Lightweight Polyethylene/Hexagonal Boron Nitride Hybrid Thermal Conductor Fabricated by Melt Compounding Plus Salt Leaching
Abstract
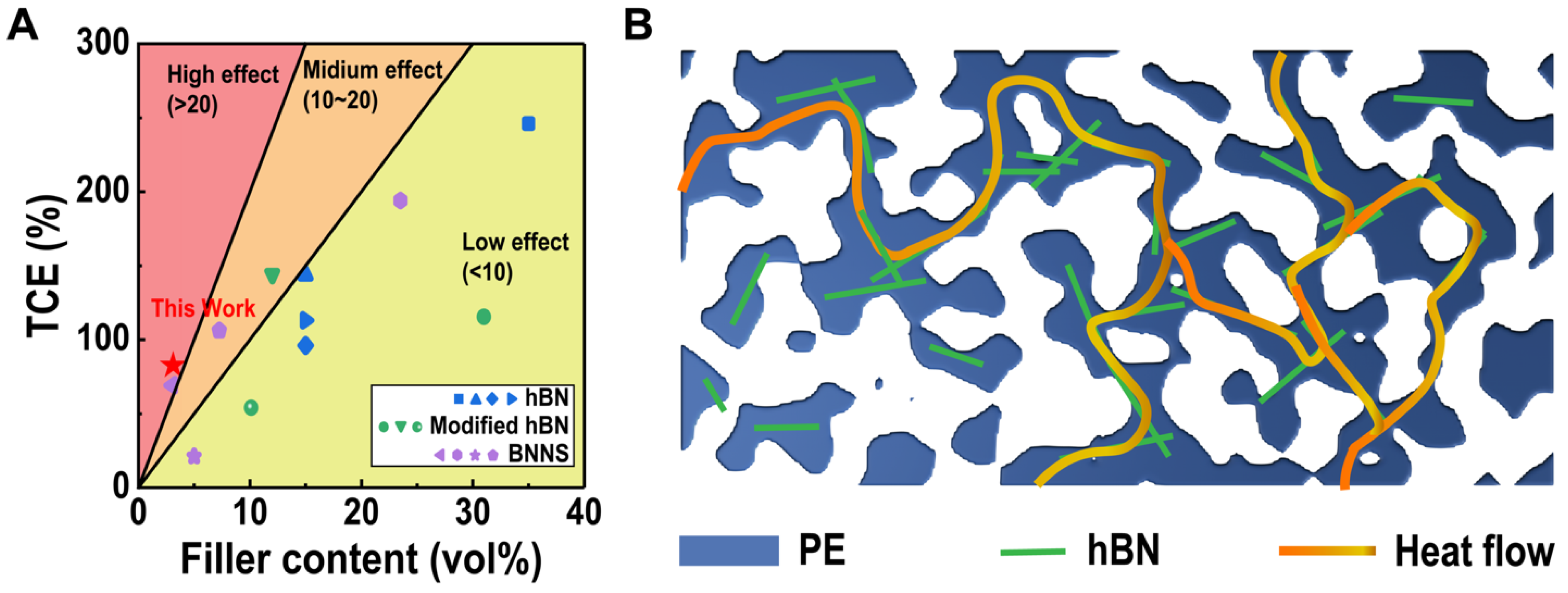
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Fabrication
2.3. Characterization
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Porous Structure Analysis
2.3.3. X-ray Diffraction (XRD)
2.3.4. Thermal Conductivity Evaluation
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zheng, B.; Lin, X.; Zhang, X.; Wu, D.; Matyjaszewski, K. Emerging functional porous polymeric and carbonaceous materials for environmental treatment and energy storage. Adv. Funct. Mater. 2020, 30, 1907006. [Google Scholar] [CrossRef]
- Vázquez Fletes, R.C.; Cisneros López, E.O.; Moscoso Sánchez, F.J.; Mendizábal, E.; González Núñez, R.; Rodrigue, D.; Ortega Gudiño, P. Morphological and mechanical properties of bilayers wood-plastic composites and foams obtained by rotational molding. Polymers 2020, 12, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junoh, H.; Jaafar, J.; Nordin, N.A.H.M.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Aziz, F.; Yusof, N. Performance of polymer electrolyte membrane for direct methanol fuel cell application: Perspective on morphological structure. Membranes 2020, 10, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.; Kim, Y.; Yang, H.; Oh, J.H. Fabrication of hierarchically porous structured PDMS composites and their application as a flexible capacitive pressure sensor. Compos. Part B Eng. 2021, 211, 108607. [Google Scholar] [CrossRef]
- Xie, Y.; Hu, J.; Li, H.; Mi, H.-Y.; Ni, G.; Zhu, X.; Jing, X.; Wang, Y.; Zheng, G.; Liu, C.; et al. Green fabrication of double-sided self-supporting triboelectric nanogenerator with high durability for energy harvesting and self-powered sensing. Nano Energy 2022, 93, 106827. [Google Scholar] [CrossRef]
