Liquid Crystal-Based Organosilicone Elastomers with Supreme Mechanical Adaptability
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization of Silicone Oils and Elastomers
2.3. Synthesis of Crosslinker
2.4. Synthesis of Crosslinker-g-MBB
2.5. Fabrication of LCMQs
3. Results and Discussion
3.1. Synthesis and Characterization of Crosslinker, Crosslinker-g-MBB and LCMQs
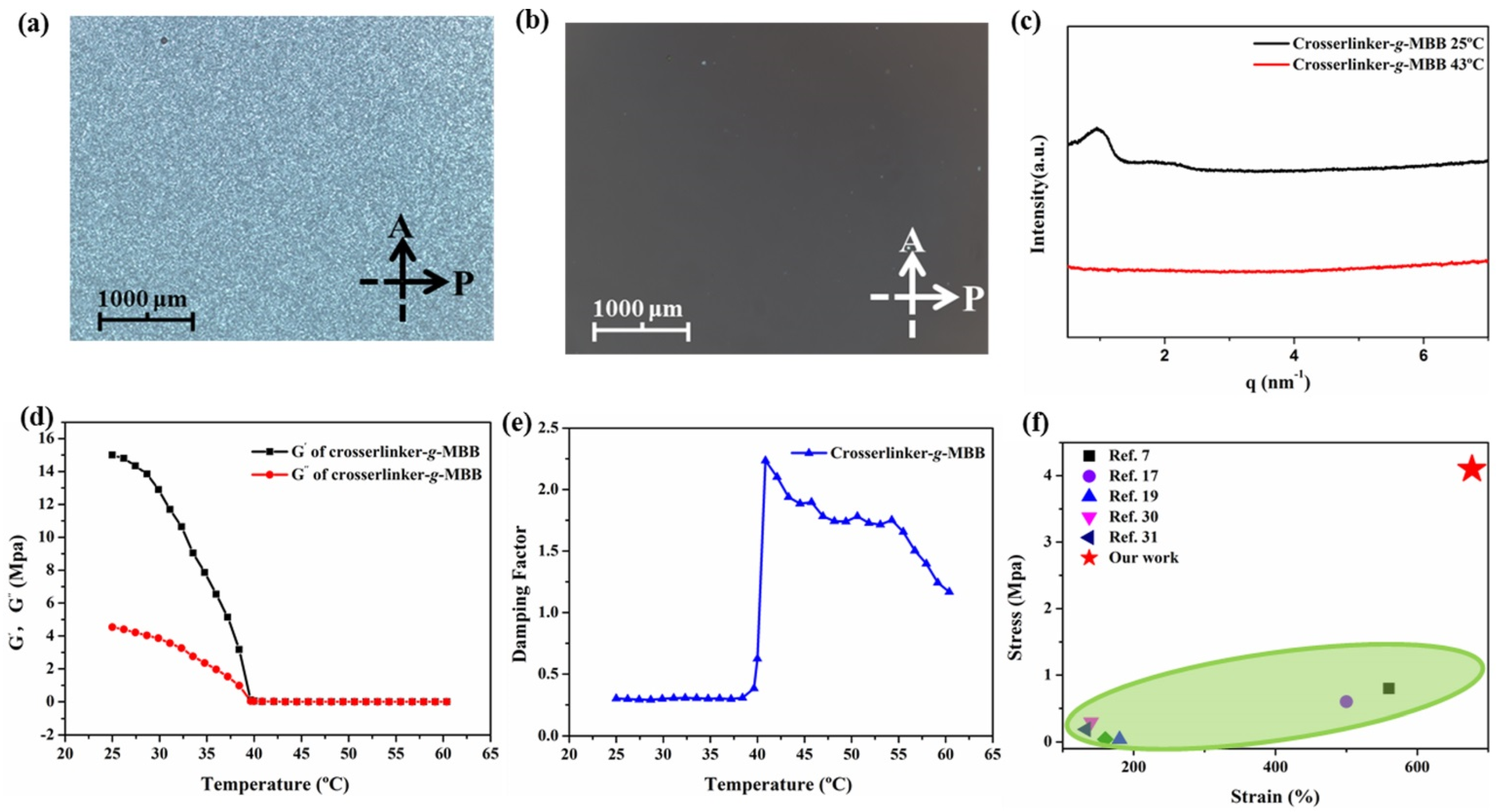
3.2. Characterization of POM, SAXS and Rheological Determination
3.3. Determination of Crosslinking Density
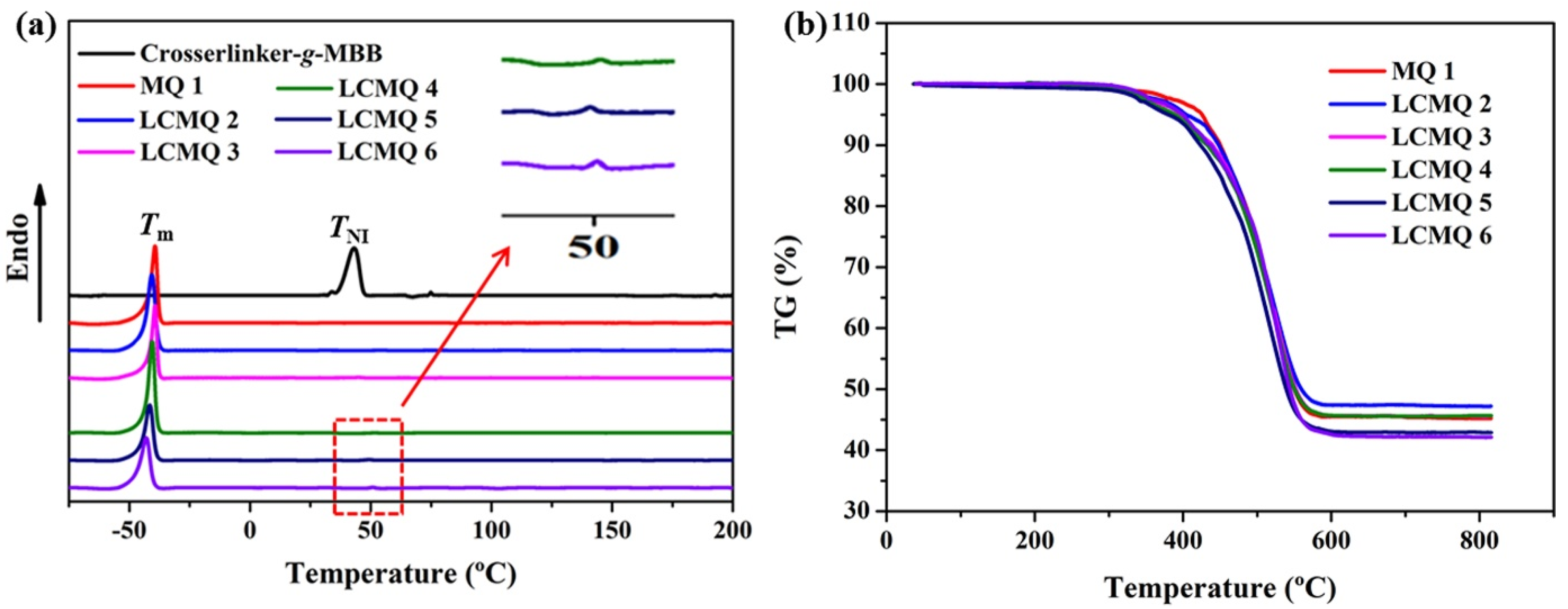
3.4. The Thermal Properties and the Thermal Stability Characterization of MQ 1 and the LCMQs
3.5. Mechanical Adaptability of MQ 1 and the LCMQs
3.6. The Viscoelastic Properties of MQ 1 and the LCMQs
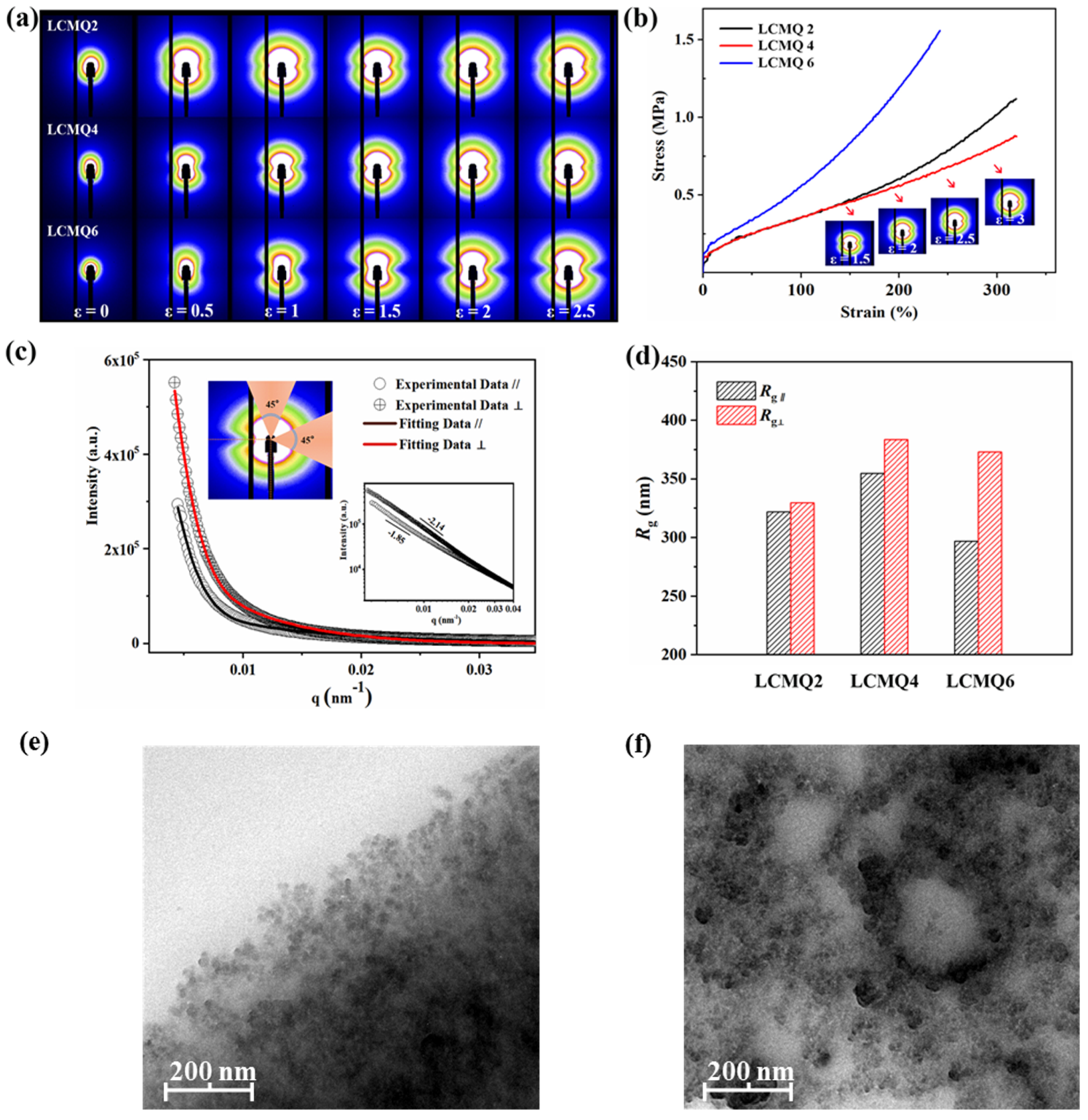
3.7. 2D USAXS Patterns of LCMQ 2, LCMQ 4 and LCMQ 6
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Someya, T.; Bao, Z.N.; Malliaras, G.G. The rise of plastic bioelectronics. Nature 2016, 540, 379–385. [Google Scholar] [CrossRef]
- Wang, S.H.; Oh, J.Y.; Xu, J.; Tran, H.; Bao, Z.A. Skin-inspired electronics: An emerging paradigm. Acc. Chem. Res. 2018, 51, 1033–1045. [Google Scholar] [CrossRef]
- Visentin, F.; Babu, S.P.M.; Meder, F.; Mazzolai, B. Selective stiffening in soft actuators by triggered phase transition of hydrogel-filled elastomers. Adv. Funct. Mater. 2021, 31, 2101121. [Google Scholar] [CrossRef]
- Ware, T.H.; McConney, M.E.; Wie, J.J.; Tondiglia, V.P.; White, T.J. Voxelated liquid crystal elastomers. Science 2015, 347, 982–984. [Google Scholar] [CrossRef] [Green Version]
- Marrucci, G. Rubber elasticity theory. A network of entangled chains. Macromolecules 2002, 14, 434–442. [Google Scholar] [CrossRef]
