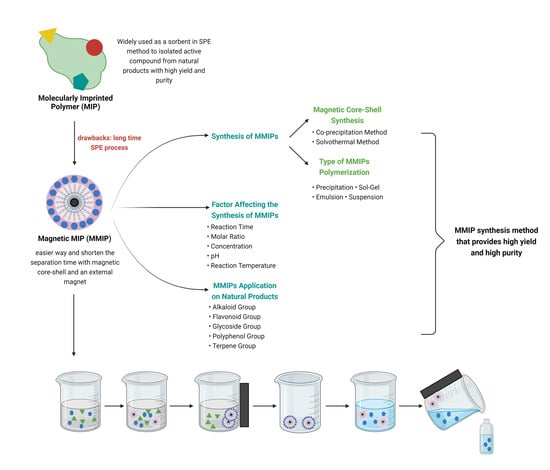
Magnetic Molecularly Imprinted Polymers: An Update on Their Use in the Separation of Active Compounds from Natural Products
Abstract
:1. Introduction
2. Materials and Methods
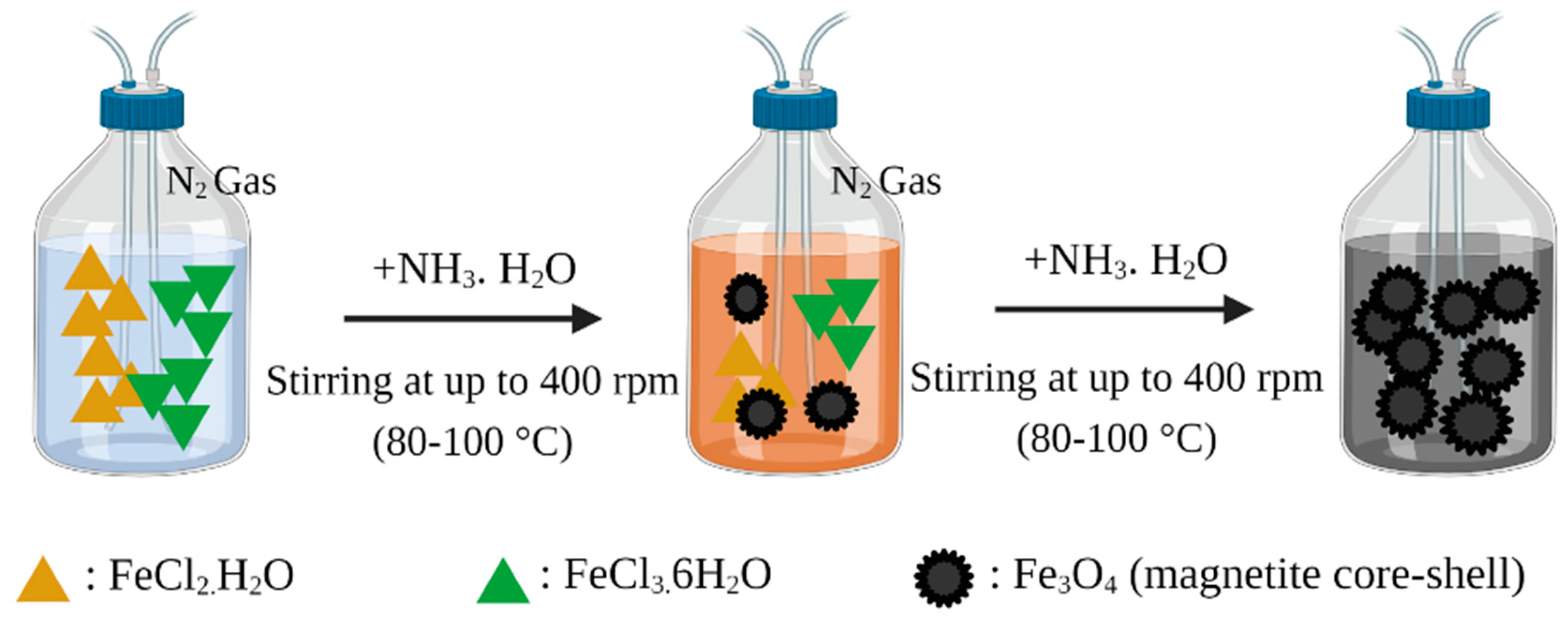
2.1. Synthesis of Magnetic Nanoparticles for MMIPs
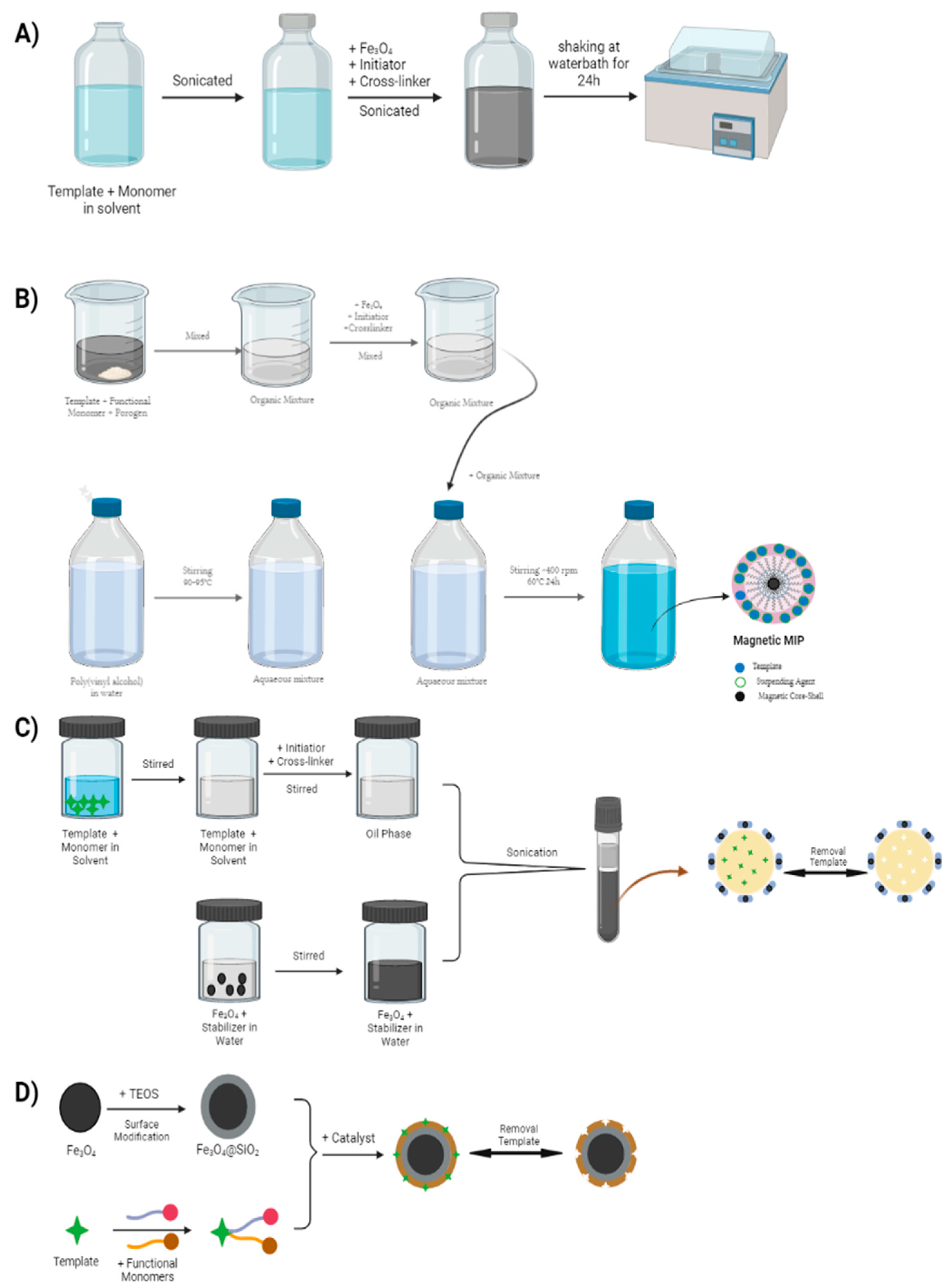
2.2. Modification of Magnetic Core-Shell Surface
2.3. Synthesis of MMIPs
2.4. Factors Affecting the Synthesis of MMIPs
2.4.1. Factors Affecting the Synthesis of Magnetic Nanoparticle Fe3O4
2.4.2. Factors Affecting the Synthesis of Magnetic Core-Shell
2.4.3. Factor Affecting the Synthesis of MIPs
3. Characterization of MMIPs
4. Application of MMIPs to Natural Products
4.1. Alkaloids
4.2. Flavonoids
4.3. Glycosides
4.4. Polyphenols
4.5. Terpenes
5. Conclusions
6. Further Perspectives
- In the development of MMIP synthesis, the choice of shell material (protective) must consider the properties of other MMIP components, such as templates and functional monomers.
- Further research is needed on the optimum conditions for coating the magnetite core with the shell so that it will be a breakthrough to obtaining a magnetic core-shell that has good resistance and stability.
- There are many methods of synthesizing MNPs; this could be an opportunity for exploring further methods to try new techniques in MMIP synthesis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Adsorption capacity |
| ACN | Acetonitrile |
| ABDV | 2,2’-Azobis(2,4-dimethylvaleronitrile) |
| AIBN | 2,2’-Azobisisobutironitrile |
| APTES | (3-Aminopropyl)triethoxysilane |
| BET | Brunauer-Emmet-Teller |
| CHCl3 | Chloroform |
| DMA | 2-(Dimethylamino) Ethyl Methacrylate |
| DMF | Dimethylformamide |
| DMSO | Dimethyl Sulfoxide |
| DSPME | Dispersive Solid-Phase Micro Extraction |
| EGDMA | Ethylene Glycol Dimethylacrylate |