- Ni, G.-L.; Zhu, X.; Mi, H.-Y.; Feng, P.-Y.; Li, J.; Jing, X.; Dong, B.; Liu, C.; Shen, C. Skinless porous films generated by supercritical CO2 foaming for high-performance complementary shaped triboelectric nanogenerators and self-powered sensors. Nano Energy 2021, 87, 106148. [Google Scholar] [CrossRef]
- Montgomery, R.B. Viscosity and thermal conductivity of air and diffusivity of water vapor in air. J. Meteorol. 1947, 4, 193–196. [Google Scholar] [CrossRef] [Green Version]
- Yeo, S.J.; Oh, M.J.; Yoo, P.J. Structurally Controlled Cellular Architectures for High-Performance Ultra-Lightweight Materials. Adv. Mater. 2019, 31, 1803670. [Google Scholar] [CrossRef]
- Ashby, M.F. The properties of foams and lattices. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2006, 364, 15–30. [Google Scholar] [CrossRef]
- Wang, X.J.; Niu, X.H.; Wang, X.X.; Qiu, X.W.; Istikomah, N.; Wang, L.B. Thermal conductivity of porous polymer materials considering pore special-shape and anisotropy. Express Polym. Lett. 2021, 15, 319–328. [Google Scholar] [CrossRef]
- Rybak, A.; Malinowski, L.; Adamus-Wlodarczyk, A.; Ulanski, P. Thermally conductive shape memory polymer composites filled with boron nitride for heat management in electrical insulation. Polymers 2021, 13, 2191. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.; Leung, S.N.; Khan, M.O.; Naguib, H.E.; Dawson, F.; Adinkrah, V. Novel thermally conductive thermoplastic/ceramic composite foams. Macromol. Mater. Eng. 2012, 297, 1014–1020. [Google Scholar] [CrossRef]
- Ding, H.; Guo, Y.; Leung, S.N. Development of thermally conductive polymer matrix composites by foaming assisted networking of micron and submicron scale hexagonal boron nitride. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Ding, H.; Leung, S.N. Modelling of effective thermal conductivity of polymer matrix composite foams with biaxially aligned filler networks. J. Cell. Plast. 2016, 52, 89–106. [Google Scholar] [CrossRef]
- Xu, T.; Zhou, S.; Cui, S.; Song, N.; Shi, L.; Ding, P. Three-dimensional carbon fiber-graphene network for improved thermal conductive properties of polyamide-imide composites. Compos. Part B Eng. 2019, 178, 107495. [Google Scholar] [CrossRef]