- Toki, S.; Che, J.; Rong, L.; Hsiao, B.S.; Amnuaypornsri, S.; Nimpaiboon, A.; Sakdapipanich, J. Entanglements and Networks to Strain-Induced Crystallization and Stress–Strain Relations in Natural Rubber and Synthetic Polyisoprene at Various Temperatures. Macromolecules 2013, 46, 5238–5248. [Google Scholar] [CrossRef]
- Wang, Z.J.; Tian, H.M.; He, Q.G.; Cai, S.Q. Reprogrammable, Reprocessible, and Self-Healable Liquid Crystal Elastomer with Exchangeable Disulfide Bonds. ACS Appl. Mater. Interfaces 2017, 9, 33119–33128. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Huo, Y.Z. Programmable Mechanical Energy Absorption and Dissipation of Liquid Crystal Elastomers: Modeling and Simulations. Adv. Eng. Mater. 2021, 2100590. [Google Scholar] [CrossRef]
- Donovan, B.R.; Fowler, H.E.; Matavulj, V.M.; White, T.J. Mechanotropic Elastomers. Angew. Chem. Int. Ed. 2019, 58, 13744–13748. [Google Scholar] [CrossRef]
- Brannum, M.T.; Auguste, A.D.; Donovan, B.R.; Godman, N.P.; Matayulj, V.M.; Steele, A.M.; Korley, L.T.J.; Wnek, G.E.; White, T.J. Deformation and Elastic Recovery of Acrylate-Based Liquid Crystalline Elastomers. Macromolecules 2019, 52, 8248–8255. [Google Scholar] [CrossRef]
- Cordoyiannis, G.; Lebar, A.; Rožič, B.; Zalar, B.; Kutnjak, Z.; Žumer, S.; Brömmel, F.; Krause, S.; Finkelmann, H. Controlling the Critical Behavior of Paranematic to Nematic Transition in Main-Chain Liquid Single-Crystal Elastomers. Macromolecules 2009, 42, 2069–2073. [Google Scholar] [CrossRef]
- Skacej, G.; Zannoni, C. Molecular Simulations Shed Light on Supersoft Elasticity in Polydomain Liquid Crystal Elastomers. Macromolecules 2014, 47, 8824–8832. [Google Scholar] [CrossRef]
- Küupfer, J.; Finkelmann, H. Liquid crystal elastomer: Influence of the orientational distribution of the crosslinks on the phase behaviour and reorientation process. Macromol. Chem. Phys. 1994, 195, 1353–1367. [Google Scholar] [CrossRef]
