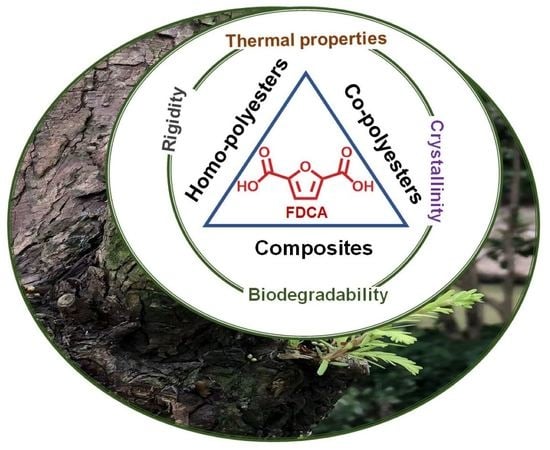
Recent Progress on Bio-Based Polyesters Derived from 2,5-Furandicarbonxylic Acid (FDCA)
Abstract
:1. Introduction
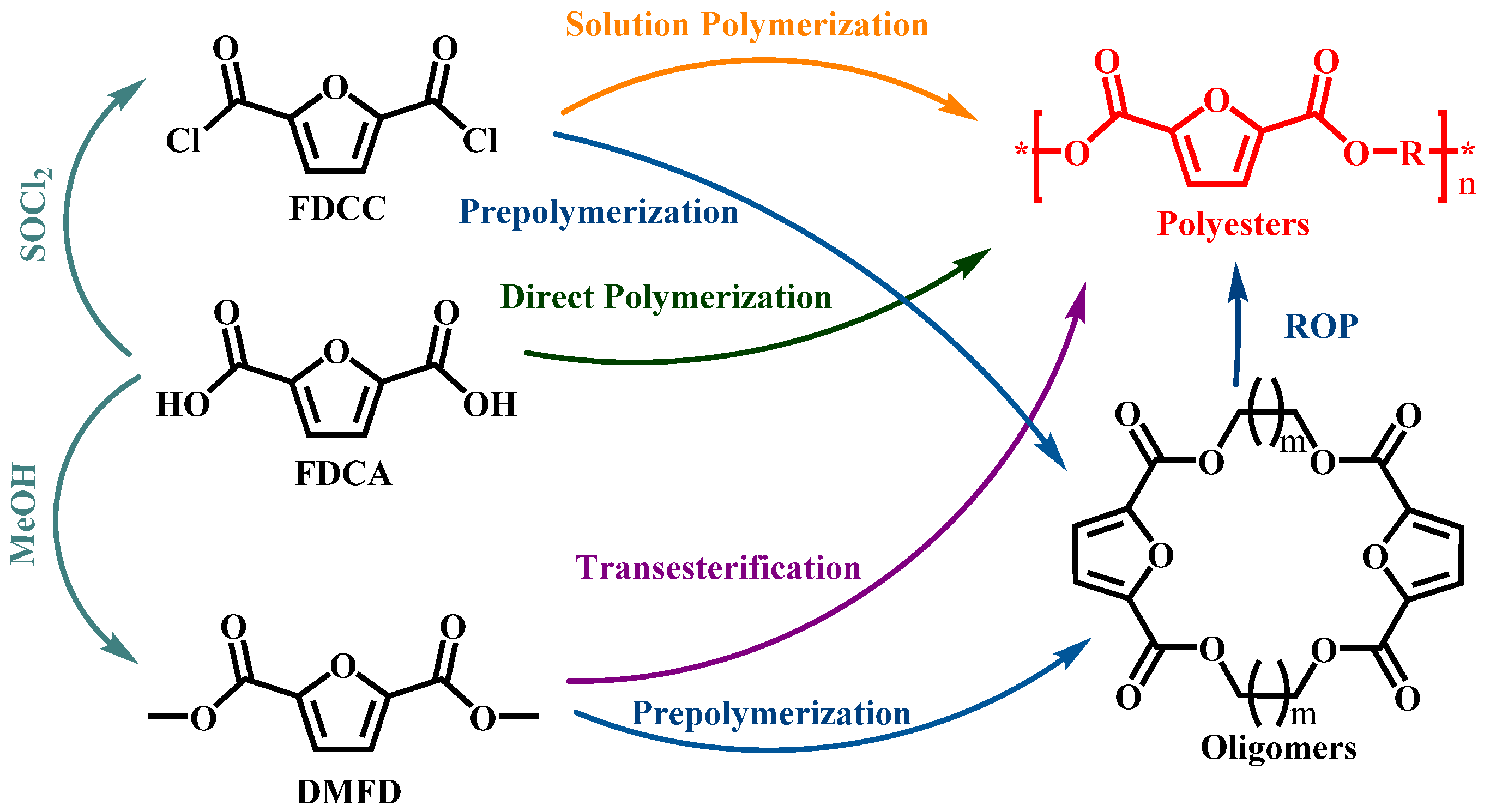
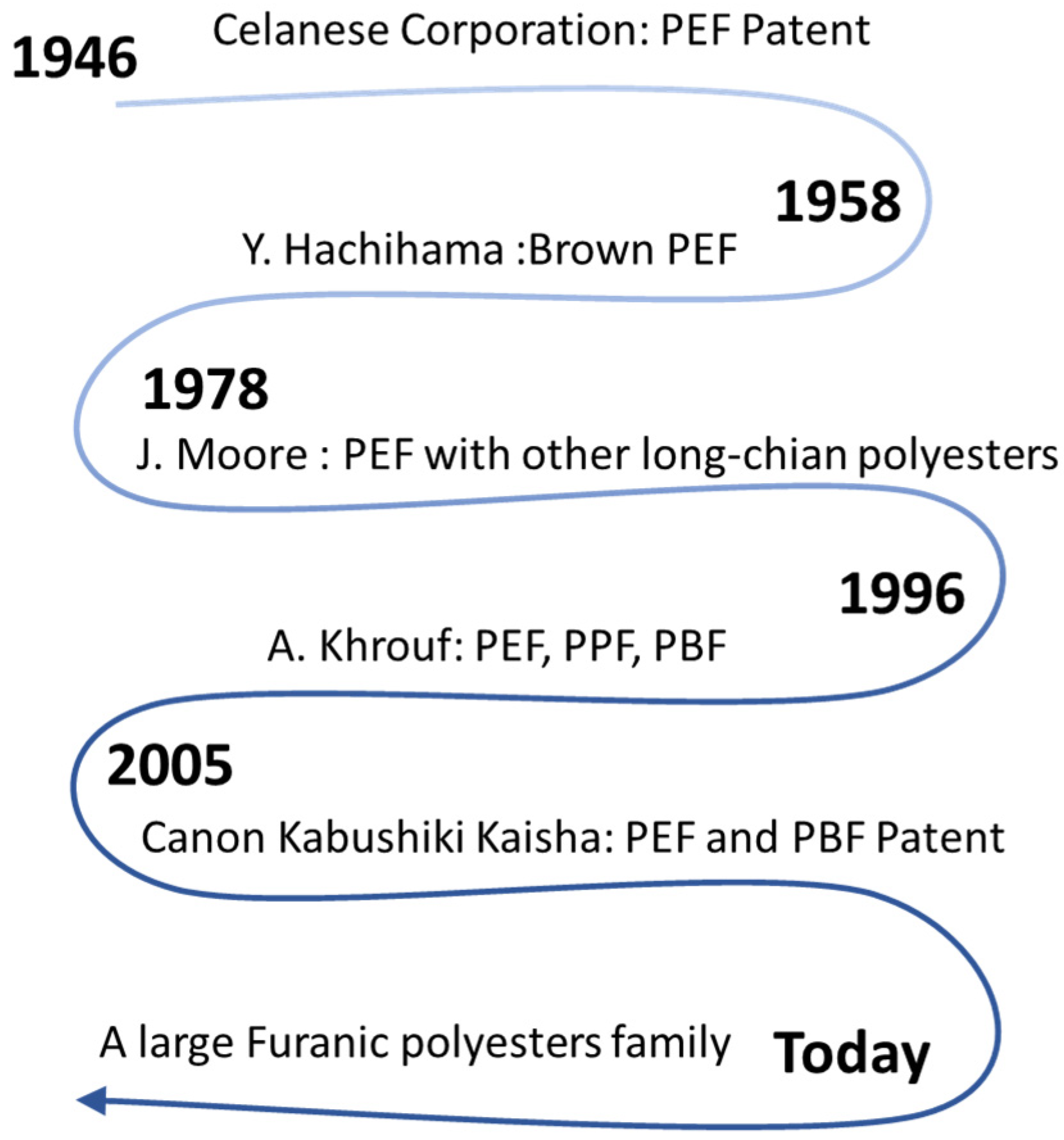
2. Synthetic Routes of Furanic Polyesters
3. Homo-Polyesters from FDCA
3.1. PEF
3.2. Furan-Polyesters with Aliphatic Diols
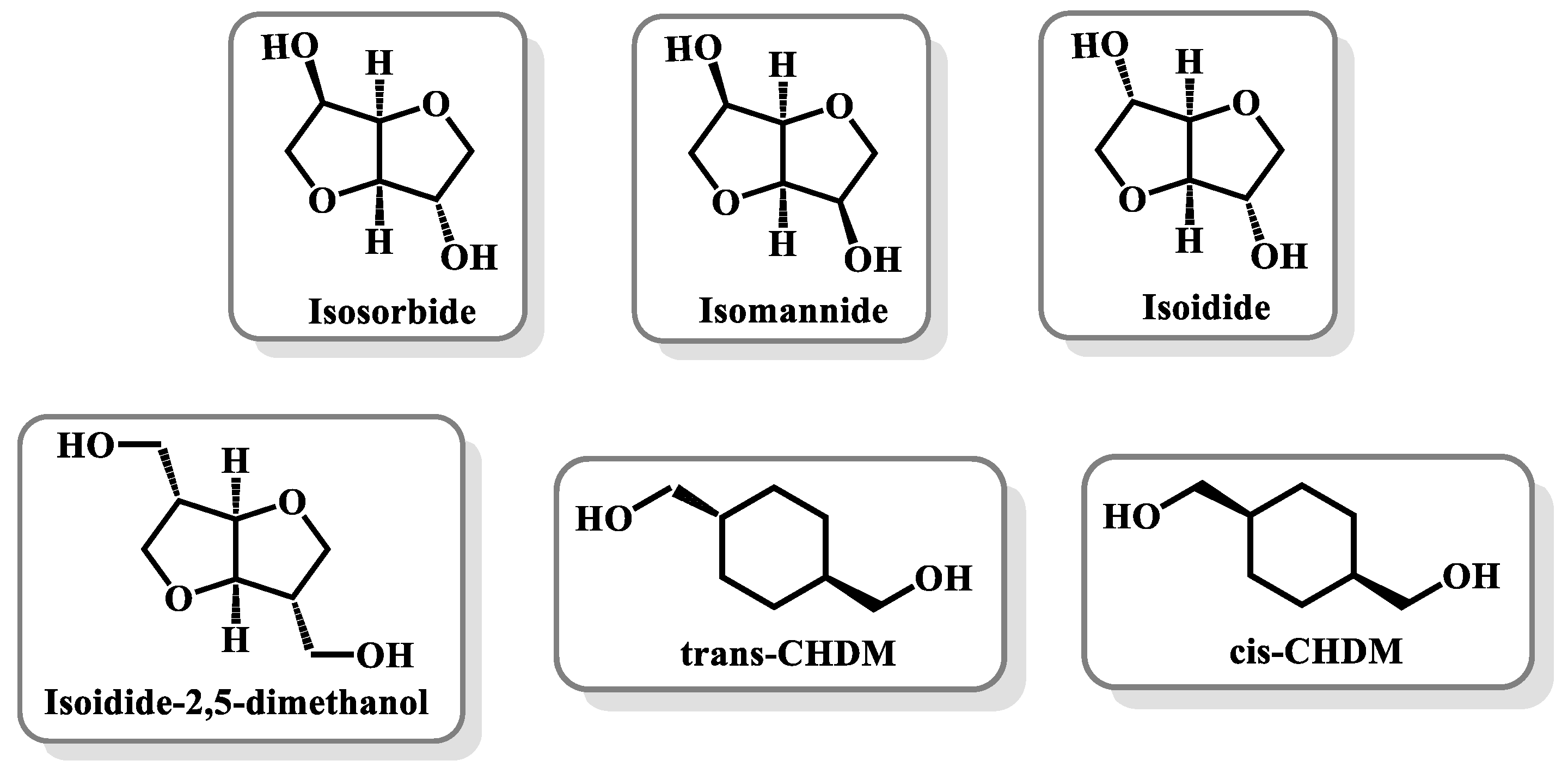
3.3. Furan-Polyesters with Rigid Diols
4. Co-Polyesters from FDCA
4.1. Balance between Rigidity and Flexibility
4.2. Introduction of Biodegradability
5. Composites of Furan-Polyesters
5.1. Inorganic and Organic Fillers
5.2. Blends of Furanoates
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vilela, C.; Sousa, A.F.; Fonseca, A.C.; Serra, A.C.; Coelho, J.F.J.; Freire, C.S.R.; Silvestre, A.J.D. The quest for sustainable polyesters—Insights into the future. Polym. Chem. 2014, 5, 3119–3141. [Google Scholar] [CrossRef]
- Fei, F.; Wang, J.G.; Zhu, J.; Wang, X.Z.; Liu, X.Q. Bio-based poly(ethylene 2,5-furancoate): No longer an alternative, but an irreplaceable one in polymer industry. ACS Sustain. Chem. Eng. 2020, 8, 8471–8485. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhim, J.W. Preparation and characterization of vacuum sputter silver coated PLA film. LWT-Food Sci. Technol. 2013, 54, 477–484. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Takahashi, K.; Taniguchi, I.; Miyamoto, M.; Kimura, Y. Melt/solid polycondensation of glycolic acid to obtain high-molecular-weight poly(glycolic acid). Polymer 2000, 41, 8725–8728. [Google Scholar] [CrossRef]
- Ye, H.M.; Wang, R.D.; Liu, J.; Xu, J.; Guo, B.H. Isomorphism in poly(butylene succinate-co-butylene fumarate) and its application as polymeric nucleating agent for poly(butylene succinate). Macromolecules 2012, 45, 5667–5675. [Google Scholar] [CrossRef]
- Gandini, A.; Coelho, D.; Gomes, M.; Reis, B.; Silvestre, A. Materials from renewable resources based on furan monomers and furan chemistry: Work in progress. J. Mater. Chem. 2009, 19, 8656–8664. [Google Scholar] [CrossRef] [Green Version]
- Gandini, A.; Silvestre, A.J.D.; Neto, C.P.; Sousa, A.F.; Gomes, M. The furan counterpart of poly(ethylene terephthalate): An alternative material based on renewable resources. J. Polym. Sci. Polym. Chem. 2009, 47, 295–298. [Google Scholar] [CrossRef]
- Gomes, M.; Gandini, A.; Silvestre, A.J.D.; Reis, B. Synthesis and characterization of poly(2,5-furan dicarboxylate)s based on a variety of diols. J. Polym. Sci. Polym. Chem. 2011, 49, 3759–3768. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, Q.; Zhang, Q.; Ye, C.; Zhou, G. A series of furan-aromatic polyesters synthesized via direct esterification method based on renewable resources. J. Polym. Sci. Polym. Chem. 2012, 50, 1026–1036. [Google Scholar] [CrossRef]
- Grosshardt, O.; Fehrenbacher, U.; Kowollik, K.; Tuebke, B.; Dingenouts, N.; Wilhelm, M. Synthesis and properties of polyester and polyamide based on furan 2,5 dycarboxylic acid. Chem. Ing. Tech. 2009, 81, 1829–1835. [Google Scholar]
- Jiang, Y.; Maniar, D.; Woortman, A.J.; Alberda van Ekenstein, G.O.; Loos, K. Enzymatic polymerization of furan-2,5-dicarboxylic acid-based furanic-aliphatic polyamides as sustainable alternatives to polyphthalamides. Biomacromolecules 2015, 16, 3674–3685. [Google Scholar] [CrossRef]
- Luo, K.; Wang, Y.; Yu, J.; Zhu, J.; Hu, Z. Semi-bio-based aromatic polyamides from 2,5-furandicarboxylic acid: Toward high-performance polymers from renewable resources. Rsc Adv. 2016, 6, 87013–87020. [Google Scholar] [CrossRef]