- Liu, B.; Li, Y.; Wang, Q.; Bai, S. Green fabrication of leather solid waste/thermoplastic polyurethanes composite: Physically de-bundling effect of solid-state shear milling on collagen bundles. Compos. Sci. Technol. 2019, 181, 107674. [Google Scholar] [CrossRef]
- Sung, K.-S.; Kim, S.-Y.; Oh, M.-K.; Kim, N. Thermal and Adhesion Properties of Fluorosilicone Adhesives Following Incorporation of Magnesium Oxide and Boron Nitride of Different Sizes and Shapes. Polymers 2022, 14, 258. [Google Scholar] [CrossRef]
- Wie, J.; Kim, J. Thermal Properties of Surface-Modified and Cross-Linked Boron Nitride/Polyethylene Glycol Composite as Phase Change Material. Polymers 2021, 13, 456. [Google Scholar] [CrossRef]
- Wang, J.; Tang, Q.; Ren, X.; Yang, Y.; Zhang, Q.; Lei, W.; Li, Z.; Jiang, T.; Shi, D. Selectively localized nanosilica particles at the phase interface of PS/PA6/nanosilica composites with co-continuous structure via reactive extrusion. Compos. Sci. Technol. 2019, 172, 125–133. [Google Scholar] [CrossRef]
- Kou, Y.; Cheng, X.; Macosko, C.W. Polymer/Graphene Composites via Spinodal Decomposition of Miscible Polymer Blends. Macromolecules 2019, 52, 7625–7637. [Google Scholar] [CrossRef]
- Ravindren, R.; Mondal, S.; Nath, K.; Das, N.C. Prediction of electrical conductivity, double percolation limit and electromagnetic interference shielding effectiveness of copper nanowire filled flexible polymer blend nanocomposites. Compos. Part B Eng. 2019, 164, 559–569. [Google Scholar] [CrossRef]
- Veeramuthu, L.; Venkatesan, M.; Benas, J.-S.; Cho, C.-J.; Lee, C.-C.; Lieu, F.-K.; Lin, J.-H.; Lee, R.-H.; Kuo, C.-C. Recent Progress in Conducting Polymer Composite/Nanofiber-Based Strain and Pressure Sensors. Polymers 2021, 13, 4281. [Google Scholar] [CrossRef] [PubMed]