- Gablier, A.; Saed, M.O.; Terentjev, E.M. Transesterification in Epoxy–Thiol Exchangeable Liquid Crystalline Elastomers. Macromolecules 2020, 53, 8642–8649. [Google Scholar] [CrossRef]
- Kragt, A.J.J.; Broer, D.J.; Schenning, A.P.H.J. Easily Processable and Programmable Responsive Semi-Interpenetrating Liquid Crystalline Polymer Network Coatings with Changing Reflectivities and Surface Topographies. Adv. Funct. Mater. 2018, 28, 1704756. [Google Scholar] [CrossRef] [Green Version]
- Marshall, J.E.; Gallagher, S.; Terentjev, E.M.; Smoukov, S.K. Anisotropic Colloidal Micromuscles from Liquid Crystal Elastomers. J. Am. Chem. Soc. 2013, 136, 474–479. [Google Scholar] [CrossRef]
- Tian, H.M.; Wang, Z.J.; Chen, Y.L.; Shao, J.Y.; Gao, T.; Cai, S.Q. Polydopamine-Coated Main-Chain Liquid Crystal Elastomer as Optically Driven Artificial Muscle. ACS Appl. Mater. Interfaces 2018, 10, 8307–8316. [Google Scholar] [CrossRef]
- Sawa, Y.; Urayama, K.; Takigawa, T.; DeSimone, A.; Teresi, L. Thermally Driven Giant Bending of Liquid Crystal Elastomer Films with Hybrid Alignment. Macromolecules 2010, 43, 4362–4369. [Google Scholar] [CrossRef]
- Bispo, M.; Guillon, D.; Donnio, B.; Finkelmann, H. Main-chain liquid crystalline elastomers: Monomer and cross-linker molecular control of the thermotropic and elastic properties. Macromolecules 2008, 41, 3098–3108. [Google Scholar] [CrossRef]
- Petr, M.; Katzman, B.-A.; DiNatale, W.; Hammond, P.T. Synthesis of a New, Low-Tg Siloxane Thermoplastic Elastomer with a Functionalizable Backbone and Its Use as a Rapid, Room Temperature Photoactuator. Macromolecules 2013, 46, 2823–2832. [Google Scholar] [CrossRef]