- Cao, M.; Zhang, C.; He, B.; Huang, M.; Jiang, S. Synthesis of 2,5-furandicarboxylic acid-based heat-resistant polyamides under existing industrialization process. Macromol. Res. 2017, 25, 722–729. [Google Scholar] [CrossRef]
- Hu, F.; La Scala, J.J.; Sadler, J.M.; Palmese, G.R. Synthesis and characterization of thermosetting furan-based epoxy systems. Macromolecules 2014, 47, 3332–3342. [Google Scholar] [CrossRef]
- Deng, J.; Liu, X.Q.; Li, C.; Jiang, Y.H.; Zhu, J. Synthesis and properties of a bio-based epoxy resin from 2,5-furandicarboxylic acid (FDCA). Rsc Adv. 2015, 5, 15930–15939. [Google Scholar] [CrossRef]
- Bao, F.; Song, Y.; Liu, Q.; Song, C.; Liu, C.; Wang, J.; Jian, X.; Xiao, J. Partial bio-based poly (aryl ether ketone) derived from 2,5-furandicarboxylic acid with enhanced processability. Polym. Degrad. Stab. 2019, 161, 309–318. [Google Scholar] [CrossRef]
- Lu, Z.; Xiaolan, L.; Yusheng, Q.; Yebo, L. A novel 2,5-furandicarboxylic acid-based bis(cyclic carbonate) for the synthesis of biobased non-isocyanate polyurethanes. Rsc Adv. 2016. [Google Scholar]
- María Nelly García, G.; Pål, B.; Marinella, L.; Stefano, T. Development and life cycle assessment of polyester binders containing 2,5-furandicarboxylic acid and their polyurethane coatings. J. Polym. Environ. 2018. [Google Scholar]
- Zhu, J.H.; Cai, J.L.; Xie, W.C.; Chen, P.H.; Gazzano, M.; Scandola, M.; Gross, R.A. Poly(butylene 2,5-furan dicarboxylate), a biobased alternative to PBT: Synthesis, physical properties, and crystal structure. Macromolecules 2013, 46, 796–804. [Google Scholar] [CrossRef]
- Eerhart, A.J.J.E.; Faaij, A.P.C.; Patel, M.K. Replacing fossil based PET with biobased PEF; process analysis, energy and GHG balance. Energy Environ. Sci. 2012, 5, 6407–6422. [Google Scholar] [CrossRef]
- Wang, J.G.; Liu, X.Q.; Jia, Z.; Sun, L.Y.; Zhu, J. Highly crystalline polyesters synthesized from furandicarboxylic acid (FDCA): Potential bio-based engineering plastic. Eur. Polym. J. 2018, 109, 379–390. [Google Scholar] [CrossRef]
- Van Putten, R.J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- de Jong, E.; Dam, M.A.; Sipos, L.; Gruter, G.-J.M. Furandicarboxylic Acid (FDCA), A Versatile Building Block for a Very Interesting Class of Polyesters. In Biobased Monomers, Polymers and Materials; Smith, P.B., Gross, R., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2012; pp. 1–13. [Google Scholar]
- Albonetti, S.; Lolli, A.; Morandi, V.; Migliori, A.; Lucarelli, C.; Cavani, F. Conversion of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over Au-based catalysts: Optimization of active phase and metal–support interaction. Appl. Catal. B 2015, 163, 520–530. [Google Scholar] [CrossRef]
- Martuscelli, E.; Pedone, C. The crystal and molecular structure of furane-α,α’-dicarboxylic acid. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 1968, 24, 175–179. [Google Scholar] [CrossRef]
- Bailey, M.; Brown, C.J. The crystal structure of terephthalic acid. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 1967, 22, 387–391. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, G.; Jiang, M.; Zhang, H.; Wang, H.; Wu, Y.; Wang, R. Bio-based polyesters with high glass-transition temperatures and gas barrier properties derived from renewable rigid tricyclic diacid or tetracyclic anhydride. Macromolecules 2020, 53, 5475–5486. [Google Scholar] [CrossRef]
