Effects of Thermal Aging on Molar Mass of Ultra-High Molar Mass Polyethylene Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Aging Experiments
2.2. Molar Mass Distribution Determination
2.3. Crystallinity Determination and Wide-Angle X-ray Scattering
2.4. Fiber Topography and Chemical Composition
2.5. Free Radical Determination
2.6. Chemical Characterization
3. Results and Discussion
3.1. Molar Mass of Thermally Aged Fibers
3.2. Crystal Morphology and Crystallinity of Aged Fibers
3.3. Topography and Oxygen Content of Aged Fibers
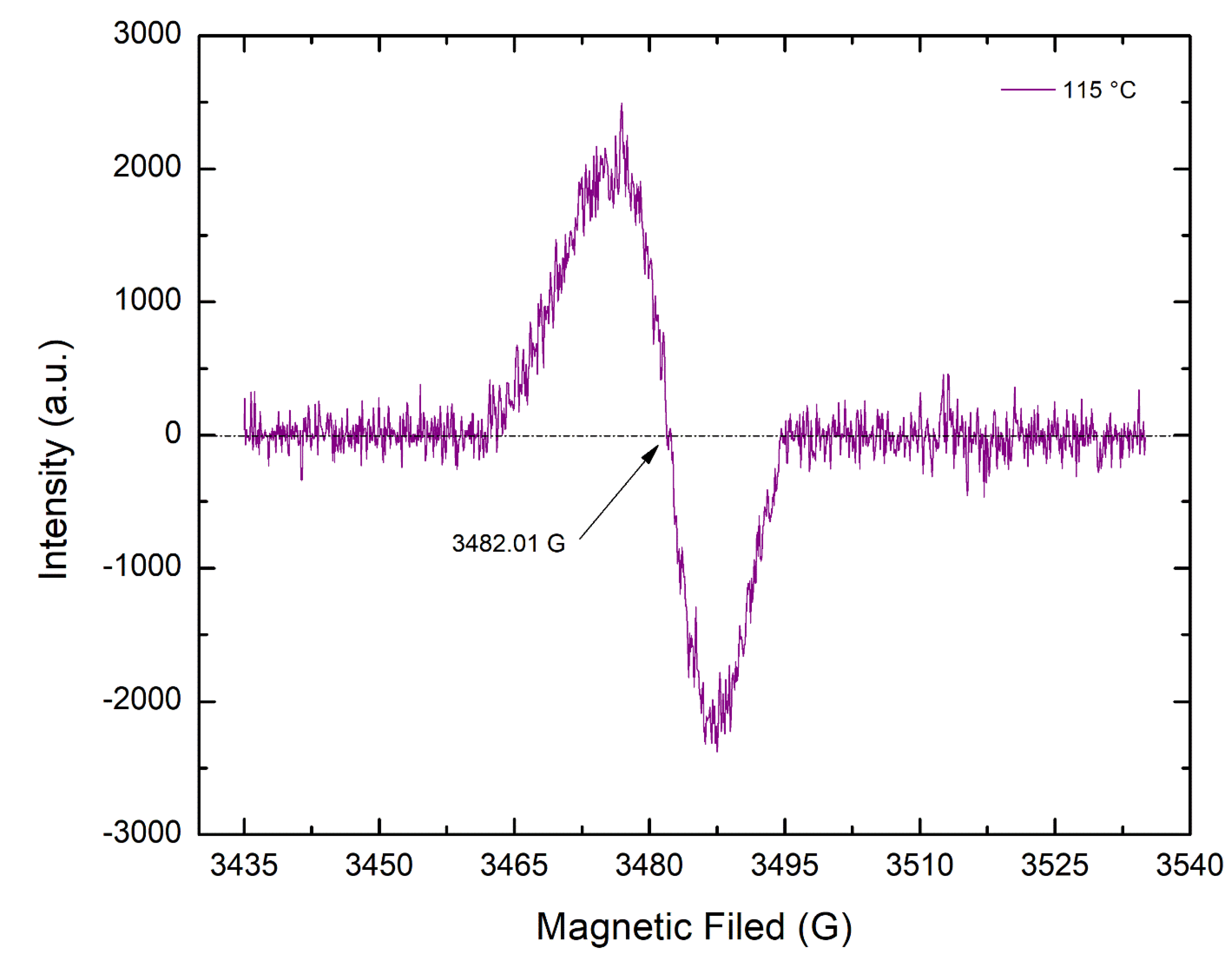
3.4. The Presence of the Polyenyl C-Centered Free Radicals in Aged Fibers
3.5. Unsaturations Present in Aged Fibers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Folgar, F. Thermoplastic matrix combat helmet with carbon-epoxy skin for ballistic performance. In Advanced Fibrous Composite Materials for Ballistic Protection; Woodhead Publishing: Cambridge, UK, 2016; Volume 15, pp. 437–456. [Google Scholar]
- Wittmann, J.; Lotz, B. Epitaxial crystallization of monoclinic and orthorhombic polyethylene phases. Polymer 1989, 30, 27–34. [Google Scholar]
- Folgar, F. Ballistic Helmets and Method of Manufacture Thereof. U.S. Patent 9,307,803B1, 12 April 2016. [Google Scholar]
- Mazelsky, B. Flexible Body Armor. U.S. Patent 5,996,115, 7 December 1999. [Google Scholar]
- Joseph, A.; Wiley, A.; Orr, R.; Schram, B.; Dawes, J.J. The impact of load carriage on measures of power and agility in tactical occupations: A critical review. Int. J. Environ. Res. Public Health 2018, 15, 88. [Google Scholar] [CrossRef] [Green Version]
- Schram, B.; Orr, R.; Pope, R.; Hinton, B.; Norris, G. Comparing the effects of different body armor systems on the occupational performance of police officers. Int. J. Environ. Res. Public Health 2018, 15, 893. [Google Scholar] [CrossRef] [Green Version]
- Crouch, I.G. Body armour—New materials, new systems. Def. Technol. 2019, 15, 241–253. [Google Scholar]
- DSM Dyneema Tech. Ultra High Molecular Weight Polyethylene Fiber from DSM Dyneema. DataSheet. 2016. Available online: https://extreemasoftslings.com/wp-content/uploads/2019/02/Dyneema-UHMWPF.pdf (accessed on 16 February 2022).
- Sanborn, B.; DiLeonardi, A.M.; Weerasooriya, T. Tensile properties of Dyneema SK76 single fibers at multiple loading rates using a direct gripping method. J. Dyn. Behav. Mater. 2015, 1, 4–14. [Google Scholar]
- James, N. Body Armor for Law Enforcement Officers: In Brief. 2016. Available online: https://ecommons.cornell.edu/bitstream/handle/1813/79529/CRS_Body_Armor_for_Law_Enforcement.pdf?sequence=1&isAllowed=y (accessed on 16 February 2022).
