbágy, M.; Rubio, F.; Yuste, L.; Rojo, F.; Ferrer, M.L.; Monte, F.D. Poly(vinyl alcohol) scaffolds with tailored morphologies for drug delivery and controlled release. Adv. Funct. Mater. 2007, 17, 3505–3513. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Hussain, I.; Brust, M.; Butler, M.F.; Rannard, S.P.; Cooper, A.I. Aligned two- and three-dimensional structures by directional freezing of polymers and nanoparticles. Nat. Mater. 2005, 4, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.H.; Wei, D.X.; Ye, H.M.; Zhang, X.; Meng, X.; Zhou, Q. Development of poly(vinyl alcohol) porous scaffold with high strength and well ciprofloxacin release efficiency. Mater. Sci. Eng. C 2016, 67, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, H.J. Thermal stability and viscoelastic properties of MF/PVAc hybrid resins on the adhesion for engineered flooring in under heating system; ONDOL. Thermochim. Acta 2006, 444, 134–140. [Google Scholar] [CrossRef]
- Rezaei, A.; Tavanai, H.; Nasirpour, A. Fabrication of electrospun almond gum/PVA nanofibers as a thermostable delivery system for vanillin. Int. J. Biol. Macromol. 2016, 91, 536–543. [Google Scholar] [CrossRef]
- Gomez, I.; Otazo, E.M.; Hernandez, H.; Rubio, E.; Varela, J.; Ramirez, M.; Barajas, I.; Gordillo, A.J. Thermal degradation study of PVA derivative with pendant phenylthionecarbamate groups by DSC/TGA and GC/MS. Polym. Degrad. Stab. 2015, 112, 132–136. [Google Scholar] [CrossRef]
- Karimi, A.; Daud, W.M.A.W. Comparison the properties of PVA/Na+-MMT nanocomposite hydrogels prepared by physical and physicochemical crosslinking. Polym. Compos. 2014, 37, 897–906. [Google Scholar] [CrossRef]
- Fei, Y.; Cui, W.; Xiong, Z.; Liu, L.; Bei, J.; Wang, S. Poly(l,l-lactide-co-glycolide)/tricalcium phosphate composite scaffold and its various changes during degradation in vitro. Polym. Degrad. Stab. 2006, 91, 3065–3073. [Google Scholar]
- Müller, F.A.; Müller, L.; Hofmann, I.; Greil, P.; Wenzel, M.M.; Staudenmaier, R. Cellulose-based scaffold materials for cartilage tissue engineering. Biomaterials 2006, 27, 3955–3963. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Li, X.; Feng, Q.; Jiao, Y.; Cui, F. Collagen-based scaffolds reinforced by chitosan fibres for bone tissue engineering. Polym. Int. 2010, 54, 1034–1040. [Google Scholar] [CrossRef]
- Fan, L.; Zhu, H.; Zheng, H.; Xu, Y.; Zhang, C. Structure and properties of blend fibers prepared from alginate and konjac glucomannan. J. Appl. Polym. Sci. 2007, 106, 3903–3907. [Google Scholar] [CrossRef]






| Sample | T50/°C | Remaining Yield/% |
|---|---|---|
| PVA-0G | 381.57 | 0.01 |
| PVA-5G | 388.86 | 4.33 |
| PVA-10G | 391.72 | 2.67 |
| PVA-20G | 377.64 | 4.59 |
| PVA-30G | 385.92 | 8.53 |
| Sample | Elasticity Modulus/MPa | Elongation at Break/% |
|---|---|---|
| PVA-0G | 91.88 ± 14.04 | 170.73 ± 13.42 |
| PVA-5G | 92.42 ± 13.08 | 186.19 ± 15.79 |
| PVA-10G | 247.55 ± 33.12 | 126.95 ± 17.92 |
| PVA-20G | 241.77 ± 17.17 | 59.30 ± 3.96 |
| PVA-30G | 215.26 ± 30.19 | 47.44 ± 7.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Wang, Y.; Yao, L.; Li, Y.; Weng, Y.; Qiu, D. Fabrication and Characterization of Gelatin/Polyvinyl Alcohol Composite Scaffold. Polymers 2022, 14, 1400. https://doi.org/10.3390/polym14071400
Sun M, Wang Y, Yao L, Li Y, Weng Y, Qiu D. Fabrication and Characterization of Gelatin/Polyvinyl Alcohol Composite Scaffold. Polymers. 2022; 14(7):1400. https://doi.org/10.3390/polym14071400
Chicago/Turabian StyleSun, Mengwen, Yajuan Wang, Lihui Yao, Ya Li, Yunxuan Weng, and Dan Qiu. 2022. "Fabrication and Characterization of Gelatin/Polyvinyl Alcohol Composite Scaffold" Polymers 14, no. 7: 1400. https://doi.org/10.3390/polym14071400
APA StyleSun, M., Wang, Y., Yao, L., Li, Y., Weng, Y., & Qiu, D. (2022). Fabrication and Characterization of Gelatin/Polyvinyl Alcohol Composite Scaffold. Polymers, 14(7), 1400. https://doi.org/10.3390/polym14071400






