Fluorophenol-Containing Hydrogen-Bond Acidic Polysiloxane for Gas Sensing-Synthesis and Characterization
Abstract
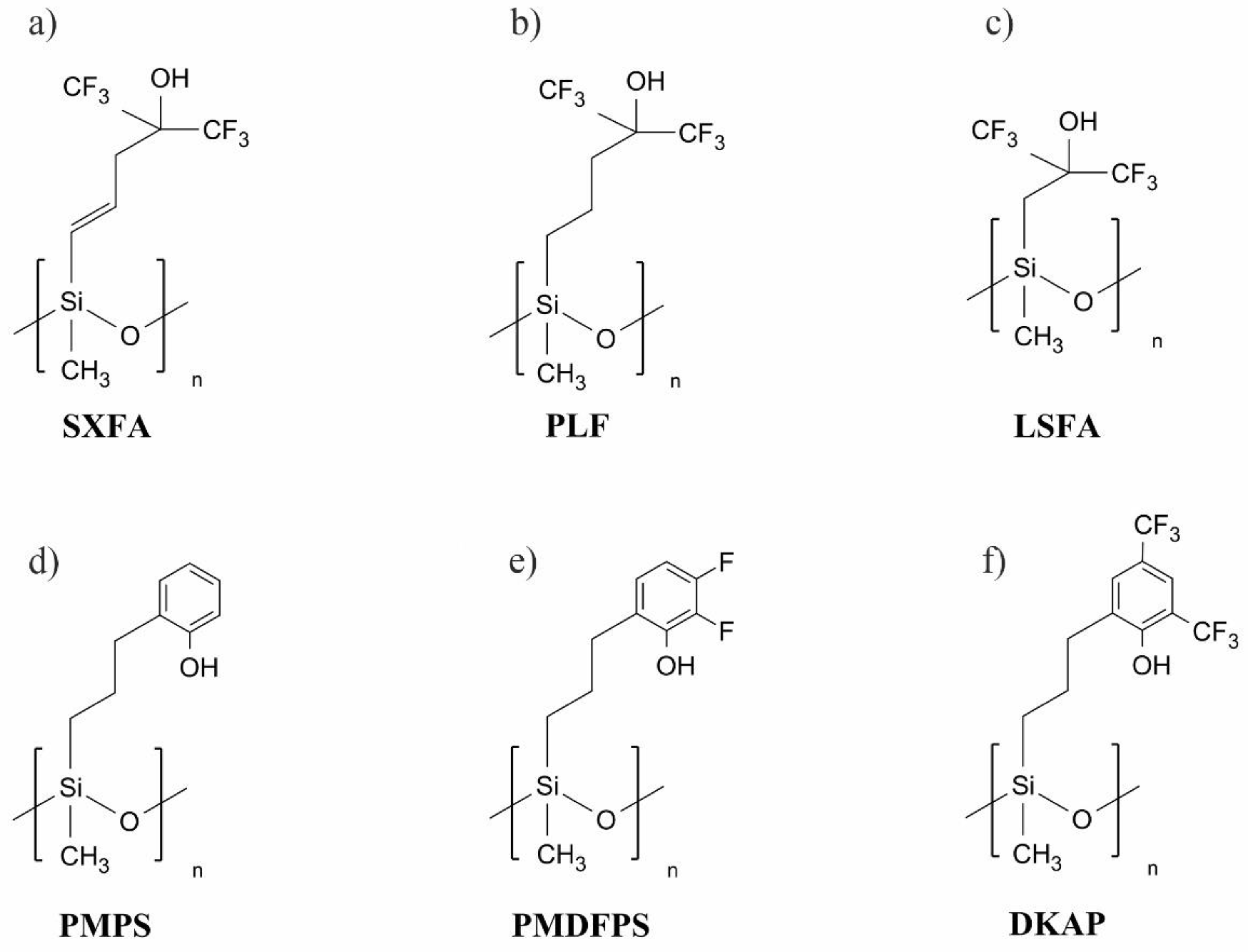
:1. Introduction
- Cs: equilibrium analyte concentration in the sensor layer;
- Cv: equilibrium analyte concentration in ambient gas.
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
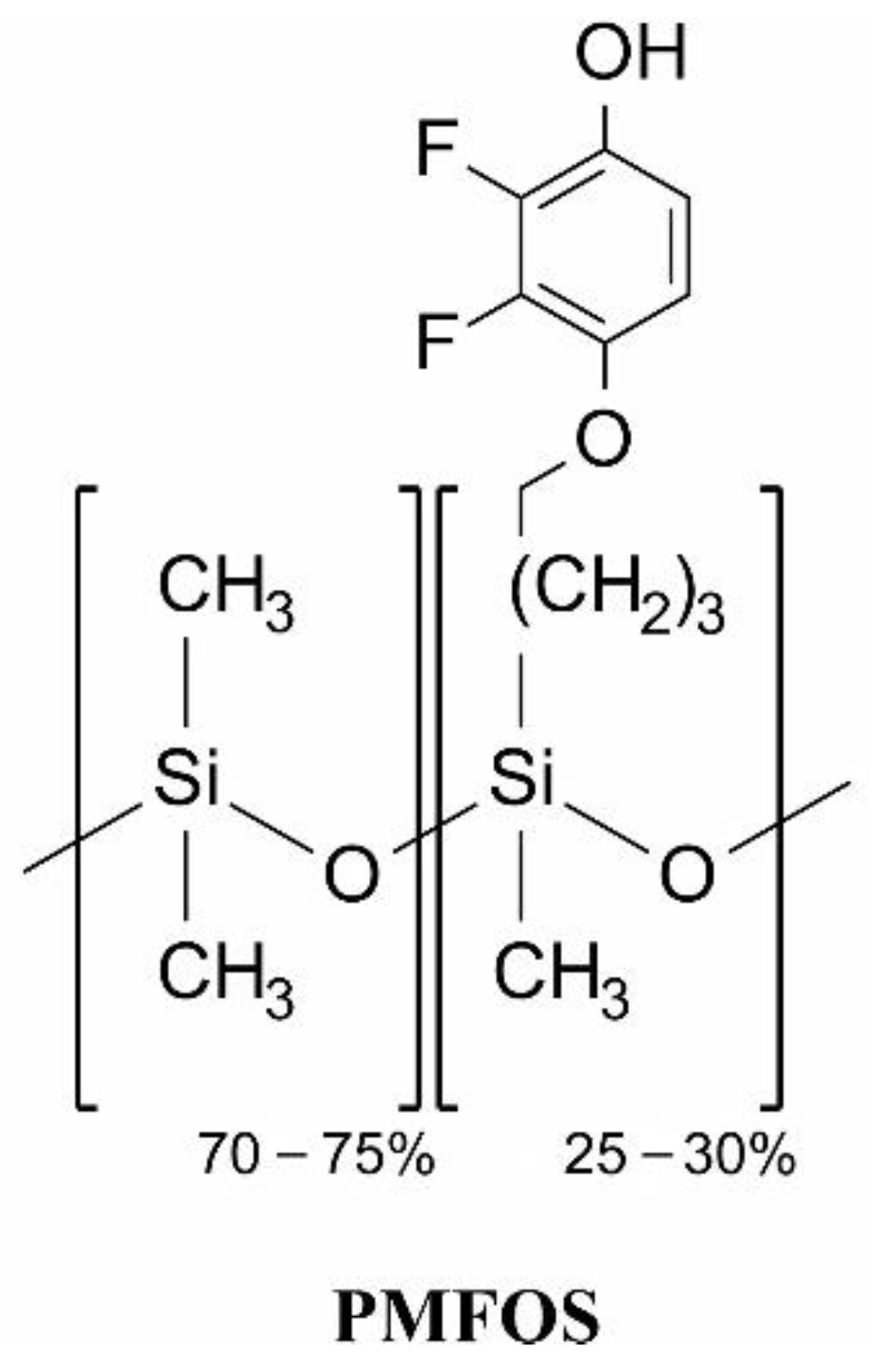
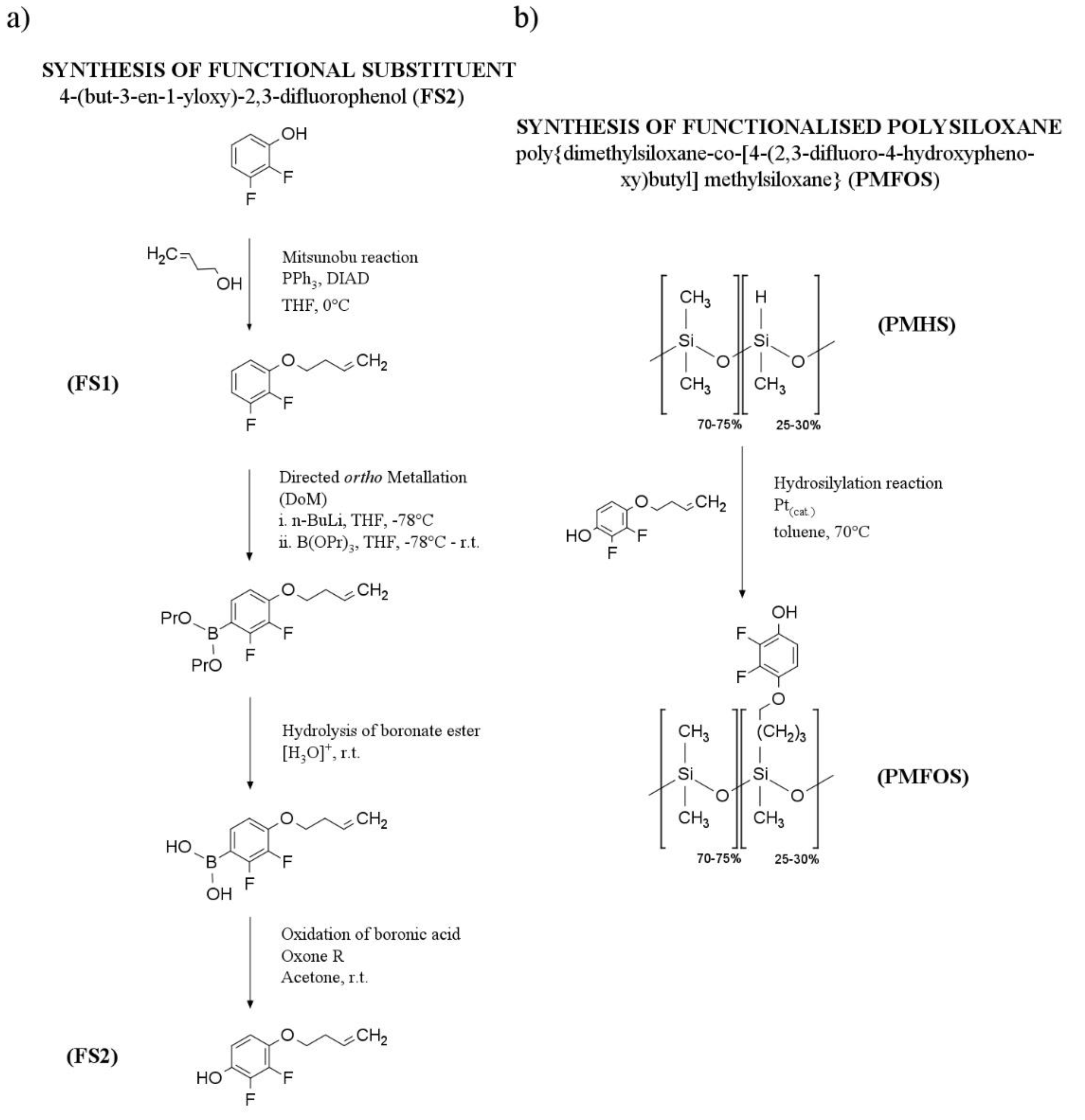
2.3. Synthesis of Functional Polysiloxane
3. Results and Discussion
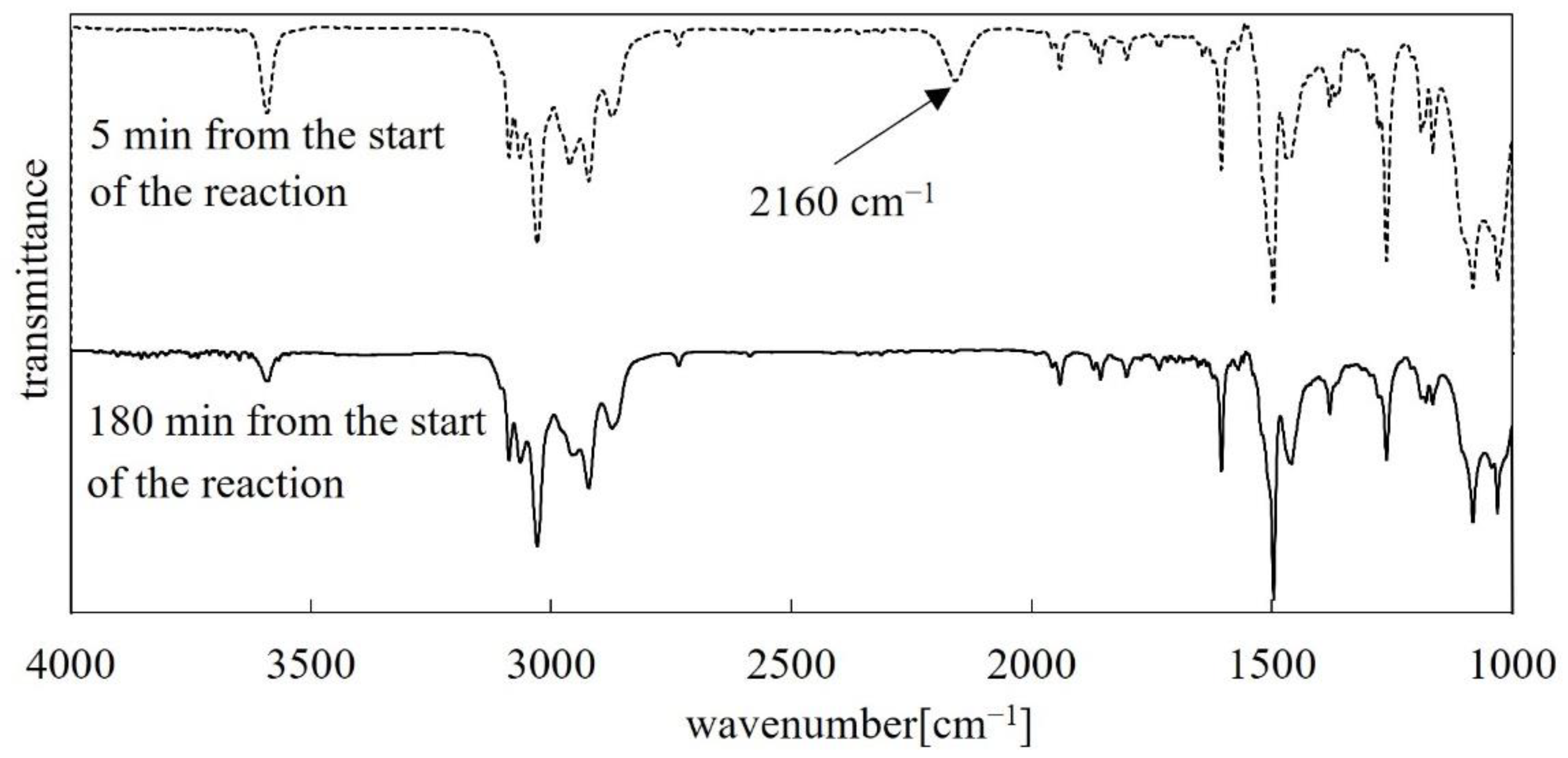
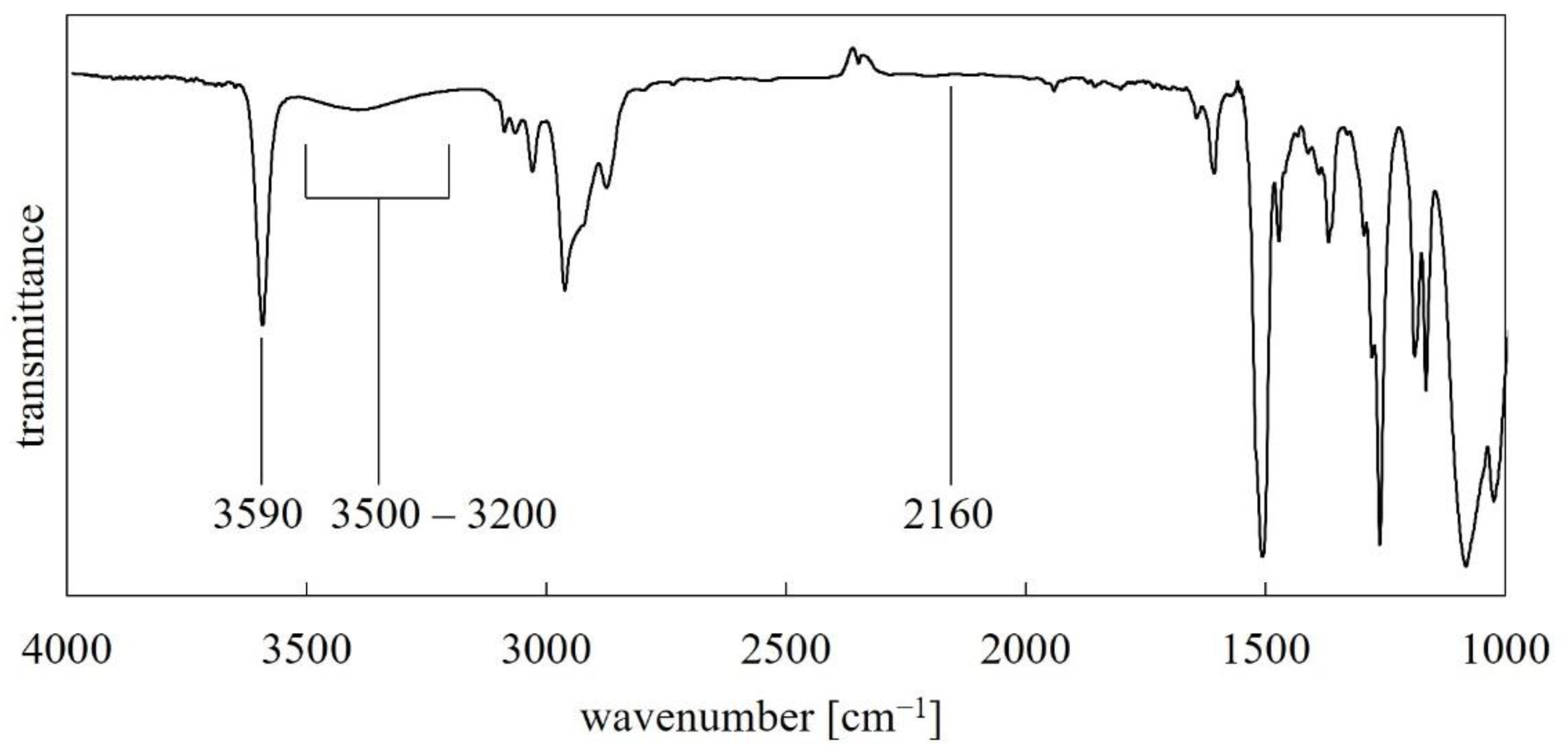
3.1. FT-IR Analysis
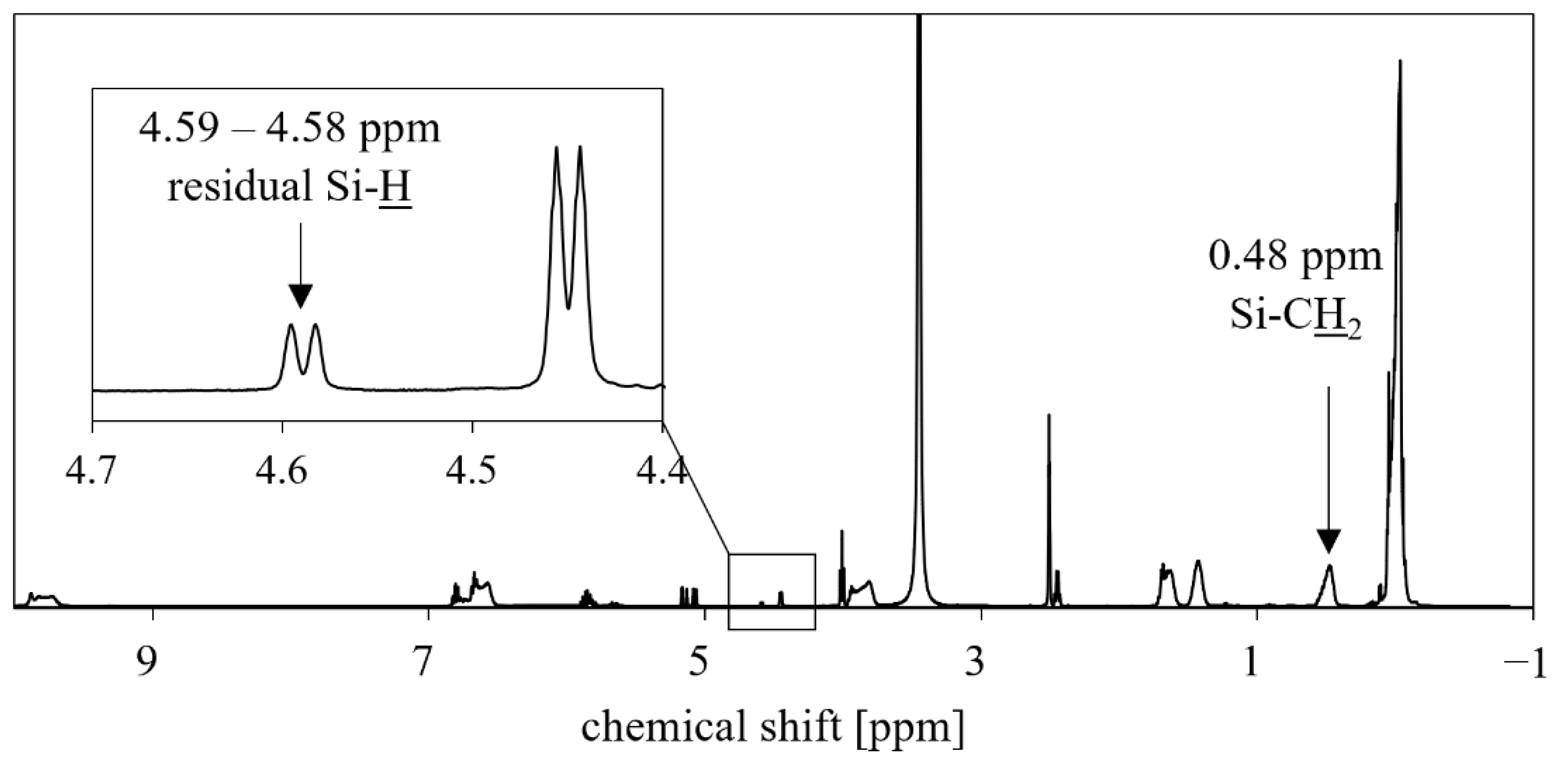
3.2. NMR Analysis
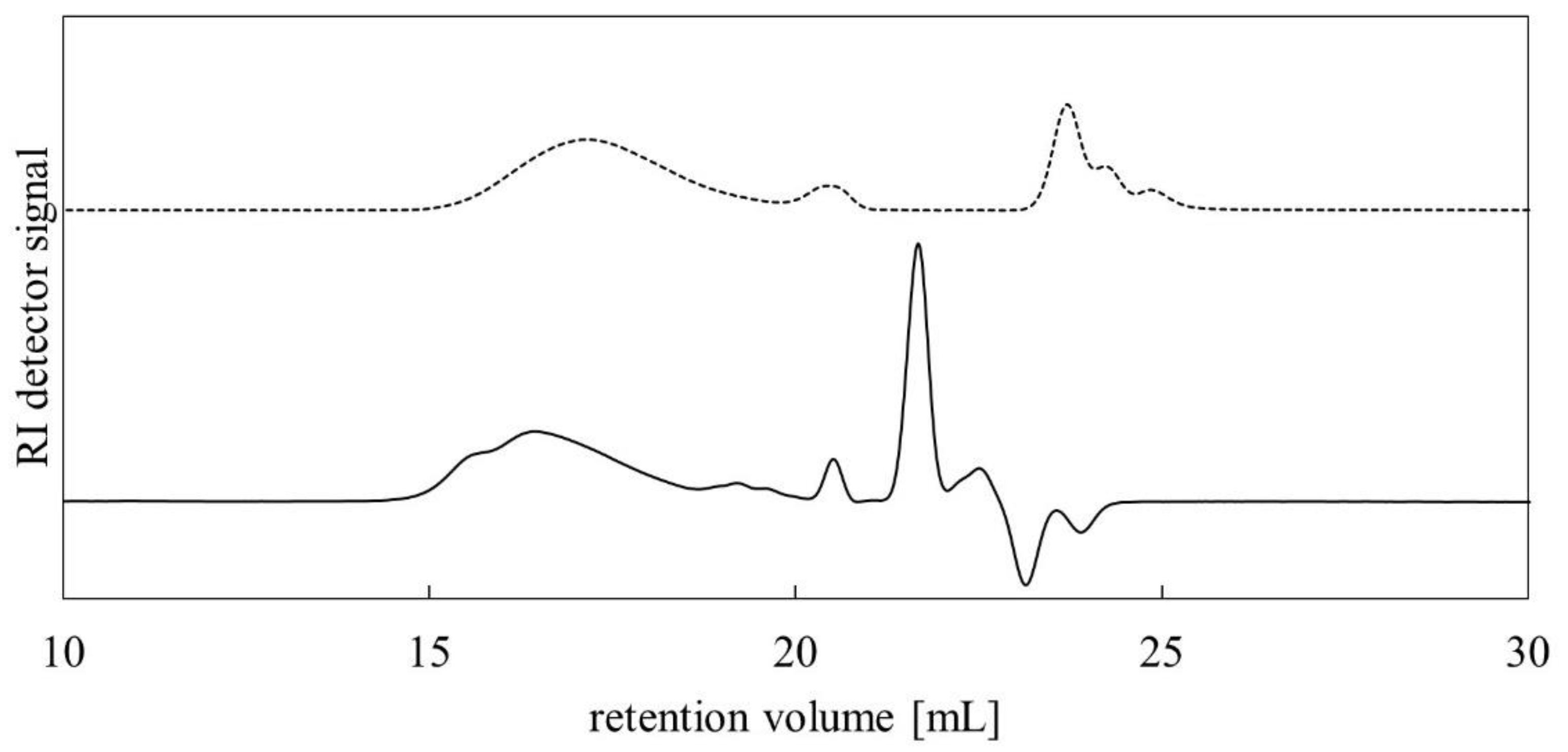
3.3. GPC Analysis
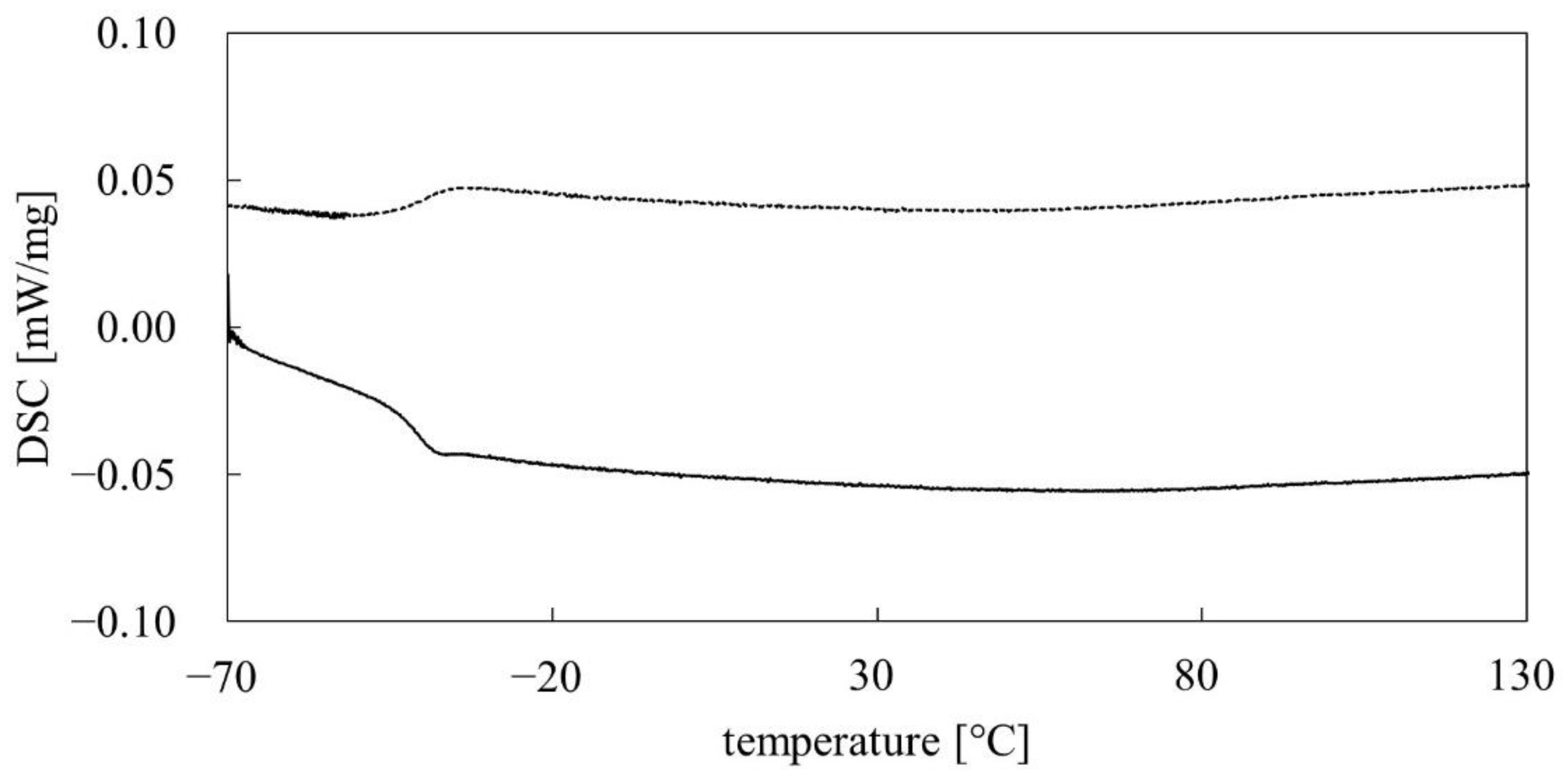
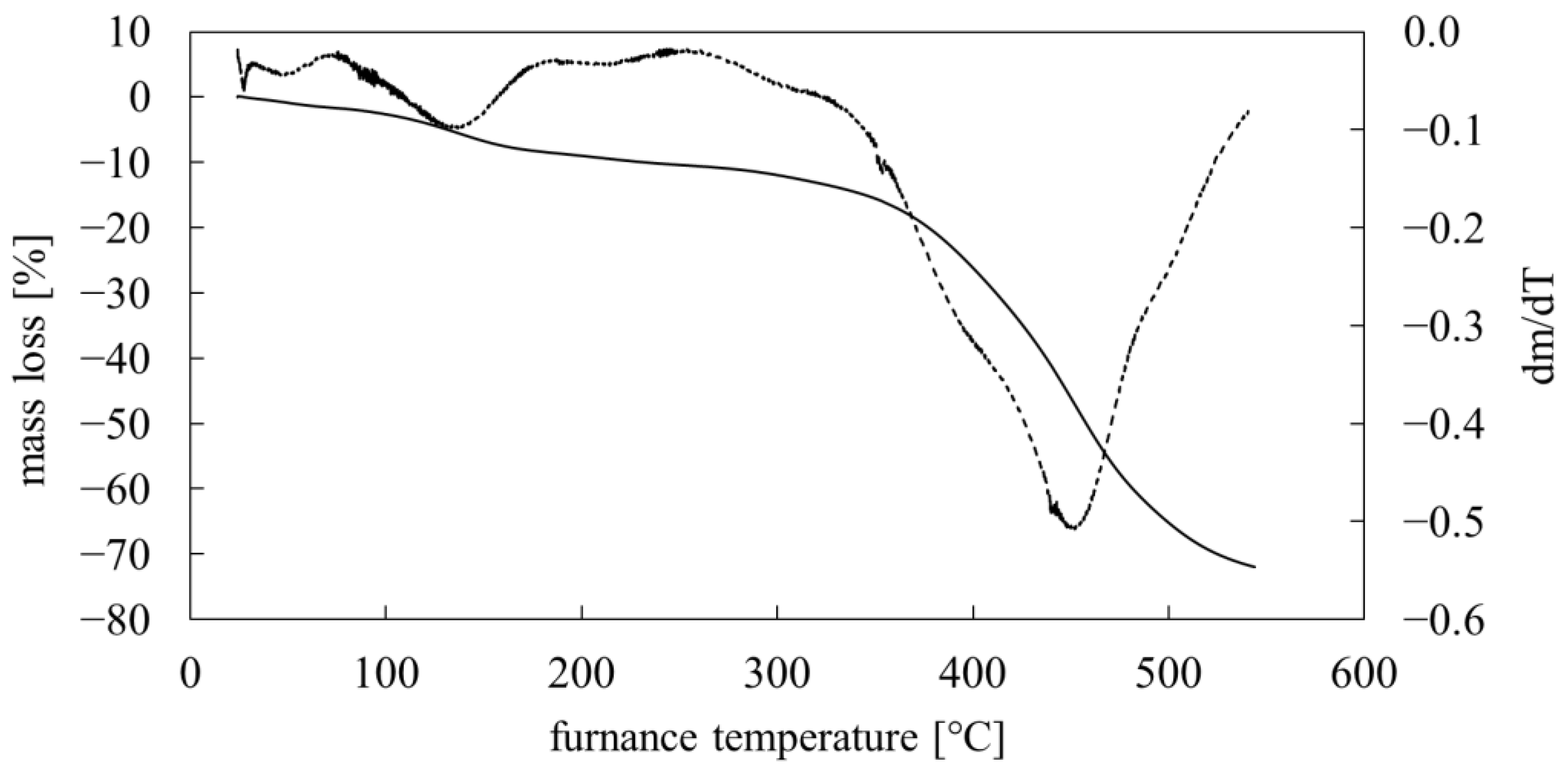
3.4. Thermal Analysis
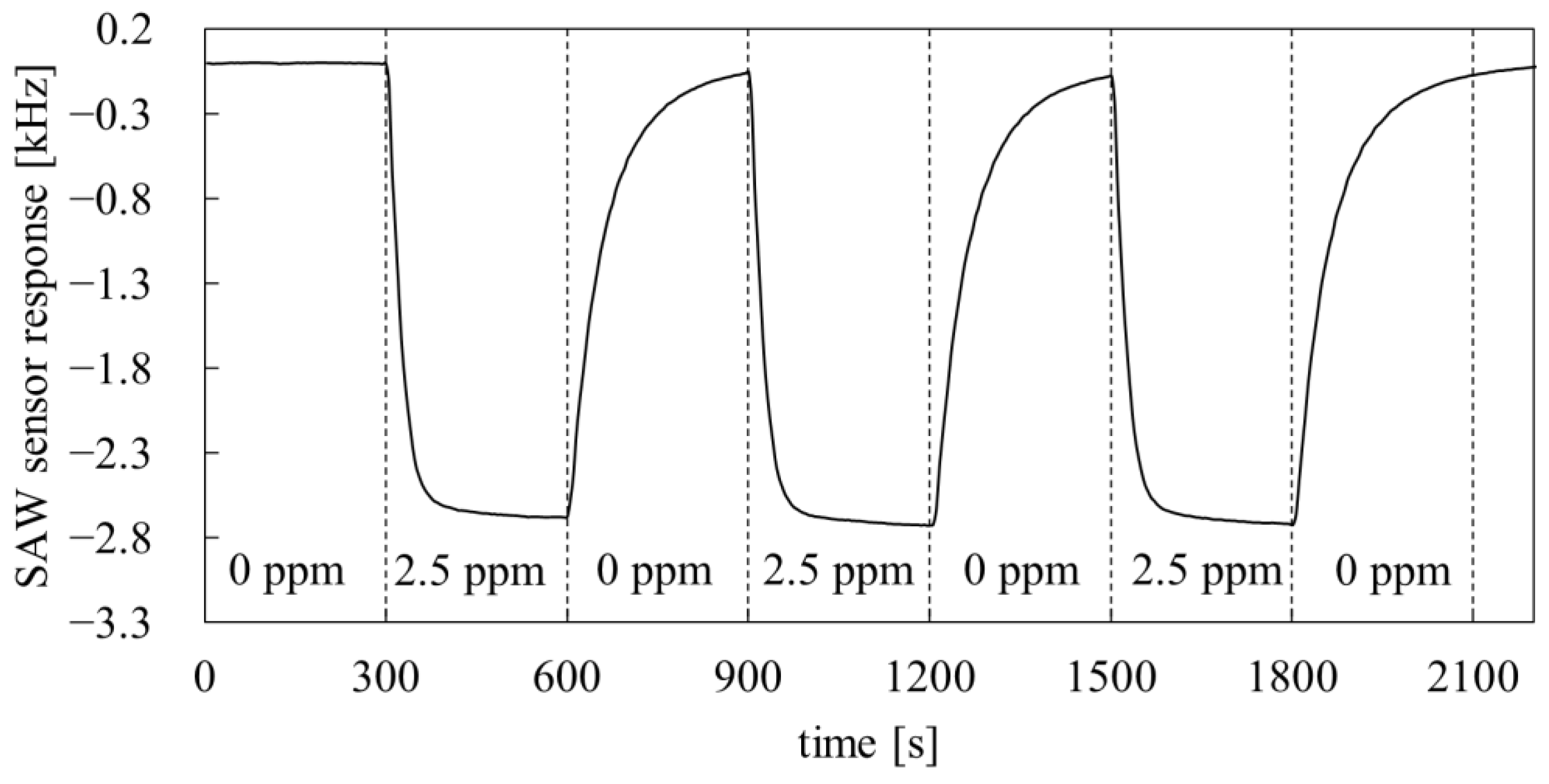
3.5. Application in SAW Sensors
4. Conclusions
- the developed synthetic route was well established and verified with instrumental methods;
- the synthetic route allows for obtaining the product with high yield (functional substituent step yield: 74%, hydrosilylation step yield: 88%);