- Yu, X.; Jia, J.; Xu, S.; Lao, K.U.; Sanford, M.J.; Ramakrishnan, R.K.; Nazarenko, S.I.; Hoye, T.R.; Coates, G.W.; DiStasio, R.A. Unraveling substituent effects on the glass transition temperatures of biorenewable polyesters. Nat. Commun. 2018, 9, 2880. [Google Scholar] [CrossRef]
- Drewitt, J.G.; Lincoln, J. Improvement in polymers. Br. Patent 621,971, 12 November 1946. [Google Scholar]
- Morales-Huerta, J.C.; de Ilarduya, A.M.; Munoz-Guerra, S. Poly(alkylene 2,5-furandicarboxylate)s (PEF and PBF) by ring opening polymerization. Polymer 2016, 87, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Knoop, R.J.I.; Vogelzang, W.; van Haveren, J.; van Es, D.S. High molecular weight poly(ethylene-2,5-furanoate); critical aspects in synthesis and mechanical property determination. J. Polym. Sci. Polym. Chem. 2013, 51, 4191–4199. [Google Scholar] [CrossRef]
- Gantillon, B.; Spitz, R.; McKenna, T.E. The solid state postcondensation of PET: A review of the physical and chemical processes taking place in the solid state. Angew. Makromol. Chem. 2004, 289, 88–105. [Google Scholar]
- Banella, M.B.; Bonucci, J.; Vannini, M.; Marchese, P.; Lorenzetti, C.; Celli, A. Insights into the synthesis of poly(ethylene 2,5-furandicarboxylate) from 2,5-furandicarboxylic acid: Steps toward environmental and food safety excellence in packaging applications. Ind. Eng. Chem. Res. 2019, 58, 8955–8962. [Google Scholar] [CrossRef]
- Qu, X.L.; Zhou, G.Y.; Wang, R.; Zhang, H.Y.; Wang, Z.P.; Jiang, M.; Tang, J. Insights into high molecular weight poly(ethylene 2,5-furandicarboxylate) with satisfactory appearance: Roles of in-situ catalysis of metal zinc. J. Ind. Eng. Chem. 2021, 99, 422–430. [Google Scholar] [CrossRef]
- Burgess, S.K.; Karvan, O.; Johnson, J.R.; Kriegel, R.M.; Koros, W.J. Oxygen sorption and transport in amorphous poly(ethylene furanoate). Polymer 2014, 55, 4748–4756. [Google Scholar] [CrossRef]
- Burgess, S.K.; Kriegel, R.M.; Koros, W.J. Carbon dioxide sorption and transport in amorphous poly(ethylene furanoate). Macromolecules 2015, 48, 2184–2193. [Google Scholar] [CrossRef]
- Burgess, S.K.; Leisen, J.E.; Kraftschik, B.E.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Chain mobility, thermal, and mechanical properties of poly(ethylene furanoate) compared to poly(ethylene terephthalate). Macromolecules 2014, 47, 1383–1391. [Google Scholar] [CrossRef]
- Sun, L.; Wang, J.; Mahmud, S.; Jiang, Y.; Zhu, J.; Liu, X. New insight into the mechanism for the excellent gas properties of poly(ethylene 2,5-furandicarboxylate) (PEF): Role of furan ring’s polarity. Eur. Polym. J. 2019, 118, 642–650. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Tsanaktsis, V.; Bikiaris, D.N. Synthesis of the bio-based polyester poly(propylene 2,5-furan dicarboxylate). Comparison of thermal behavior and solid state structure with its terephthalate and naphthalate homologues. Polymer 2015, 62, 28–38. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Papageorgiou, G.Z.; Bikiaris, D.N. A facile method to synthesize high-molecular-weight biobased polyesters from 2,5-furandicarboxylic acid and long-chain diols. J. Polym. Sci. Polym. Chem. 2015, 53, 2617–2632. [Google Scholar] [CrossRef]
- Avantium. Avantium Technology & Markets Day: “Entering the Commercialization Phase for PEF”. Available online: https://www.avantium.com/press-releases/avantium-technology-markets-day-entering-the-commercialization-phase-for-pef/ (accessed on 25 July 2018).
