Ionic Liquid Agar–Alginate Beads as a Sustainable Phenol Adsorbent
Abstract
:1. Introduction
2. Experimental Study
2.1. Materials
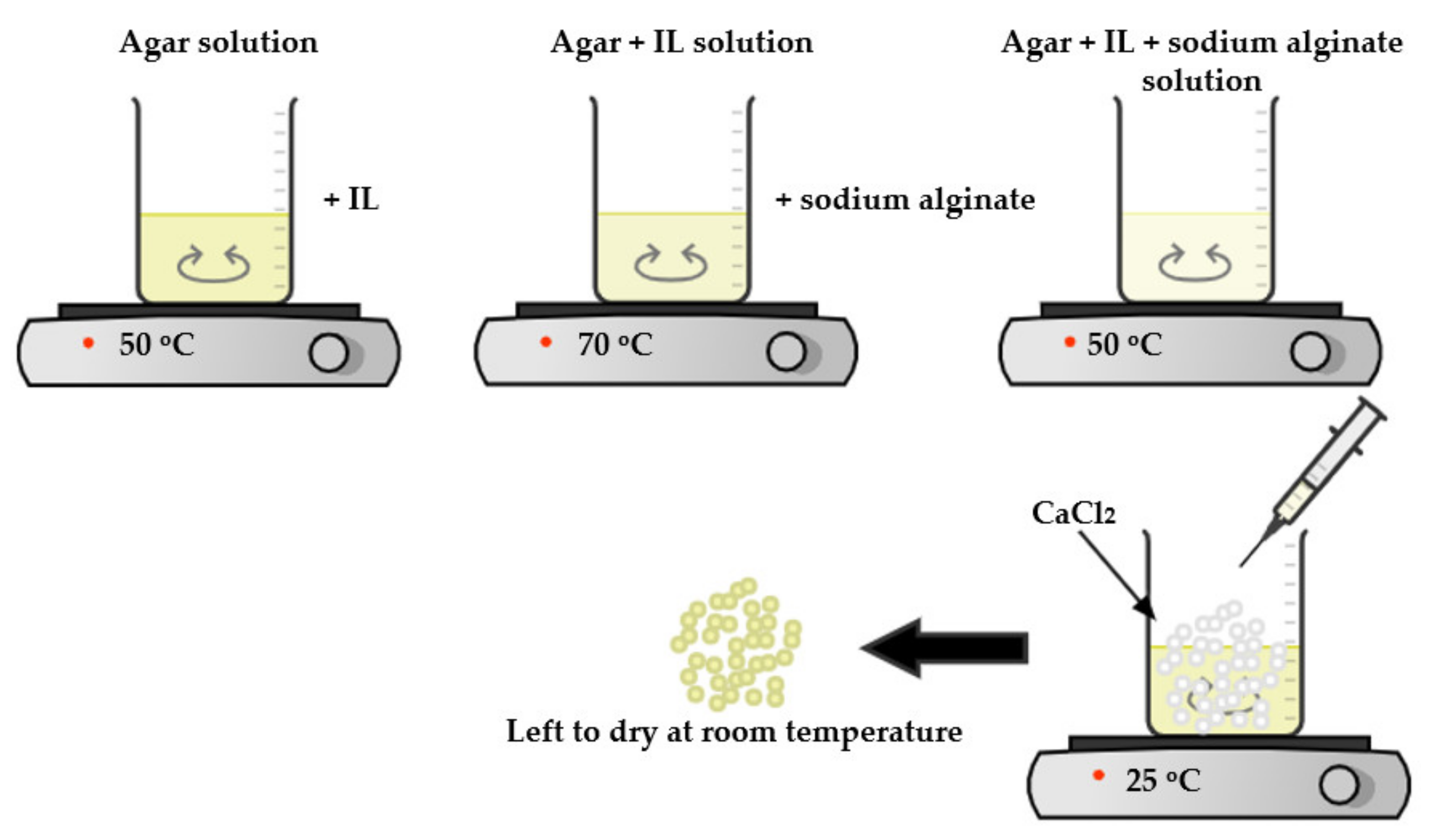
2.2. Preparation of Solutions
2.3. Preparation of IL–Agar–Alginate Beads
2.4. Characterization and Instruments
2.5. Adsorption Study
2.6. Desorption/Regeneration Study
3. Results and Discussion
3.1. Characterization
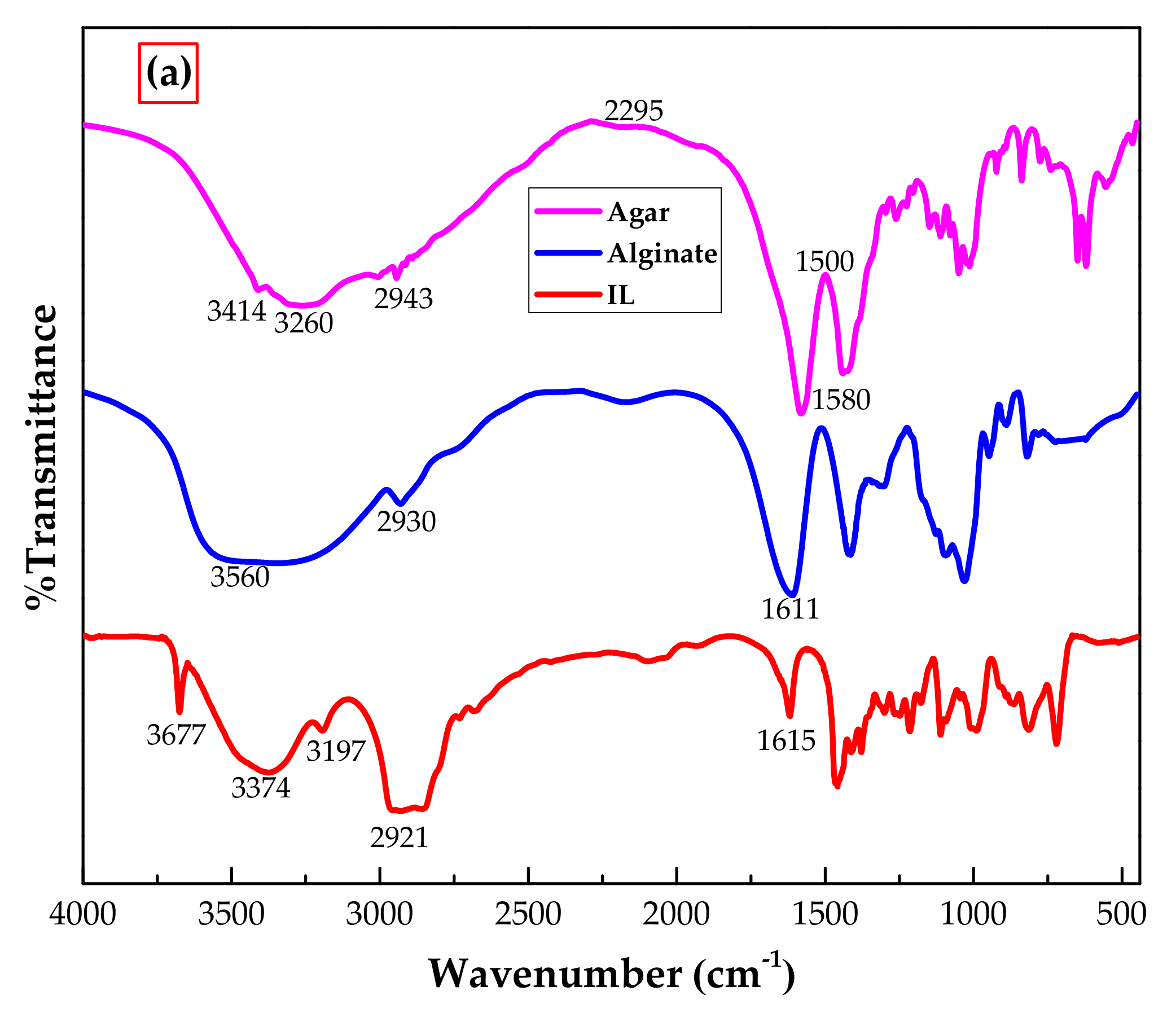
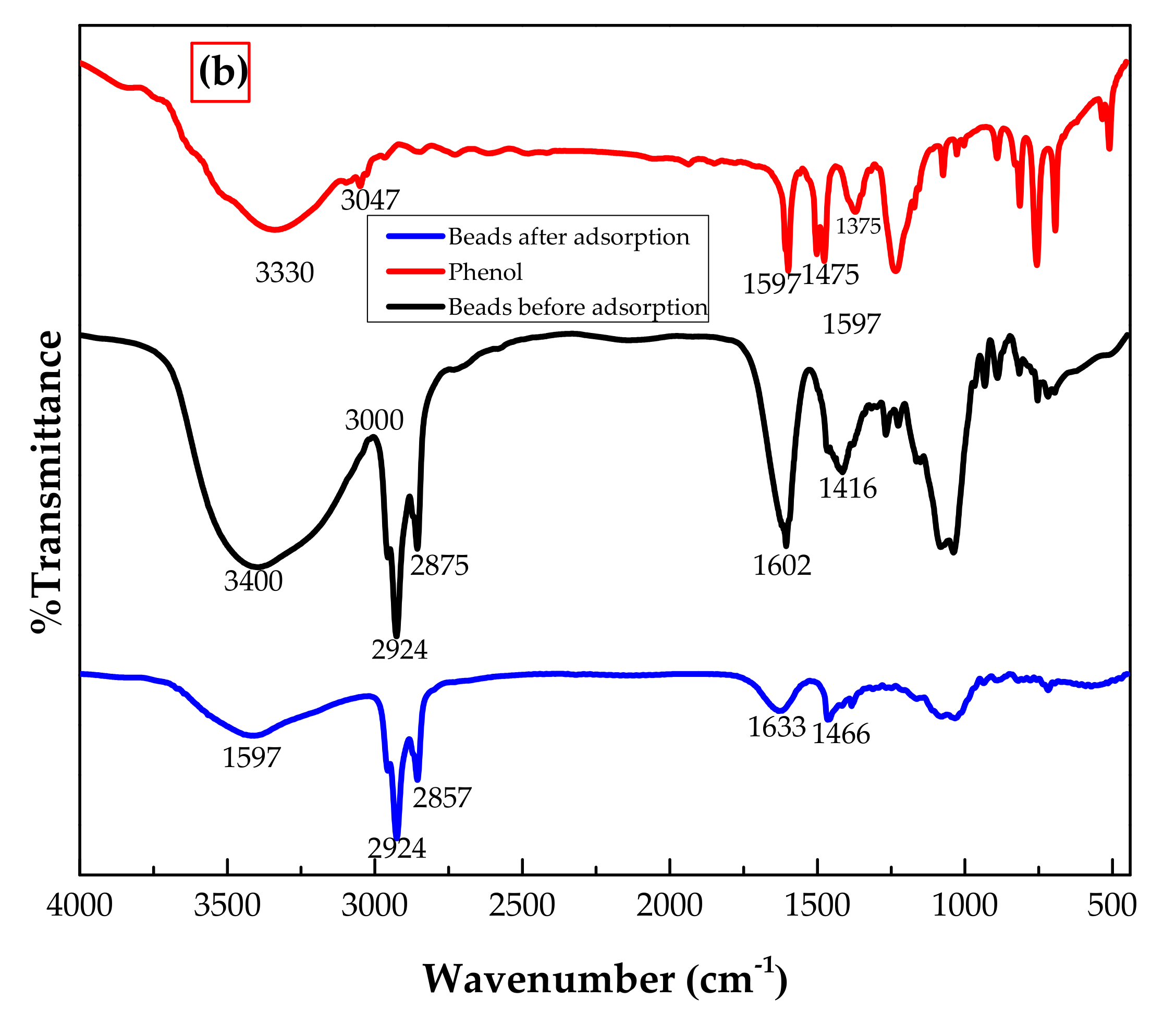