- the determined thermal properties of PMFOS (Tg and decomposition temperature) meet the requirements for materials intended for use in gas sensors based on acoustoelectric transducers;
- the research on the dynamic properties of the PMFOS sensors has shown that they are characterized by short response time to DMMP vapor (several seconds). The response time at this level is appropriate for the detection of highly toxic substances.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Puton, J.; Namiesnik, J. Ion mobility spectrometry: Current status and application for chemical warfare agents detection. TrAC Trends Anal. Chem. 2016, 85, 10–20. [Google Scholar] [CrossRef]
- Seto, Y.; Kanamori-Kataoka, M.; Tsuge, K.; Ohsawa, I.; Matsushita, K.; Sekiguchi, H.; Itoi, T.; Iura, K.; Sano, Y.; Yamashiro, S. Sensing technology for chemical-warfare agents and its evaluation using authentic agents. Sens. Actuators B Chem. 2005, 108, 193–197. [Google Scholar] [CrossRef]
- Kelly, J.T.; Qualley, A.; Hughes, G.T.; Rubenstein, M.H.; Malloy, T.A.; Piatkowski, T. Improving Quantification of tabun, sarin, soman, cyclosarin, and sulfur mustard by focusing agents: A field portable gas chromatography-mass spectrometry study. J. Chromatogr. A 2021, 1636, 461784. [Google Scholar] [CrossRef]