- Maltsev, A. PEF Challenges PET to Battle. Available online: https://ethz.ch/en/news-and-events/eth-news/news/2018/07/pef-for-pet.html (accessed on 3 August 2018).
- Rosenboom, J.G.; Hohl, D.K.; Fleckenstein, P.; Storti, G.; Morbidelli, M. Bottle-grade polyethylene furanoate from ring-opening polymerisation of cyclic oligomers. Nat. Commun. 2018, 9, 2701. [Google Scholar] [CrossRef] [Green Version]
- Tsanaktsis, V.; Vouvoudi, E.; Papageorgiou, G.Z.; Papageorgiou, D.G.; Chrissafis, K.; Bikiaris, D.N. Thermal degradation kinetics and decomposition mechanism of polyesters based on 2,5-furandicarboxylic acid and low molecular weight aliphatic diols. J. Anal. Appl. Pyrolysis 2015, 112, 369–378. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Terzopoulou, Z.; Bikiaris, D.N. Production of bio-based 2,5-furan dicarboxylate polyesters: Recent progress and critical aspects in their synthesis and thermal properties. Eur. Polym. J. 2016, 83, 202–229. [Google Scholar] [CrossRef]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.S.R.; Gruter, G.-J.M.; Coelho, J.F.J.; Silvestre, A.J.D. Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: A tribute to furan excellency. Polym.Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Jiang, Y.; Woortman, A.J.J.; Van Ekenstein, G.O.R.A.; Loos, K. A biocatalytic approach towards sustainable furanic–aliphatic polyesters. Polym. Chem. 2015, 6, 5198–5211. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.P.; Yu, X.F.; Xu, J.; Pang, Y. Synthesis and crystallinity of poly(butylene 2,5-furandicarboxylate). Polymer 2012, 53, 4145–4151. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Vogelzang, W.; Knoop, R.J.I.; Frissen, A.E.; van Haveren, J.; van Es, D.S. Biobased furandicarboxylic acids (FDCAs): Effects of isomeric substitution on polyester synthesis and properties. Green Chem. 2014, 16, 1957–1966. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Zhang, C.L.; Yang, F.; Weng, Y.X. Gas barrier properties of furan-based polyester films analyzed experimentally and by molecular simulations. Polymer 2021, 233, 124200. [Google Scholar] [CrossRef]
- Fenouillot, F.; Rousseau, A.; Colomines, G.; Saint-Loup, R.; Pascault, J.P. Polymers from renewable 1,4:3,6-dianhydrohexitols (isosorbide, isomannide and isoidide): A review. Prog. Polym. Sci. 2010, 35, 578–622. [Google Scholar] [CrossRef]
- Okada, M.; Tsunoda, K.; Tachikawa, K.; Aoi, K. Biodegradable polymers based on renewable resources. IV. Enzymatic degradation of polyesters composed of 1,4:3.6-dianhydro-D-glucitol and aliphatic dicarboxylic acid moieties. J. Appl. Polym. Sci. 2000, 77, 338–346. [Google Scholar] [CrossRef]
- Caouthar, A.; Roger, P.; Tessier, M.; Chatti, S.; Blais, J.C.; Bortolussi, M. Synthesis and characterization of new polyamides derived from di(4-cyanophenyl)isosorbide. Eur. Polym. J. 2007, 43, 220–230. [Google Scholar] [CrossRef]
- Chatti, S.; Schwarz, G.; Kricheldorf, H.R. Cyclic and noncyclic polycarbonates of isosorbide (1,4:3,6-dianhydro-D-glucitol). Macromolecules 2006, 39, 9064–9070. [Google Scholar] [CrossRef]
- Lomelí-Rodríguez, M.; Corpas-Martínez, J.R.; Willis, S.; Mulholland, R.; Lopez-Sanchez, J.A. Synthesis and Characterization of Renewable Polyester Coil Coatings from Biomass-Derived Isosorbide, FDCA, 1,5-Pentanediol, Succinic Acid, and 1,3-Propanediol. Polymers 2018, 10, 600. [Google Scholar] [CrossRef] [Green Version]
- Terzopoulou, Z.; Kasmi, N.; Tsanaktsis, V.; Doulakas, N.; Bikiaris, D.N.; Achilias, D.S.; Papageorgiou, G.Z. Synthesis and Characterization of Bio-Based Polyesters: Poly(2-methyl-1,3-propylene-2,5-furanoate), Poly(isosorbide-2,5-furanoate), Poly(1,4-cyclohexanedimethylene-2,5-furanoate). Materials 2017, 10, 801. [Google Scholar] [CrossRef] [Green Version]
- Chebbi, Y.; Kasmi, N.; Majdoub, M.; Cerruti, P.; Scarinzi, G.; Malinconico, M.; Dal Poggetto, G.; Papageorgiou, G.Z.; Bikiaris, D.N. Synthesis, Characterization, and Biodegradability of Novel Fully Biobased Poly(decamethylene-co-isosorbide 2,5-furandicarboxylate) Copolyesters with Enhanced Mechanical Properties. ACS Sustain. Chem. Eng. 2019, 7, 5501–5514. [Google Scholar] [CrossRef]
- Chen, J.; Lin, Y.; Chen, Y.; Koning, C.E.; Wu, J.; Wang, H. Low-crystallinity to highly amorphous copolyesters with high glass transition temperatures based on rigid carbohydrate-derived building blocks. Polym. Int. 2021, 70, 536–545. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Liu, S.; Wang, G. Synthesis and characterization of poly(isosorbide-co-butylene 2,5-furandicarboxylate) copolyesters. Eur. Polym. J. 2019, 115, 70–75. [Google Scholar] [CrossRef]
- Kasmi, N.; Majdoub, M.; Papageorgiou, G.Z.; Bikiaris, D.N. Synthesis and crystallization of new fully renewable resources-based copolyesters: Poly(1,4-cyclohexanedimethanol-co-isosorbide 2,5-furandicarboxylate). Polym. Degrad. Stab. 2018, 152, 177–190. [Google Scholar] [CrossRef]
- Wu, J.; Eduard, P.; Jasinska-Walc, L.; Rozanski, A.; Noordover, B.A.J.; van Es, D.S.; Koning, C.E. Fully Isohexide-Based Polyesters: Synthesis, Characterization, and Structure–Properties Relations. Macromolecules 2013, 46, 384–394. [Google Scholar] [CrossRef]
- Jacquel, N.; Saint-Loup, R.; Pascault, J.-P.; Rousseau, A.; Fenouillot, F. Bio-based alternatives in the synthesis of aliphatic–aromatic polyesters dedicated to biodegradable film applications. Polymer 2015, 59, 234–242. [Google Scholar] [CrossRef]