3.1.1. FTIR Analysis
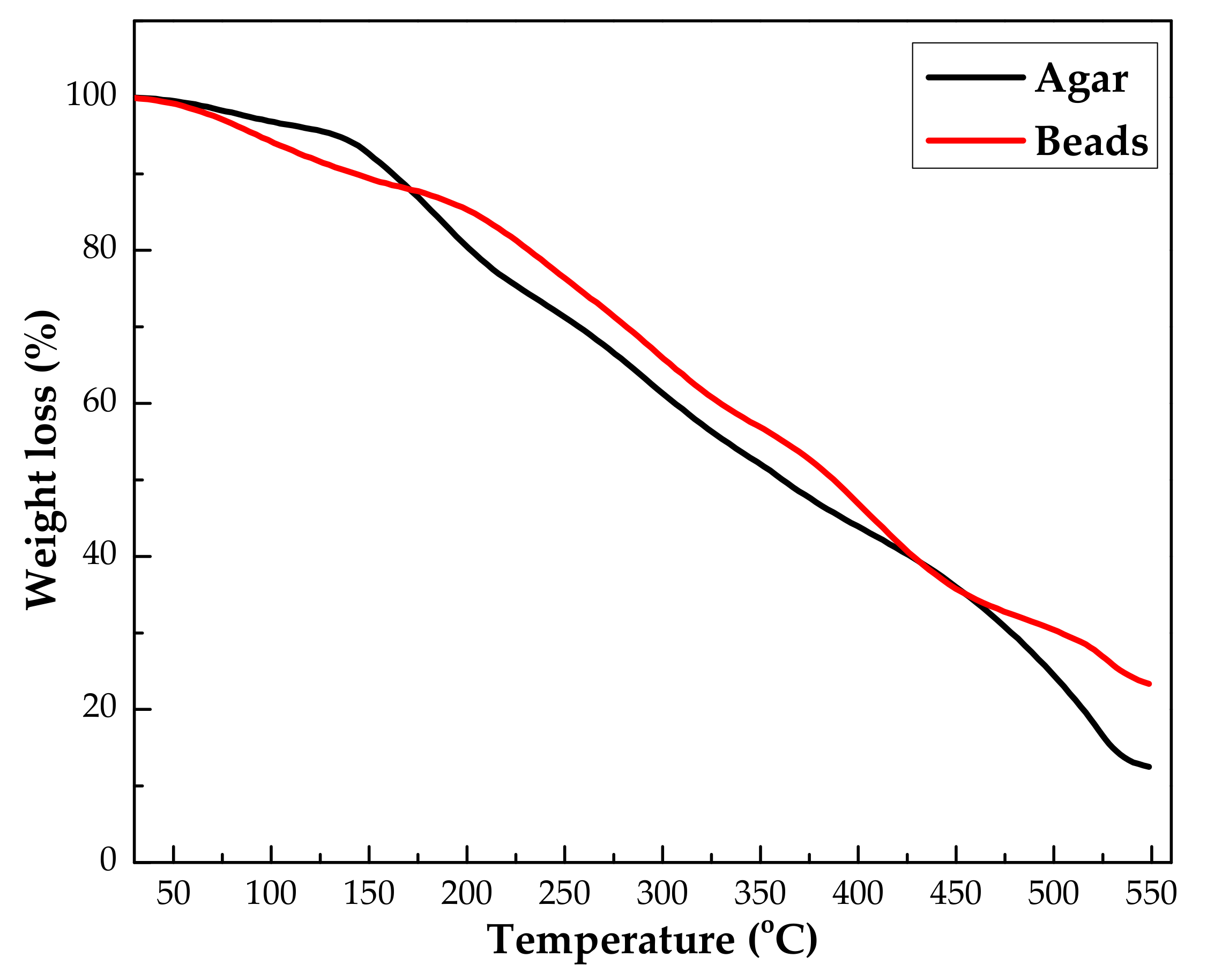
3.1.2. Thermogravimetric Analysis
3.1.3. SEM–EDX Analysis
3.1.4. The pH of Point Zero Charge Analysis (pHpzc)
3.2. Phenol Adsorption Study
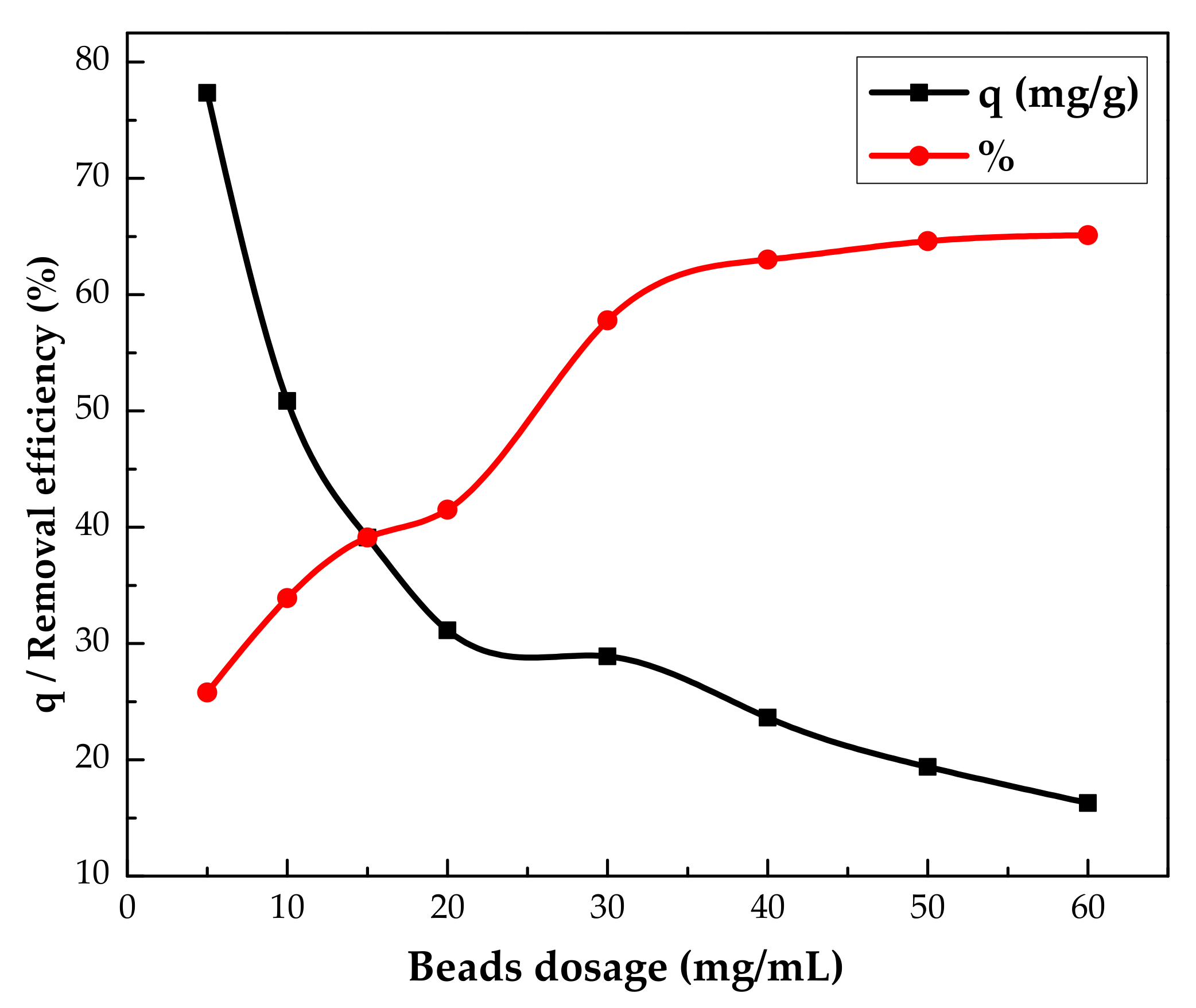
3.2.1. Effect of IL/Agar Ratio and Adsorbent Dosage
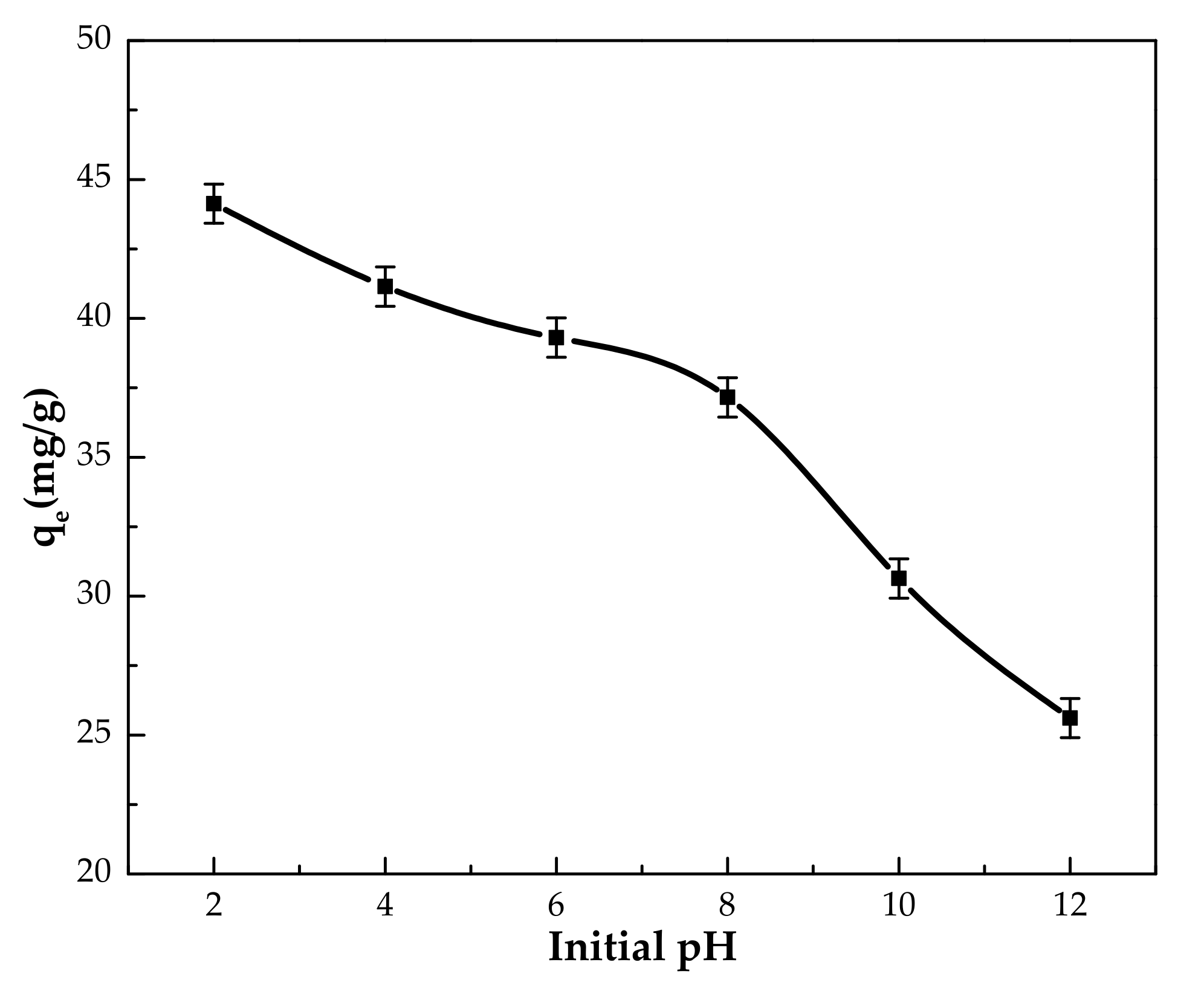
3.2.2. Effect of pH
3.2.3. Effect of Contact Time
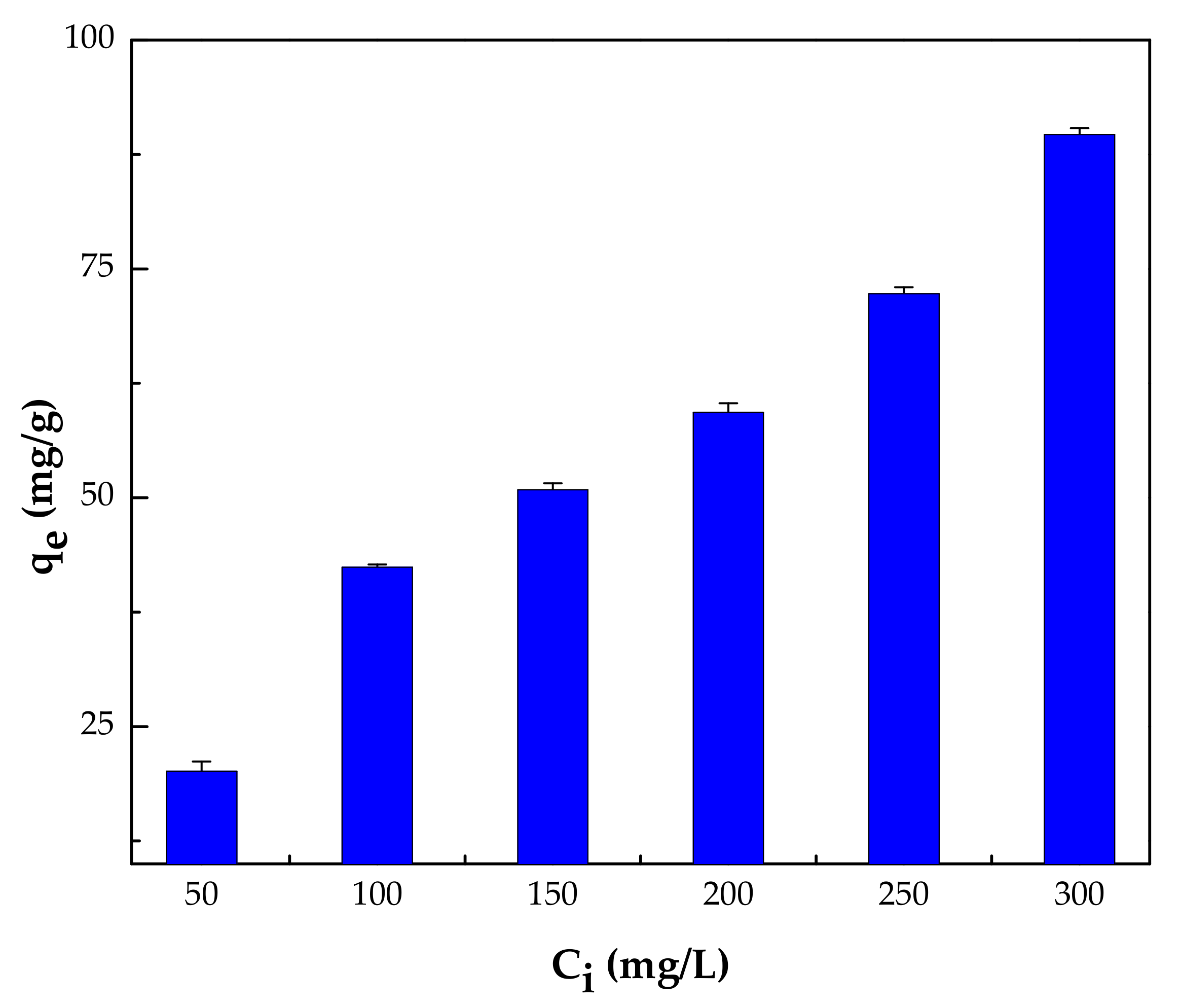
3.2.4. Effect of Initial Concentration