- Giannoukos, S.; Brkić, B.; Taylor, S.; Marshall, A.; Verbeck, G.F. Chemical Sniffing Instrumentation for Security Applications. Chem. Rev. 2016, 116, 8146–8172. [Google Scholar] [CrossRef]
- Ji, X.; Yao, W.; Peng, J.; Ren, N.; Zhou, J.; Huang, Y. Evaluation of Cu-ZSM-5 zeolites as QCM sensor coatings for DMMP detection. Sens. Actuators B Chem. 2012, 166, 50–55. [Google Scholar] [CrossRef]
- Raj, V.B.; Singh, H.; Nimal, A.; Sharma, M.; Gupta, V. Oxide thin films (ZnO, TeO2, SnO2, and TiO2) based surface acoustic wave (SAW) E-nose for the detection of chemical warfare agents. Sens. Actuators B Chem. 2013, 178, 636–647. [Google Scholar] [CrossRef]
- Sayago, I.; Matatagui, D.; Fernández, M.J.; Fontecha, J.L.; Jurewicz, I.; Garriga, R.; Muñoz, E. Graphene oxide as sensitive layer in Love-wave surface acoustic wave sensors for the detection of chemical warfare agent simulants. Talanta 2016, 148, 393–400. [Google Scholar] [CrossRef]