- Chin, J.; Forster, A.; Rice, K. Chapter 10: Field and Laboratory Aging Effects on Poly (p-phenylene benzobisoxazole) Fibers Used in Body Armor. In Polymer Degradation and Performance; ACS Publications: Washington, DC, USA, 2009; pp. 113–120. [Google Scholar]
- Hart, S.V. Report to the Attorney General on Body Armor Safety Initiative Testing and Activities; Office of Justice Programs, Department of Justice: Washington, DC, USA, 2004.
- Office of Justice Programs, US Department of Justice. Third Status Report to the Attorney General on Body Armor Safety Initiative Testing and Activities; National Institute of Justice (NIJ): Washington, DC, USA, 2005.
- Messin, G.H.; Rice, K.D.; Riley, M.A.; Watson, S.S.; Sieber, J.R.; Forster, A.L. Effect of moisture on copolymer fibers based on 5-amino-2-(p-aminophenyl)-benzimidazole. Polym. Degrad. Stab. 2011, 96, 1847–1857. [Google Scholar]
- Forster, A.L.; Pintus, P.; Messin, G.H.; Riley, M.A.; Petit, S.; Rossiter, W.; Chin, J.; Rice, K.D. Hydrolytic stability of polybenzobisoxazole and polyterephthalamide body armor. Polym. Degrad. Stab. 2011, 96, 247–254. [Google Scholar]
- Forster, A.L.; Forster, A.M.; Chin, J.W.; Peng, J.S.; Lin, C.C.; Petit, S.; Kang, K.L.; Paulter, N.; Riley, M.A.; Rice, K.D. Long-term stability of UHMWPE fibers. Polym. Degrad. Stab. 2015, 114, 45–51. [Google Scholar]
- Costa, L.; Luda, M.; Trossarelli, L. Ultra-high molecular weight polyethylene: I. Mechano-oxidative degradation. Polym. Degrad. Stab. 1997, 55, 329–338. [Google Scholar]
- Costa, L.; Luda, M.; Trossarelli, L. Ultra high molecular weight polyethylene—II. Thermal-and photo-oxidation. Polym. Degrad. Stab. 1997, 58, 41–54. [Google Scholar]
- Costa, L.; Bracco, P. Mechanisms of cross-linking, oxidative degradation, and stabilization of UHMWPE. In UHMWPE Biomaterials Handbook; Elsevier: Amsterdam, The Netherlands, 2016; pp. 467–487. [Google Scholar]
- Lacoste, J.; Carlsson, D. Gamma-, photo-, and thermally-initiated oxidation of linear low density polyethylene: A quantitative comparison of oxidation products. J. Polym. Sci. A Polym. Chem. 1992, 30, 493–500. [Google Scholar]
- Lacoste, J.; Deslandes, Y.; Black, P.; Carlsson, D. Surface and bulk analyses of the oxidation of polyolefins. Polym. Degrad. Stab. 1995, 49, 21–28. [Google Scholar]
- Chin, J.W.; Byrd, E.; Clerici, C.; Forster, A.L.; Oudina, M.; Sung, L.P.; Rice, K.D. Chemical and Physical Characterization of Poly(p-phenylene-2,6-benzobisoxazole) Fibers Used in Body Armor: Temperature and Humidity Aging; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2006.