| EtOAc | Ethyl Acetate |
| Fe3O4 | Ferrosoferric Oxide |
| FT-IR | Fourier-Transform Infrared |
| HEC | Hydroxyethyl Cellulose |
| HPLC-UV | High Performance Liquid Chromatography–Ultraviolet |
| JCPDS | Joint Committee on Powder Diffraction Standards |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| MeOH | Methanol |
| MIPs | Molecularly Imprinted Polymers |
| MMA | Methyl Metacrylate |
| MMIP-SPE | Magnetic Molecularly Imprinted Polymer–Solid Phase Extraction |
| MMIPs | Magnetic Molecularly Imprinted Polymers |
| MNIPs | Magnetic Non-Imprinted Polymers |
| MNPs | Magnetic Nanoparticles |
| MPS | 3-(Trimethoxysilyl) Propyl Methacrylate |
| NIPs | Non-Imprinted Polymers |
| OA | Oleic Acid |
| P | Purity |
| PEG | Polyethylene Glycol |
| PCMS | Polychloromethylstyrene |
| PA | Protocatechuic Acid |
| SEM | Scanning Electron Microscopy |
| SiO2 | Silicon Oxide |
| SPE | Solid Phase Extraction |
| TEM | Transmission Electron Microscopy |
| TEOS | Tetraethyl Orthosilicate |
| TGA | Thermogravimetric Analysis |
| THF | Tetrahydrofuran |
| TRIM | Trimethylolpropane Trimethacrylate |
| VSM | Vibration Sample Magnetometer |
| XRD | X-Ray Diffraction |
| Y | Yield |
References
- Fernando, W.G.D. Plants: An international scientific open access journal to publish all facets of plants, their functions and interactions with the environment and other living organisms. Plants 2012, 1, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Dhir, A.; Talwar, S.; Chakraborty, D.; Kaur, P. What drives brand love for natural products? The moderating role of household size. J. Retail. Consum. Serv. 2021, 58, 102329. [Google Scholar] [CrossRef]
- Allied Market Research Natural Food & Drinks Market. 2020. Available online: https://www.alliedmarketresearch.com/natural-food-and-drinks-market (accessed on 20 January 2022).
