Adulterant Detection in Peppermint Oil by Means of Holographic Photopolymers Based on Composite Materials with Liquid Crystal
Abstract
1. Introduction
2. Peppermint Oil Composition
3. Obtaining Pure Peppermint Oil
4. Peppermint Oil Adulteration
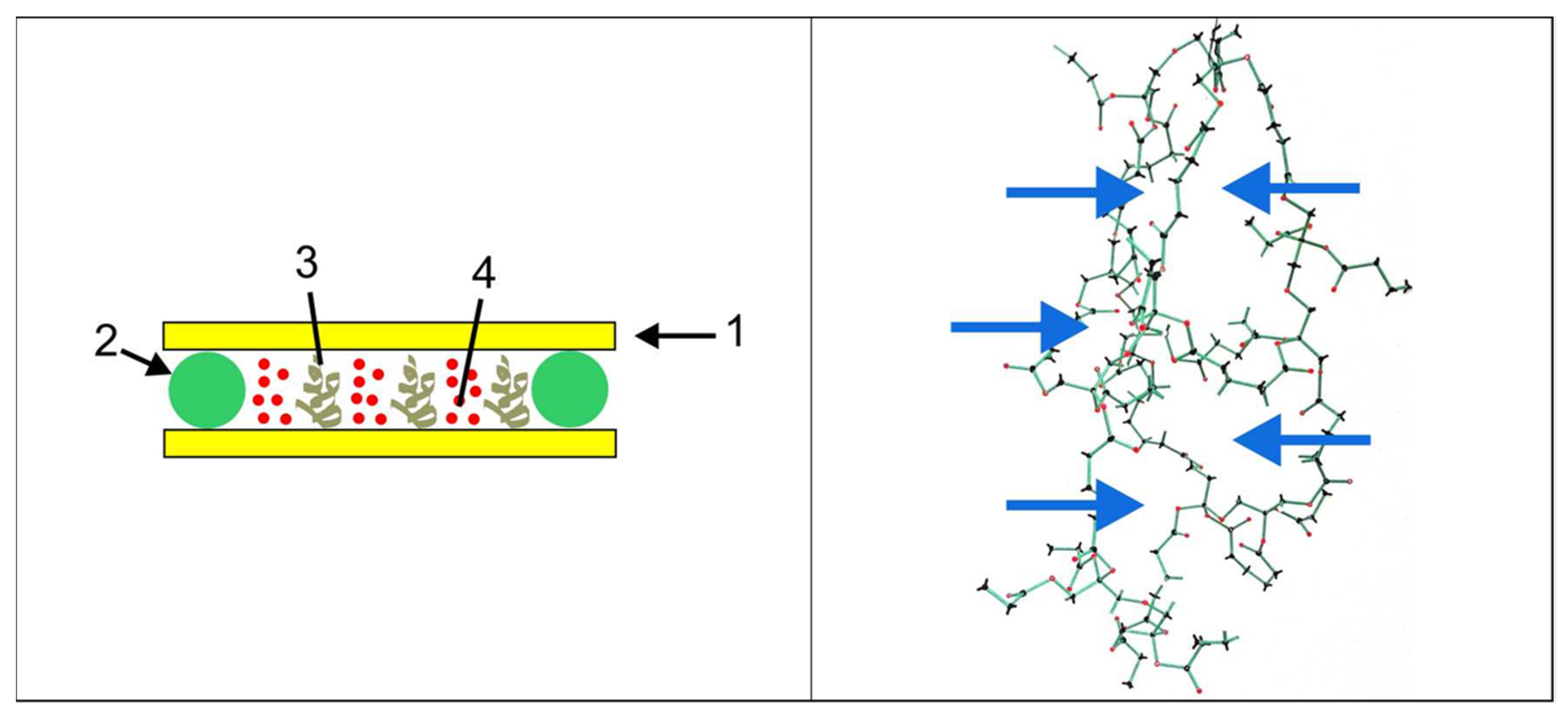
5. Photopolymer Formulation and Sample Preparation
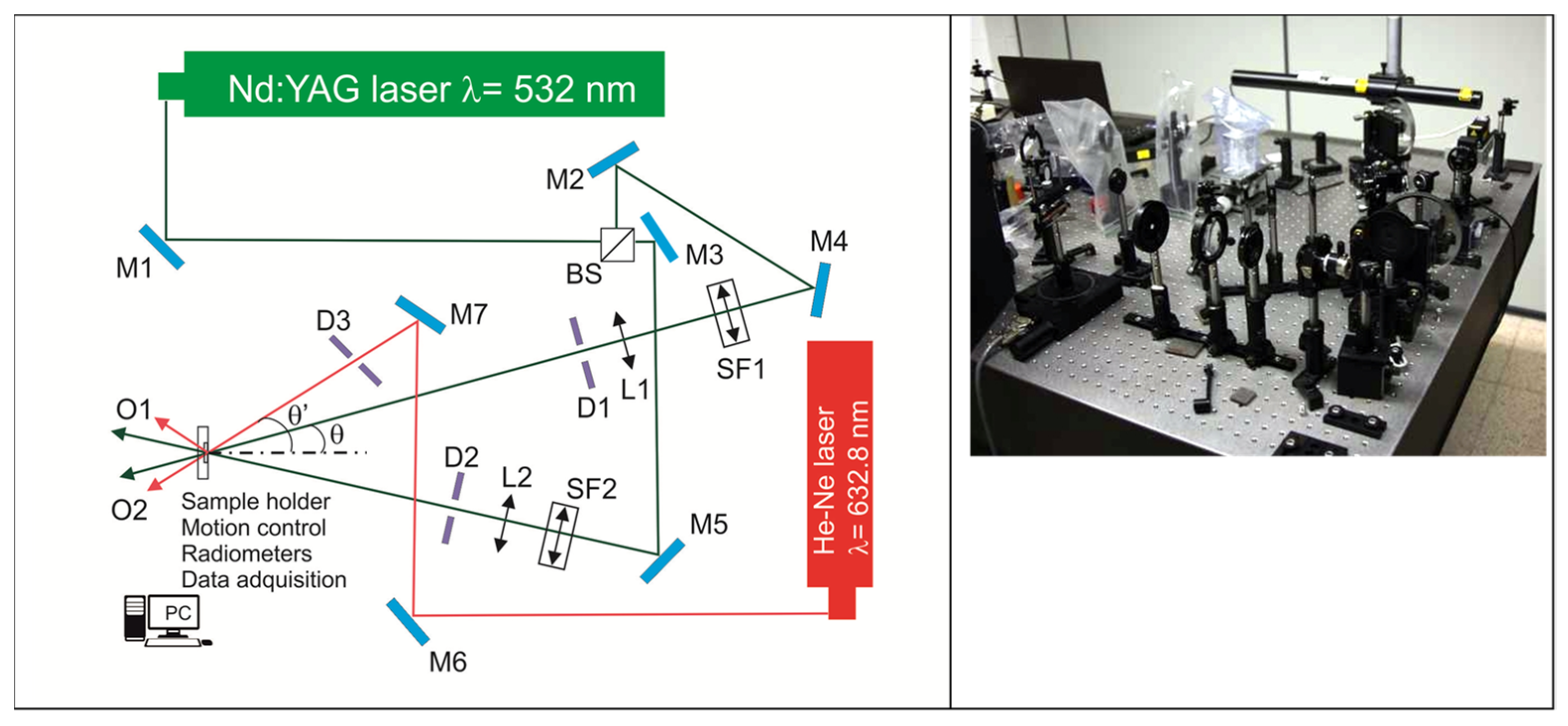