- He, Q.G.; Wang, Z.J.; Wang, Y.; Song, Z.Q.; Cai, S.Q. Recyclable and Self-Repairable Fluid-Driven Liquid Crystal Elastomer Actuator. ACS Appl. Mater. Interfaces 2020, 12, 35464–35474. [Google Scholar] [CrossRef] [PubMed]
- Boothby, J.M.; Ware, T.H. Dual-responsive, shape-switching bilayers enabled by liquid crystal elastomers. Soft Matter 2017, 13, 4349–4356. [Google Scholar] [CrossRef] [PubMed]
- Urayama, K. Selected issues in liquid crystal elastomers and gels. Macromolecules 2007, 40, 2277–2288. [Google Scholar] [CrossRef]
- White, T.J.; Broer, D.J. Programmable and adaptive mechanics with liquid crystal polymer networks and elastomers. Nat. Mater. 2015, 14, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Buguin, A.; Li, M.-H.; Silberzan, P.; Ladoux, B.; Keller, P. Micro-Actuators: When Artificial Muscles Made of Nematic Liquid Crystal Elastomers Meet Soft Lithography. J. Am. Chem. Soc. 2006, 128, 1088–1089. [Google Scholar] [CrossRef]
- Wang, Y.C.; Dang, A.L.; Zhang, Z.F.; Yin, R.; Gao, Y.C.; Feng, L.; Yang, S. Repeatable and Reprogrammable Shape Morphing from Photoresponsive Gold Nanorod/Liquid Crystal Elastomers. Adv. Mater. 2020, 32, 2004270. [Google Scholar] [CrossRef]
- Traugutt, N.A.; Mistry, D.; Luo, C.; Yu, K.; Ge, Q.; Yakacki, C.M. Liquid-Crystal-Elastomer-Based Dissipative Structures by Digital Light Processing 3D Printing. Adv. Mater. 2020, 32, 2000797. [Google Scholar] [CrossRef]
- Martinez, A.; Clement, A.; Gao, J.F.; Kocherzat, J.; Tabrizi, M.; Shankar, M.R. Thermomechanically active electrodes power work-dense soft actuators. Soft Matter 2021, 17, 1521–1529. [Google Scholar] [CrossRef]
- Marshall, J.E.; Terentjev, E.M. Photo-sensitivity of dye-doped liquid crystal elastomers. Soft Matter 2013, 9, 8547–8551. [Google Scholar] [CrossRef]