3.2.5. Effect of Temperature
3.3. Adsorption Models
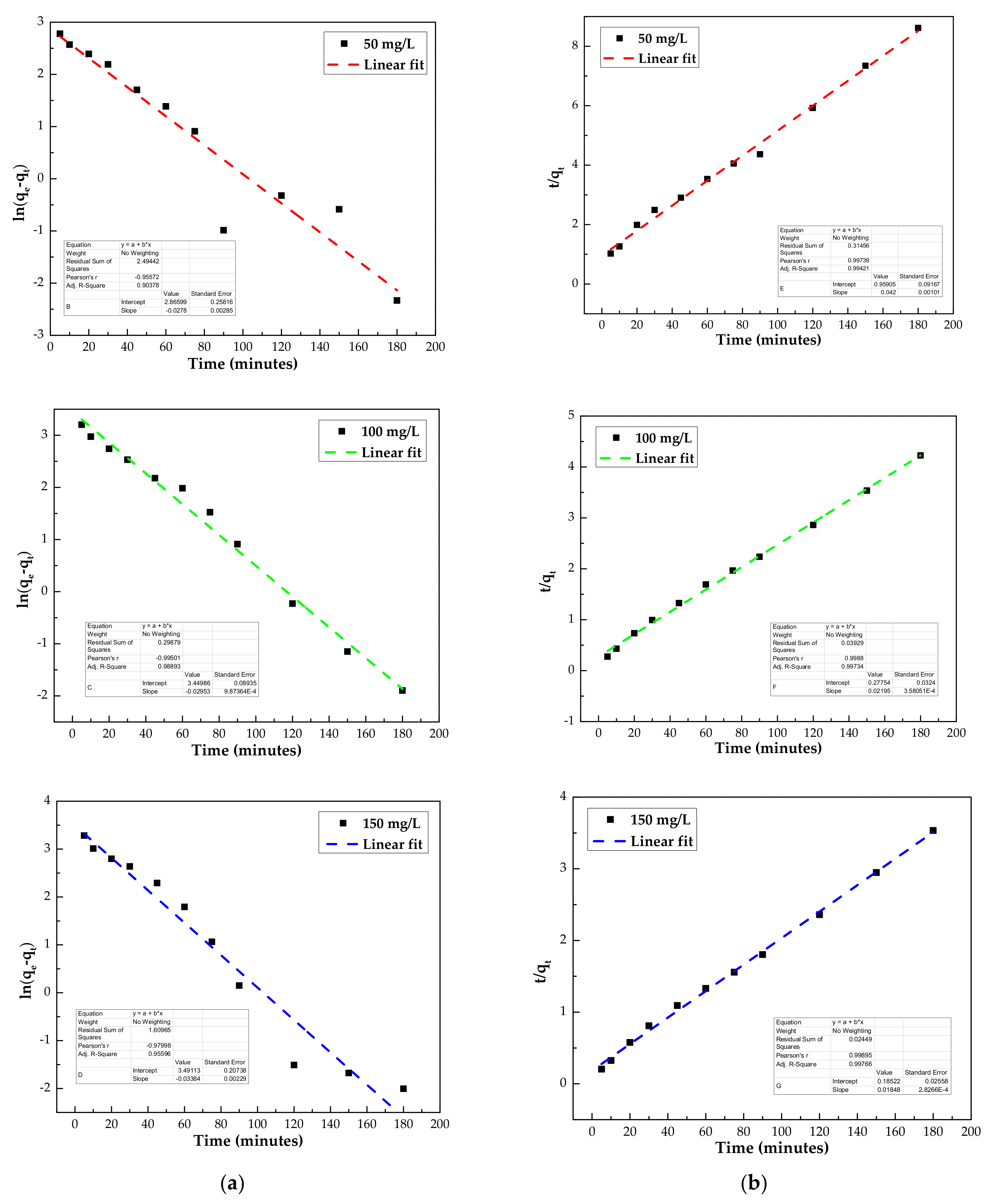
3.3.1. Adsorption Kinetics
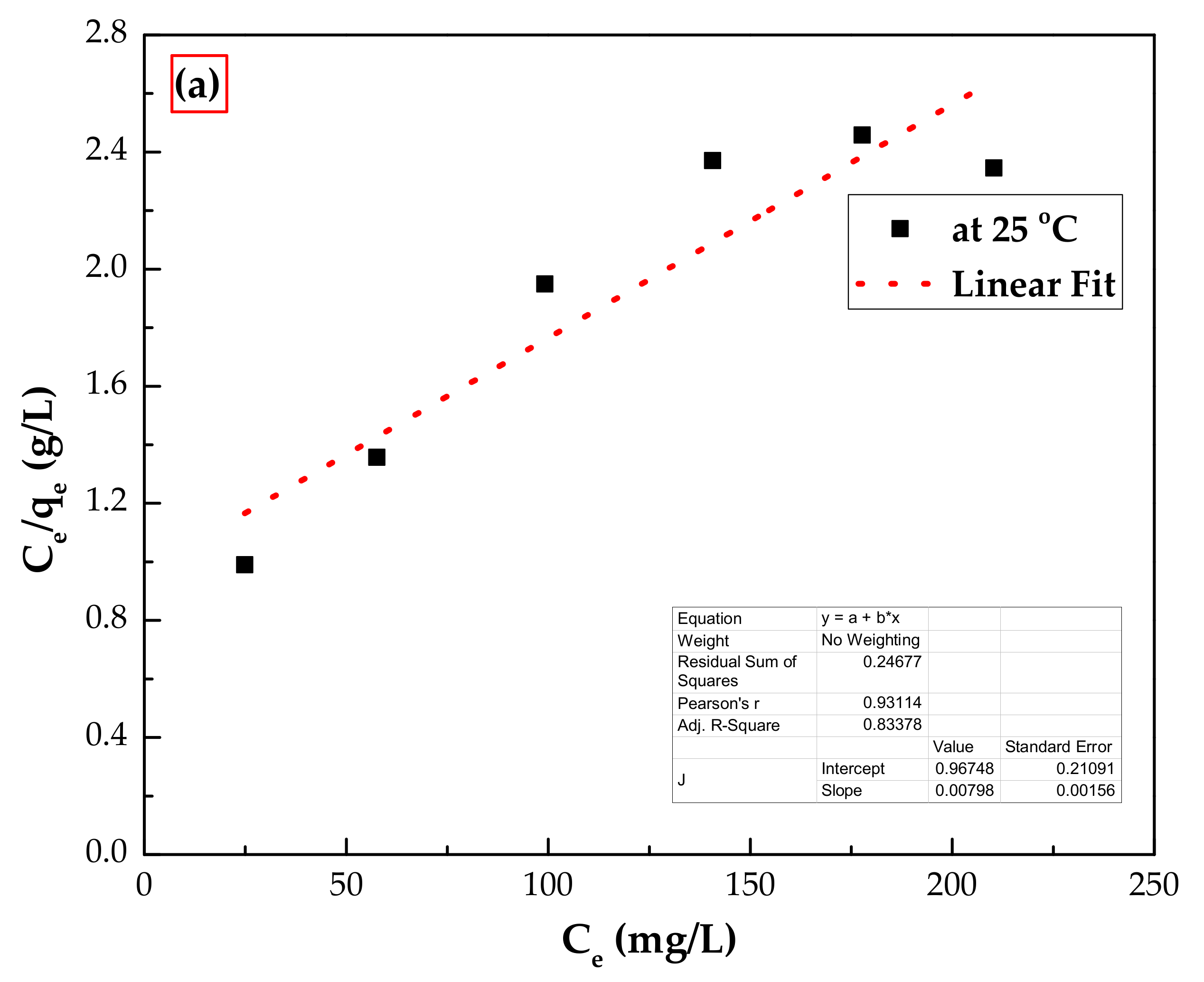
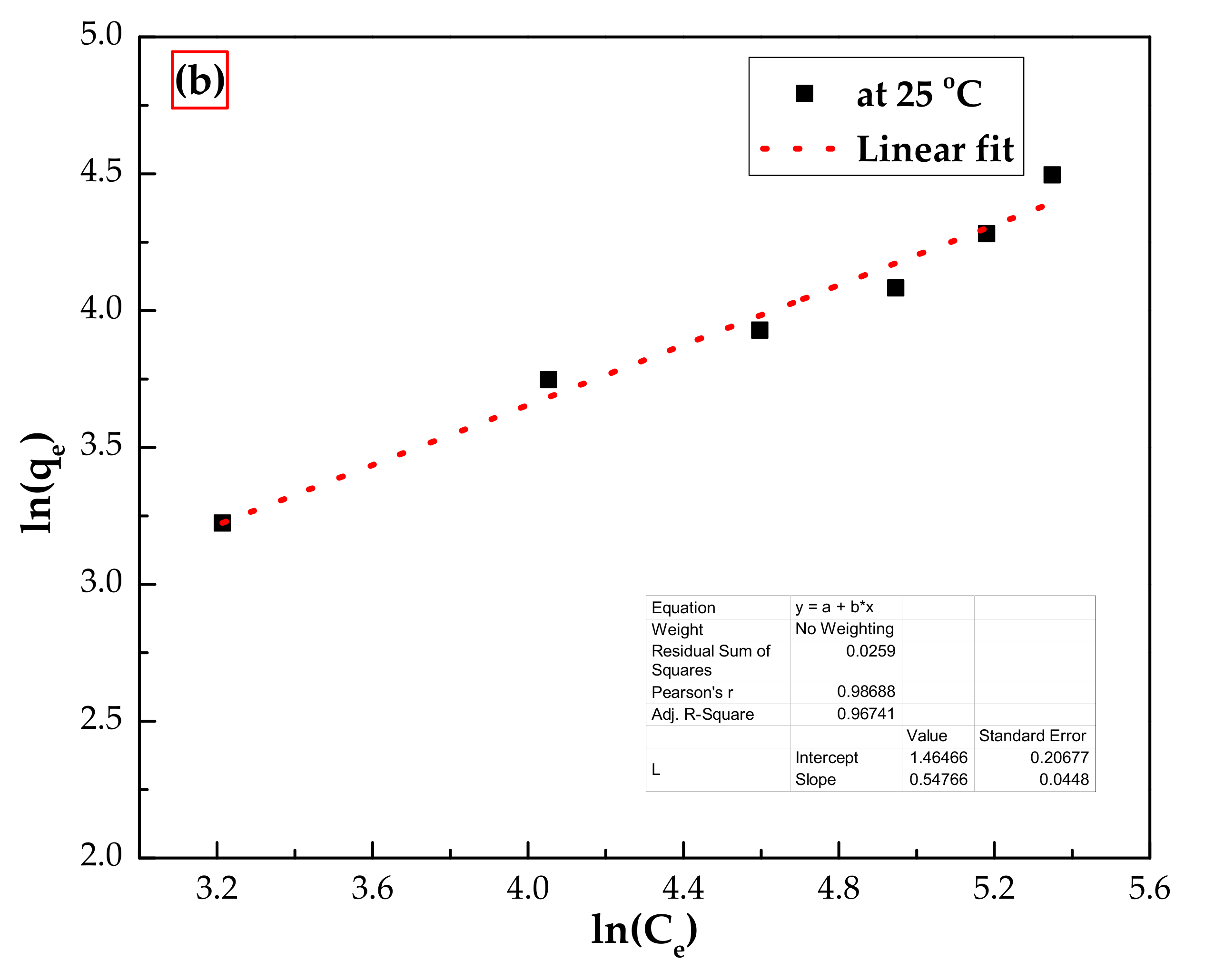
3.3.2. Adsorption Isotherms
3.4. Thermodynamic Analysis
3.5. Recyclability and Reusability
3.6. Comparative Study with Literature Values
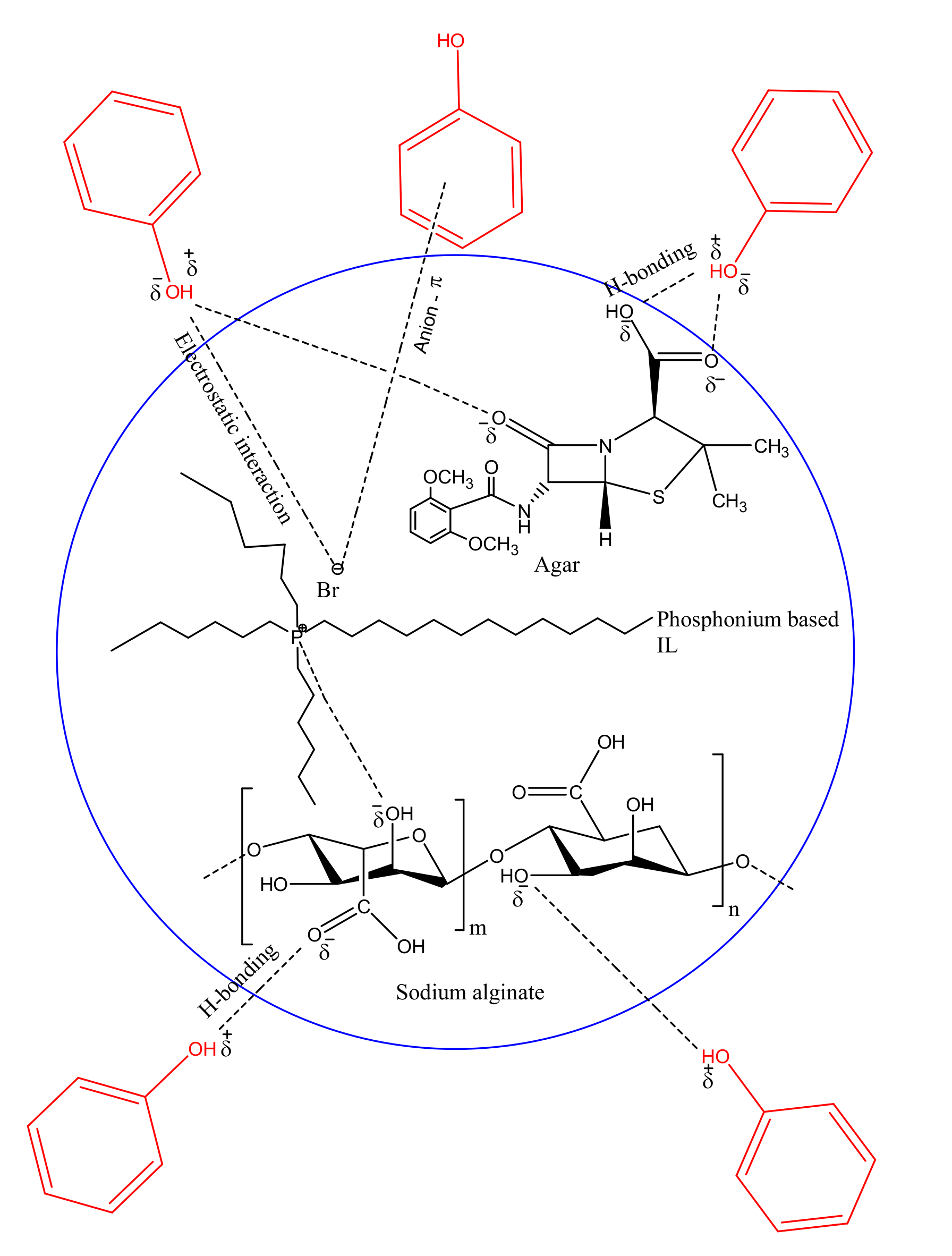
3.7. Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, S.; Zhang, F.; Li, D.; Jiao, P. Highly efficient and selective removal of cadmium from aqueous solutions based on magnetic graphitic carbon nitride materials with molecularly imprinted polymers. J. Mol. Struct. 2020, 1221, 128887. [Google Scholar] [CrossRef]
- Chang, J.; Mei, J.; Jia, W.; Chen, J.; Li, X.; Ji, B.; Wu, H. Treatment of heavily polluted river water by tidal-operated biofilters with organic/inorganic media: Evaluation of performance and bacterial community. Bioresour. Technol. 2019, 279, 34–42. [Google Scholar] [CrossRef]
- Dolaksiz, Y.E.; Temel, F.; Tabakci, M. Adsorption of phenolic compounds onto calix[4]arene-bonded silica gels from aqueous solutions. React. Funct. Polym. 2018, 126, 27–35. [Google Scholar] [CrossRef]
- Brandes, R.; Newton, B.; Owens, M.; Southerland, E. Technical Support Document for Water Quality-Based Toxics Control; Office of Water Enforcement and Permits; Environmental Protection Agency: Washington, DC, USA, 1985.
