Employing Cellulose Nanofiber-Based Hydrogels for Burn Dressing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrogel Fabrication
2.3. Characterizations
2.3.1. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.3.2. Gel Fraction
2.3.3. Field Emission Scanning Electron Microscope (FE-SEM)
2.3.4. Rheological Characterization
2.3.5. Drug Uploading
2.3.6. Drug Release Mechanism
2.3.7. Biocompatibility of Hydrogels
2.4. Statistical Analysis
3. Results
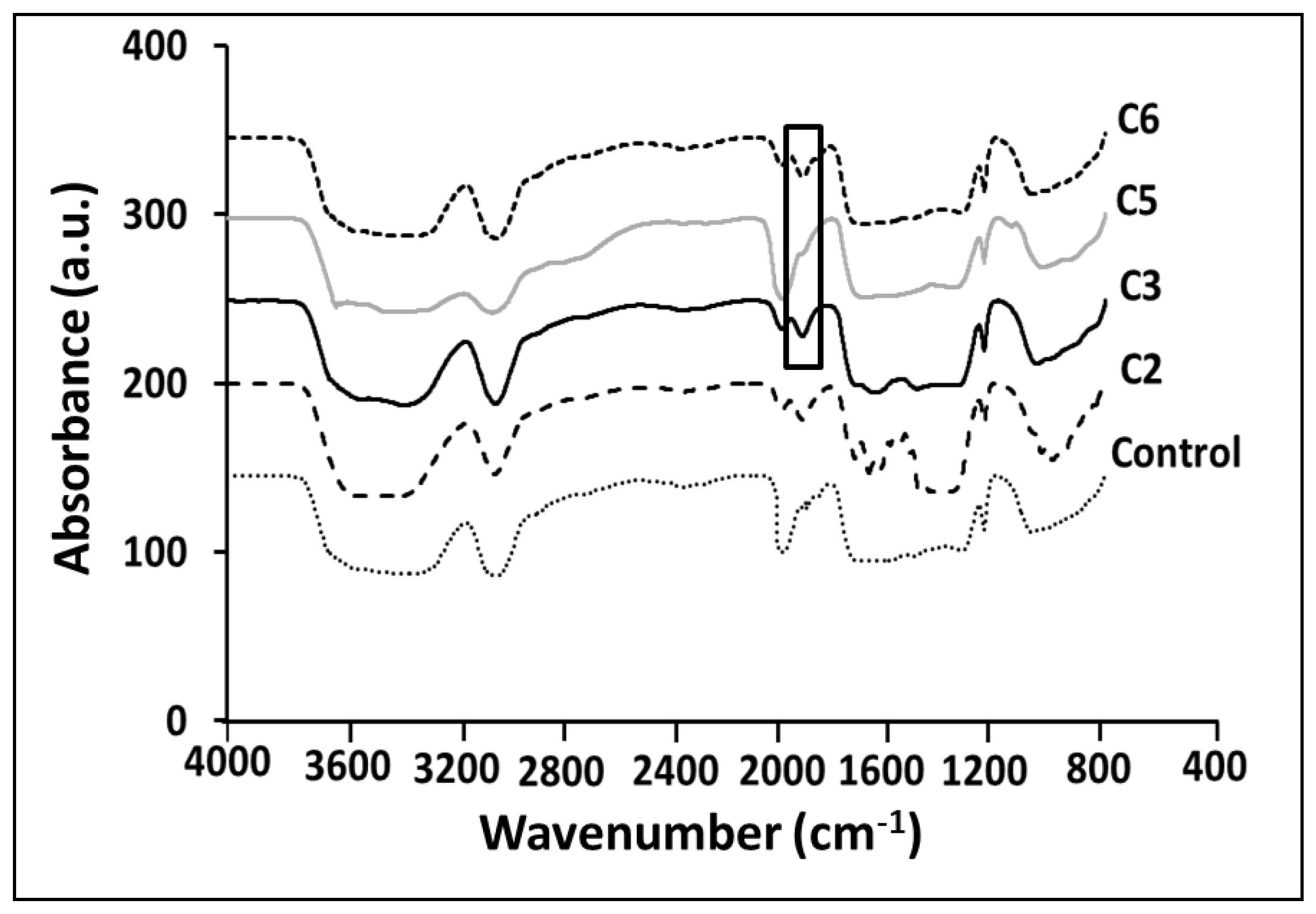
3.1. ATR-FTIR
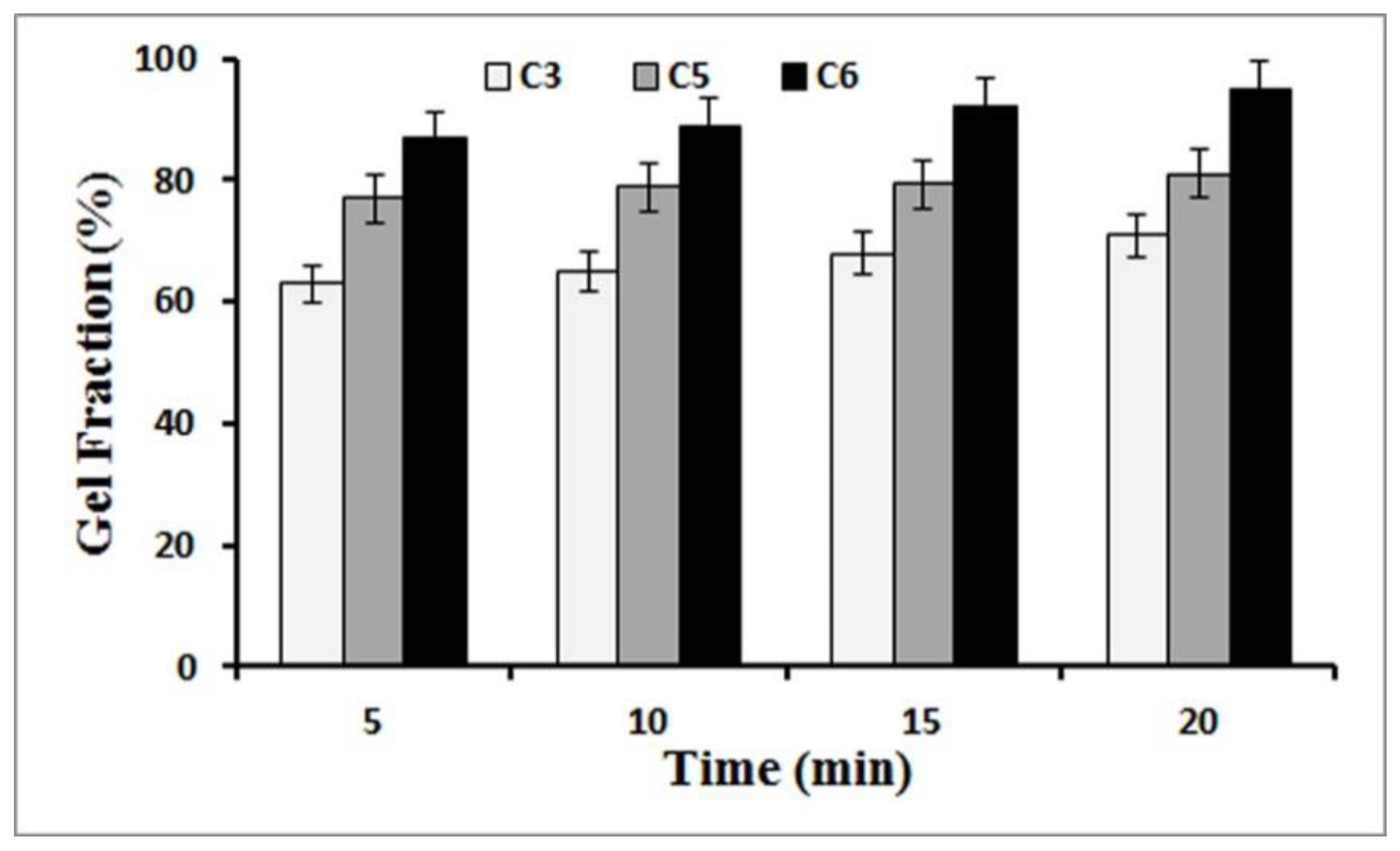
3.2. Gel Fraction
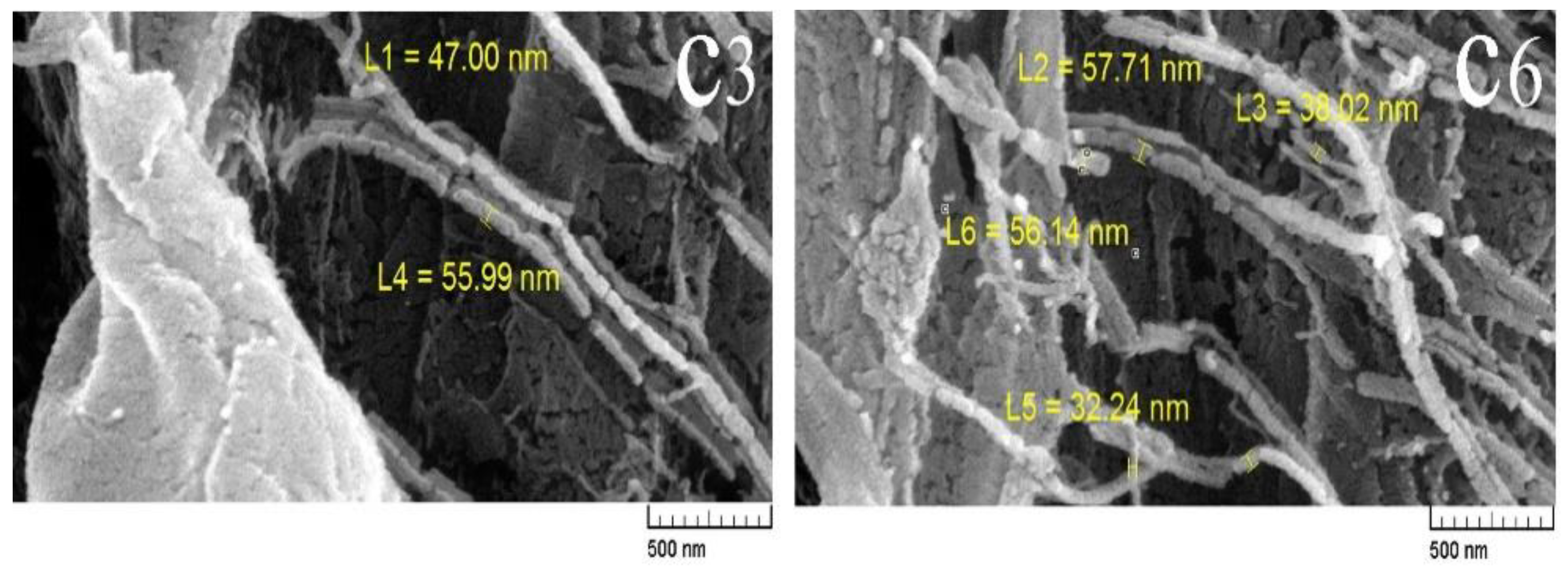
3.3. Field Emission Scanning Electron Microscopes (FE-SEM)
3.4. Rheological Properties of Hydrogels
3.5. Biocompatibility
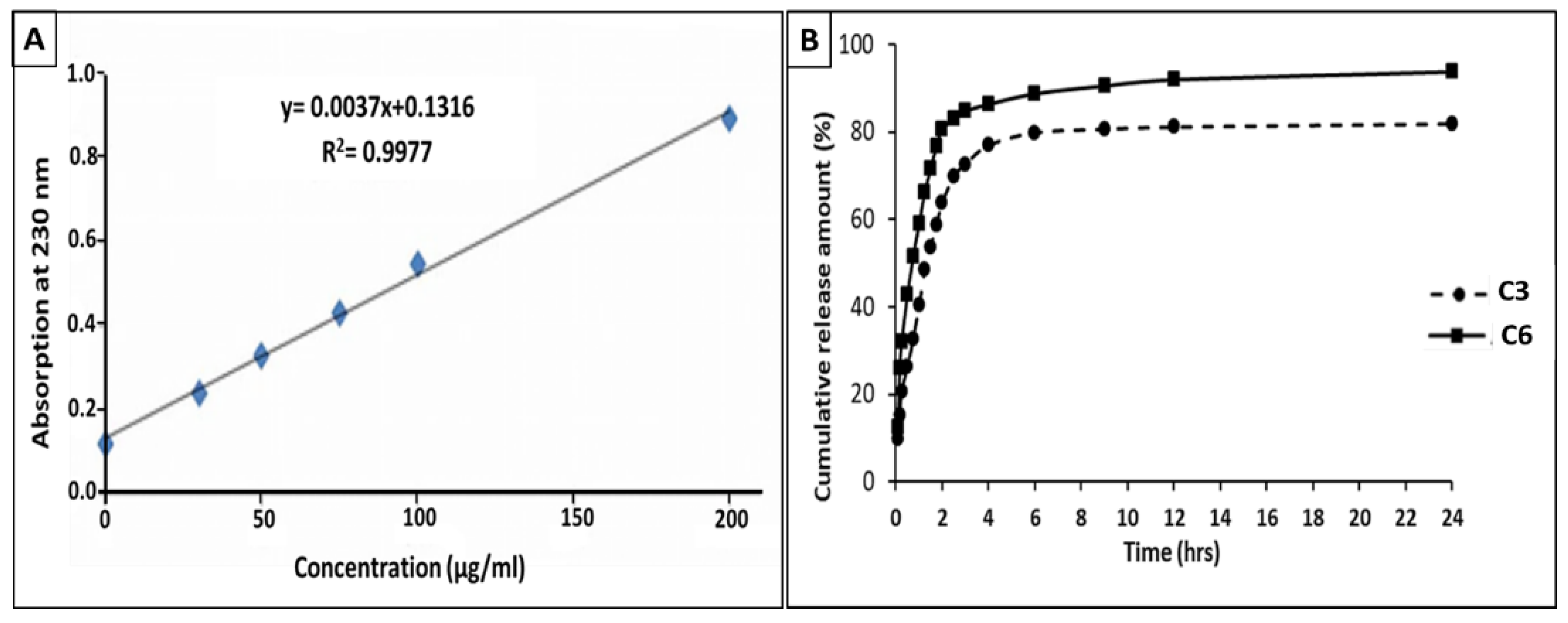
3.6. Drug Release
3.7. Investigation of Drug Release Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound dressings and comparative effectiveness data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharskar, G. A review on hydrogel. World J. Pharm. Pharm. Sci. 2020, 9, 1288–1298. [Google Scholar] [CrossRef]
- Gibas, I.; Janik, H. Review: Synthetic polymer hydrogels for biomedical application. Chem. Chem. Technol. 2010, 4, 297–304. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings–A review. BioMed 2015, 5, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.E.; Spencer, J.M. Clinical aspects of full-thickness wound healing. Clin. Dermatol. 