- Thomsen, D.L.; Keller, P.; Naciri, J.; Pink, R.; Jeon, H.; Shenoy, D.; Ratna, B.R. Liquid crystal elastomers with mechanical properties of a muscle. Macromolecules 2001, 34, 5868–5875. [Google Scholar] [CrossRef]
- Rogez, D.; Krause, S.; Martinoty, P. Main-chain liquid-crystal elastomers versus side-chain liquid-crystal elastomers: Similarities and differences in their mechanical properties. Soft Matter 2018, 14, 6449–6462. [Google Scholar] [CrossRef] [PubMed]
- Kundler, I.; Finkelmann, H. Strain-Induced Director Reorientation in Nematic Liquid Single-Crystal Elastomers. Macromol. Rapid Commun. 1995, 16, 679–686. [Google Scholar] [CrossRef]
- Garcia-Marquez, A.R.; Heinrich, B.; Beyer, N.; Guillon, D.; Donnio, B. Mesomorphism and Shape-Memory Behavior of Main-Chain Liquid-Crystalline Co-Elastomers: Modulation by the Chemical Composition. Macromolecules 2014, 47, 5198–5210. [Google Scholar] [CrossRef]
- Ware, T.H.; Perry, Z.P.; Middleton, C.M.; Iacono, S.T.; White, T.J. Programmable Liquid Crystal Elastomers Prepared by Thiol–Ene Photopolymerization. ACS Macro Lett. 2015, 4, 942–946. [Google Scholar] [CrossRef]
- Cai, G.P.; Weber, W.P. Synthesis and chemical modification of poly(divinylsiloxane). Polymer 2002, 43, 1753–1759. [Google Scholar] [CrossRef]
- Yang, H.; Liu, M.-X.; Yao, Y.-W.; Tao, P.-Y.; Lin, B.-P.; Keller, P.; Zhang, X.-Q.; Sun, Y.; Guo, L.-X. Polysiloxane-Based Liquid Crystalline Polymers and Elastomers Prepared by Thiol–Ene Chemistry. Macromolecules 2013, 46, 3406–3416. [Google Scholar] [CrossRef]
- Nguyen, K.D.Q.; Megone, W.V.; Kong, D.; Gautrot, J.E. Ultrafast diffusion-controlled thiol–ene based crosslinking of silicone elastomers with tailored mechanical properties for biomedical applications. Polym. Chem. 2016, 7, 5281–5293. [Google Scholar] [CrossRef]