- Verma, S.; Singh, S.P. Current and future status of herbal medicines. Vet. World 2008, 1, 347–350. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Müller-Kuhrt, L. Putting nature back into drug discovery. Nat. Biotechnol. 2003, 21, 602. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta-Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [Green Version]
- Weinmann, S.; Roll, S.; Schwarzbach, C.; Vauth, C.; Willich, S.N. Effects of Ginkgo biloba in dementia: Systematic review and meta-analysis. BMC Geriatr. 2010, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- Tintore, M.; Vidal-Jordana, A.; Sastre-Garriga, J. Treatment of multiple sclerosis—success from bench to bedside. Nat. Rev. Neurol. 2019, 15, 53–58. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Kumar, A.; Patil, D.; Rajamohanan, P.R.; Ahmad, A. Isolation, Purification and Characterization of Vinblastine and Vincristine from Endophytic Fungus Fusarium oxysporum Isolated from Catharanthus roseus. PLoS ONE 2013, 8, e71805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakim, A.; Andayani, Y.; Deana Rahayuan, B. Isolation of Ethyl P-Methoxy Cinnamate from Kaemferia galanga L. J. Phys. Conf. Ser. 2018, 1095, 4–7. [Google Scholar] [CrossRef]
- Lin, L.C.; Pai, Y.F.; Tsai, T.H. Isolation of Luteolin and Luteolin-7-O-glucoside from Dendranthema morifolium Ramat Tzvel and Their Pharmacokinetics in Rats. J. Agric. Food Chem. 2015, 63, 7700–7706. [Google Scholar] [CrossRef] [PubMed]
- Jokić, S.; Rajić, M.; Bilić, B.; Molnar, M. Supercritical Extraction of Scopoletin from Helichrysum italicum (Roth) G. Don Flowers. Phytochem. Anal. 2016, 5, 290–295. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Yoga Latha, L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. African J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Mosbach, K. Molecular imprinting: Synthetic materials as substitutes for biological antibodies and receptors. Chem. Mater. 2008, 20, 859–868. [Google Scholar] [CrossRef]
- Baggiani, C.; Anfossi, L.; Giovannoli, C. Solid phase extraction of food contaminants using molecular imprinted polymers. Anal. Chim. Acta 2007, 591, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Puoci, F.; Cirillo, G.; Curcio, M.; Iemma, F.; Spizzirri, U.G.; Picci, N. Molecularly imprinted solid phase extraction for the selective HPLC determination of α-tocopherol in bay leaves. Anal. Chim. Acta 2007, 593, 164–170. [Google Scholar] [CrossRef]
- Scorrano, S.; Mergola, L.; del Sole, R.; Vasapollo, G. Synthesis of molecularly imprinted polymers for amino acid derivates by using different functional monomers. Int. J. Mol. Sci. 2011, 12, 1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bossi, A.; Bonini, F.; Turner, A.P.F.; Piletsky, S.A. Molecularly I mprinted polymers for the recognition of proteins: The state of the art. Biosens. Bioelectron. 2007, 22, 1131–1137. [Google Scholar] [CrossRef]
- Shi, H.; Tsal, W.B.; Garrison, M.D.; Ferrari, S.; Ratner, B.D. Template-imprinted nanostructured surfaces for protein recognition. Nature 1999, 398, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, S. Molecular imprinting: A versatile tool for separation, sensors and catalysis. Adv. Polym. Sci. 2007, 206, 191–210. [Google Scholar] [CrossRef]
- Piletsky, S.A.; Piletskaya, E.V.; Elgersma, A.V.; Yano, K.; Karube, I.; Parhometz, Y.P.; El’skaya, A.V. Atrazine sensing by molecularly imprinted membranes. Biosens. Bioelectron. 1995, 10, 959–964. [Google Scholar] [CrossRef]
- Puoci, F.; Iemma, F.; Picci, N. Stimuli-Responsive Molecularly Imprinted Polymers for Drug Delivery: A Review. Curr. Drug Deliv. 2008, 5, 85–96. [Google Scholar] [CrossRef]
- Andersson, L.I. Molecular imprinting: Developments and applications in the analytical chemistry field. J. Chromatogr. B Biomed. Sci. Appl. 2000, 745, 3–13. [Google Scholar] [CrossRef]
- Sellergren, B. Imprinted chiral stationary phases in high-performance liquid chromatography. J. Chromatogr. A 2001, 906, 227–252. [Google Scholar] [CrossRef]
- Tamayo, F.G.; Titirici, M.M.; Martin-Esteban, A.; Sellergren, B. Synthesis and evaluation of new propazine-imprinted polymer formats for use as stationary phases in liquid chromatography. In Proceedings of the Analytica Chimica Acta, Stockholm, Sweden, 29 June 2005; Volume 542. [Google Scholar]
- Hu, S.G.; Li, L.; He, X.W. Solid-phase extraction of esculetin from the ash bark of Chinese traditional medicine by using molecularly imprinted polymers. J. Chromatogr. A 2005, 1062, 31–37. [Google Scholar] [CrossRef]
- Kitahara, K.I.; Yoshihama, I.; Hanada, T.; Kokuba, H.; Arai, S. Synthesis of monodispersed molecularly imprinted polymer particles for high-performance liquid chromatographic separation of cholesterol using templating polymerization in porous silica gel bound with cholesterol molecules on its surface. J. Chromatogr. A 2010, 1217, 7249–7254. [Google Scholar] [CrossRef]
- Saad, E.M.; El Gohary, N.A.; Abdel-Halim, M.; Handoussa, H.; Mohamed El Nashar, R.; Mizaikoff, B. Molecularly Imprinted Polymers for Selective Extraction of Rosmarinic Acid from Rosmarinus Officinalis L; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; Volume 335, ISBN 2001005044622. [Google Scholar]
- Hasanah, A.N.; Andriana, G.; Pratiwi, R.; Megantara, S. Synthesis of molecular imprinting solid phase extraction (MI-SPE) for quercetin purification as active compound in plant extract. Eurasian J. Anal. Chem. 2018, 13, 36–43. [Google Scholar]
- Wang, L.; Fu, W.; Shen, Y.; Tan, H.; Xu, H.; McPhee, D.J. Molecularly imprinted polymers for selective extraction of Oblongifolin C from garcinia Yunnanensis Hu. Molecules 2017, 22, 508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosny, H.; El Gohary, N.; Saad, E.; Handoussa, H.; El Nashar, R.M. Isolation of sinapic acid from broccoli using molecularly imprinted polymers. J. Sep. Sci. 2018, 41, 1164–1172. [Google Scholar] [CrossRef]