2007, 25, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Strecker-McGraw, M.K.; Jones, T.R.; Baer, D.G. Soft tissue wounds and principles of healing. Emerg. Medic. Clinic. N. Am. 2007, 25, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Xu, W.; Liu, K.; Li, T.; Zhang, W.; Dong, Y.; Lv, J.; Wang, W.; Sun, J.; Li, M.; Wang, M.; et al. An in situ hydrogel based on carboxymethyl chitosan and sodium alginate dialdehyde for corneal wound healing after alkali burn. J. Biomed. Mater. Res. Part A 2019, 107, 742–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naves, L.B.; Almedia, L. Wound Healing Dressing and Some Composites Such as Zeolite, TiO2, Chitosan and PLGA: A Review. Int. J. Metall. Mater. Eng. 2015, 9, 242–246. [Google Scholar] [CrossRef]
- Liu, R.; Dai, L.; Si, C.; Zeng, Z. Antibacterial and hemostatic hydrogel via nanocomposite from cellulose nanofibers. Carbohydr. Polym. 2018, 195, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Dargaville, T.R.; Farrugia, B.L.; Broadbent, J.A.; Pace, S.; Upton, Z.; Voelcker, N.H. Sensors and imaging for wound healing: A review. Biosens. Bioelectron. 2013, 41, 30–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alven, S.; Nqoro, X.; Aderibigbe, B.A. Polymer-based materials loaded with curcumin for wound healing applications. Polymers 2020, 12, 2286. [Google Scholar] [CrossRef]
- Farina, J.A.; Rosique, M.J.; Rosique, R.G. Curbing inflammation in burn patients. Int. J. Inflamm. 2013, 2013, 715645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable cellulose-based hydrogels: Design and applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Lu, T.; Li, Q.; Chen, W.; Yu, H. Composite aerogels based on dialdehyde nanocellulose and collagen for potential applications as wound dressing and tissue engineering scaffold. Compos. Sci. Technol. 