- Kim, S.T.; Finkelmann, H. Cholesteric liquid single-crystal elastomers (LSCE) obtained by the anisotropic deswelling method. Macromol. Rapid Commun. 2001, 22, 429–433. [Google Scholar] [CrossRef]
- Dvornic, P.R.; Lenz, R.W. Exactly alternating silarylene-siloxane polymers. 9. Relationships between polymer structure and glass transition temperature. Macromolecules 2002, 25, 3769–3778. [Google Scholar] [CrossRef]
- Tang, Y.; Tsiang, R. Rheological, extractive and thermal studies of the room temperature vulcanized polydimethylsiloxane. Polymer 1999, 40, 6135–6146. [Google Scholar] [CrossRef]
- Wang, J.J.; Feng, L.J.; Lei, A.L.; Yan, A.J.; Wang, X.J. Thermal stability and mechanical properties of room temperature vulcanized silicone rubbers. J. Appl. Polym. Sci. 2012, 125, 505–511. [Google Scholar] [CrossRef]
- Azoug, A.; Vasconcellos, V.; Dooling, J.; Saed, M.; Yakacki, C.M.; Nguyen, T.D. Viscoelasticity of the polydomain-monodomain transition in main-chain liquid crystal elastomers. Polymer 2016, 98, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Ware, T.H.; Biggins, J.S.; Shick, A.F.; Warner, M.; White, T.J. Localized soft elasticity in liquid crystal elastomers. Nat. Commun. 2016, 7, 10781. [Google Scholar] [CrossRef] [PubMed]
- Herbert, K.M.; Fowler, H.E.; McCracken, J.M.; Schlafmann, K.R.; Koch, J.A.; White, T.J. Synthesis and alignment of liquid crystalline elastomers. Nat. Rev. Mater. 2021, 7, 23–38. [Google Scholar] [CrossRef]



| No. | 1 (g) | Crosslinker (g) | MBB (g) | H2000 (Fumed Silica) (g) |
|---|---|---|---|---|
| MQ 1 | 20 a | 0.20 | 0 | 30 |
| LCMQ 1 | 20 a | 0.41 | 0.21 | 30 |
| LCMQ 3 | 20 a | 0.61 | 0.42 | 30 |
| LCMQ 4 | 20 a | 1.03 | 0.84 | 30 |
| LCMQ 5 | 20 b | 1.03 | 0.84 | 30 |