- Saad, E.M.; Madbouly, A.; Ayoub, N.; Mohamed El Nashar, R. Preparation and application of molecularly imprinted polymer for isolation of chicoric acid from Chicorium intybus L. medicinal plant. Anal. Chim. Acta 2015, 877, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Tsermentseli, S.K.; Manesiotis, P.; Assimopoulou, A.N.; Papageorgiou, V.P. Molecularly imprinted polymers for the isolation of bioactive naphthoquinones from plant extracts. J. Chromatogr. A 2013, 1315, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.; Claude, B.; Morin, P.; Max, J.P.; Pena, R.; Ribet, J.P. Synthesis and study of a molecularly imprinted polymer for the specific extraction of indole alkaloids from Catharanthus roseus extracts. Anal. Chim. Acta 2011, 683, 198–205. [Google Scholar] [CrossRef]
- Cirillo, G.; Curcio, M.; Parisi, O.I.; Puoci, F.; Iemma, F.; Spizzirri, U.G.; Restuccia, D.; Picci, N. Molecularly imprinted polymers for the selective extraction of glycyrrhizic acid from liquorice roots. Food Chem. 2011, 125, 1058–1063. [Google Scholar] [CrossRef]
- Huang, M.; Pang, W.; Zhang, J.; Lin, S.; Hu, J. A target analogue imprinted polymer for the recognition of antiplatelet active ingredients in Radix Salviae Miltiorrhizae by LC/MS/MS. J. Pharm. Biomed. Anal. 2012, 58, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Fang, Q.; Feng, B.; Sun, S.; Du, W.; Amut, E.; Xiao, A.; Chang, C. Matrine-imprinted monolithic stationary phase for extraction and purification of matrine from Sophorae flavescentis Ait. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 894–900. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, M.; Fu, Q.; Wang, L.; Wang, D.; Zhang, K.; Xia, Z.; Gao, D. Novel dual functional monomers based molecularly imprinted polymers for selective extraction of myricetin from herbal medicines. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1097–1098, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lin, H.; Zhang, J.; She, Y.; Zhou, X.; Li, X.; Cui, Y.; Wang, J.; Rabah, T.; Shao, Y. Extraction and Identification of Matrine-Type Alkaloids from Sophora Moorcroftiana Using Double-Templated Molecularly Imprinted Polymers with HPLC–MS/MS; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; Volume 41, ISBN 1389361241. [Google Scholar]
- Gao, D.; Yang, F.; Xia, Z.; Zhang, Q. Molecularly imprinted polymer for the selective extraction of luteolin from Chrysanthemum morifolium Ramat. J. Sep. Sci. 2016, 39, 3002–3010. [Google Scholar] [CrossRef] [PubMed]
- Kitabatake, T.; Tabo, H.; Matsunaga, H.; Haginaka, J. Preparation of monodisperse curcumin-imprinted polymer by precipitation polymerization and its application for the extraction of curcuminoids from Curcuma longa L. Anal. Bioanal. Chem. 2013, 405, 6555–6561. [Google Scholar] [CrossRef] [PubMed]
- Funaya, N.; Haginaka, J. Matrine- and oxymatrine-imprinted monodisperse polymers prepared by precipitation polymerization and their applications for the selective extraction of matrine-type alkaloids from Sophora flavescens Aiton. J. Chromatogr. A 2012, 1248, 18–23. [Google Scholar] [CrossRef]
- Bi, W.; Tian, M.; Row, K.H. Separation of phenolic acids from natural plant extracts using molecularly imprinted anion-exchange polymer confined ionic liquids. J. Chromatogr. A 2012, 1232, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.F.; Wang, G.Y.; Shi, Y.P. Molecularly imprinted polymer microspheres for solid-phase extraction of protocatechuic acid in Rhizoma homalomenae. J. Sep. Sci. 2011, 34, 2602–2610. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, Y.; Huang, M.; Xu, R.; Zeng, H.; Nie, C.; Kong, J. Development and characterization of molecularly imprinted polymers for the selective enrichment of podophyllotoxin from traditional Chinese medicines. Anal. Chim. Acta 2011, 695, 63–72. [Google Scholar] [CrossRef]
- Yin, X.; Liu, Q.; Jiang, Y.; Luo, Y. Development of andrographolide molecularly imprinted polymer for solid-phase extraction. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2011, 79, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Y.; Ju, Z.; Niu, L.; Gong, Z.; Xu, Z. Molecularly imprinted polymers fabricated by Pickering emulsion polymerization for the selective adsorption and separation of quercetin from Spina Gleditsiae. New J. Chem. 2019, 43, 14747–14755. [Google Scholar] [CrossRef]
- Gomes, C.; Sadoyan, G.; Dias, R.C.S.; Costa, M.R.P.F.N. Development of molecularly imprinted polymers to target polyphenols present in plant extracts. Processes 2017, 5, 72. [Google Scholar] [CrossRef] [Green Version]
- Lai, J.P.; He, X.W.; Jiang, Y.; Chen, F. Preparative separation and determination of matrine from the Chinese medicinal plant Sophora flavescens Ait by molecularly imprinted solid-phase extraction. Anal. Bioanal. Chem. 2003, 375, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S. Application of magnetic molecularly imprinted polymer as a versatile and highly selective tool in food and environmental analysis: Recent developments and trends. TrAC-Trends Anal. Chem. 2017, 90, 89–106. [Google Scholar] [CrossRef]
- Dinc, M.; Esen, C.; Mizaikoff, B. Recent advances on core–shell magnetic molecularly imprinted polymers for biomacromolecules. TrAC-Trends Anal. Chem. 2019, 114, 202–217. [Google Scholar] [CrossRef]