2014, 94, 132–138. [Google Scholar] [CrossRef]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The use of natural polymers in tissue engineering: A focus on electrospun extracellular matrix analogues. Polymers 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Babu, R.P.; O’connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Bai, Y.; Chen, X.; Liu, W. A review on raw materials, commercial production and properties of lyocell fiber. J. Bioresour. Manag. 2020, 5, 16–25. [Google Scholar] [CrossRef]
- Joseph, B.; Sagarika, V.K.; Sabu, C.; Kalarikkal, N.; Thomas, S. Cellulose nanocomposites: Fabrication and biomedical applications. J. Bioresour. Bioprod. 2020, 5, 223–237. [Google Scholar] [CrossRef]
- Khanjanzadeh, H.; Behrooz, R.; Bahramifar, N.; Gindl-Altmutter, W.; Bacher, M.; Edler, M.; Griesser, T. Surface chemical functionalization of cellulose nanocrystals by 3-aminopropyltriethoxysilane. Intern. J. Bio. Macro. 2018, 106, 1288–1296. [Google Scholar] [CrossRef]
- Sepahvand, S.; Jonoobi, M.; Ashori, A.; Gauvin, F.; Brouwers, H.J.H.; Oksman, K.; Yu, Q. A promising process to modify cellulose nanofibers for carbon dioxide (CO2) adsorption. Carbohydr. Polym. 2020, 230, 115571. [Google Scholar] [CrossRef] [PubMed]
- Sepahvand, S.; Bahmani, M.; Ashori, A.; Pirayesh, H.; Yu, Q.; Dafchahi, M.N. Preparation and characterization of air nanofilters based on cellulose nanofibers. Int. J. Biol. Macromol. 2021, 182, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Moritz, S.; Wiegand, C.; Wesarg, F.; Hessler, N.; Müller, F.A.; Kralisch, D.; Hipler, U.; Fischer, D. Active wound dressings based on bacterial nanocellulose as drug delivery system for octenidine. Int. J. Pharm. 