6. Holographic Setup
7. Results
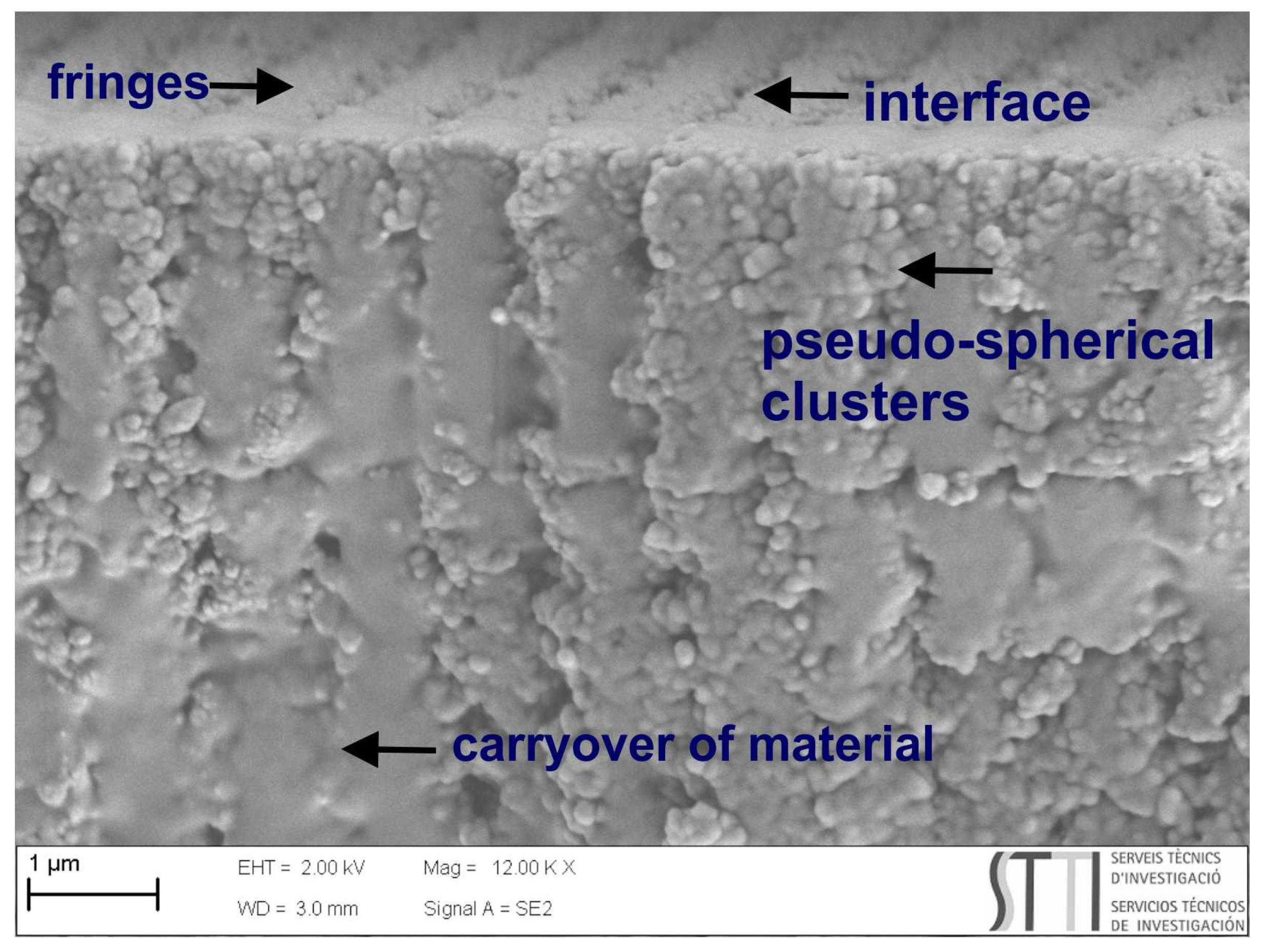
7.1. Compatibility of Peppermint Oil with the HPDLC
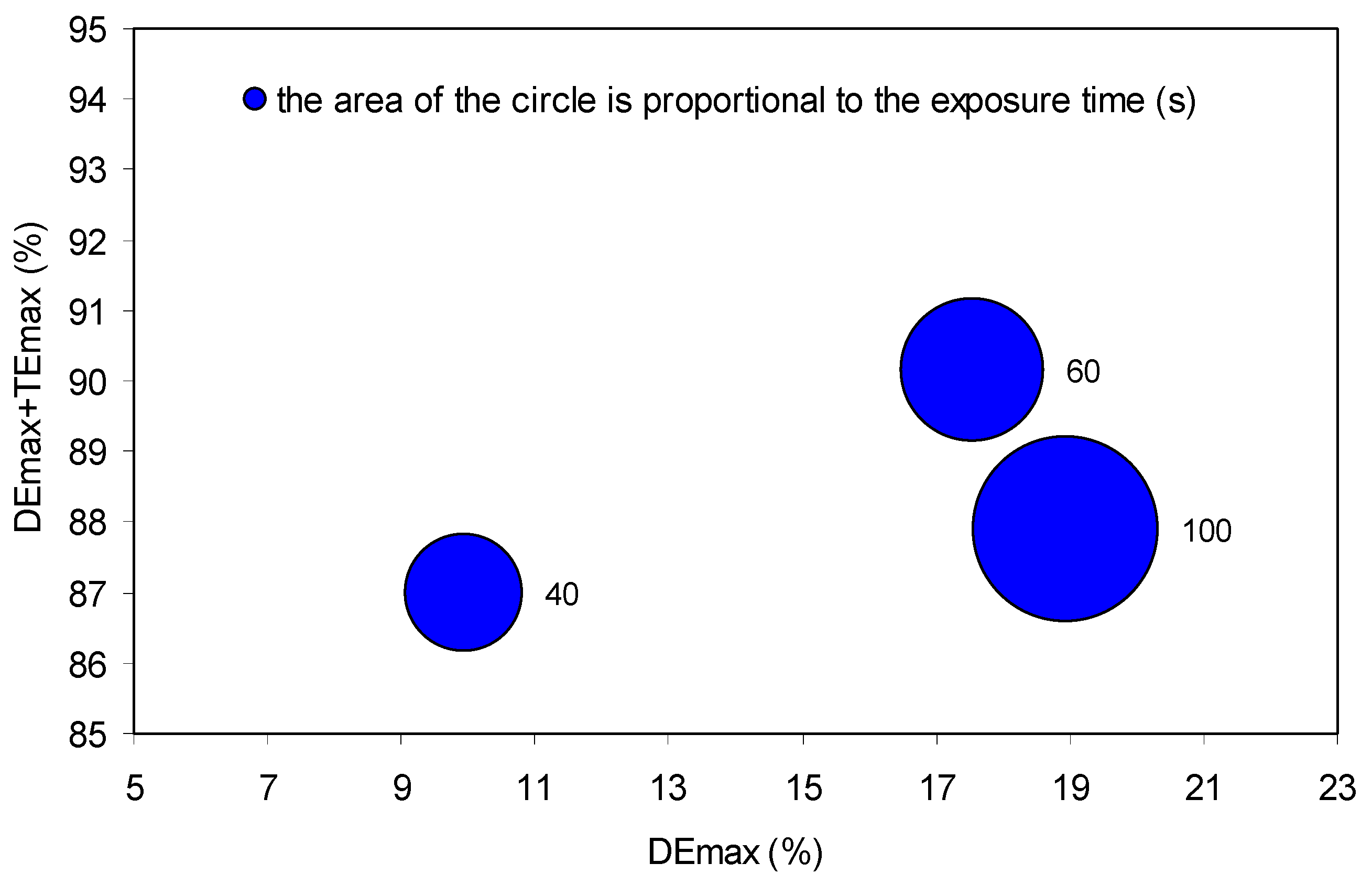
7.2. Recording Time Selection
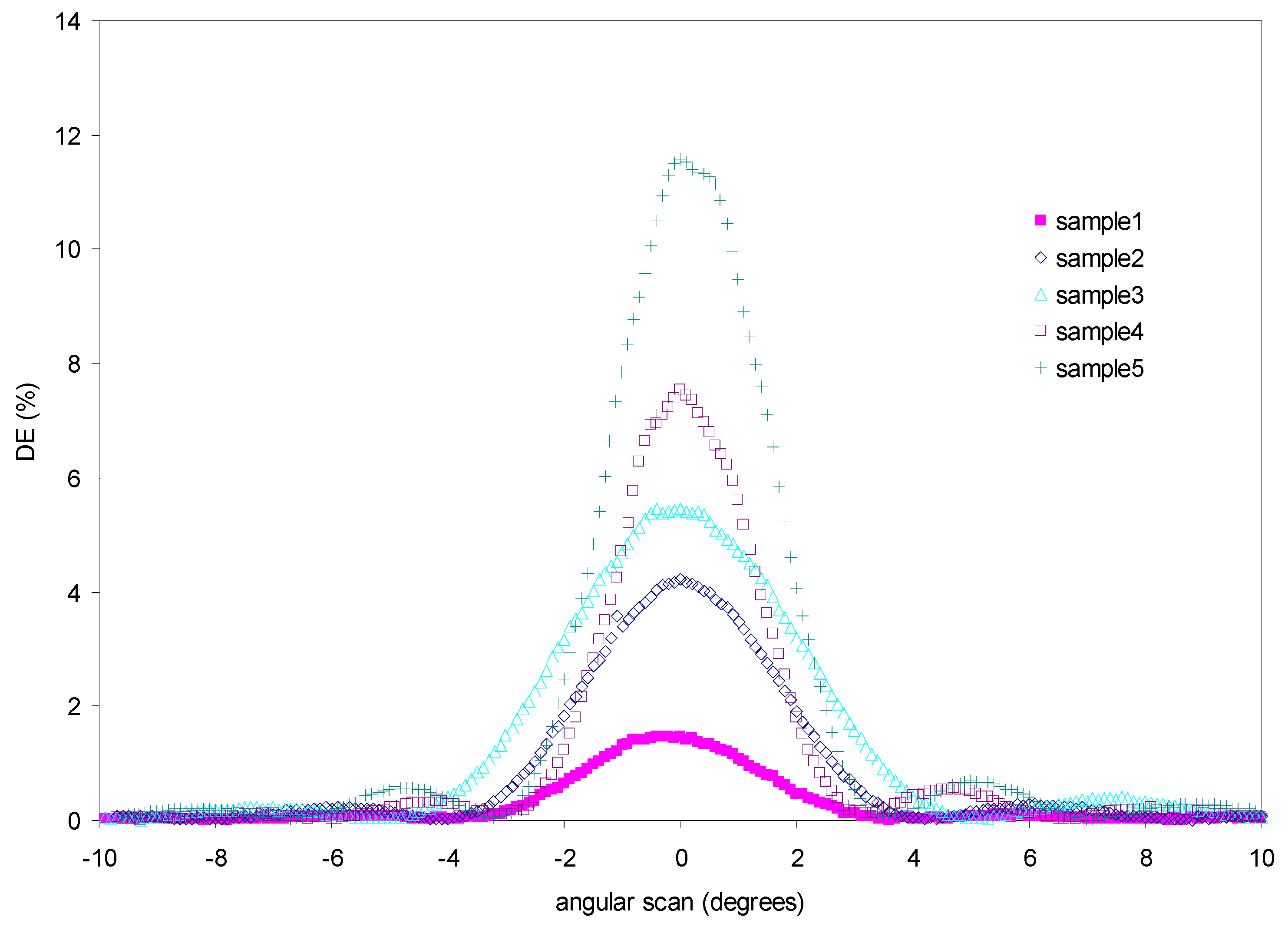
7.3. Influence of the Triethyl Citrate Concentration in the Recording of the Diffraction Grating
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manion, C.R.; Widder, R.M. Essentials of Essential Oils. Am. J. Health Pharm. 2017, 74, e153–e162. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Z.A.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential Oils: Extraction Techniques, Pharmaceutical And Therapeutic Potential-A Review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Sachan, A.K.; Das, D.R.; Gangwar, S.S. An Overview on Menthae Piperitae (Peppermint Oil) Review Article an Overview on Menthae Piperitae (Peppermint Oil). Int. J. Pharmaceut. Chem. Biol. Sci. 2013, 3, 834–838. [Google Scholar]
- A Review on Peppermint Oil. Available online: https://www.researchgate.net/publication/237842903_A_REVIEW_ON_PEPPERMINT_OIL (accessed on 3 December 2021).