- Marischal, L.; Cayla, A.; Lemort, G.; Campagne, C.; Devaux, É. Selection of Immiscible Polymer Blends Filled with Carbon Nanotubes for Heating Applications. Polymers 2019, 11, 1827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghelinejad, M.; Leung, S.N. Fabrication of open-cell thermoelectric polymer nanocomposites by template-assisted multi-walled carbon nanotubes coating. Compos. Part B Eng. 2018, 145, 100–107. [Google Scholar] [CrossRef]
- Shahidi, K.; Rodrigue, D. Production of Composite Membranes by Coupling Coating and Melt Extrusion/Salt Leaching. Ind. Eng. Chem. Res. 2017, 56, 1306–1315. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kummara, M.R.; Kamal, T.; Alghyamah, A.-A.A.; Iftikhar, F.J.; Bano, B.; Khan, N.; Afridi, M.A.; Han, S.S. Advances in the scaffolds fabrication techniques using biocompatible polymers and their biomedical application: A technical and statistical review. J. Saudi Chem. Soc. 2020, 24, 186–215. [Google Scholar] [CrossRef]
- Kutcherov, V.; Håkansson, B.; Ross, R.G.; Bäckström, G. Experimental test of theories for the effective thermal conductivity of a dispersed composite. J. Appl. Phys. 1992, 71, 1732–1736. [Google Scholar] [CrossRef]
- Guo, F.; Zhao, J.; Li, F.; Kong, D.; Guo, H.; Wang, X.; Hu, H.; Zong, L.; Xu, J. Polar crystalline phases of PVDF induced by interaction with functionalized boron nitride nanosheets. CrystEngComm 2020, 22, 6207–6215. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Linares-Fernandez, L.; Doehne, E.; Sebastian, E. Effects of ferrocyanide ions on NaCl crystallization in porous stone. J. Cryst. Growth 2002, 243, 503–516. [Google Scholar] [CrossRef] [Green Version]
- Addala, S.; Bouhdjer, L.; Chala, A.; Bouhdjar, A.; Halimi, O.; Boudine, B.; Sebais, M. Structural and optical properties of a NaCl single crystal doped with CuO nanocrystals. Chin. Phys. B 2013, 22, 098103. [Google Scholar] [CrossRef]