- Liu, S.; Wang, J.; Huang, W.; Tan, X.; Dong, H.; Goodman, B.A.; Du, H.; Lei, F.; Diao, K. Adsorption of phenolic compounds from water by a novel ethylenediamine rosin-based resin: Interaction models and adsorption mechanisms. Chemosphere 2018, 214, 821–829. [Google Scholar] [CrossRef]
- Garcia, I.; Venceslada, J.; Pena, P.; Gomez, E. Biodegradation of phenol compounds in vinasse using aspergillus terreus and geotrichum candidum. Water Resour. 1997, 31, 2005–2011. [Google Scholar] [CrossRef]
- Norouzi, M.; Fazeli, A.; Tavakoli, O. Phenol contaminated water treatment by photocatalytic degradation on electrospun Ag/TiO2 nanofibers: Optimization by the response surface method. J. Water Process. Eng. 2020, 37, 101489. [Google Scholar] [CrossRef]
- Zhao, L.; Xiao, D.; Liu, Y.; Xu, H.; Nan, H.; Li, D.; Kan, Y.; Cao, X. Biochar as simultaneous shelter, adsorbent, pH buffer, and substrate of Pseudomonas citronellolis to promote biodegradation of high concentrations of phenol in wastewater. Water Res. 2020, 172, 115494. [Google Scholar] [CrossRef] [PubMed]
- Arancibia-Miranda, N.; Manquián-Cerda, K.; Pizarro, C.; Maldonado, T.; Hernandez, J.M.S.; Escudey, M.; Bolan, N.; Sarkar, B. Mechanistic insights into simultaneous removal of copper, cadmium and arsenic from water by iron oxide-functionalized magnetic imogolite nanocomposites. J. Hazard. Mater. 2020, 398, 122940. [Google Scholar] [CrossRef] [PubMed]
- Weidner, E.; Ciesielczyk, F. Removal of Hazardous Oxyanions from the Environment Using Metal-Oxide-Based Materials. Materials 2019, 12, 927. [Google Scholar] [CrossRef] [Green Version]
- Santonastaso, G.; Erto, A.; Bortone, I.; Chianese, S.; Di Nardo, A.; Musmarra, D. Experimental and simulation study of the restoration of a thallium (I)-contaminated aquifer by Permeable Adsorptive Barriers (PABs). Sci. Total Environ. 2018, 630, 62–71. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Bai, G.; Yang, J.; Huang, J.; Fei, Z.; Liu, Q.; Zhang, Z.; Chen, X.; Tang, J.; Cui, M.; et al. Facile improvement of amine dispersion in KIT-1 with the alkyl chains template for enhanced CO2 adsorption capacity. J. Solid State Chem. 2020, 290, 121531. [Google Scholar] [CrossRef]
- Elhag, M.; Abdelwahab, H.E.; Mostafa, M.A.; Nasr, A.Z.; El Sadek, M.M. Synthesis and characterization of chitosan-pyrazoloquinoxaline Schiff bases for Cr (VI) removal from wastewater. Int. J. Biol. Macromol. 2020, 163, 2180–2188. [Google Scholar] [CrossRef] [PubMed]
- Isawi, H. Using Zeolite/Polyvinyl alcohol/sodium alginate nanocomposite beads for removal of some heavy metals from wastewater. Arab. J. Chem. 2020, 13, 5691–5716. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Y.; Dong, X.; Hu, G.; Hua, S.; Miao, J.; Zhou, D. Extraction of Phenols from Water with Functionalized Ionic Liquids. Ind. Eng. Chem. Res. 2014, 53, 20024–20031. [Google Scholar] [CrossRef]
- Archana, V.; Begum, K.M.S.; Anantharaman, N. Studies on removal of phenol using ionic liquid immobilized polymeric micro-capsules. Arab. J. Chem. 2016, 9, 371–382. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, X.; Lan, Z.; Xu, H. Unique extraction of U (VI), Pu (IV), Am (III) and Eu (III) from nitric acid medium using a Phosphonium based ionic liquid consisting of novel anion, 1, 1, 1, 5, 5, 5-hexafluoro-2, 4-pentanedionate-dual solvent behavior. Sep. Purif. Technol. 2021, 262, 118309. [Google Scholar] [CrossRef]
- Rooney, D.; Jacquemin, J.; Gardas, R. Thermophysical properties of ionic liquids. Ion. Liq. 2009, 290, 85–212. [Google Scholar]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Ullah, Z.; Bustam, M.A.; Man, Z.; Khan, A.S. Phosphonium-based ionic liquids and their application in separation of dye from aqueous solution. ARPN J. Eng. Appl. Sci. 2016, 11, 1653–1659. [Google Scholar]
- Rasheed, I.; Tabassum, A.; Khan, U.; Rehman, A. Fourier Transform Infrared (FT-IR) Spectroscopy of Agar from Red Seaweeds of Karachi Coast. Int. J. Biol. Biotechnol. 2019, 16, 59–63. [Google Scholar]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between Alginate and Chitosan Biopolymers Characterized Using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef]
- Trikkaliotis, D.G.; Christoforidis, A.K.; Mitropoulos, A.C.; Kyzas, G.Z. Adsorption of copper ions onto chitosan/poly(vinyl alcohol) beads functionalized with poly(ethylene glycol). Carbohydr. Polym. 2020, 234, 115890. [Google Scholar] [CrossRef] [PubMed]
- Nasrullah, A.; Bhat, A.; Naeem, A.; Isa, M.H.; Danish, M. High surface area mesoporous activated carbon-alginate beads for efficient removal of methylene blue. Int. J. Biol. Macromol. 2017, 107, 1792–1799. [Google Scholar] [CrossRef] [PubMed]
- Awual, M.R.; Hasan, M.M.; Iqbal, J.; Islam, A.; Islam, M.A.; Asiri, A.M.; Rahman, M.M. Naked-eye lead (II) capturing from contaminated water using innovative large-pore facial composite materials. Microchem. J. 2020, 154, 104585. [Google Scholar] [CrossRef]
- Shah, N.S.; Khan, J.A.; Sayed, M.; Iqbal, J.; Khan, Z.U.H.; Muhammad, N.; Polychronopoulou, K.; Hussain, S.; Imran, M.; Murtaza, B.; et al. Nano-zerovalent copper as a Fenton-like catalyst for the degradation of ciprofloxacin in aqueous solution. J. Water Process. Eng. 2020, 37, 101325. [Google Scholar] [CrossRef]