- Wu, J.; Eduard, P.; Thiyagarajan, S.; Noordover, B.A.; van Es, D.S.; Koning, C.E. Semi-aromatic polyesters based on a carbohydrate-derived rigid diol for engineering plastics. ChemSusChem 2015, 8, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matos, M.; Sousa, A.F.; Silvestre, A.J.D. Improving the thermal properties of poly(2,5-furandicarboxylate)s using cyclohexylene moieties: A comparative study. Macromol. Chem. Phys. 2016, 218, 1600492. [Google Scholar] [CrossRef]
- Celli, A.; Marchese, P.; Sisti, L.; Dumand, D.; Sullalti, S.; Totaro, G. Effect of 1,4-cyclohexylene units on thermal properties of poly(1,4-cyclohexylenedimethylene adipate) and similar aliphatic polyesters. Polym. Int. 2013, 62, 1210–1217. [Google Scholar] [CrossRef]
- Celli, A.; Marchese, P.; Sullalti, S.; Berti, C.; Barbiroli, G. Eco-friendly poly(butylene 1,4-cyclohexanedicarboxylate): Relationships between stereochemistry and crystallization behavior. Macromol. Chem. Phys. 2011, 212, 1524–1534. [Google Scholar] [CrossRef]
- Wang, J.G.; Liu, X.Q.; Jia, Z.; Sun, L.Y.; Zhang, Y.J.; Zhu, J. Modification of poly(ethylene 2,5-furandicarboxylate) (PEF) with 1, 4-cyclohexanedimethanol: Influence of stereochemistry of 1,4-cyclohexylene units. Polymer 2018, 137, 173–185. [Google Scholar] [CrossRef]
- Carman, H.S.; Killman, J.I.; Crawford, E.D.; Jenkins, J.C.; Eastman Chemical Company. Polyester Compositions Containing Furandicarboxylic Acid or an Ester Thereof, and 2,2,4,4-tetramethyl-1,3-cyclobutanediol. U.S. Patent 2,013,055,862, 18 April 2013. [Google Scholar]
- Ghosh, T.; Mahajan, K.; Narayan-Sarathy, S.; Balgacem, M.N.; Gopalakrishnan, P. 2,5-Furan Dicarboxylic Acid-based Polyesters Prepared from Biomass. U.S. Patent 20,130,171,397A1, 4 July 2013. [Google Scholar]
- Zaidi, S.; Soares, M.J.; Bougarech, A.; Thiyagarajan, S.; Guigo, N.; Abid, S.; Abid, M.; Silvestre, A.J.D.; Sousa, A.F. Unravelling the para- and ortho-benzene substituent effect on the glass transition of renewable wholly (hetero-)aromatic polyesters bearing 2,5-furandicarboxylic moieties. Eur. Polym. J. 2021, 150, 110413. [Google Scholar] [CrossRef]
- Bengs, H.; Schoenfeld, A.; Boehm, G.; Weis, S.; Clauss, J. Biodegradable Polymers Based on Natural and Renewable Raw Materials Especially Isosorbite. U.S. Patent 6,342,300 B1, 29 January 2002. [Google Scholar]
- van Berkel, J.G.; Guigo, N.; Kolstad, J.J.; Sipos, L.; Wang, B.; Dam, M.A.; Sbirrazzuoli, N. Isothermal crystallization kinetics of poly (ethylene 2,5-furandicarboxylate). Macromol. Mater. Eng. 2015, 300, 466–474. [Google Scholar] [CrossRef]
- Ma, J.P.; Pang, Y.; Wang, M.; Xu, J.; Ma, H.; Nie, X. The copolymerization reactivity of diols with 2,5-furandicarboxylic acid for furan-based copolyester materials. J. Mater. Chem. 2012, 22, 3457–3461. [Google Scholar] [CrossRef]
- Hong, S.; Min, K.D.; Nam, B.U.; Park, O.O. High molecular weight bio furan-based co-polyesters for food packaging applications: Synthesis, characterization and solid-state polymerization. Green Chem. 2016, 18, 5142–5150. [Google Scholar] [CrossRef]
- Wang, J.G.; Liu, X.Q.; Zhang, Y.J.; Liu, F.; Zhu, J. Modification of poly(ethylene 2,5-furandicarboxylate) with 1,4-cyclohexanedimethylene: Influence of composition on mechanical and barrier properties. Polymer 2016, 103, 1–8. [Google Scholar] [CrossRef]
- Wang, X.S.; Wang, Q.Y.; Liu, S.Y.; Wang, G.Y. Biobased copolyesters: Synthesis, structure, thermal and mechanical properties of poly(ethylene 2,5-furandicarboxylate-co-ethylene 1,4-cyclohexanedicarboxylate. Polym. Degrad. Stab. 2018, 154, 96–102. [Google Scholar] [CrossRef]
- Diao, L.C.; Su, K.M.; Li, Z.H.; Ding, C.K. Furan-based co-polyesters with enhanced thermal properties: Poly(1,4-butylene-co-1,4-cyclohexanedimethylene-2,5-furandicarboxylic acid). Rsc Adv. 2016, 6, 27632–27639. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, J.G.; Sun, L.Y.; Liu, F.; Zhu, J.; Liu, X.Q. Copolyesters developed from bio-based 2,5-furandicarboxylic acid: Synthesis, sequence distribution, mechanical, and barrier properties of poly(propylene-co-1,4-cyclohexanedimethylene 2,5-furandicarboxylate)s. J. Appl. Polym. Sci. 2019, 136, 47291. [Google Scholar] [CrossRef]
- Kim, T.; Koo, J.M.; Ryu, M.H.; Jeon, H.; Kim, S.M.; Park, S.A.; Oh, D.X.; Park, J.; Hwang, S.Y. Sustainable terpolyester of high T-g based on bio heterocyclic monomer of dimethyl furan-2,5-dicarboxylate and isosorbide. Polymer 2017, 132, 122–132. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zhu, J.; Jiang, Y. Copolyesters based on 2,5-furandicarboxylic acid (FDCA): Effect of 2,2,4,4-tetramethyl-1,3-cyclobutanediol units on their properties. Polymers 2017, 9, 305. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.Q.; Jiang, M.; Zhang, Q.; Wang, R.; Zhou, G.Y. Biobased multiblock copolymers: Synthesis, properties and shape memory performance of poly(ethylene 2,5-furandicarboxylate)-b-ly(ethylene glycol). Polym. Degrad. Stab. 2017, 144, 121–127. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, M.; Wu, Y.; Li, L.; Wang, Z.; Wang, R.; Zhou, G. Development of completely furfural-based renewable polyesters with controllable properties. Green Chem. 2021, 23, 2437–2448. [Google Scholar] [CrossRef]
- Pang, C.; Jiang, X.; Yu, Y.; Chen, L.; Ma, J.; Gao, H. Copolymerization of natural camphor-derived rigid diol with various dicarboxylic acids: Access to biobased polyesters with various properties. ACS Macro Lett. 2019, 8, 1442–1448. [Google Scholar] [CrossRef]
- Shen, A.; Wang, J.; Ma, S.; Fei, X.; Zhang, X.; Zhu, J.; Liu, X. Completely amorphous high thermal resistant copolyesters from bio-based 2,5-furandicarboxylic acid. J. Appl. Polym. Sci. 2021, 138, 50627. [Google Scholar] [CrossRef]