- Guo, F.; Shen, X.; Zhou, J.; Liu, D.; Zheng, Q.; Yang, J.; Jia, B.; Lau, A.K.T.; Kim, J.K. Highly thermally conductive dielectric nanocomposites with synergistic alignments of graphene and boron nitride nanosheets. Adv. Funct. Mater. 2020, 30, 1910826. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J. Fabrication of Fe3O4 coated boron nitride nanoplatelets by liquid-phase exfoliation for thermally enhanced epoxy composites via magnetic alignment. Compos. Sci. Technol. 2020, 188, 107961. [Google Scholar] [CrossRef]
- Jiang, F.; Song, N.; Ouyang, R.; Ding, P. Wall density-controlled thermal conductive and mechanical properties of three-dimensional vertically aligned boron nitride network-based polymeric composites. ACS Appl. Mater. Interfaces 2021, 13, 7556–7566. [Google Scholar] [CrossRef]
- Srivastava, D.; Menon, M.; Cho, K. Anisotropic nanomechanics of boron nitride nanotubes: Nanostructured “skin” effect. Phys. Rev. B 2001, 63, 195413. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Schenning, A.H.P.J.; Shen, L.; Bastiaansen, C.W.M. The Role of Polyethylene Wax on the Thermal Conductivity of Transparent Ultradrawn Polyethylene Films. Macromolecules 2020, 53, 5599–5603. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Yang, G.; Su, F.; Feng, Y.; Ji, Y.; Liu, D.; Yin, R.; Liu, C.; Shen, C. Multilayer polyethylene/hexagonal boron nitride composites showing high neutron shielding efficiency and thermal conductivity. Compos. Commun. 2020, 19, 147–153. [Google Scholar] [CrossRef]
- Hasanzadeh, R.; Azdast, T.; Doniavi, A. Thermal Conductivity of Low-Density Polyethylene Foams Part II: Deep Investigation using Response Surface Methodology. J. Therm. Sci. 2020, 29, 159–168. [Google Scholar] [CrossRef]
- Rezgar, H.; Taher, A.; Ali, D.; Richard, E.L. Thermal Conductivity of Low Density Polyethylene Foams Part I: Comprehensive Study of Theoretical Models. J. Therm. Sci. 2019, 28, 745–754. [Google Scholar] [CrossRef]
- Alvarez-Lainez, M.; Rodriguez-Perez, M.A.; De Saja, J.A. Thermal conductivity of open-cell polyolefin foams. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 212–221. [Google Scholar] [CrossRef]