- Cui, Y.; Ding, L.; Ding, J. Recent advances of magnetic molecularly imprinted materials: From materials design to complex sample pretreatment. TrAC-Trends Anal. Chem. 2022, 147, 116514. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Yu, X. Magnetic Molecularly Imprinted Polymers: Synthesis and Applications in the Selective Extraction of Antibiotics. Front. Chem. 2021, 9, 555. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Pang, L.; Geng, P.; Mi, F.; Hu, C.; Peng, F.; Guan, M. Application progress of magnetic molecularly imprinted polymer chemical sensors in the detection of biomarkers. Analyst 2022, 147, 571–586. [Google Scholar] [CrossRef]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic Nanoparticles: Design and Characterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Kong, X.; Wang, X.; He, X.; Chen, L.; Zhang, Y. Preparation and characterization of uniformly sized molecularly imprinted polymers functionalized with core-shell magnetic nanoparticles for the recognition and enrichment of protein. J. Mater. Chem. 2011, 21, 17863–17871. [Google Scholar] [CrossRef]
- Gu, X.H.; Xu, R.; Yuan, G.L.; Lu, H.; Gu, B.R.; Xie, H.P. Preparation of chlorogenic acid surface-imprinted magnetic nanoparticles and their usage in separation of Traditional Chinese Medicine. Anal. Chim. Acta 2010, 675, 64–70. [Google Scholar] [CrossRef]
- Lu, F.; Li, H.; Sun, M.; Fan, L.; Qiu, H.; Li, X.; Luo, C. Flow injection chemiluminescence sensor based on core-shell magnetic molecularly imprinted nanoparticles for determination of sulfadiazine. Anal. Chim. Acta 2012, 718, 84–91. [Google Scholar] [CrossRef]
- Zhu, W.; Jiang, G.; Xu, L.; Li, B.; Cai, Q.; Jiang, H.; Zhou, X. Facile and controllable one-step fabrication of molecularly imprinted polymer membrane by magnetic field directed self-assembly for electrochemical sensing of glutathione. Anal. Chim. Acta 2015, 886, 37–47. [Google Scholar] [CrossRef]
- Xie, X.; Wei, F.; Chen, L.; Wang, S. Preparation of molecularly imprinted polymers based on magnetic nanoparticles for the selective extraction of protocatechuic acid from plant extracts. J. Sep. Sci. 2015, 38, 1046–1052. [Google Scholar] [CrossRef]
- Shao, M.; Ning, F.; Zhao, J.; Wei, M.; Evans, D.G.; Duan, X. Magnetic molecularly imprinted specific solid-phase extraction for determination of dihydroquercetin from Larix griffithiana using HPLC. J. Sep. Sci. 2020, 43, 2301–2310. [Google Scholar] [CrossRef]
- Zhong, M.; Wang, Y.H.; Wang, L.; Long, R.Q.; Chen, C.L. Preparation and application of magnetic molecularly imprinted polymers for the isolation of chelerythrine from Macleaya cordata. J. Sep. Sci. 2018, 41, 3318–3327. [Google Scholar] [CrossRef]
- Xie, L.; Jiang, R.; Zhu, F.; Liu, H.; Ouyang, G. Application of functionalized magnetic nanoparticles in sample preparation. Anal. Bioanal. Chem. 2014, 406, 377–399. [Google Scholar] [CrossRef]
- Liu, L.; Shi, S.; Zhao, H.; Yu, J.; Jiang, X.; Chen, X. Selective fishing and analysis of xanthine oxidase binders from two Fabaceae species by coupling enzyme functionalized core-shell magnetic nanoparticles with HPLC-MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 945–946, 163–170. [Google Scholar] [CrossRef]
- Kang, X.; Deng, L.; Quan, T.; Gao, M.; Zhang, K.; Xia, Z.; Gao, D. Selective extraction of quinolizidine alkaloids from Sophora flavescens Aiton root using tailor-made deep eutectic solvents and magnetic molecularly imprinted polymers. Sep. Purif. Technol. 2021, 261, 118282. [Google Scholar] [CrossRef]
- Ma, X.; Lin, H.; He, Y.; She, Y.; Wang, M.; Abd El-Aty, A.M.; Afifi, N.A.; Han, J.; Zhou, X.; Wang, J.; et al. Magnetic Molecularly Imprinted Polymers Doped with Graphene Oxide for the Selective Recognition and Extraction of Four Flavonoids from Rhododendron Species; Elsevier B.V.: Amsterdam, The Netherlands, 2019; Volume 1598, ISBN 1389361241. [Google Scholar]
- Liu, J.; Wang, W.; Xie, Y.; Huang, Y.; Liu, Y.; Liu, X.; Zhao, R.; Liu, G.; Chen, Y. A novel polychloromethylstyrene coated superparamagnetic surface molecularly imprinted core-shell nanoparticle for bisphenol A. J. Mater. Chem. 2011, 21, 9232–9238. [Google Scholar] [CrossRef]
- Qiao, F.; Gao, M.; Yan, H. Molecularly imprinted ionic liquid magnetic microspheres for the rapid isolation of organochlorine pesticides in environmental water. J. Sep. Sci. 2016, 39, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.F.; Xie, X.Y.; Shi, Y.P. Magnetic molecularly imprinted polymer for the detection of rhaponticin in Chinese patent medicines. J. Chromatogr. A 2012, 1252, 8–14. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef] [PubMed]
- Söylemez, M.A.; Okan, M.; Güven, O.; Barsbay, M. Synthesis of well-defined molecularly imprinted bulk polymers for the removal of azo dyes from water resources. Curr. Res. Green Sustain. Chem. 2021, 4, 100196. [Google Scholar] [CrossRef]
- Wang, X.; Fang, Q.; Liu, S.; Chen, L. Preparation of a magnetic molecularly imprinted polymer with pseudo template for rapid simultaneous determination of cyromazine and melamine in bio-matrix samples. Anal. Bioanal. Chem. 2012, 404, 1555–1564. [Google Scholar] [CrossRef]
- Dai, J.; He, J.; Xie, A.; Gao, L.; Pan, J.; Chen, X.; Zhou, Z.; Wei, X.; Yan, Y. Novel pitaya-inspired well-defined core-shell nanospheres with ultrathin surface imprinted nanofilm from magnetic mesoporous nanosilica for highly efficient chloramphenicol removal. Chem. Eng. J. 2016, 284, 812–822. [Google Scholar] [CrossRef]
- Hang, H.; Li, C.; Pan, J.; Li, L.; Dai, J.; Dai, X.; Yu, P.; Feng, Y. Selective separation of lambdacyhalothrin by porous/magnetic molecularly imprinted polymers prepared by Pickering emulsion polymerization. J. Sep. Sci. 2013, 36, 3285–3294. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Hao, Y.; Zhao, S.; Zhang, L.; Cui, X.; Liu, D.; Tang, Y.; Zheng, Y. Novel magnetic multi-template molecularly imprinted polymers for specific separation and determination of three endocrine disrupting compounds simultaneously in environmental water samples. RSC Adv. 2014, 4, 56798–56808. [Google Scholar] [CrossRef]