- Authentication of Natural Peppermint Oil|Perfumer & Flavorist. Available online: https://www.perfumerflavorist.com/flavor/ingredients/article/21856932/authentication-of-natural-peppermint-oil (accessed on 3 December 2021).
- Sikarwar, S.; Yadav, B.C. Opto-Electronic Humidity Sensor: A Review. Sens. Actuators A Phys. 2015, 233, 54–70. [Google Scholar] [CrossRef]
- Chen, D.; Yuan, Y.J. Thin-Film Sensors for Detection of Formaldehyde: A Review. IEEE Sens. J. 2015, 15, 6749–6760. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Zhao, Y.; Lv, R.Q. A Review for Optical Sensors Based on Photonic Crystal Cavities. Sens. Actuators A Phys. 2015, 233, 374–389. [Google Scholar] [CrossRef]
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S.; Kim, T.W. Recent Advances in Energy-Saving Chemiresistive Gas Sensors: A Review. Nano Energy 2021, 79, 105369. [Google Scholar] [CrossRef]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon Nanotube Chemical Sensors. Chem. Rev. 2018, 119, 599–663. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, X.; Yuan, L.; Yu, J. A Review of Higherature Electrochemical Sensors Based on Stabilized Zirconia. Solid State Ion. 2015, 283, 91–102. [Google Scholar] [CrossRef]
- Yetisen, A.K. Holographic Metal Ion Sensors; Springer: Cham, Swizerland, 2015; pp. 85–99. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Naydenova, I.; Da Cruz Vasconcellos, F.; Blyth, J.; Lowe, C.R. Holographic Sensors: Three-Dimensional Analyte-Sensitive Nanostructures and Their Applications. Chem. Rev. 2014, 114, 10654–10696. [Google Scholar] [CrossRef]
- Naydenova, I.; Grand, J.; Mikulchyk, T.; Martin, S.; Toal, V.; Georgieva, V.; Thomas, S.; Mintova, S. Hybrid Sensors Fabricated by Inkjet Printing and Holographic Patterning. Chem. Mater. 2015, 27, 6097–6101. [Google Scholar] [CrossRef]
- Vezouviou, E.; Lowe, C.R. A near Infrared Holographic Glucose Sensor. Biosens. Bioelectron. 2015, 68, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Barachevsky, V.A. The Current Status of the Development of Light-Sensitive Media for Holography (a Review). OptSp 2018, 124, 373–407. [Google Scholar] [CrossRef]
- Guo, J.; Gleeson, M.R.; Sheridan, J.T. A Review of the Optimisation of Photopolymer Materials for Holographic Data Storage. Phys. Res. Int. 2012, 2012, 803439. [Google Scholar] [CrossRef]
- Mikulchyk, T.; Martin, S.; Naydenova, I. Investigation of the Sensitivity to Humidity of an Acrylamide-Based Photopolymer Containing N-Phenylglycine as a Photoinitiator. Opt. Mater. 2014, 37, 810–815. [Google Scholar] [CrossRef][Green Version]
- Leite, E.; Naydenova, I.; Mintova, S.; Leclercq, L.; Toal, V. Photopolymerizable Nanocomposites for Holographic Recording and Sensor Application. Appl. Opt. 2010, 49, 3652–3660. [Google Scholar] [CrossRef]