- Tiwari, A.; Soni, A.; Bajpai, A.K. Nanoparticles Loaded Alginate Beads as Potential Adsorbent for Removal of Phenol from Aqueous Solution. Synth. React. Inorg. Met. Nano Metal Chem. 2012, 42, 1158–1166. [Google Scholar] [CrossRef]
- Sposito, G. On points of zero charge. Environ. Sci. Technol. 1998, 32, 2815–2819. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Alothman, Z.; Ghfar, A.A. Novel Metal–Organic Framework (MOF) Based Composite Material for the Sequestration of U(VI) and Th(IV) Metal Ions from Aqueous Environment. ACS Appl. Mater. Interfaces 2017, 9, 36026–36037. [Google Scholar] [CrossRef]
- Daneshvar, E.; Vazirzadeh, A.; Niazi, A.; Kousha, M.; Naushad, M.; Bhatnagar, A. Desorption of methylene blue dye from brown macroalga: Effects of operating parameters, isotherm study and kinetic modeling. J. Clean. Prod. 2017, 152, 443–453. [Google Scholar] [CrossRef]
- Kumari, H.J.; Krishnamoorthy, P.; Arumugam, T.; Radhakrishnan, S.; Vasudevan, D. An efficient removal of crystal violet dye from waste water by adsorption onto TLAC/Chitosan composite: A novel low cost adsorbent. Int. J. Biol. Macromol. 2017, 96, 324–333. [Google Scholar] [CrossRef]
- Kumar, I.A.; Viswanathan, N. Micro-encapsulation and hydrothermal tuning of amine decorated magnetic alginate hybrid beads for nitrate and phosphate remediation. J. Taiwan Inst. Chem. Eng. 2019, 102, 283–296. [Google Scholar] [CrossRef]
- Ayati, A.; Tanhaei, B.; Sillanpää, M. Lead (II)-ion removal by ethylenediaminetetraacetic acid ligand functionalized magnetic chitosan–aluminum oxide–iron oxide nanoadsorbents and microadsorbents: Equilibrium, kinetics, and thermodynamics. J. Appl. Polym. Sci. 2017, 134, 4. [Google Scholar] [CrossRef]
- Naushad, M.; Alqadami, A.A.; Alothman, Z.; Alsohaimi, I.H.; Algamdi, M.S.; Aldawsari, A.M. Adsorption kinetics, isotherm and reusability studies for the removal of cationic dye from aqueous medium using arginine modified activated carbon. J. Mol. Liq. 2019, 293, 111442. [Google Scholar] [CrossRef]
- Belhouchat, N.; Zaghouane-Boudiaf, H.; Viseras, C. Removal of anionic and cationic dyes from aqueous solution with activated organo-bentonite/sodium alginate encapsulated beads. Appl. Clay Sci. 2017, 135, 9–15. [Google Scholar] [CrossRef]
- Sari, A.; Tuzen, M. Biosorption of total chromium from aqueous solution by red algae (Ceramium virgatum): Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 160, 349–355. [Google Scholar] [CrossRef]
- Blanchard, G.; Maunaye, M.; Martin, G. Removal of heavy metals from waters by means of natural zeolites. Water Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Al-Salehin, P.Z.; Moeinpour, F.; Mohseni-Shahri, F.S. Adsorption isotherm and thermodynamic studies of As(III) removal from aqueous solutions using used cigarette filter ash. Appl. Water Sci. 2019, 9, 172. [Google Scholar] [CrossRef] [Green Version]
- Priya, D.S.; Sureshkumar, M.V. Synthesis of Borassus flabellifer fruit husk activated carbon filter for phenol removal from wastewater. Int. J. Environ. Sci. Technol. 2019, 17, 829–842. [Google Scholar] [CrossRef]
- Dong, R.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Removal of phenol from aqueous solution using acid-modified Pseudomonas putida-sepiolite/ZIF-8 bio-nanocomposites. Chemosphere 2019, 239, 124708. [Google Scholar] [CrossRef]
- Mandal, A.; Mukhopadhyay, P.; Das, S.K. The study of adsorption efficiency of rice husk ash for removal of phenol from wastewater with low initial phenol concentration. SN Appl. Sci. 2019, 1, 192. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, S.; Igwegbe, C. Adsorptive removal of phenol and aniline by modified bentonite: Adsorption isotherm and kinetics study. Appl. Water Sci. 2018, 8, 170. [Google Scholar] [CrossRef] [Green Version]
- Ge, M.; Wang, X.; Du, M.; Liang, G.; Hu, G.; SM, J.A. Adsorption Analyses of Phenol from Aqueous Solutions Using Magadiite Modified with Organo-Functional Groups: Kinetic and Equilibrium Studies. Materials 2018, 12, 96. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Balomajumder, C. Simultaneous removal of Cr (VI) and phenol from binary solution using Bacillus sp. immobilized onto tea waste biomass. J. Water Process. Eng. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Cheng, W.P.; Gao, W.; Cui, X.; Ma, J.H.; Li, R.F. Phenol adsorption equilibrium and kinetics on zeolite X/activated carbon composite. J. Taiwan Inst. Chem. Eng. 2016, 62, 192–198. [Google Scholar] [CrossRef]
- Yang, C.; Qian, Y.; Zhang, L.; Feng, J. Solvent extraction process development and on-site trial-plant for phenol removal from industrial coal-gasification wastewater. Chem. Eng. J. 2006, 117, 179–185. [Google Scholar] [CrossRef]
- Mihoc, G.; Ianoş, R.; Păcurariu, C. Adsorption of phenol and p-chlorophenol from aqueous solutions by magnetic nanopowder. Water Sci. Technol. 2014, 69, 385–391. [Google Scholar] [CrossRef]
- Yoon, S.U.; Mahanty, B.; Ha, H.M.; Kim, C.G. Phenol adsorption on surface-functionalized iron oxide nanoparticles: Modeling of the kinetics, isotherm, and mechanism. J. Nanopart. Res. 2016, 18, 170. [Google Scholar] [CrossRef]
- Khan, A.S.; Ibrahim, T.H.; Khamis, M.I.; Nancarrow, P.; Iqbal, J.; AlNashef, I.; Jabbar, N.A.; Hassan, M.F.; Mjalli, F.S. Preparation of sustainable activated carbon-alginate beads impregnated with ionic liquid for phenol decontamination. J. Clean. Prod. 2021, 321, 128899. [Google Scholar] [CrossRef]