- Pan, X.; Shen, L.; Schenning, A.P.H.J.; Bastiaansen, C.W.M. Transparent, High-Thermal-Conductivity Ultradrawn Polyethylene/Graphene Nanocomposite Films. Adv. Mater. 2019, 31, 1904348. [Google Scholar] [CrossRef]
- Liu, P.; Li, X.; Min, P.; Chang, X.; Shu, C.; Ding, Y.; Yu, Z.-Z. 3D lamellar-structured graphene aerogels for thermal interface composites with high through-plane thermal conductivity and fracture toughness. Nano-Micro Lett. 2021, 13, 1–15. [Google Scholar] [CrossRef]
- Leung, S.N. Thermally conductive polymer composites and nanocomposites: Processing-structure-property relationships. Compos. Part B Eng. 2018, 150, 78–92. [Google Scholar] [CrossRef]
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal conductivity of polymer-based composites: Fundamentals and applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
- Guerra, V.; Wan, C.; McNally, T. Thermal conductivity of 2D nano-structured boron nitride (BN) and its composites with polymers. Prog. Mater. Sci. 2019, 100, 170–186. [Google Scholar] [CrossRef]
- Wang, Z.; Priego, P.; Meziani, M.J.; Wirth, K.; Bhattacharya, S.; Rao, A.; Wang, P.; Sun, Y.-P. Dispersion of high-quality boron nitride nanosheets in polyethylene for nanocomposites of superior thermal transport properties. Nanoscale Adv. 2020, 2, 2507–2513. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Qi, S.; An, Q.; Zhao, H.; Liu, N. Thermal conductivity of boron nitride reinforced polyethylene composites. Mater. Res. Bull. 2007, 42, 1863–1873. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, L.; Wu, H.; Guo, S. Enhanced thermally conductivity and mechanical properties of polyethylene (PE)/boron nitride (BN) composites through multistage stretching extrusion. Compos. Sci. Technol. 2013, 89, 24–28. [Google Scholar] [CrossRef]
- Zhong, S.-L.; Zhou, Z.-Y.; Zhang, K.; Shi, Y.-D.; Chen, Y.-F.; Chen, X.-D.; Zeng, J.-B.; Wang, M. Formation of thermally conductive networks in isotactic polypropylene/hexagonal boron nitride composites via “Bridge Effect” of multi-wall carbon nanotubes and graphene nanoplatelets. RSC Advances 2016, 6, 98571–98580. [Google Scholar] [CrossRef]