- Shian Li, M.; Trong Wu, S.; Ying-Guey Fuh, A.; Lai, N.D.; Lin, J.H.; Huang, Y.Y.; Hsu, C.C. Sensor for Monitoring the Vibration of a Laser Beam Based on Holographic Polymer Dispersed Liquid Crystal Films. Opt. Express 2010, 18, 26300–26306. [Google Scholar] [CrossRef]
- Jie, Y.; Jihong, Z. Fingerprint Sensor Using a Polymer Dispersed Liquid Crystal Holographic Lens. Appl. Opt. 2010, 49, 4763–4766. [Google Scholar] [CrossRef]
- Mora, M.P.; Ramírez, M.G.; Brocal, F.; Ortuño, M.; Beléndez, A.; Pascual, I. Influence of Tert-Butylthiol and Tetrahydrofuran on the Holographic Characteristics of a Polymer Dispersed Liquid Crystal: A Research Line Toward a Specific Sensor for Natural Gas and Liquefied Petroleum Gas. Polymers 2019, 11, 254. [Google Scholar] [CrossRef]
- Guan, W.; Li, S.; Yan, R.; Tang, S.; Quan, C. Comparison of Essential Oils of Clove Buds Extracted with Supercritical Carbon Dioxide and Other Three Traditional Extraction Methods. Food Chem. 2007, 101, 1558–1564. [Google Scholar] [CrossRef]
- Peredo-Luna, H.; Palou-García, E.; López-Malo, A. Aceites Esenciales: Métodos de Extracción. Temas Sel. Ing. Aliment. 2009, 3, 8. [Google Scholar]
- Greenstein, G.R. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. Ref. Rev. 2007, 21, 40. [Google Scholar] [CrossRef]
- Advanced Organic Chemistry: Reaction Mechanisms-Reinhard Bruckner-Google Livres. Available online: https://books.google.es/books?hl=fr&lr=&id=1nT6c3TQL8wC&oi=fnd&pg=PP2&dq=advanced+organic+chemistry.+Reactions,+mechanisms,+and+structure.+5th+ed.+New+York:+John+Wiley+%26+Sons%3B+2001&ots=NE3oKmc_xx&sig=XAMbyPeoxpsygIRfpNFVv6HU63Y#v=onepage&q&f=false (accessed on 3 December 2021).
- Boren, K.E.; Young, D.G.; Woolley, C.L.; Smith, B.L.; Carlson, R.E. Detecting Essential Oil Adulteration. J. Environ. Anal. Chem. 2015, 2, 1000132. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. CRC Handbook of Chemistry and Physics, 87th ed.; David, R., Ed.; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2006; ISBN 0-8493-0487-3. [Google Scholar] [CrossRef]
- Estimation of Menthone, Menthofuran, Menthyl Acetate and Menthol in Peppermint Oil by Capillary Gas Chromatography. Available online: https://agris.fao.org/agris-search/search.do?recordID=US201302578217 (accessed on 3 December 2021).
- Areias, F.M.; Valentão, P.; Andrade, P.B.; Ferreres, F.; Seabra, R.M. Phenolic Fingerprint of Peppermint Leaves. Food Chem. 2001, 73, 307–311. [Google Scholar] [CrossRef]
- Meng, S.; Duran, H.; Hu, J.; Kyu, T.; Natarajan, L.V.; Tondiglia, V.P.; Sutherland, R.L.; Bunning, T.J. Influence of Photopolymerization Reaction Kinetics on Diffraction Efficiency of H-PDLC Undergoing Photopatterning Reaction in Mixtures of Acrylic Monomer/Nematic Liquid Crystals. Macromolecules 2007, 40, 3190–3197. [Google Scholar] [CrossRef]