- Zhao, B.; He, M.; Chen, B.; Hu, B. Novel ion imprinted magnetic mesoporous silica for selective magnetic solid phase extraction of trace Cd followed by graphite furnace atomic absorption spectrometry detection. Spectrochim. Acta-Part B At. Spectrosc. 2015, 107, 115–124. [Google Scholar] [CrossRef]
- Liu, X.; Yu, D.; Yu, Y.; Ji, S. Preparation of a magnetic molecularly imprinted polymer for selective recognition of rhodamine B. Appl. Surf. Sci. 2014, 320, 138–145. [Google Scholar] [CrossRef]
- Lofgreen, J.E.; Ozin, G.A. Controlling morphology and porosity to improve performance of molecularly imprinted sol-gel silica. Chem. Soc. Rev. 2014, 43, 911–933. [Google Scholar] [CrossRef]
- He, Y.; Huang, Y.; Jin, Y.; Liu, X.; Liu, G.; Zhao, R. Well-defined nanostructured surface-imprinted polymers for highly selective magnetic separation of fluoroquinolones in human urine. ACS Appl. Mater. Interfaces 2014, 6, 9634–9642. [Google Scholar] [CrossRef]
- Mahajan, R.; Suriyanarayanan, S.; Nicholls, I.A. Improved solvothermal synthesis of γ-Fe2O3 magnetic nanoparticles for SiO2 coating. Nanomaterials 2021, 11, 1889. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Liu, X.; Qiu, G.; Wu, H.; Yi, R.; Zhang, N.; Xu, J. Solvothermal synthesis and characterization of size-controlled Fe3O4 nanoparticles. J. Alloys Compd. 2008, 458, 487–491. [Google Scholar] [CrossRef]
- Saragi, T.; Depi, B.L.; Butarbutar, S.; Permana, B. The impact of synthesis temperature on magnetite nanoparticles size synthesized by co-precipitation method. J. Phys. Conf. Ser. 2018, 1013, 012190. [Google Scholar] [CrossRef]
- Binh, V.T.; Purcell, S.T.; Semet, V.; Feschet, F. Nanotips and nanomagnetism. Appl. Surf. Sci. 1998, 130–132, 803–814. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Liu, J.; Liu, M.; Han, X.; Peng, Y.; Tian, X.; Liu, J.; Zhang, S. Synthesis of molecularly imprinted polymer via emulsion polymerization for application in solanesol separation. Appl. Sci. 2020, 10, 2868. [Google Scholar] [CrossRef] [Green Version]
- Barros, L.A.; Custodio, R.; Rath, S. Design of a new molecularly imprinted polymer selective for hydrochlorothiazide based on theoretical predictions using Gibbs free energy. J. Braz. Chem. Soc. 2016, 27, 2300–2311. [Google Scholar] [CrossRef]
- Song, X.; Wang, J.; Zhu, J. Effect of porogenic solvent on selective performance of molecularly imprinted polymer for quercetin. Mater. Res. 2009, 12, 299–304. [Google Scholar] [CrossRef] [Green Version]
- Foroughirad, S.; Arabzadeh, N.; Mohammadi, A.; Khosravi, A. Synthesis and characterization of novel water-compatible magnetic molecularly imprinted polymer for tartrazine. J. Chin. Adv. Mater. Soc. 2018, 6, 706–721. [Google Scholar] [CrossRef]
- Su, Y.; Qiu, B.; Chang, C.; Li, X.; Zhang, M.; Zhou, B.; Yang, Y. Separation of bovine hemoglobin using novel magnetic molecular imprinted nanoparticles. RSC Adv. 2018, 8, 6192–6199. [Google Scholar] [CrossRef] [Green Version]
- Ma, R.T.; Shi, Y.P. Magnetic molecularly imprinted polymer for the selective extraction of quercetagetin from Calendula officinalis extract. Talanta 2015, 134, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Huang, P.; Fu, Q.; Du, W.; Sun, S.; Li, Y.; Liu, M.; Chang, C. Preparation of monolithic imprinted stationary phase for clenbuterol by in situ polymerization and application in biological samples pretreatment. Chromatographia 2011, 74, 693–701. [Google Scholar] [CrossRef]
- Zhuang, L.; Zhang, W.; Zhao, Y.; Shen, H.; Lin, H.; Liang, J. Preparation and characterization of Fe3O4 particles with novel nanosheets morphology and magnetochromatic property by a modified solvothermal method. Sci. Rep. 2015, 5, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Nie, J.; Li, J.; Liu, H.; Yan, Z.; Kuang, L. Synthesis and characterization of core–shell magnetic molecularly imprinted polymers for selective recognition and determination of quercetin in apple samples. Food Chem. 2019, 287, 100–106. [Google Scholar] [CrossRef]
- Sadegh, N.; Asfaram, A.; Javadian, H.; Haddadi, H.; Sharifpour, E. Ultrasound-assisted solid phase microextraction-HPLC method based on Fe3O4@SiO2-NH2-molecularly imprinted polymer magnetic nano-sorbent for rapid and efficient extraction of harmaline from Peganum harmala extract. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1171, 122640. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Nie, J.; Liu, H.; Kuang, L.; Xu, G. Synthesis and characterization of magnetic molecularly imprinted polymers for effective extraction and determination of kaempferol from apple samples. J. Chromatogr. A 2020, 1630, 461531. [Google Scholar] [CrossRef]
- Xie, L.; Guo, J.; Zhang, Y.; Hu, Y.; You, Q.; Shi, S. Novel molecular imprinted polymers over magnetic mesoporous silica microspheres for selective and efficient determination of protocatechuic acid in Syzygium aromaticum. Food Chem. 2015, 178, 18–25. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, Q.; Liu, H.; Chen, X.; Zhang, D.; Lai, X.; Li, B.; Yang, X. Amido surface-functionalized magnetic molecularly imprinted polymers for the efficient extraction of Sibiskoside from Sibiraea angustata. J. Chromatogr. B 2019, 1109, 90–98. [Google Scholar] [CrossRef]
- Wang, D.D.; Gao, D.; Xu, W.J.; Li, F.; Yin, M.N.; Fu, Q.F.; Xia, Z.N. Magnetic molecularly imprinted polymer for the selective extraction of hesperetin from the dried pericarp of Citrus reticulata Blanco. Talanta 2018, 184, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Tabaraki, R.; Sadeghinejad, N. Preparation and application of magnetic molecularly imprinted polymers for rutin determination in green tea. Chem. Pap. 2020, 74, 1937–1944. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, X.; Lin, H.; Abd El-Aty, A.M.; Rabah, T.; Liu, X.; Yu, Z.; Yong, Y.; Ju, X.; She, Y. Magnetic molecularly imprinted specific solid-phase extraction for determination of dihydroquercetin from Larix griffithiana using HPLC. J. Sep. Sci. 2020, 43, 2301–2310. [Google Scholar] [CrossRef]