- Zhu, Q.; Shih, W.-H.; Shih, W.Y. Enhanced dimethyl methylphosphonate (DMMP) detection sensitivity by lead magnesium niobate-lead titanate/copper piezoelectric microcantilever sensors via Young’s modulus change. Sens. Actuators B Chem. 2013, 182, 147–155. [Google Scholar] [CrossRef]
- Thomas, G.; Spitzer, D. Double-side microcantilevers as a key to understand the adsorption mechanisms and kinetics of chemical warfare agents on vertically-aligned TiO2 nanotubes. J. Hazard. Mater. 2021, 406, 124672. [Google Scholar] [CrossRef]
- Henry, W. Experiments on the quantity of gases absorbed by water, at different temperatures, and under different pressures. Philos. Trans. R. Soc. 1803, 93, 29–43. [Google Scholar] [CrossRef] [Green Version]
- Grate, J.W. Hydrogen-Bond Acidic Polymers for Chemical Vapor Sensing. Chem. Rev. 2008, 108, 726–745. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Wang, Y.; Du, X.; Cheng, L.; Wu, P.; Jiang, Y. The different sensitive behaviors of a hydrogen-bond acidic polymer-coated SAW sensor for chemical warfare agents and their simulants. Sensors 2015, 15, 18302–18314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Du, X.; Li, Y.; Long, Y.; Qiu, D.; Tai, H.; Tang, X.; Jiang, Y. A simple route to functionalize siloxane polymers for DMMP sensing. J. Appl. Polym. Sci. 2013, 130, 4516–4520. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, G.; Guo, T.; Liu, X.; Zhang, C.; Yang, J.; Cao, B.; Zhang, C.; Wang, W. Environmental characteristics of surface acoustic wave devices for sensing organophosphorus vapor. Sens. Actuators B Chem. 2020, 315, 127986. [Google Scholar] [CrossRef]
- Martin, S.D.; Poole, C.F.; Abraham, M.H. Synthesis and gas chromatographic evaluation of high-temperature hydrogen-bond acid stationary phase. J. Chromatogr. A 1998, 805, 217–235. [Google Scholar] [CrossRef]
- Houser, E.; Mlsna, T.; Nguyen, V.; Chung, R.; Mowery, R.; McGill, R. Rational materials design of sorbent coatings for explosives: Applications with chemical sensors. Talanta 2001, 54, 469–485. [Google Scholar] [CrossRef]
- Du, X.; Ying, Z.; Jiang, Y.; Liu, Z.; Yang, T.; Xie, G. Synthesis and evaluation of a new polysiloxane as SAW sensor coatings for DMMP detection. Sens. Actuators B Chem. 2008, 134, 409–413. [Google Scholar] [CrossRef]
- He, W.; Liu, Z.; Du, X.; Jiang, Y.; Xiao, D. Analytical application of poly{methyl[3-(2-hydroxy-3,4-difluoro)phenyl]propyl siloxane} as a QCM coating for DMMP detection. Talanta 2008, 76, 698–702. [Google Scholar] [CrossRef]
- Du, X.; Wang, Z.; Huang, J.; Tao, S.; Tang, X.; Jiang, Y. A new polysiloxane coating on QCM sensor for DMMP vapor detection. J. Mater. Sci. 2009, 44, 5872–5876. [Google Scholar] [CrossRef]
- Wang, Y.; Du, X.; Long, Y.; Tang, X.; Tai, H.; Jiang, Y. The response comparison of a hydrogen-bond acidic polymer to sarin, soman and dimethyl methyl phosphonate based on a surface acoustic wave sensor. Anal. Methods 2014, 6, 1951–1955. [Google Scholar] [CrossRef]
- Dietrich, A.; Mejía, E. Investigations on the hydrosilylation of allyl cyanide: Synthesis and characterization of cyanopropyl-functionalized silicones. Eur. Polym. J. 2020, 122, 109377. [Google Scholar] [CrossRef]
- Grabka, M.; Jasek, K.; Pasternak, M. Application of polymethyl[4-(2,3-difluoro-4-hydroxyphenoxy)butyl] siloxane in surface acoustic wave gas sensors for dimethyl methylphosphonate detection. Sens. Actuators B Chem. 2021, 329, 129216. [Google Scholar] [CrossRef]