- Liu, Y.J.; Sun, X.W. Holographic Polymer-Dispersed Liquid Crystals: Materials, Formation, and Applications. Adv. Optoelectron. 2008, 2008, 684349. [Google Scholar] [CrossRef]
- Natarajan, L.V.; Brown, D.P.; Wofford, J.M.; Tondiglia, V.P.; Sutherland, R.L.; Lloyd, P.F.; Bunning, T.J. Holographic Polymer Dispersed Liquid Crystal Reflection Gratings Formed by Visible Light Initiated Thiol-Ene Photopolymerization. Polymer 2006, 47, 4411–4420. [Google Scholar] [CrossRef]
- Fukuda, Y.; Tomita, Y. Computer Simulation of Bragg Grating Formation in Holographic Polymer-Dispersed Liquid Crystals Based on the Density Functional Theory. In Optical Modelling and Design II; International Society for Optics and Photonics: Bellingham, WA, USA, 2012; Volume 8429, pp. 376–390. [Google Scholar] [CrossRef]
- Francés, J.; Bleda, S.; Puerto, D.; Gallego, S.; Márquez, A.; Neipp, C.; Pascual, I.; Beléndez, A. Accurate, Efficient and Rigorous Numerical Analysis of 3D H-PDLC Gratings. Materials 2020, 13, 3725. [Google Scholar] [CrossRef]
- Massenot, S.; Kaiser, J.L.; Chevallier, R.; Renotte, Y. Study of the Dynamic Formation of Transmission Gratings Recorded in Photopolymers and Holographic Polymer-Dispersed Liquid Crystals. Appl. Opt. 2004, 43, 5489–5497. [Google Scholar] [CrossRef]
- Kogelnik, H. Coupled Wave Theory for Thick Hologram Gratings. Bell Syst. Tech. J. 1969, 48, 2909–2947. [Google Scholar] [CrossRef]
- Gallego, S.; Neipp, C.; Estepa, L.A.; Ortuño, M.; Márquez, A.; Francés, J.; Pascual, I.; Beléndez, A. Volume Holograms in Photopolymers: Comparison between Analytical and Rigorous Theories. Materials 2012, 5, 1373–1388. [Google Scholar] [CrossRef]
- Gallego, S.; Ortuño, M.; Neipp, C.; Márquez, A.; Beléndez, A.; Pascual, I.; Kelly, J.V.; Sheridan, J.T. Physical and Effective Optical Thickness of Holographic Diffraction Gratings Recorded in Photopolymers. Opt. Express 2005, 13, 1939–1947. [Google Scholar] [CrossRef] [PubMed]


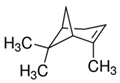
| (+)-α-Pinene | Sabinene | (−)-β-Pinene | β-Myrcene |
 |  |  | 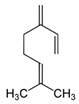 |
| 3-Octanol | R-(+)-Limonene | Eucaliptol | Isopregol |
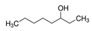 | 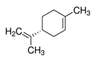 |  | 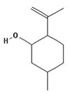 |
| (−)-Isopulegol | Piperitone | (−)-Menthol | (−)-Menthone |
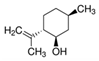 | 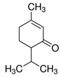 | 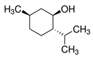 | 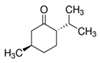 |
| (+)-Isomenthone | Cis-3-Hexenyl isovalerate | (+)-Pulegon | (+)-2-Carene |
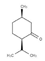 | 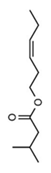 | 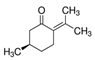 |  |
| Peak Number | TR (min) | Area Component | Area (%) | Qual (%) |