- Witt, U.; Einig, T.; Yamamoto, M.; Kleeberg, I.; Deckwer, W.D.; Muller, R.J. Biodegradation of aliphatic-aromatic copolyesters: Evaluation of the final biodegradability and ecotoxicological impact of degradation intermediates. Chemosphere 2001, 44, 289–299. [Google Scholar] [CrossRef]
- Shah, A.A.; Eguchi, T.; Mayumi, D.; Kato, S.; Shintani, N.; Kamini, N.R.; Nakajima-Kambe, T. Purification and properties of novel aliphatic-aromatic co-polyesters degrading enzymes from newly isolated Roseateles depolymerans strain TB-87. Polym. Degrad. Stab. 2013, 98, 609–618. [Google Scholar] [CrossRef]
- Nakajimakambe, T.; Toyoshima, K.; Saito, C.; Takaguchi, H.; Akutsushigeno, Y.; Sato, M.; Miyama, K.; Nomura, N.; Uchiyama, H. Rapid monomerization of poly(butylene succinate)-co-(butylene adipate) by Leptothrix sp. J. Biosci. Bioeng. 2009, 108, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Kasuya, K.; Ishii, N.; Inoue, Y.; Yazawa, K.; Tagaya, T.; Yotsumoto, T.; Kazahaya, J.; Nagai, D. Characterization of a mesophilic aliphatic–aromatic copolyester-degrading fungus. Polym. Degrad. Stab. 2009, 94, 1190–1196. [Google Scholar] [CrossRef]
- Wu, D.; Archana, S.; Rajiv, S.; Minna, H. Nano-graphene oxide functionalized bioactive poly(lactic acid) and poly(ε-caprolactone) nanofibrous scaffolds. Materials 2018, 11, 566. [Google Scholar] [CrossRef] [Green Version]
- Kashif, M.; Yun, B.M.; Lee, K.S.; Chang, Y.W. Biodegradable shape-memory poly(ε-caprolactone)/polyhedral oligomeric silsequioxane nanocomposites: Sustained drug release and hydrolytic degradation. Mater. Lett. 2016, 166, 125–128. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.S.; Liu, S.Y.; Wang, Q.Y.; Li, J.G.; Wang, G.Y. Synthesis and characterization of poly(ethylene 2,5-furandicarboxylate-co-epsilon-ecaprolactone) copolyesters. Eur. Polym. J. 2018, 109, 191–197. [Google Scholar] [CrossRef]
- Kasmi, N.; Wahbi, M.; Papadopoulos, L.; Terzopoulou, Z.; Guigo, N.; Sbirrazzuoli, N.; Papageorgiou, G.Z.; Bikiaris, D.N. Synthesis and characterization of two new biobased poly(pentylene 2,5-furandicarboxylate-co-caprolactone) and poly(hexamethylene 2,5-furandicarboxylate-co-caprolactone) copolyesters with enhanced enzymatic hydrolysis properties. Polym. Degrad. Stab. 2019, 160, 242–263. [Google Scholar] [CrossRef]
- Zheng, M.Y.; Zang, X.L.; Wang, G.X.; Wang, P.L.; Lu, B.; Ji, J.H. Poly(butylene 2,5-furandicarboxylate-epsilon-caprolactone): A new bio-based elastomer with high strength and biodegradability. Express Polym. Lett. 2017, 11, 611–621. [Google Scholar] [CrossRef]
- Morales-Huerta, J.C.; de Ilarduya, A.M.; Munoz-Guerra, S. Blocky poly(-caprolactone-co-butylene 2,5-furandicarboxylate) copolyesters via enzymatic ring opening polymerization. J. Polym. Sci. Polym. Chem. 2018, 56, 290–299. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, R.; Ying, W.B.; Kong, Z.; Wang, K.; Wang, J.; Zhu, J. Biodegradable elastomer from 2,5-furandicarboxylic acid and ε-caprolactone: Effect of crystallization on elasticity. ACS Sustain. Chem. Eng. 2019, 7, 17778–17788. [Google Scholar] [CrossRef]
- Li, F.X.; Xu, X.J.; Hao, Q.H.; Li, Q.B.; Yu, J.Y.; Cao, A.M. Effects of comonomer sequential structure on thermal and crystallization behaviors of biodegradable poly(butylene succinate-co-butylene terephthalate)s. J. Polym. Sci. B Polym. Phys. 2006, 44, 1635–1644. [Google Scholar] [CrossRef]
- Sun, Y.J.; Wu, L.B.; Bu, Z.Y.; Li, B.G.; Li, N.X.; Dai, J.M. Synthesis and thermomechanical and rheological properties of biodegradable long-chain branched poly(butylene succinate-co-butylene terephthalate) copolyesters. Ind. Eng. Chem. Res. 2014, 53, 10380–10386. [Google Scholar] [CrossRef]
- Luo, S.L.; Li, F.X.; Yu, J.Y. The thermal, mechanical and viscoelastic properties of poly(butylene succinate-co-terephthalate) (PBST) copolyesters with high content of BT units. J. Polym. Res. 2011, 18, 393–400. [Google Scholar] [CrossRef]
- Moraleshuerta, J.C.; Ciulik, C.; De Ilarduya, A.M.; Munozguerra, S. Fully bio-based aromatic–aliphatic copolyesters: Poly(butylene furandicarboxylate-co-succinate)s obtained by ring opening polymerization. Polym. Chem. 2017, 8, 748–760. [Google Scholar] [CrossRef] [Green Version]
- Lomeli-Rodriguez, M.; Martin-Molina, M.; Jimenez-Pardo, M.; Nasim-Afzal, Z.; Cauet, S.I.; Davies, T.E.; Rivera-Toledo, M.; Lopez-Sanchez, J.A. Synthesis and kinetic modeling of biomass-derived renewable polyesters. J. Polym. Sci. Polym. Chem. 2016, 54, 2876–2887. [Google Scholar] [CrossRef]
- Wu, L.; Mincheva, R.; Xu, Y.; Raquez, J.M.; Dubois, P. High molecular weight poly(butylene succinate-co-butylene furandicarboxylate) copolyesters: From catalyzed polycondensation reaction to thermomechanical properties. Biomacromolecules 2012, 13, 2973–2981. [Google Scholar] [CrossRef]
- Peng, S.; Wu, L.; Li, B.G.; Dubois, P. Hydrolytic and compost degradation of biobased PBSF and PBAF copolyesters with 40–60 mol% BF unit. Polym. Degrad. Stab. 2017, 146, 223–228. [Google Scholar] [CrossRef]
- Peng, S.B.; Wu, B.S.; Wu, L.B.; Li, B.G.; Dubois, P. Hydrolytic degradation of biobased poly(butylene succinate-co-furandicarboxylate) and poly(butylene adipate-co-furandicarboxylate) copolyesters under mild conditions. J. Appl. Polym. Sci. 2017, 134, 44674. [Google Scholar] [CrossRef]
- Hua, H.; Zhang, R.Y.; Wang, J.G.; Ying, W.B.; Zhu, J. Fully bio-based poly(propylene succinate-co-propylene furandicarboxylate) copolyesters with proper mechanical, degradation and barrier properties for green packaging applications. Eur. Polym. J. 2018, 102, 101–110. [Google Scholar] [CrossRef]
- Witt, U.; Yamamoto, M.; Seeliger, U.; Müller, R.J.; Warzelhan, V. Biodegradable polymeric materials—not the origin but the chemical structure determines biodegradability. Angew. Chem. Int. Ed. 1999, 38, 1438–1442. [Google Scholar] [CrossRef]