- Cheewawuttipong, W.; Tanoue, S.; Uematsu, H.; Iemoto, Y. Thermal conductivity of polypropylene composites with hybrid fillers of boron nitride and vapor-grown carbon fiber. Polym. Compos. 2016, 37, 936–942. [Google Scholar] [CrossRef]
- Shin, J.W.; Lee, J.-W.; Yu, S.; Baek, B.K.; Hong, J.P.; Seo, Y.; Kim, W.N.; Hong, S.M.; Koo, C.M. Polyethylene/boron-containing composites for radiation shielding. Thermochim. Acta 2014, 585, 5–9. [Google Scholar] [CrossRef]
- Chen, L.; Xu, H.-F.; He, S.-J.; Du, Y.-H.; Yu, N.-J.; Du, X.-Z.; Lin, J.; Nazarenko, S. Thermal conductivity performance of polypropylene composites filled with polydopamine-functionalized hexagonal boron nitride. PLoS ONE 2017, 12, e0170523. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, H.; Guo, S. Effect of interfacial interaction on morphology and properties of polyethylene/boron nitride thermally conductive composites. Polym. Plast. Technol. Eng. 2015, 54, 1097–1105. [Google Scholar] [CrossRef]
- Seyhan, A.T.; Göncü, Y.; Durukan, O.; Akay, A.; Ay, N. Silanization of boron nitride nanosheets (BNNSs) through microfluidization and their use for producing thermally conductive and electrically insulating polymer nanocomposites. J. Solid State Chem. 2017, 249, 98–107. [Google Scholar] [CrossRef]
- Quiles-Díaz, S.; Martínez-Rubí, Y.; Guan, J.; Kim, K.S.; Couillard, M.; Salavagione, H.J.; Gómez-Fatou, M.A.; Simard, B. Enhanced Thermal Conductivity in Polymer Nanocomposites via Covalent Functionalization of Boron Nitride Nanotubes with Short Polyethylene Chains for Heat-Transfer Applications. ACS Appl. Nano Mater. 2018, 2, 440–451. [Google Scholar] [CrossRef]
- Li, J.-l.; Yin, J.-h.; Ji, T.; Feng, Y.; Liu, Y.-y.; Zhao, H.; Li, Y.-p.; Zhu, C.-c.; Yue, D.; Su, B. Microstructure evolution effect on high-temperature thermal conductivity of LDPE/BNNS investigated by in-situ SAXS. Mater. Lett. 2019, 234, 74–78. [Google Scholar] [CrossRef]