2014, 471, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Afshar, A.; Yang, H.; Portela, C.M.; Kochmann, D.M.; Di Leo, C.V.; Greer, J.R. Electrochemically reconfigurable architected materials. Nature 2019, 573, 205–213. [Google Scholar] [CrossRef]
- Abazari, M.F.; Gholizadeh, S.; Karizi, S.Z.; Birgani, N.H.; Abazari, D.; Paknia, S.; Derakhshandeh, H.; Allahyari, Z.; Amini, S.M.; Hamidi, M.; et al. Recent Advances in Cellulose-Based Structures as the Wound-Healing Biomaterials: A Clinically Oriented Review. Appl. Sci. 2021, 11, 7769. [Google Scholar] [CrossRef]
- Li, S.-M.; Jia, N.; Ma, M.-G.; Zhang, Z.; Liu, Q.-H.; Sun, R.-C. Cellulose–silver nanocomposites: Microwave-assisted synthesis, characterization, their thermal stability, and antimicrobial property. Carbohydr. Polym. 2011, 86, 441–447. [Google Scholar] [CrossRef]
- Yang, T.; Long, H.; Malkoch, M.; Gamstedt, E.K.; Berglund, L.; Hult, A. Characterization of well-defined poly(ethylene glycol) hydrogels prepared by Thiol-ene chemistry. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4044–4054. [Google Scholar] [CrossRef]
- Trovatti, E.; Freire, C.S.R.; Pinto, P.C.; Almeida, I.F.; Costa, P.; Silvestre, A.J.D.; Neto, C.P.; Rosado, C. Bacterial cellulose membranes applied in topical and transdermal delivery of lidocaine hydrochloride and ibuprofen: In vitro diffusion studies. Int. J. Pharm. 2012, 435, 8387. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Li, J.; Wei, W.; Zuo, G.; Su, T.; Pan, X.; Zhang, J.; Dong, W. Cationic Salecan-based hydrogels for release of 5- fluorouracil. RSC Adv. 2017, 7, 14337–14347. [Google Scholar] [CrossRef] [Green Version]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian & Anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Robles, E.; Urruzola, I.; Labidi, J.; Serrano, L. Surface-modified nano-cellulose as reinforcement in poly (lactic acid) to conform new composites. Ind. Crops Prod. 2015, 71, 44–53. [Google Scholar] [CrossRef]
- Barique, M.A.; Tsuchida, E.; Ohira, A.; Tashiro, K. Effect of elevated temperatures on the states of water and their correlation with the proton conductivity of Nafion. ACS Omega 2018, 3, 349–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciolacu, D.; Ciolacu, F.; Popa, V.I. Amorphous cellulose-structure and characterization. Cellul. Chem. Technol. 2011, 45, 13. [Google Scholar]
- Hokkanen, S.; Bhatnagar, A.; Repo, E.; Lou, S.; Sillanpää, M. Calcium hydroxyapatite microfibrillated cellulose composite as a potential adsorbent for the removal of Cr (VI) from aqueous solution. Chem. Eng. J. 2016, 283, 445–452. [Google Scholar] [CrossRef]
- Mulyadi, A.; Deng, Y. Surface modification of cellulose nanofibrils by maleated styrene block copolymer and their composite reinforcement application. Cellulose 2016, 23, 519–528. [Google Scholar] [CrossRef]
- Abdel-Mohsen, S.A.; El-Emary, T.I. New pyrazolo [3, 4-b] pyridines: Synthesis and antimicrobial Activity. Der Pharma Chem. 2018, 10, 44–51. Available online: www.derpharmachemica.com (accessed on 15 October 2021).
- Mohsen, M.; Gomaa, E.; Mazaid, N.A. Mohammed, Synthesis and characterization of organic montmorillonite-polyvinyl alcohol-co-polyacrylic nanocomposite hydrogel for heavy metal uptake in water. AIMS Mater. Sci. 2017, 4, 1122–1139. [Google Scholar] [CrossRef]