- Xie, L.; Guo, J.; Zhang, Y.; Shi, S. Efficient determination of protocatechuic acid in fruit juices by selective and rapid magnetic molecular imprinted solid phase extraction coupled with HPLC. J. Agric. Food Chem. 2014, 62, 8221–8228. [Google Scholar] [CrossRef]
- Chen, F.F.; Xie, X.Y.; Shi, Y.P. Preparation of magnetic molecularly imprinted polymer for selective recognition of resveratrol in wine. J. Chromatogr. A 2013, 1300, 112–118. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Qin, B.; Zhang, B.; Ma, J.G.; Hu, Y.Q.; Han, L.; He, M.F.; Liu, C.Y. Specific enrichment of caffeic acid from Taraxacum mon-golicum Hand.-Mazz. by pH and magnetic dual-responsive molecularly imprinted polymers. Anal. Chim. Acta 2020, 1096, 193–202. [Google Scholar] [CrossRef]
- Gao, D.; Wang, D.D.; Fu, Q.F.; Wang, L.J.; Zhang, K.L.; Yang, F.Q.; Xia, Z.N. Preparation and evaluation of magnetic molecularly imprinted polymers for the specific enrichment of phloridzin. Talanta 2018, 178, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Munhoz, V.M.; Longhini, R.; Souza, J.R.P.; Zequi, J.A.C.; Mello, E.V.S.L.; Lopes, G.C.; Mello, J.C.P. Extraction of flavonoids from Tagetes patula: Process optimization and screening for biological activity. Rev. Bras. Farmacogn. 2014, 24, 576–583. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Jin, J.; Dai, N.; Han, N.; Han, J.; Bao, B. Anti-inflammatory effects, nuclear magnetic resonance identification, and high-performance liquid chromatography isolation of the total flavonoids from Artemisia frigida. J. Food Drug Anal. 2016, 24, 385–391. [Google Scholar] [CrossRef] [Green Version]
- Marzouk, M.M. Flavonoid constituents and cytotoxic activity of Erucaria hispanica (L.) Druce growing wild in Egypt. Arab. J. Chem. 2016, 9, S411–S415. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.Y.; Li, Q.; Bi, K.S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Feng, S.; Li, M.; Zhao, D.; Li, X.; Zhang, L.; Wang, Z.; Gao, N. Simultaneous Determination of 10 Anthraquinones in Rhubarb Based on HPLC-Q-HR/MS. Chin. Herb. Med. 2017, 9, 388–395. [Google Scholar] [CrossRef]
- Matsuda, H.; Morikawa, T.; Toguchida, I.; Park, J.Y.; Harima, S.; Yoshikawa, M. Antioxidant constituents from rhubarb: Structural requirements of stilbenes for the activity and structures of two new anthraquinone glucosides. Bioorg. Med. Chem. 2001, 9, 41–50. [Google Scholar] [CrossRef]



| Type of Polymerization | Sample | Template Molecule | Functional Monomer | Crosslinker | Initiator | Porogen Solvent | Ref. |
|---|---|---|---|---|---|---|---|
| Bulk | Rosmarinus officinalis L. | Rosmarinic Acid | 4-Vinyl pyridine | EGDMA | AIBN | DMSO | [31] |
| Plectranthus scutellarioides | Quercetin | Acrylamide | EGDMA | AIBN | MeOH | [32] | |
| Gracinia yunnanensis Hu. | Oblongiofolin C | Acrylamide | EGDMA | AIBN | DMSO | [33] | |
| Broccoli (Botrtis italica L.) | Sinapic Acid | 4-Vinyl pyridine | EGDMA | N/A | DMSO | [34] | |
| Chicory herb (Chicorium intybus L.) | Chicoric Acid | 4-Vinyl pyridine | EGDMA | AIBN | DMSO | [35] | |
| Alkana tinctoria Roots | Shikonin | 2-Diethylaminoethyl methacrylate | EGDMA | ABDV | CHCl3 | [36] | |
| Catharanthus roseus | Catharanthine | Methacrylic Acid | EGDMA | AIBN | ACN | [37] | |
| Liquorice roots (Glycirrhiza glabra) | Glycyrrhizic Acid | 2-Hydroxyethylmetacrylate | EGDMA | AIBN | DMF | [38] | |
| In situ | Radix Salviae Miltiorrhizae | Propyl Gallate | 4-Vinyl pyridine | EGDMA | AIBN | EtOAc | [39] |
| Sophorae flavescentis Ait | Matrine | Methacrylic Acid | EGDMA | AIBN | Toluene | [40] | |
| Precipitation | Carthamus tinctorius L. | Myricetin | 4-Vinyl pyridine | EGDMA | AIBN | MeOH/ACN (1:2 v/v) | [41] |
| Sophora moorcroftiana | Matrine and Oxymatrine | Methacrylic Acid | EGDMA | AIBN | ACN | [42] | |
| Chrysanthemum morifolium Ramat | Luteolin | Acrylamide | EGDMA | AIBN | ACN/DMSO (19:1 v/v) | [43] | |
| Curcuma longa L. | Curcumin | Methacrylic Acid | Divinylbenzene | AIBN | ACN/Toluene (3:1 v/v) | [44] | |
| Sophora flavescens Aiton | Matrine and Oxymatrine | Methacrylic Acid | Divinylbenzene | AIBN | ACN/Toluene (3:1 v/v) | [45] | |
| Salicornia herbacea L. | Phenolic Acid | Methacrylic Acid | EGDMA | AIBN | Alcohol/Water (9:1 v/v) | [46] | |
| Rhizoma homalomenae | Protocatechuic Acid | Acrylamide | EGDMA | AIBN | ACN | [47] | |
| Traditional Chinese Medicine | Podophylotoxin | Acrylamide | EGDMA | AIBN | ACN | [48] | |
| Andrographis paniculata | Andrographolide | Acrylamide | EGDMA | AIBN | ACN/Toluene (3:1 v/v) | [49] | |
| Emulsion | Spina gleditsiae | Quercetin | 4-Vinyl pyridine | Divinyl Benzene | AIBN | Water-THF | [50] |
| Suspension | Vegetable Extract | Polydatin | 4-Vinyl pyridine | EGDMA | AIBN | Water/MeOH | [51] |
| Sophora flavescens Ait | Matrine | Methacrylic Acid | EGDMA | AIBN | CHCl3 | [52] |
| Factor | Effect [84] |
|---|---|
| Reaction time | Shorter reaction time, smaller size of nanoparticles [84] |
| Molar ratio of FeCl3 and protective agents | Higher molar ratio, smaller size of nanoparticles [84] |
| Initial concentration of FeCl3 | Higher concentration, larger size of nanoparticles [84] |
| pH | Higher pH value, smaller size of nanoparticles [84] |
| Reaction temperature | Higher temperature, larger size of nanoparticles [85] |
| Physical Characterization | Objective |
|---|---|
| Brunauer–Emmet–Teller (BET) | To analyze the porosity and specific surface area [91] |
| Fourier-transform infrared (FT-IR) | To determine the functional group of the molecular structure and the presence of Fe-O bond stretching vibration at 590 cm−1 [92] |