- Borjas-Ramos, J.J.; Ramos-de-Valle, L.F.; Neira-Velázquez, M.G.; Hernández-Hernández, E.; Saucedo-Salazar, E.M.; Soria-Argüello, G. Thermal Conductivity of Nanocomposites Based in High Density Polyethylene and Surface Modified Hexagonal Boron Nitride via Cold Ethylene Plasma. Plasma Chem. Plasma Processing 2018, 38, 429–441. [Google Scholar] [CrossRef]
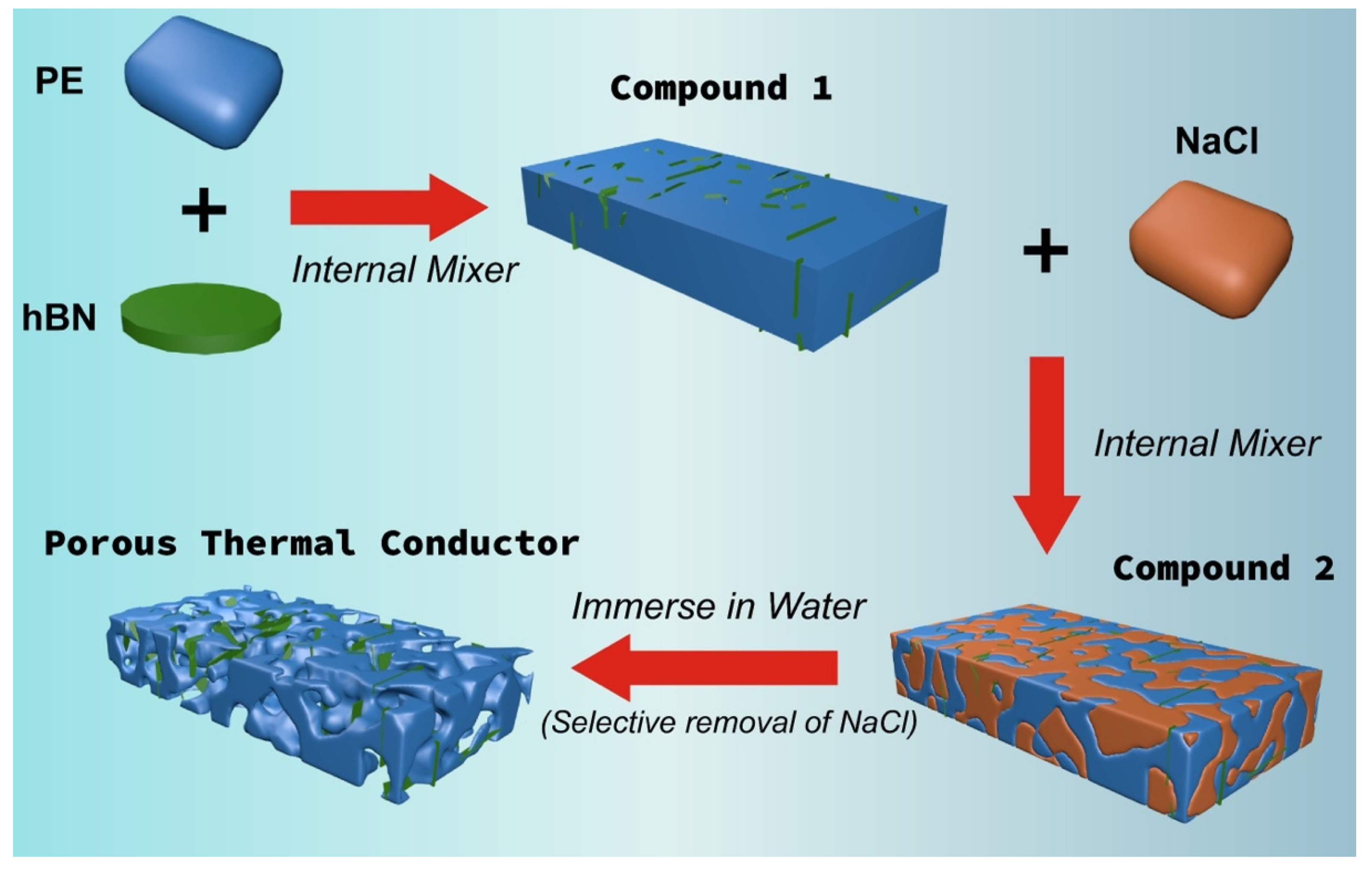
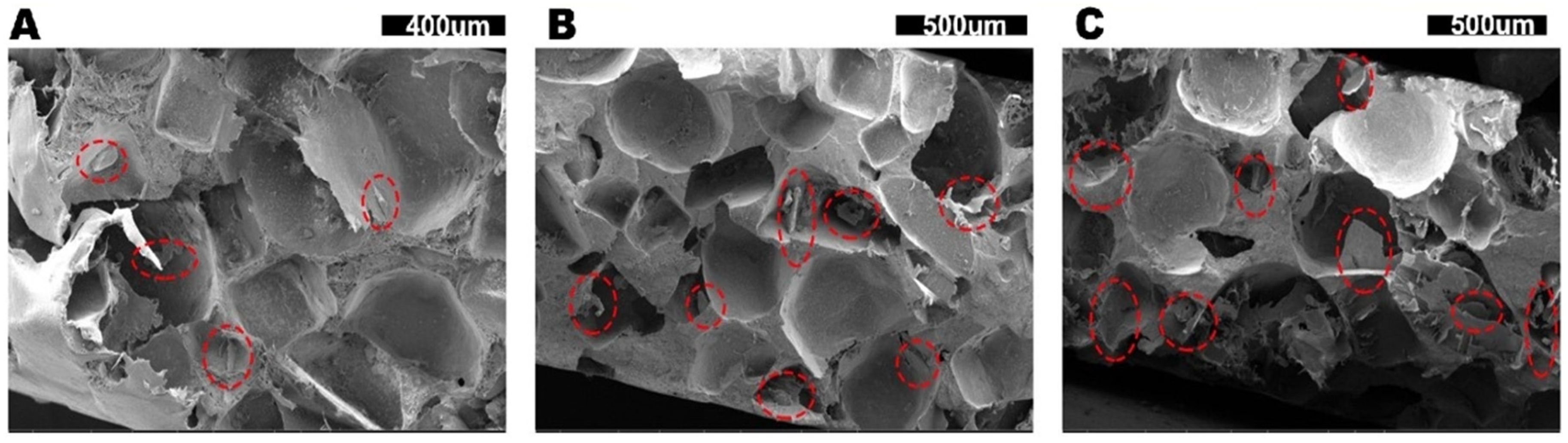
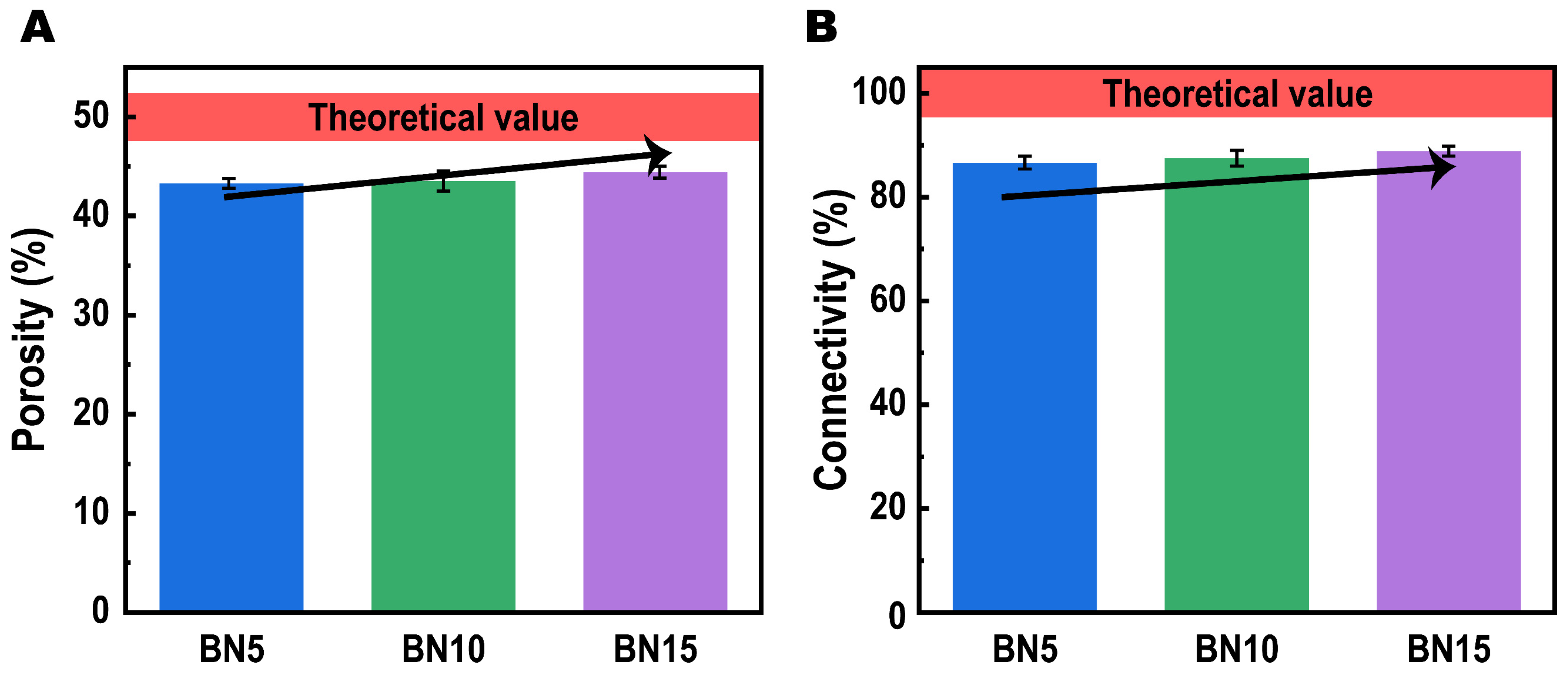
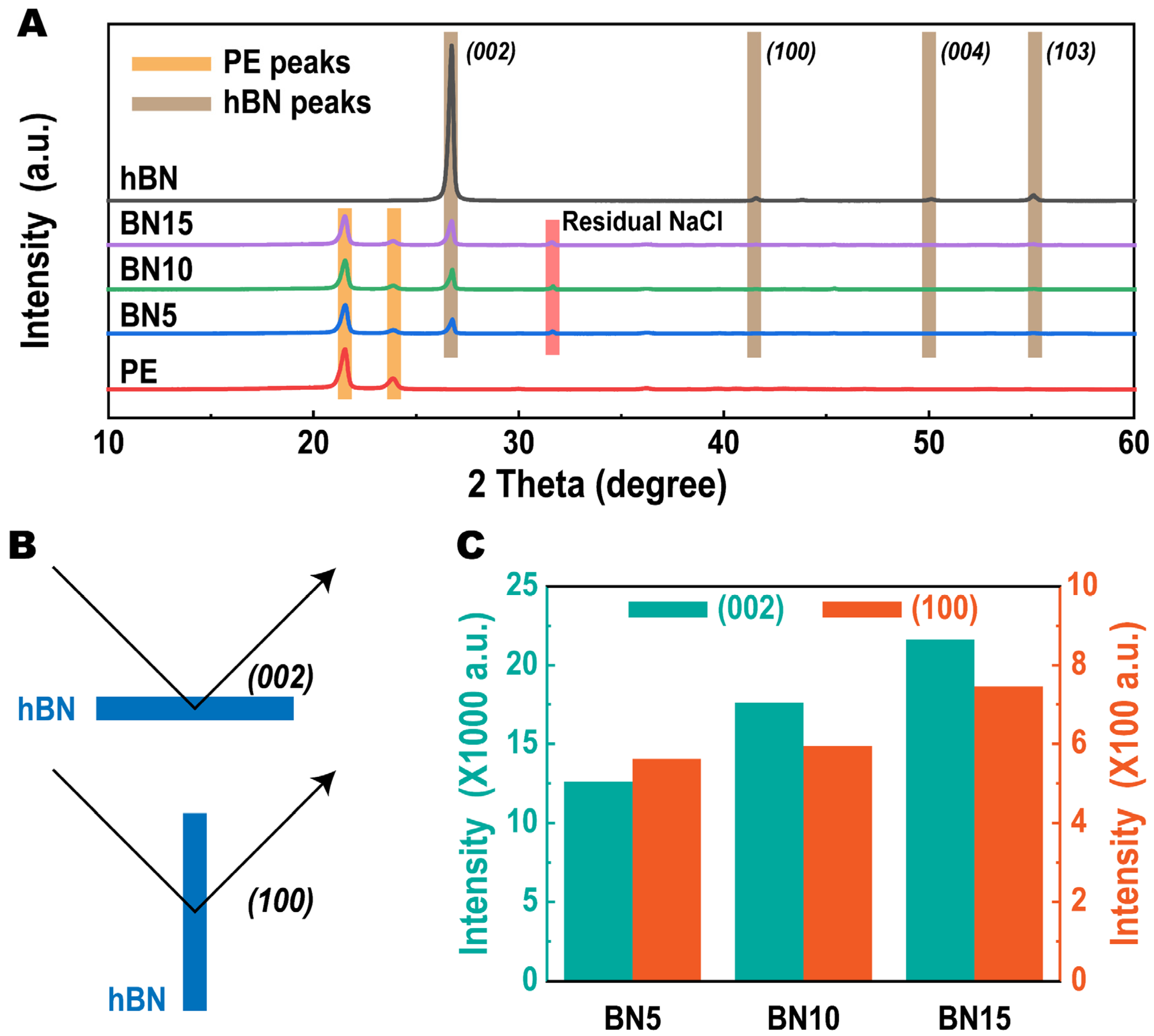


| Sample Code | Composition (wt%) | Apparent Volume Fraction of hBN in PTCs (vol%) | Apparent Density (g/cm3) | ||
|---|---|---|---|---|---|
| PE | hBN | Salt | |||
| 5–50 | 47.5 | 2.5 | 50 | 1.5 | 0.71 ± 0.01 |
| 10–50 | 45 | 5 | 50 | 3.1 | 0.75 ± 0.02 |
| 15–50 | 42.5 | 7.5 | 50 | 4.7 | 0.78 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pi, H.-J.; Liu, X.-X.; Liao, J.-Y.; Zhou, Y.-Y.; Meng, C. Lightweight Polyethylene/Hexagonal Boron Nitride Hybrid Thermal Conductor Fabricated by Melt Compounding Plus Salt Leaching. Polymers 2022, 14, 852. https://doi.org/10.3390/polym14050852
Pi H-J, Liu X-X, Liao J-Y, Zhou Y-Y, Meng C. Lightweight Polyethylene/Hexagonal Boron Nitride Hybrid Thermal Conductor Fabricated by Melt Compounding Plus Salt Leaching. Polymers. 2022; 14(5):852. https://doi.org/10.3390/polym14050852
Chicago/Turabian StylePi, He-Jie, Xiao-Xiao Liu, Jian-Yu Liao, Yue-Yun Zhou, and Cong Meng. 2022. "Lightweight Polyethylene/Hexagonal Boron Nitride Hybrid Thermal Conductor Fabricated by Melt Compounding Plus Salt Leaching" Polymers 14, no. 5: 852. https://doi.org/10.3390/polym14050852
APA StylePi, H.-J., Liu, X.-X., Liao, J.-Y., Zhou, Y.-Y., & Meng, C. (2022). Lightweight Polyethylene/Hexagonal Boron Nitride Hybrid Thermal Conductor Fabricated by Melt Compounding Plus Salt Leaching. Polymers, 14(5), 852. https://doi.org/10.3390/polym14050852