- Dehmani, Y.; Alrashdi, A.A.; Lgaz, H.; Lamhasni, T.; Abouarnadasse, S.; Chung, I.-M. Removal of phenol from aqueous solution by adsorption onto hematite (α-Fe2O3): Mechanism exploration from both experimental and theoretical studies. Arab. J. Chem. 2020, 13, 5474–5486. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Cho, H.-H.; Poster, D.L.; Ball, W.P. Evidence for a Pore-Filling Mechanism in the Adsorption of Aromatic Hydrocarbons to a Natural Wood Char. Environ. Sci. Technol. 2007, 41, 1212–1217. [Google Scholar] [CrossRef]
- Ye, H.; Liu, Y.; Zhang, X.; Di, D. DFT study on hydrogen-bonding adsorption mechanism of rutin onto macroporous adsorption resins functionalized with amino, hydroxyl, and carboxyl groups. Struct. Chem. 2013, 24, 1443–1449. [Google Scholar] [CrossRef] [Green Version]
- Paiman, S.H.; Rahman, M.A.; Uchikoshi, T.; Abdullah, N.; Othman, M.H.D.; Jaafar, J.; Abas, K.H.; Ismail, A.F. Functionalization effect of Fe-type MOF for methylene blue adsorption. J. Saudi Chem. Soc. 2020, 24, 896–905. [Google Scholar] [CrossRef]

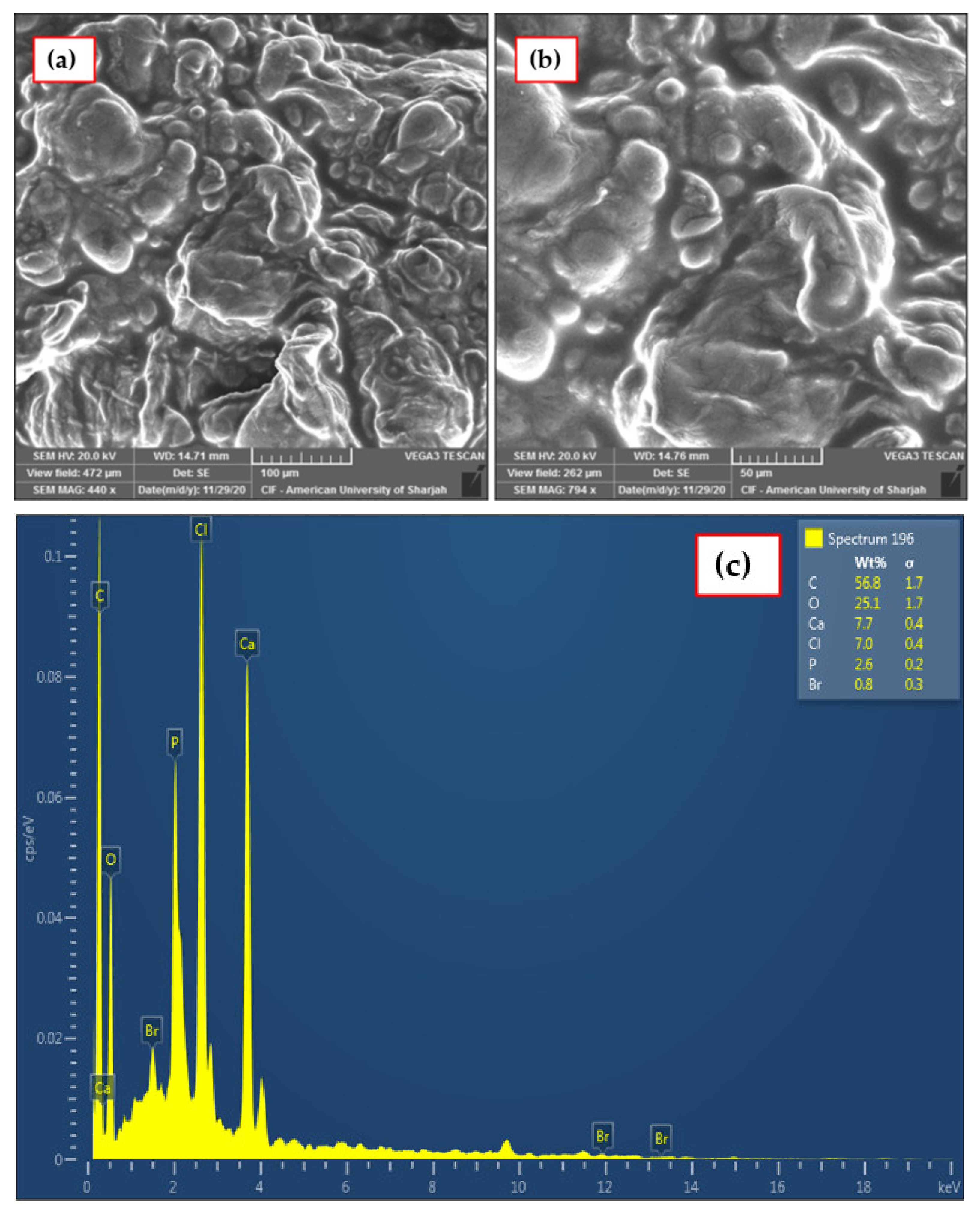





| Element | Apparent Concentration | wt.% |
|---|---|---|
| C | 0.55 | 56.83 ± 1.74 |
| O | 0.37 | 25.07 ± 1.65 |
| P | 0.15 | 2.59 ± 0.25 |
| Cl | 0.28 | 7.01 ± 0.36 |
| Ca | 0.31 | 7.68 ± 0.40 |
| Br | 0.03 | 0.84 ± 0.28 |
| Total | 100 |
| Kinetic Models | Parameters | 50 mg/L | 100 mg/L | 150 mg/L |
|---|---|---|---|---|
| Pseudo-first order | qe (mg/g) | 17.6 | 31.5 | 32.8 |
| k1 (min−1) | 0.028 | 0.029 | 0.034 | |
| R2 | 0.913 | 0.990 | 0.960 | |
| Pseudo-second order | qe (mg/g) | 23.5 | 45.4 | 53.8 |
| k2 (g/mg min−1) | 0.002 | 0.002 | 0.002 | |
| R2 | 0.996 | 0.998 | 0.998 |
| Isotherm Model | Parameters | At 25 °C |
|---|---|---|
| Langmuir model | qm (mg/g) | 159 |
| KL (L/mg) | 0.005 | |
| RL | 0.432 | |
| R2 | 0.835 | |
| Freundlich model | Kf (mg/g)(L/mg)1/n | 2.17 |
| N | 1.46 | |
| R2 | 0.956 |
| Ci (mg/L) | H° (kJ mol−1) | S° (J mol−1 K−1) | G° (kJ mol−1) | ||
|---|---|---|---|---|---|
| 298 K | 308 K | 318 K | |||
| 150 | −23.5 | −78.8 | −0.023 | 0.765 | 1.555 |
| Cycle | Hot Water | Methanol | ||
|---|---|---|---|---|
| q (mg/g) | %R | q (mg/g) | %R | |
| 1 | 50.85 | 33.9 | 37.17 | 24.78 |
| 2 | 45.62 | 30.41 | 33.79 | 22.53 |
| 3 | 45.34 | 30.25 | 30.55 | 20.37 |
| 4 | 45.18 | 30.12 | 24.62 | 16.42 |
| Adsorbent | qe (mg/g) | pH | Temp. (°C) | Ref. |
|---|---|---|---|---|
| Borassus flabellifer fruit husk activated carbon (Pyrolysis) | 13.97 | 2 | - | [39] |
| Borassus flabellifer fruit husk activated carbon (H2SO4 activation) | 13.42 | 4 | - | [39] |
| Microorganism P. putida and acid-modified CESEP/ZIF-8 | 5.96 | - | - | [40] |
| Rice husk | 7.89 | 9 | 35 | [41] |
| Acid-modified bentonite | 6.8 | 4 | 20–25 | [42] |
| Organo-functional groups | 45.26 | 7–10 | - | [43] |
| Tea waste biomass | 9.49 | 5–9 | - | [44] |
| Zeolite | 4.31 | 3.3–8.6 | 40 | [45] |
| Solvent extraction | 3.45 | 7.5–8.5 | 40–60 | [46] |
| Magnetic iron oxide nanopowder (MNM) | 2.536 | - | - | [47] |
| Methyl acrylic acid (MAA)-coated iron oxide nanoparticles (NPs) | 950 | 7 | - | [48] |
| Activated carbon alginate beads impregnated with IL | 78.8 | 2 | - | [49] |
| α-Fe2O3 | 16.17 | 2–6 | 60 | [50] |
| IL–agar–alginate beads (Ci = 150 mg/L) | 16.28 | 2 | 25 | This work |
| Adsorbent | Kinetics Parameters | (%) R | Ref. | ||
|---|---|---|---|---|---|
| k | qe | R2 | |||
| Borassus flabellifer fruit husk activated carbon (Pyrolysis) | 0.022 | 4.06 | 0.99 | 83 | [39] |
| Borassus flabellifer fruit husk activated carbon (H2SO4 activation) | 0.024 | 4.38 | 0.99 | 80 | [39] |
| Microorganism P. putida and acid-modified CESEP/ZIF-8 | 0.002 | 5.82 | 0.99 | 77 | [40] |
| Rice husk | 0.063 | 2.42 | 0.99 | 95 | [41] |
| Acid-modified bentonite | 0.05 | 4.58 | 0.99 | 94.2 | [42] |
| Organo-functional groups | 0.000 | 47.11 | 0.99 | 95 | [43] |
| Tea waste biomass | 0.004 | 9.64 | 0.89 | 82 | [44] |
| Zeolite | 0.02 | 13.87 | 0.99 | - | [45] |
| Magnetic iron oxide nanopowder (MNM) | 0.002 | 2.94 | 0.98 | - | [47] |
| Methyl acrylic acid (MAA)-coated iron oxide nanoparticles (NPs) | - | 1 | 0.99 | 90 | [48] |
| Activated carbon alginate beads impregnated with IL | 0.000 | 53.07 | 0.99 | - | [49] |
| α-Fe2O3 | 0.23 | 10.66 | 0.99 | - | [50] |
| IL–agar–alginate beads (Ci = 150 mg/L, beads dosage = 1 mg/mL) | 0.002 | 53.8 | 0.99 | 65 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasir, N.; Khan, A.S.; Hassan, M.F.; Ibrahim, T.H.; Khamis, M.I.; Nancarrow, P. Ionic Liquid Agar–Alginate Beads as a Sustainable Phenol Adsorbent. Polymers 2022, 14, 984. https://doi.org/10.3390/polym14050984
Yasir N, Khan AS, Hassan MF, Ibrahim TH, Khamis MI, Nancarrow P. Ionic Liquid Agar–Alginate Beads as a Sustainable Phenol Adsorbent. Polymers. 2022; 14(5):984. https://doi.org/10.3390/polym14050984
Chicago/Turabian StyleYasir, Nihal, Amir Sada Khan, Muhammad Faheem Hassan, Taleb H. Ibrahim, Mustafa I. Khamis, and Paul Nancarrow. 2022. "Ionic Liquid Agar–Alginate Beads as a Sustainable Phenol Adsorbent" Polymers 14, no. 5: 984. https://doi.org/10.3390/polym14050984
APA StyleYasir, N., Khan, A. S., Hassan, M. F., Ibrahim, T. H., Khamis, M. I., & Nancarrow, P. (2022). Ionic Liquid Agar–Alginate Beads as a Sustainable Phenol Adsorbent. Polymers, 14(5), 984. https://doi.org/10.3390/polym14050984