- Tsinas, Z.; Forster, A.L.; Al-Sheikhly, M. Oxidation reactions in kink banded regions of UHMMPE fiber-based laminates used in body armor: A mechanistic study. Polym. Degrad. Stab. 2018, 154, 103–114. [Google Scholar]
- Dijkstra, D.; Pennings, A. The role of taut tie molecules on the mechanical properties of gel-spun UHMWPE fibres. Polym. Bull. 1988, 19, 73–80. [Google Scholar]
- Klein, P.; Woods, D.; Ward, I. The effect of electron irradiation on the structure and mechanical properties of highly drawn polyethylene fibers. J. Polym. Sci. B Polym. Phys. 1987, 25, 1359–1379. [Google Scholar]
- Wilding, M.A.; Ward, I.M. Routes to improved creep behaviour in drawn linear polyethylene. Plast. Rubber Process. Appl. 1981, 1, 167–172. [Google Scholar]
- Deng, M.; Latour, R.; Drews, M.; Shalaby, S.W. Effects of gamma irradiation, irradiation environment, and postirradiation aging on thermal and tensile properties of ultrahigh molecular weight polyethylene fibers. J. Appl. Polym. Sci. 1996, 61, 2075–2084. [Google Scholar]
- De Boer, J.; Pennings, A. Crosslinking of ultra-high strength polyethylene fibers by means of γ-radiation. Polym. Bull. 1981, 5, 317–324. [Google Scholar]
- Forster, A.L.; Tsinas, Z.; Al-Sheikhly, M. Effect of irradiation and detection of long-lived polyenyl radicals in highly crystalline ultra-high molar mass polyethylene (UHMMPE) fibers. Polymers 2019, 11, 924. [Google Scholar] [CrossRef] [Green Version]
- Kerber, R.W. Schnabel: Polymer Degradation, Principles and Practical Applications. Carl Hanser Verlag, München 1981. 227 Seiten, Preis: DM 68,—. Ber. Bunsenges. Phys. Chem. 1983, 87, 838–839. [Google Scholar]
- Zhao, L.; Song, P.a.; Cao, Z.; Fang, Z.; Guo, Z. Thermal stability and rheological behaviors of high-density polyethylene/fullerene nanocomposites. J. Nanomater. 2012, 2012, 340962. [Google Scholar]
- Nyden, M.R.; Stoliarov, S.I.; Westmoreland, P.R.; Guo, Z.; Jee, C. Applications of reactive molecular dynamics to the study of the thermal decomposition of polymers and nanoscale structures. Mater. Sci. Eng. A 2004, 365, 114–121. [Google Scholar]
- Stoliarov, S.I.; Lyon, R.E.; Nyden, M.R. A reactive molecular dynamics model of thermal decomposition in polymers. II. Polyisobutylene. Polymer 2004, 45, 8613–8621. [Google Scholar]
- Knyazev, V.D. Effects of Chain Length on the Rates of C−C Bond Dissociation in Linear Alkanes and Polyethylene. J. Phys. Chem. A 2007, 111, 3875–3883. [Google Scholar]
- Chabba, S.; Van Es, M.; Van Klinken, E.; Jongedijk, M.; Vanek, D.; Gijsman, P.; Van Der Waals, A. Accelerated aging study of ultra high molecular weight polyethylene yarn and unidirectional composites for ballistic applications. J. Mater. Sci. 2007, 42, 2891–2893. [Google Scholar]
- Jung, M.R.; Balazs, G.H.; Work, T.M.; Jones, T.T.; Orski, S.V.; Rodriguez, C.V.; Beers, K.L.; Brignac, K.C.; Hyrenbach, K.D.; Jensen, B.A.; et al. Polymer identification of plastic debris ingested by pelagic-phase sea turtles in the central Pacific. Environ. Sci. Technol. 2018, 52, 11535–11544. [Google Scholar]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, V.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.-J. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2018, 127, 704–716. [Google Scholar]
- ASTM D 6474-12; Standard Test Method for Determining Molecular Weight Distribution and Molecular Weight Averages of Polyolefins by High Temperature Gel Permeation Chromatography. Document Center Inc.: Belmont, CA, USA, 2012.