- Grabka, M.; Jasek, K.; Witkiewicz, Z. Inverse gas chromatographic evaluation of polysiloxanes containing phenolic and fluorophenolic functional groups for use in gas sensors. Talanta 2021, 234, 122711. [Google Scholar] [CrossRef]
- Wang, W.; He, S.; Li, S.; Liu, M.; Pan, Y. Advances in SXFA-coated SAW chemical sensors for organophosphorous compound detection. Sensors 2011, 11, 1526–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, C.; Rebière, D.; Déjous, C.; Pistré, J.; Chastaing, E.; Planade, R. A love-wave gas sensor coated with functionalized polysiloxane for sensing organophosphorus compounds. Sens. Actuators B Chem. 2001, 76, 86–94. [Google Scholar] [CrossRef]
- Grate, J.W.; Kaganove, S.N.; Patrash, S.J.; Craig, R.; Bliss, M. Hybrid organic/inorganic copolymers with strongly hydrogen-bond acidic properties for acoustic wave and optical sensors. Chem. Mater. 1997, 9, 1201–1207. [Google Scholar] [CrossRef]
- Holmes, M.A.; Mackay, M.E.; Giunta, R.K. Nanoparticles for dewetting suppression of thin polymer films used in chemical sensors. J. Nanopart. Res. 2007, 9, 753–763. [Google Scholar] [CrossRef]