|---|---|---|---|---|
| 1 | 7.44 | α-Pinene | 0.73 | 97 |
| 2 | 8.54 | Sabinene | 0.34 | 91 |
| 3 | 8.62 | β-Pinene | 0.86 | 94 |
| 4 | 9.02 | β-Myrcene | 0.38 | 91 |
| 5 | 9.15 | 3-Octanol | 0.39 | 83 |
| 6 | 10.11 | R-(+)-Limonene | 4.54 | 94 |
| 7 | 10.18 | Eucaliptol | 4.43 | 98 |
| 9 | 13.53 | Isopregol | 1.67 | 99 |
| 10 | 13.79 | Isomenthone | 17.81 | 97 |
| 12 | 14.09 | Menthone | 11.57 | 96 |
| 13 | 14.38 | Menthol (±) | 45.8 | 91 |
| 14 | 14.45 | Isopulegol | 1.04 | 60 |
| 15 | 14.64 | Menthol | 0.68 | 91 |
| 16 | 16.08 | Isoleric Acid | 0.42 | 83 |
| 17 | 16.21 | Pulegon | 1.29 | 97 |
| 18 | 16.63 | Piperitone | 0.87 | 95 |
| 19 | 17.71 | (+)-2-Carene | 6.96 | 91 |
| Physical Parameters | Peppermint Oil | Triethyl Citrate |
|---|---|---|
| Density 25 °C (g/cm3) | 0.893–0.905 | 1.14 |
| nD20 | 1.459–1.465 | 1.442 |
| Bp (°C) | 215 | 235 (150mmHg) |
| Color | Colorless to pale yellow | Colorless |
| Optical rotation | −18 to −32 | 0 |
| Component | Concentration (wt%) |
|---|---|
| DPHPA | 52.77 |
| QYPDLC-036 | 36.27 |
| YET | 0.05 |
| NPG | 0.47 |
| OA | 10.44 |
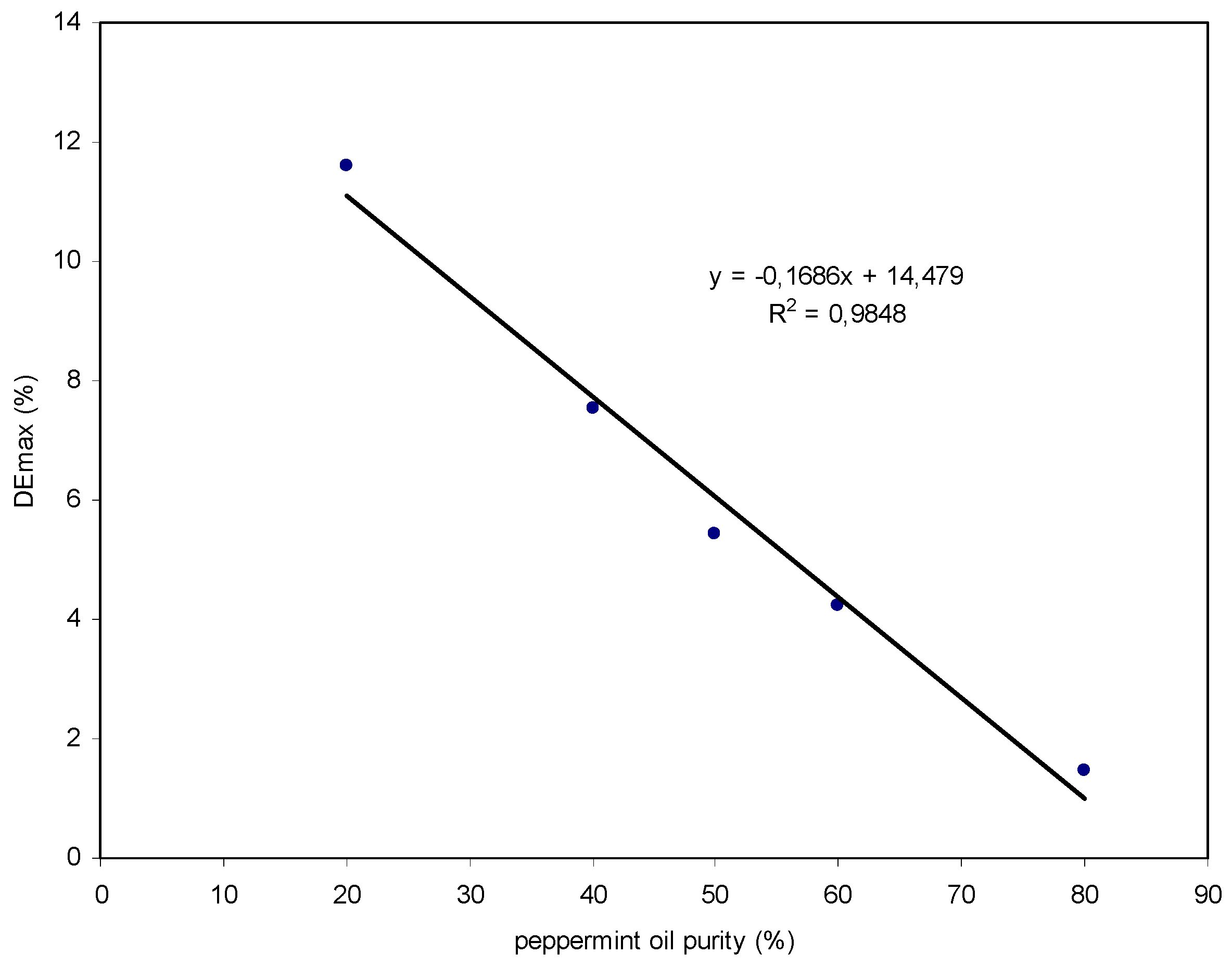
| Sample | Purity of Peppermint Oil (%) | DE Max (%) | V Peppermint Oil (μL) | V Triethyl Citrate (μL) |
|---|---|---|---|---|
| Sample 1 | 80 | 1.5 | 40 | 10 |
| Sample 2 | 60 | 4.2 | 30 | 20 |
| Sample 3 | 50 | 5.4 | 25 | 25 |
| Sample 4 | 40 | 7.5 | 20 | 30 |
| Sample 5 | 20 | 11.6 | 10 | 40 |
| Sample | Purity of Peppermint Oil (%) | DE Max (%) |
|---|---|---|
| Sample 1 | 80 | 1.5 |
| Sample 2 | 60 | 4.2 |
| Sample 3 | 50 | 5.4 |
| Sample 4 | 40 | 7.5 |
| Sample 5 | 20 | 11.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miloua, W.; Ortuño, M.; Navarro-Fuster, V.; Beléndez, A.; Pascual, I. Adulterant Detection in Peppermint Oil by Means of Holographic Photopolymers Based on Composite Materials with Liquid Crystal. Polymers 2022, 14, 1061. https://doi.org/10.3390/polym14051061
Miloua W, Ortuño M, Navarro-Fuster V, Beléndez A, Pascual I. Adulterant Detection in Peppermint Oil by Means of Holographic Photopolymers Based on Composite Materials with Liquid Crystal. Polymers. 2022; 14(5):1061. https://doi.org/10.3390/polym14051061
Chicago/Turabian StyleMiloua, Wafaa, Manuel Ortuño, Víctor Navarro-Fuster, Augusto Beléndez, and Inmaculada Pascual. 2022. "Adulterant Detection in Peppermint Oil by Means of Holographic Photopolymers Based on Composite Materials with Liquid Crystal" Polymers 14, no. 5: 1061. https://doi.org/10.3390/polym14051061
APA StyleMiloua, W., Ortuño, M., Navarro-Fuster, V., Beléndez, A., & Pascual, I. (2022). Adulterant Detection in Peppermint Oil by Means of Holographic Photopolymers Based on Composite Materials with Liquid Crystal. Polymers, 14(5), 1061. https://doi.org/10.3390/polym14051061