- Roe, R.-J.; Roe, R. Methods of X-ray and Neutron Scattering in Polymer Science; Oxford University Press: New York, NY, USA, 2000; Volume 739. [Google Scholar]
- Russell, K.; Hunter, B.; Heyding, R. Monoclinic polyethylene revisited. Polymer 1997, 38, 1409–1414. [Google Scholar]
- Kwon, Y.; Boller, A.; Pyda, M.; Wunderlich, B. Melting and heat capacity of gel-spun, ultra-high molar mass polyethylene fibers. Polymer 2000, 41, 6237–6249. [Google Scholar]
- Joo, Y.; Han, O.; Lee, H.-K.; Song, J. Characterization of ultra high molecular weight polyethyelene nascent reactor powders by X-ray diffraction and solid state NMR. Polymer 2000, 41, 1355–1368. [Google Scholar]
- Stadler, F.J.; Kaschta, J.; Münstedt, H. Dynamic-mechanical behavior of polyethylenes and ethene-/α-olefin-copolymers. Part I. α′-Relaxation. Polymer 2005, 46, 10311–10320. [Google Scholar]
- Yeh, J.-T.; Lin, S.-C.; Tu, C.-W.; Hsie, K.-H.; Chang, F.-C. Investigation of the drawing mechanism of UHMWPE fibers. J. Mater. Sci. 2008, 43, 4892–4900. [Google Scholar]