- Soluch, W. SAW synchronous multimode resonator with gold electrodes on quartz. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2008, 55, 1391–1393. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabka, M.; Kula, P.; Szala, M.; Jasek, K.; Czerwiński, M. Fluorophenol-Containing Hydrogen-Bond Acidic Polysiloxane for Gas Sensing-Synthesis and Characterization. Polymers 2022, 14, 1147. https://doi.org/10.3390/polym14061147
Grabka M, Kula P, Szala M, Jasek K, Czerwiński M. Fluorophenol-Containing Hydrogen-Bond Acidic Polysiloxane for Gas Sensing-Synthesis and Characterization. Polymers. 2022; 14(6):1147. https://doi.org/10.3390/polym14061147
Chicago/Turabian StyleGrabka, Michał, Przemysław Kula, Mateusz Szala, Krzysztof Jasek, and Michał Czerwiński. 2022. "Fluorophenol-Containing Hydrogen-Bond Acidic Polysiloxane for Gas Sensing-Synthesis and Characterization" Polymers 14, no. 6: 1147. https://doi.org/10.3390/polym14061147
APA StyleGrabka, M., Kula, P., Szala, M., Jasek, K., & Czerwiński, M. (2022). Fluorophenol-Containing Hydrogen-Bond Acidic Polysiloxane for Gas Sensing-Synthesis and Characterization. Polymers, 14(6), 1147. https://doi.org/10.3390/polym14061147