| Thermogravimetric analysis (TGA) | To analyze thermal stability and composition of a material [68] |
| Scanning electron microscopy (SEM) | To confirm molecule size distribution and morphology [93] |
| Vibration sample magnetometer (VSM) | To measure the magnetic separation ability of MMIPs, based on the relationship between magnetization and magnetic field strength [93] |
| X-ray diffraction (XRD) | To analyze the diffraction spectroscopy of materials [84] |
| Compound Group | Type of Polymerization | Sample | Analyte | Magnetic Materials | Monomer; Crosslinker; Initiator; Template | Yield/Purity (Y%/P%) and Adsorption Capacity (AC) | Ref. |
|---|---|---|---|---|---|---|---|
| Alkaloid | Precipitation Polymerization | Sophora flavescens Root | Quinolizidine Alkaloids | Fe3O4@SiO2@MPS | Acrylamide 1.0 mmol; EGDMA 4.0 mmol; AIBN 0.3 mmol; Oxymatrine 0.2 mmol | Y: 80.21–89.15% and 85.33–95.28% AC: 110.8 and 63.4 mg/g | [68] |
| Sol–Gel Polymerization | Peganum harmala extract | Harmaline | Fe3O4@SiO2 | Methacrylic Acid 1.3 mmol; EGDMA 18.6 mmol; AIBN 0.4 mmol; Harmaline 0.3 mmol | Y: >90% AC: 45.31 mg/g | [97] | |
| Surface Imprinting Polymerization | Macleaya cordata | Chelerythrine | Fe3O4@SiO2@MPS | Methacrylic Acid 0.04 mmol; EGDMA 2.0 mmol; AIBN 1.2 mmol; Chelerythrine 0.1 mmol | Y: not mentioned in the article AC: 7.96 mg/g | [65] | |
| Flavonoid | Precipitation Polymerization | Rhododendron species | Farrerol, Taxifolin, Kaempferol, Hyperin | Fe3O4@SiO2-GO@MPS | 4-Vinylpyridine 4.0 mmol; EGDMA 20 mmol; AIBN 0.1 mmol; Farrerol 0.1 mmol | Y: not mentioned in the article AC: 10.04–20.66 mg/g | [69] |
| Surface Imprinting Polymerization | Citrus reticulata Blanco | Hesperitin | Fe3O4@SiO2@MPS | N-Isopropylacrylamide 0.078 mmol; EGDMA 0.78 mmol; AIBN 0.06 mmol; Hesperetin 0.026 mmol | Y: 90.5–96.9% AC: 16.648 mg/g | [101] | |
| Surface Imprinting Polymerization | Apple Samples | Kaempferol | Fe3O4@SiO2 | Acrylamide 1.0 mmol; EGDMA 6.0 mmol; AIBN 0.2 mmol; Kaempferol 0.2 mmol | Y: not mentioned in the article AC: 3.84 mg/g | [98] | |
| Suspension Polymerization | Green Tea | Rutin | Fe3O4 | Methacrylic Acid 4.0 mmol; EGDMA 25.0 mmol; AIBN 1.0 mmol; Rutin 0.5 mmol | Y: not mentioned in the article AC: 2.43 mg/g | [102] | |
| Precipitation Polymerization | Larix griffithiana | Dihydro-quercetin | Fe3O4@SiO2 | 4-Viniylpyridine 0.065 mmol; EGDMA 0.41 mmol; AIBN 0.15 mmol; Dihydroquercetin 0.016 mmol | Y: 76.64–101.8% AC: 7.56 mg/g | [103] | |
| Glycoside | Suspension Polymerization | Chinese Patent Medicines | Rhaponticin | Fe3O4 | Acrylamide 6.0 mmol; EGDMA 30.0 mmol; AIBN 0.6 mmol; Rhaponticin 1.0 mmol | Y: 77.82–91.00% AC: not mentioned in the article | [72] |
| Polyphenol | Precipitation Polymerization | Homalomena occulta, Cynomorium songaricum | Protocatechuic Acid (PA) | Fe3O4@SiO2-CH=CH2 | Acrylamide 5.0 mmol; EGDMA 30.0 mmol; AIBN 0.6 mmol; PA 1.0 mmol | Y: 86.3–122% AC: not mentioned in the article | [63] |
| Surface Imprinting Polymerization | Fruit Juice (apple, pineapple, orange, peach juice) | Protocatechuic Acid (PA) | Fe3O4@SiO2@MPS | 4-Vinylpyridine 1.0 mmol; EGDMA 5.0 mmol; AIBN 0.1 mmol; PA 0.25 mmol | Y: 92–107% AC: 7.5 mg/g | [104] | |
| Surface Imprinting Polymerization | Wine | Rhapontigenin (resveratrol dummy analogues) | Fe3O4@SiO2@MPS | Acrylamide 0.5 mmol; EGDMA 3.0 mmol; AIBN 0.1 mmol; Rhapotingenin 0.1 mmol | Y: 79.3–90.6% AC: 5.33 mg/g | [105] | |
| Surface Imprinting Polymerization | Syxigium aromaticum | Protocatechuic Acid (PA) | Fe3O4@SiO2 | 4-Vinylpyridine 1.0 mmol; EGDMA 5.0 mmol; AIBN 0.1 mmol; PA 0.25 mmol | Y: 29.3 µg/g extract AC: 11.9 mg/g | [99] | |
| Suspension Polymerization | Traditional Chinese Medicine (TCM) | Chlorogenic Acid | Fe3O4@SiO2@MPS | Methacrylic Acid 3.0 mmol; TRIM 5.0 mmol; AIBN 0.15 mmol; Chlorogenic Acid 0.25 mmol | P: 80.58% AC: 5.07 mg/g | [60] | |
| Surface Imprinting Polymerization | Taraxacum mon-golicum Hand.-Mazz | Caffeic Acid | Fe3O4@SiO2@MPS | 4-Vinylpyridine 0.2 mmol and 2-(Dimethylamino) Ethyl Methacrylate (DMA) 0.2 mmol; EGDMA 2.0 mmol; AIBN 0.1 mmol; Caffeic Acid 0.1 mmol | Y: 90.47–98.97% AC: 11.5 mg/g | [106] | |
| Surface Imprinting Polymerization | Malus doumeri (Bois) A. | Phloridzin | Fe3O4@SiO2@NH2 | Fe3O4@SiO2@NH2 0.13 mmol; EGDMA 0.85 mmol; AIBN 0.05 mmol; Phloridzin 0.08 mmol | Y: 81.45–90.27% AC: | [107] | |
| Terpene | Surface Imprinting Polymerization | Sibiraea angustata | Sibiskoside | Fe3O4@SiO2 | 4-Vinyl Benzoic Acid 0.2 mmol; EGDMA 1.0 mmol; AIBN 0.12 mmol; Sibiskoside 0.05 mmol | Y: 6.0 mg/g extract AC: 13.75 mg/g | [100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariani, M.D.; Zuhrotun, A.; Manesiotis, P.; Hasanah, A.N. Magnetic Molecularly Imprinted Polymers: An Update on Their Use in the Separation of Active Compounds from Natural Products. Polymers 2022, 14, 1389. https://doi.org/10.3390/polym14071389
Ariani MD, Zuhrotun A, Manesiotis P, Hasanah AN. Magnetic Molecularly Imprinted Polymers: An Update on Their Use in the Separation of Active Compounds from Natural Products. Polymers. 2022; 14(7):1389. https://doi.org/10.3390/polym14071389
Chicago/Turabian StyleAriani, Marisa Dwi, Ade Zuhrotun, Panagiotis Manesiotis, and Aliya Nur Hasanah. 2022. "Magnetic Molecularly Imprinted Polymers: An Update on Their Use in the Separation of Active Compounds from Natural Products" Polymers 14, no. 7: 1389. https://doi.org/10.3390/polym14071389
APA StyleAriani, M. D., Zuhrotun, A., Manesiotis, P., & Hasanah, A. N. (2022). Magnetic Molecularly Imprinted Polymers: An Update on Their Use in the Separation of Active Compounds from Natural Products. Polymers, 14(7), 1389. https://doi.org/10.3390/polym14071389