- Takahashi, Y.; Ishida, T.; Furusaka, M. Monoclinic-to-orthorhombic transformation in polyethylene. J. Polym. Sci. B Polym. Phys. 1988, 26, 2267–2277. [Google Scholar]
- Hu, W.; Buzin, A.; Lin, J.S.; Wunderlich, B. Annealing behavior of gel-spun polyethylene fibers at temperatures lower than needed for significant shrinkage. J. Polym. Sci. B Polym. Phys. 2003, 41, 403–417. [Google Scholar]
- Berger, L.; Kausch, H.; Plummer, C. Structure and deformation mechanisms in UHMWPE-fibres. Polymer 2003, 44, 5877–5884. [Google Scholar]
- Li, C.-S.; Zhan, M.-S.; Huang, X.-C.; Zhou, H. Degradation behavior of ultra-high molecular weight polyethylene fibers under artificial accelerated weathering. Polym. Test. 2012, 31, 938–943. [Google Scholar]
- Tsinas, Z. Towards an Understanding of the Degradation Mechanisms of UHMWPE-Based Soft Ballistic Inserts. Ph.D. Thesis, University of Maryland, Colege Park, MD, USA, 2016. [Google Scholar]
- Kasser, M.J.; Silverman, J.; Al-Sheikhly, M. EPR simulation of polyenyl radicals in ultrahigh molecular weight polyethylene. Macromolecules 2010, 43, 8862–8867. [Google Scholar]