- García-Astrain, C.; González, K.; Gurrea, T.; Guaresti, O.; Algar, I.; Eceiza, A.; Gabilondo, N. Maleimide-Grafted Cellulose Nanocrystals as Cross-Linkers for Bionanocomposite Hydrogels. Carbohydr. Polym. 2016, 149, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Rudraraju, V.S.; Wyandt, C.M. Rheological characterization of Microcrystalline Cellulose/Sodiumcarboxymethyl cellulose hydrogels using a controlled stress rheometer: Part I. Int. J. Pharm. 2005, 292, 53–61. [Google Scholar] [CrossRef]
- Khondkar, D.; Tester, R.F.; Hudson, N.; Karkalas, J.; Morrow, J. Rheological behaviour of uncross-linked and cross-linked gelatinised waxy maize starch with pectin gels. Food Hydrocoll. 2007, 21, 1296–1301. [Google Scholar] [CrossRef]
- Shimojo, A.A.; Pires, A.; Lichy, R.; Santana, M.H. The performance of crosslinking with divinyl sulfone as controlled by the interplay between the chemical modification and conformation of hyaluronic acid. J. Braz. Chem. Soc. 2015, 26, 506–512. [Google Scholar] [CrossRef]
- Paakko, M.; Ankerfors, M.; Kosonen, H.; Nykanen, A.; Ahola, S.; Osterberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.T. Lindstrom, Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Shetye, S.P.; Godbole, A.; Bhilegaokar, S.; Gajare, P. Hydrogels: Introduction, preparation, characterization and applications. IJRM Hum. 2015, 1, 47–71. Available online: http://ijrm.humanjournals.com/wp-content/uploads/2018/08/3.Shivani-P.-Shetye-Dr.-Ajeet-Godbole-Dr.-Shilpa-Bhilegaokar-Pankaj-Gajare.pdf (accessed on 15 October 2015).