- Witt, U.; Muller, R.J.; Deckwer, W.D. Biodegradation of polyester copolymers containing aromatic-compounds. J. Macromol. 1995, A32, 851–856. [Google Scholar] [CrossRef]
- Papadopoulos, L.; Magaziotis, A.; Nerantzaki, M.; Terzopoulou, Z.; Papageorgiou, G.Z.; Bikiaris, D.N. Synthesis and characterization of novel poly(ethylene furanoate-co-adipate) random copolyesters with enhanced biodegradability. Polym. Degrad. Stab. 2018, 156, 32–42. [Google Scholar] [CrossRef]
- Zhou, W.D.; Wang, X.W.; Yang, B.; Xu, Y.; Zhang, W.; Zhang, Y.J.; Ji, J.H. Synthesis, physical properties and enzymatic degradation of bio-based poly(butylene adipate-co-butylene furandicarboxylate) copolyesters. Polym. Degrad. Stab. 2013, 98, 2177–2183. [Google Scholar] [CrossRef]
- Wu, B.S.; Xu, Y.T.; Bu, Z.Y.; Wu, L.B.; Li, B.G.; Dubois, P. Biobased poly(butylene 2,5-furandicarboxylate) and poly(butylene adipate-co-butylene 2,5-furandicarboxylate)s: From synthesis using highly purified 2,5-furandicarboxylic acid to thermo-mechanical properties. Polymer 2014, 55, 3648–3655. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, R.Y.; Wang, J.G.; Ying, W.B.; Shi, L.; Yao, C.K.; Kong, Z.Y.; Wang, K.; Zhu, J. A mild method to prepare high molecular weight poly(butylene furandicarboxylate-co-glycolate) copolyesters: Effects of the glycolate content on thermal, mechanical, and barrier properties and biodegradability. Green Chem. 2019, 21, 3013–3022. [Google Scholar] [CrossRef]
- Soccio, M.; Costa, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Salatelli, E.; Manaresi, P.; Munari, A. Novel fully biobased poly(butylene 2,5-furanoate/diglycolate) copolymers containing ether linkages: Structure-property relationships. Eur. Polym. J. 2016, 81, 397–412. [Google Scholar] [CrossRef]
- Wu, H.L.; Wen, B.B.; Zhou, H.; Zhou, J.D.; Yu, Z.L.; Cui, L.Y.; Huang, T.; Cao, F. Synthesis and degradability of copolyesters of 2,5-furandicarboxylic acid, lactic acid, and ethylene glycol. Polym. Degrad. Stab. 2015, 121, 100–104. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, J.G.; Sun, L.Y.; Zhu, J.; Liu, X.Q. Fully bio-based polyesters derived from 2,5-furandicarboxylic acid (2,5-FDCA) and dodecanedioic acid (DDCA): From semicrystalline thermoplastic to amorphous elastomer. J. Appl. Polym. Sci. 2018, 135, 46076. [Google Scholar] [CrossRef]
- Soares, M.J.; Dannecker, P.K.; Vilela, C.; Bastos, J.; Meier, M.A.R.; Sousa, A.F. Poly(1,20-eicosanediyl 2,5-furandicarboxylate), a biodegradable polyester from renewable resources. Eur. Polym. J. 2017, 90, 301–311. [Google Scholar] [CrossRef]
- Weinberger, S.; Canadell, J.; Quartinello, F.; Yeniad, B.; Arias, A.; Pellis, A.; Guebitz, G.M. Enzymatic degradation of poly(ethylene 2,5-furanoate) powders and amorphous films. Catalysts 2017, 7, 318. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Jiang, M.; Wu, Y.; Li, L.; Wang, Z.; Wang, R.; Zhou, G. Development of high-molecular-weight fully renewable biopolyesters based on oxabicyclic diacid and 2,5-furandicarboxylic acid: Promising as packaging and medical materials. ACS Sustain. Chem. Eng. 2021, 9, 6799–6809. [Google Scholar] [CrossRef]
- Kim, H.; Kim, T.; Choi, S.; Jeon, H.; Oh, D.X.; Park, J.; Eom, Y.; Hwang, S.Y.; Koo, J.M. Remarkable elasticity and enzymatic degradation of bio-based poly(butylene adipate-co-furanoate): Replacing terephthalate. Green Chem. 2020, 22, 7778–7787. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Litchfield, D.W.; Baird, D.G. The role of nanoclay in the generation of poly(ethylene terephthalate) fibers with improved modulus and tenacity. Polymer 2008, 49, 5027–5036. [Google Scholar] [CrossRef]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene–polymer nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Ray, S.S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar]
- Moniruzzaman, M.; Winey, K.I. Polymer nanocomposites containing carbon nanotubes. Macromolecules 2006, 39, 5194–5205. [Google Scholar] [CrossRef]
- Lotti, N.; Munari, A.; Gigli, M.; Gazzano, M.; Tsanaktsis, V.; Bikiaris, D.N.; Papageorgiou, G.Z. Thermal and structural response of in situ prepared biobased poly(ethylene 2,5-furan dicarboxylate) nanocomposites. Polymer 2016, 103, 288–298. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Tarani, E.; Kasmi, N.; Papadopoulos, L.; Chrissafis, K.; Papageorgiou, D.G.; Papageorgiou, G.Z.; Bikiaris, D.N. Thermal decomposition kinetics and mechanism of in-situ prepared bio-based poly(propylene 2,5-furan dicarboxylate)/graphene nanocomposites. Molecules 2019, 24, 1717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paszkiewicz, S.; Janowska, I.; Pawlikowska, D.; Szymczyk, A.; Irska, I.; Lisiecki, S.; Stanik, R.; Gude, M.; Piesowicz, E. New functional nanocomposites based on poly(trimethylene 2,5-furanoate) and few layer graphene prepared by in situ polymerization. Express Polym. Lett. 2018, 12, 530–542. [Google Scholar] [CrossRef]
- Achilias, D.S.; Chondroyiannis, A.; Nerantzaki, M.; Adam, K.V.; Terzopoulou, Z.; Papageorgiou, G.Z.; Bikiaris, D.N. Solid state polymerization of poly(ethylene furanoate) and its nanocomposites with SiO2 and TiO2. Macromol. Mater. Eng. 2017, 302, 1700012. [Google Scholar] [CrossRef]
- Zhou, G.; Li, L.; Jiang, M.; Wang, G.; Wang, R.; Wu, G.; Zhou, G. Renewable poly(butene 2, 5-furan dicarboxylate) nanocomposites constructed by TiO2 nanocubes: Synthesis, crystallization, and properties. Polym. Degrad. Stab. 2021, 189, 109591. [Google Scholar] [CrossRef]