- Tantipattarakul, S.; Vaughan, A.S.; Andritsch, T. Ageing behaviour of a polyethylene blend: Influence of chemical defects and morphology on charge transport. High Volt. 2020, 5, 270–279. [Google Scholar]
- Johnson, W.; Lyons, B. Radiolytic formation and decay of trans-vinylene unsaturation in polyethylene: Fourier transform infra-red measurements. Radiat. Phys. Chem. 1995, 46, 829–832. [Google Scholar]
- Dole, M.; Milner, D.; Williams, T.F. Irradiation of polyethylene. II. Kinetics of unsaturation effects. J. Am. Chem. Soc. 1958, 80, 1580–1588. [Google Scholar]
- Jahan, M.S. ESR insights into macroradicals in UHMWPE. In UHMWPE Biomaterials Handbook, 2nd ed.; Academic Press: Boston, MA, USA, 2009; pp. 433–450. [Google Scholar]
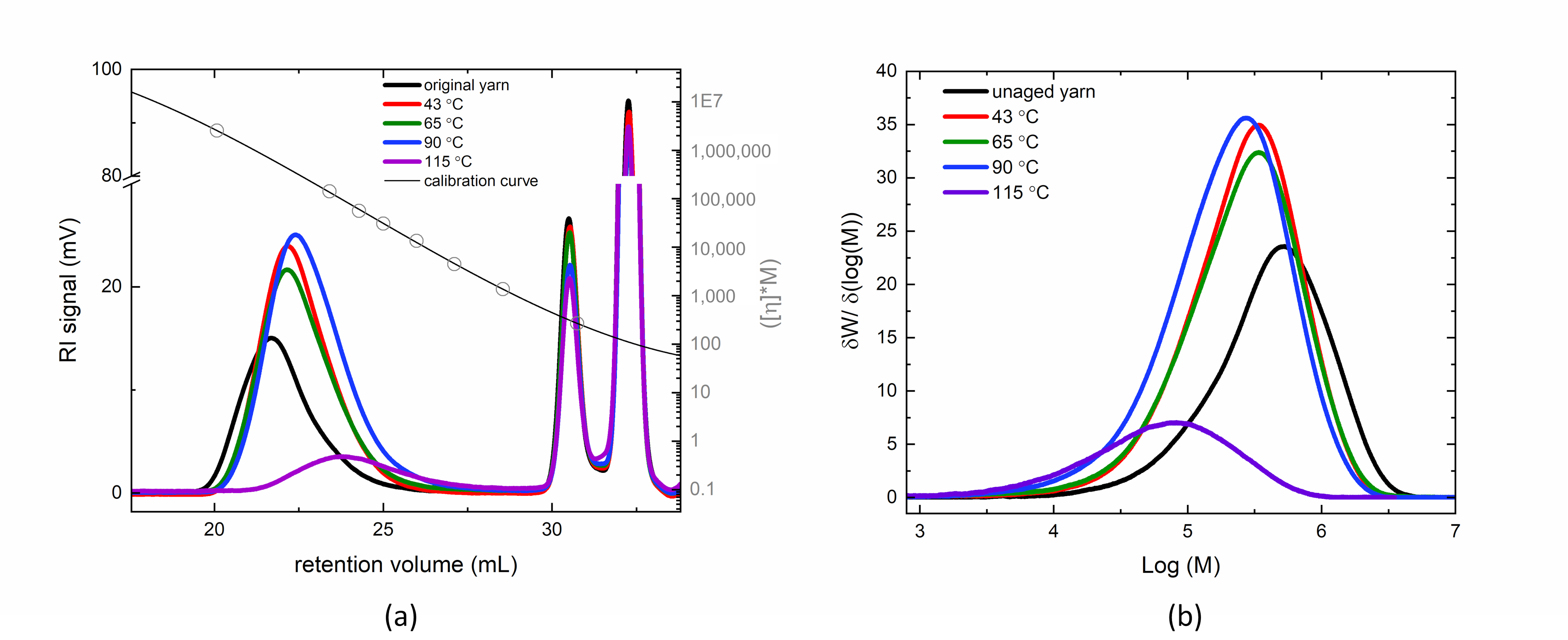
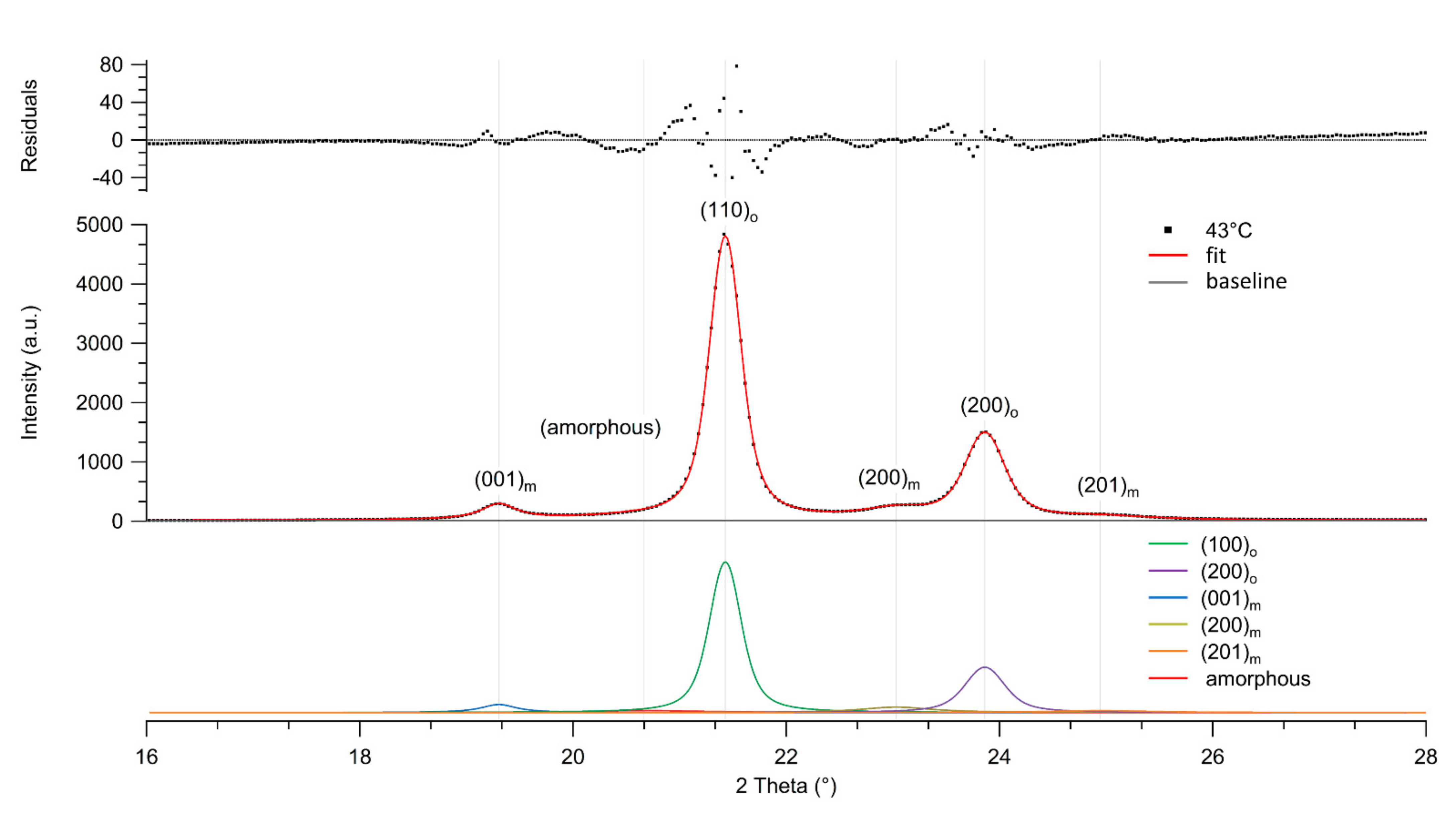
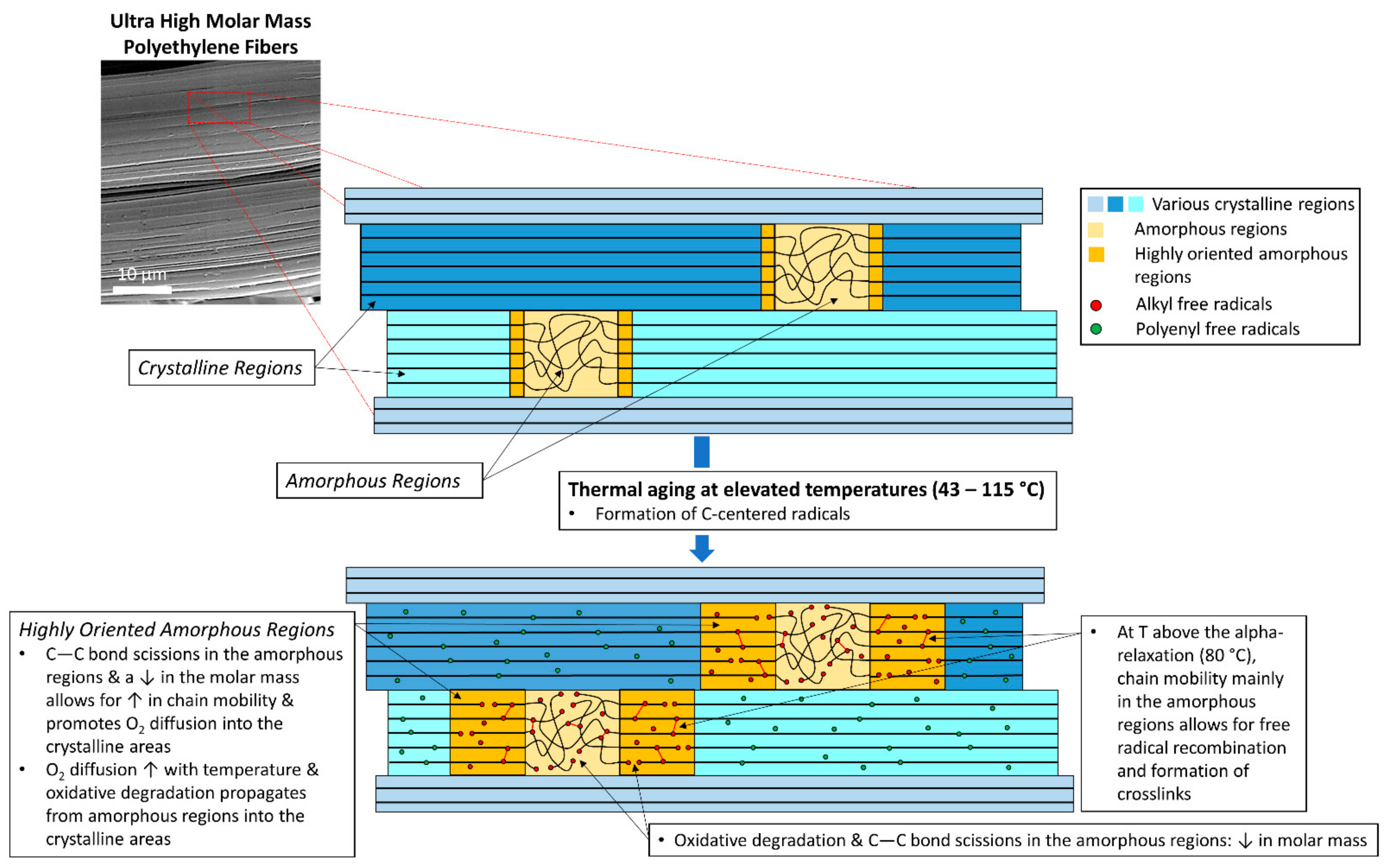
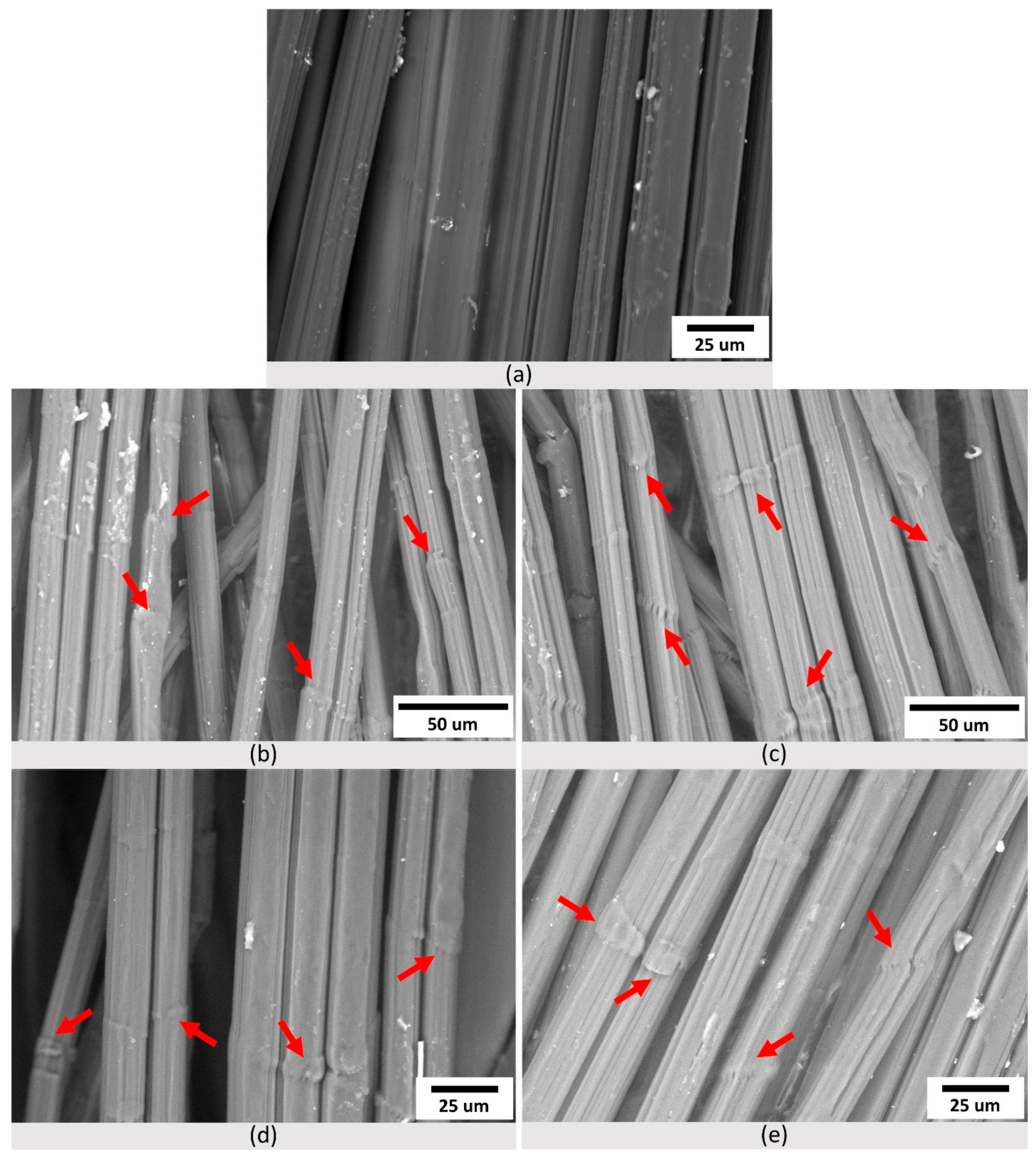

| Sample | Mn (× 10−5) (g/mol) | Mw (× 10−5) (g/mol) | Mz (× 10−5) (g/mol) | Ð |
|---|---|---|---|---|
| Unaged | 2.31 ± 0.88 | 6.53 ± 1.04 | 9.69 ± 1.30 | 3.2 ± 1.2 |