- Behnia, N.; Pirouzfar, V. Effect of operating pressure and pyrolysis conditions on the performance of carbon membranes for CO2/CH4 and O2/N2 separation derived from polybenzimidazole/Matrimid and UIP-S precursor blends. Polym. Bull. 2018, 75, 4341–4358. [Google Scholar] [CrossRef]
- Rodríguez, R.; Alvarez-Lorenzo, C.; Concheiro, A. Cationic cellulose hydrogels: Kinetics of the cross-linking process and characterization as pH-/ion-sensitive drug delivery systems. J. Control. Release 2003, 86, 253–265. [Google Scholar] [CrossRef]
- Wilhelms, T.A.; Schulze, D.; Alupeil, C.I.; Rohrer, C.; Abel, M.; Wiegand, C.; Hipler, U.C. Release of polyhexamethylene biguanide hydrochloride (PHMB) from a hydroballanced cellulose wound dressing with PHMB. In Proceedings of the 17th Conference of the European Wound Management Association, Glasgow, UK, 2–4 May 2007. [Google Scholar]
- Amin, M.C.I.M.; Ahmad, N.; Halib, N.; Ahmad, I. Synthesis and characterization of thermo-and pH-responsive bacterial cellulose/acrylic acid hydrogels for drug delivery. Carbohydr. Polym. 2012, 88, 465–473. [Google Scholar] [CrossRef]

| Groups | CNF Concentration (wt.%) | CA Concentration (wt.%) | HEC Concentration (wt.%) |
|---|---|---|---|
| C1 | 1 | 10 | 1 |
| C2 | 2 | 10 | 1 |
| C3 | 3 | 10 | 1 |
| C4 | 1 | 20 | 1 |
| C5 | 2 | 20 | 1 |
| C6 | 3 | 20 | 1 |
| Sample | Linear Formula | Diffusion Coefficient (n) | Regression Coefficient (R2) |
|---|---|---|---|
| C3 | y = 0.5294X + 1.7899 | 0.5394 | 0.9994 |
| C6 | y = 0.5352X + 1.6911 | 0.5171 | 0.9966 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basti, A.T.K.; Jonoobi, M.; Sepahvand, S.; Ashori, A.; Siracusa, V.; Rabie, D.; Mekonnen, T.H.; Naeijian, F. Employing Cellulose Nanofiber-Based Hydrogels for Burn Dressing. Polymers 2022, 14, 1207. https://doi.org/10.3390/polym14061207
Basti ATK, Jonoobi M, Sepahvand S, Ashori A, Siracusa V, Rabie D, Mekonnen TH, Naeijian F. Employing Cellulose Nanofiber-Based Hydrogels for Burn Dressing. Polymers. 2022; 14(6):1207. https://doi.org/10.3390/polym14061207
Chicago/Turabian StyleBasti, Aliakbar Tofangchi Kalle, Mehdi Jonoobi, Sima Sepahvand, Alireza Ashori, Valentina Siracusa, Davood Rabie, Tizazu H. Mekonnen, and Fatemeh Naeijian. 2022. "Employing Cellulose Nanofiber-Based Hydrogels for Burn Dressing" Polymers 14, no. 6: 1207. https://doi.org/10.3390/polym14061207
APA StyleBasti, A. T. K., Jonoobi, M., Sepahvand, S., Ashori, A., Siracusa, V., Rabie, D., Mekonnen, T. H., & Naeijian, F. (2022). Employing Cellulose Nanofiber-Based Hydrogels for Burn Dressing. Polymers, 14(6), 1207. https://doi.org/10.3390/polym14061207