| LCMQ 6 | 20 c | 1.03 | 0.84 | 30 |
| No. | Proportion of Crosslinking Chains (%) | Proportion of Dangling Chains (%) | Proportion of Free Chains (%) | Crosslinking Density (×10−4 mol/mL) |
|---|---|---|---|---|
| MQ 1 | 36.10 | 41.59 | 22.31 | 0.7 |
| LCMQ 1 | 26.88 | 42.16 | 30.97 | 0.71 |
| LCMQ 3 | 27.73 | 42.69 | 29.58 | 0.69 |
| LCMQ 4 | 32.43 | 43.39 | 24.19 | 0.70 |
| LCMQ 5 | 56.84 | 26.07 | 17.09 | 0.87 |
| LCMQ 6 | 37.6 | 29.42 | 32.98 | 1.01 |
| No. | Tensile Strength (MPa) | Elongation at Break (%) | Shore Hardness (HA) |
|---|---|---|---|
| MQ 1 | 3.57 | 533.55 | 14 |
| LCMQ 1 | 4.15 | 677.18 | 13 |
| LCMQ 3 | 3.23 | 648.94 | 12 |
| LCMQ 4 | 2.16 | 601.06 | 14 |
| LCMQ 5 | 3.25 | 541.69 | 22 |
| LCMQ 6 | 1.56 | 241.90 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Xiong, Y.; Hao, J.; Zhang, H.; Cheng, X.; Wang, H.; Chen, W.; Zhou, C. Liquid Crystal-Based Organosilicone Elastomers with Supreme Mechanical Adaptability. Polymers 2022, 14, 789. https://doi.org/10.3390/polym14040789
Liu Z, Xiong Y, Hao J, Zhang H, Cheng X, Wang H, Chen W, Zhou C. Liquid Crystal-Based Organosilicone Elastomers with Supreme Mechanical Adaptability. Polymers. 2022; 14(4):789. https://doi.org/10.3390/polym14040789
Chicago/Turabian StyleLiu, Zhe, Yuqi Xiong, Jinghao Hao, Hao Zhang, Xiao Cheng, Hua Wang, Wei Chen, and Chuanjian Zhou. 2022. "Liquid Crystal-Based Organosilicone Elastomers with Supreme Mechanical Adaptability" Polymers 14, no. 4: 789. https://doi.org/10.3390/polym14040789
APA StyleLiu, Z., Xiong, Y., Hao, J., Zhang, H., Cheng, X., Wang, H., Chen, W., & Zhou, C. (2022). Liquid Crystal-Based Organosilicone Elastomers with Supreme Mechanical Adaptability. Polymers, 14(4), 789. https://doi.org/10.3390/polym14040789