| 43 °C, 81 w | 2.02 ± 0.40 | 5.66 ± 1.25 | 8.91 ± 1.89 | 2.8 ± 0.4 |
| 65 °C, 43 w | 1.74 ± 0.21 | 5.95 ± 0.37 | 10.3 ± 0.31 | 3.5 ± 0.6 |
| 90 °C, 17 w | 1.02 ± 0.17 | 5.12 ± 0.38 | 9.34 ± 0.80 | 5.2 ± 1.0 |
| 115 °C, 8 w | 0.19 ± 0.10 | 1.52 ± 0.49 | 4.94 ± 1.97 | 8.0 ± 3.4 |
| Thermal Treatment | Amorphous (%) | Orthorhombic Crystals (%) | Monoclinic Crystals (%) | Ortho/Mono Ratio | Crystallinity WAXS (%) | |||
|---|---|---|---|---|---|---|---|---|
| (110) | (200) | (010) | (200) | (210) | ||||
| Unaged | 3.1 | 68.3 | 23.6 | 2.0 | 2.8 | 0.2 | 18.5 | 96.9 |
| 43 °C, 81 w | 3.7 | 60.1 | 23.1 | 4.3 | 5.9 | 2.9 | 6.3 | 96.3 |
| 65 °C, 43 w | 2.8 | 63.1 | 25.7 | 2.2 | 4.4 | 1.8 | 10.5 | 97.2 |
| 90 °C, 17 w | 2.4 | 65.3 | 27.5 | 0.9 | 3.9 | - | 19.0 | 97.6 |
| 115 °C, 8 w | 12.5 | 61.5 | 23.6 | 0.6 | 1.8 | - | 34.8 | 87.5 |
| Thermal Treatment | Oxygen (At. %) | Carbon (At. %) | O/C Ratio |
|---|---|---|---|
| Unaged | 2.93 ± 0.20 | 97.07 ± 0.20 | 0.03 |
| 65 °C, 43 w | 6.64 ± 0.12 | 93.37 ± 0.12 | 0.07 |
| 90 °C, 17 w | 6.27 ± 0.23 | 93.74 ± 0.23 | 0.07 |
| 115 °C, 8 w | 7.59 ± 0.31 | 92.33 ± 0.27 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsinas, Z.; Orski, S.V.; Bentley, V.R.C.; Gonzalez Lopez, L.; Al-Sheikhly, M.; Forster, A.L. Effects of Thermal Aging on Molar Mass of Ultra-High Molar Mass Polyethylene Fibers. Polymers 2022, 14, 1324. https://doi.org/10.3390/polym14071324
Tsinas Z, Orski SV, Bentley VRC, Gonzalez Lopez L, Al-Sheikhly M, Forster AL. Effects of Thermal Aging on Molar Mass of Ultra-High Molar Mass Polyethylene Fibers. Polymers. 2022; 14(7):1324. https://doi.org/10.3390/polym14071324
Chicago/Turabian StyleTsinas, Zois, Sara V. Orski, Viviana R. C. Bentley, Lorelis Gonzalez Lopez, Mohamad Al-Sheikhly, and Amanda L. Forster. 2022. "Effects of Thermal Aging on Molar Mass of Ultra-High Molar Mass Polyethylene Fibers" Polymers 14, no. 7: 1324. https://doi.org/10.3390/polym14071324
APA StyleTsinas, Z., Orski, S. V., Bentley, V. R. C., Gonzalez Lopez, L., Al-Sheikhly, M., & Forster, A. L. (2022). Effects of Thermal Aging on Molar Mass of Ultra-High Molar Mass Polyethylene Fibers. Polymers, 14(7), 1324. https://doi.org/10.3390/polym14071324