- Pavlidou, S.; Papaspyrides, C.D. A review on polymerelayered silicate nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Stoeffler, K.; Lafleur, P.G.; Denault, J. Thermal decomposition of various alkyl onium organoclays: Effect on polyethylene terephthalate nanocomposites’ properties. Polym. Degrad. Stab. 2008, 93, 1332–1350. [Google Scholar] [CrossRef]
- Yuan, X.; Li, C.; Guan, G.; Xiao, Y.; Zhang, D. Thermal degradation investigation of poly(ethylene terephthalate)/fibrous silicate nanocomposites. Polym. Degrad. Stab. 2008, 93, 466–475. [Google Scholar] [CrossRef]
- Martino, L.; Niknam, V.; Guigo, N.; van Berkel, J.G.; Sbirrazzuoli, N. Morphology and thermal properties of novel clay-based poly(ethylene 2,5-furandicarboxylate) (PEF) nanocomposites. Rsc Adv. 2016, 6, 59800–59807. [Google Scholar] [CrossRef]
- Papadopoulos, L.; Terzopoulou, Z.; Bikiaris, D.N.; Patsiaoura, D.; Chrissafis, K.; Papageorgiou, D.G.; Papageorgiou, G.Z. Synthesis and characterization of in-situ-prepared nanocomposites based on poly(propylene 2,5-furan dicarboxylate) and aluminosilicate clays. Polymers 2018, 10, 937. [Google Scholar] [CrossRef] [Green Version]
- Codou, A.; Guigo, N.; van Berkel, J.G.; de Jong, E.; Sbirrazzuoli, N. Preparation and characterization of poly(ethylene 2,5-furandicarboxylate/nanocrystalline cellulose composites via solvent casting. J. Polym. Eng. 2017, 37, 869–878. [Google Scholar] [CrossRef]
- Nair, S.S.; Zhu, J.Y.; Deng, Y.; Ragauskas, A.J. High performance green barriers based on nanocellulose. Sustain. Chem. Processes 2014, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Abdul Khalil, H.; Bhat, Y.; Ireana, A.F. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Heux, L.; Chauve, G.; Bonini, C. Nonflocculating and chiral-nematic self-ordering of cellulose microcrystals suspensions in nonpolar solvents. Langmuir 2000, 16, 8210–8212. [Google Scholar] [CrossRef]
- Codou, A.; Guigo, N.; van Berkel, J.G.; de Jong, E.; Sbirrazzuoli, N. Preparation and crystallization behavior of poly(ethylene 2,5-furandicarboxylate)/cellulose composites by twin screw extrusion. Carbohydr. Polym. 2017, 174, 1026–1033. [Google Scholar] [CrossRef]
- Matos, M.; F. Sousa, A.; H.C.S. Silva, N.; S.R. Freire, C.; Andrade, M.; Mendes, A.; J.D. Silvestre, A. Furanoate-based nanocomposites: A case study using poly(butylene 2,5-furanoate) and poly(butylene 2,5-furanoate)-co-(butylene diglycolate) and bacterial cellulose. Polymers 2018, 10, 810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martino, L.; Guigo, N.; Berkel, J.G.V.; Sbirrazzuoli, N. Influence of organically modified montmorillonite and sepiolite clayson the physical properties of bio-based poly(ethylene 2,5-furandicarboxylate). Compos. Part B-Eng. 2016, 110, 96–105. [Google Scholar] [CrossRef]
- Safapour, S.; Seyed-Esfahani, M.; Auriemma, F.; de Ballesteros, O.R.; Vollaro, P.; Di Girolamo, R.; De Rosa, C.; Khosroshahi, A. Reactive blending as a tool for obtaining poly(ethylene terephthalate)-based engineering materials with tailored properties. Polymer 2010, 51, 4340–4350. [Google Scholar] [CrossRef]
- Poulopoulou, N.; Kasmi, N.; Bikiaris, D.N.; Papageorgiou, D.G.; Floudas, G.; Papageorgiou, G.Z. Sustainable polymers from renewable resources: Polymer blends of furan-based polyesters. Macromol. Mater. Eng. 2018, 303, 1800153. [Google Scholar] [CrossRef]
- Poulopoulou, N.; Kantoutsis, G.; Bikiaris, D.N.; Achilias, D.S.; Kapnisti, M.; Papageorgiou, G.Z. Biobased engineering thermoplastics: Poly(butylene 2,5-furandicarboxylate) blends. Polymers 2019, 11, 937. [Google Scholar] [CrossRef] [Green Version]
- Poulopoulou, N.; Pipertzis, A.; Kasmi, N.; Bikiaris, D.N.; Papageorgiou, D.G.; Floudas, G.; Papageorgiou, G.Z. Green polymeric materials: On the dynamic homogeneity and miscibility of furan-based polyester blends. Polymer 2019, 174, 187–199. [Google Scholar] [CrossRef]
- Poulopoulou, N.; Smyrnioti, D.; Nikolaidis, G.N.; Tsitsimaka, I.; Christodoulou, E.; Bikiaris, D.N.; Charitopoulou, M.A.; Achilias, D.S.; Kapnisti, M.; Papageorgiou, G.Z. Sustainable plastics from biomass: Blends of polyesters based on 2,5-furandicarboxylic acid. Polymers 2020, 12, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulopoulou, N.; Kasmi, N.; Siampani, M.; Terzopoulou, Z.N.; Bikiaris, D.N.; Achilias, D.S.; Papageorgiou, D.G.; Papageorgiou, G.Z. Exploring next-generation engineering bioplastics: Poly(alkylene furanoate)/poly(alkylene terephthalate) (PAF/PAT) blends. Polymers 2019, 11, 556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, Y.; Zhang, R.; Huang, J.; Wang, J.; Jiang, Y.; Hu, G.H.; Yang, J.; Zhu, J. Tensile property balanced and gas barrier improved PLA by blending with bio-based poly(butylene 2,5-furan dicarboxylate). ACS Sustain. Chem. Eng. 2017, 5, 9244–9253. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, R.Y.; Huang, J.C.; Wang, J.G.; Zhang, J.W.; Rayand, N.; Hu, G.H.; Yang, J.; Zhu, J. Retroreflection in binary bio-based PLA/PBF blends. Polymer 2017, 125, 138–143. [Google Scholar] [CrossRef]
- Cai, Q.Q.; Bai, T.W.; Zhang, H.J.; Yao, X.X.; Ling, J.; Zhu, W.P. Catalyst-free synthesis of polyesters via conventional melt polycondensation. Mater. Today 2021, 51, 155–164. [Google Scholar] [CrossRef]
- Maaskant, E.; van Es, D.S. Unexpected susceptibility of poly(ethylene furanoate) to UV irradiation: A warning light for furandicarboxylic acid? ACS Macro Lett. 2021, 10, 1616–1621. [Google Scholar] [CrossRef]


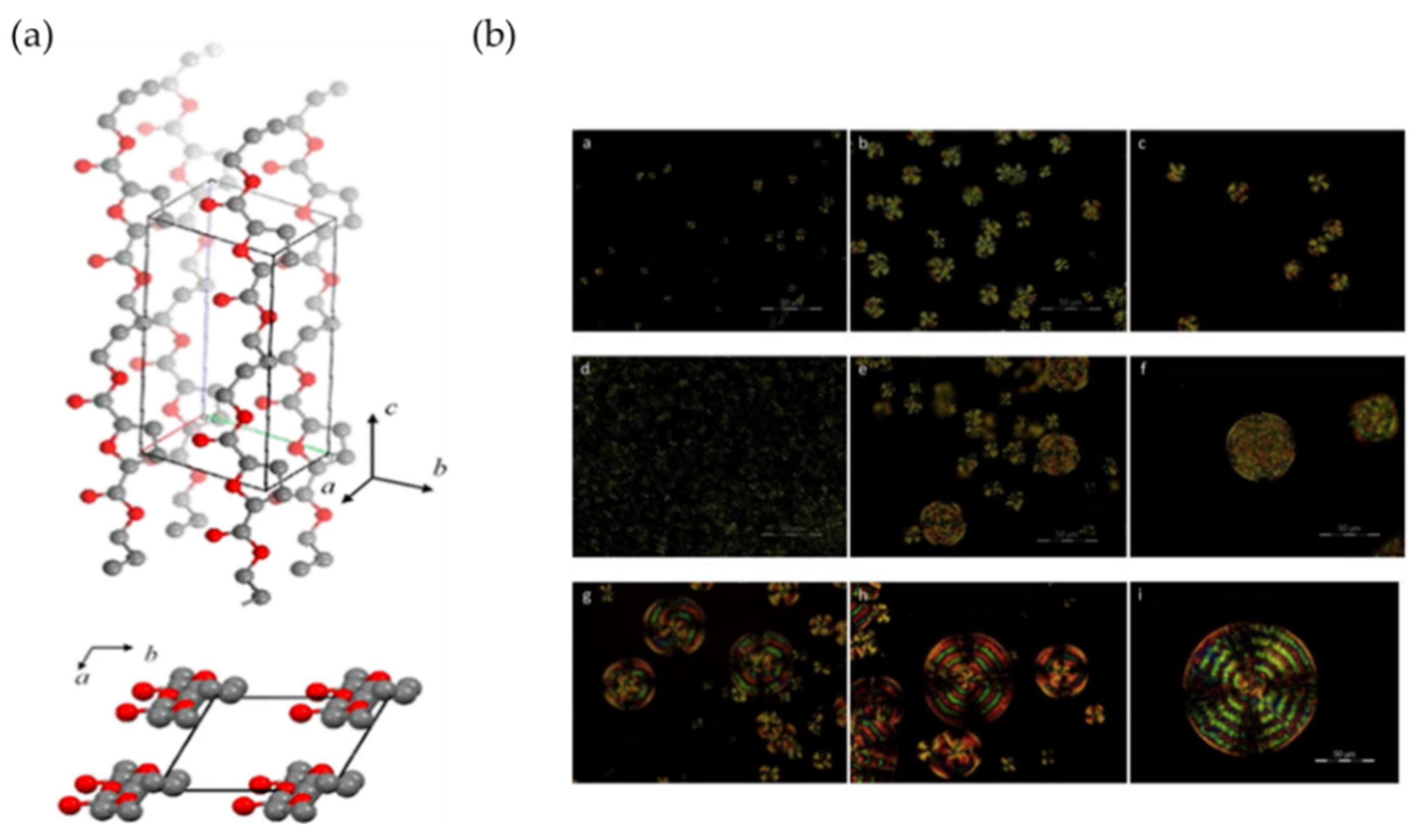
| Polyester | Synthetic Method | Tg /°C | Tm /°C | Tdmax /°C | Tensile Modulus (GPa) | Tensile Strength (MPa) | Mn (103 g/mol) | Ref. |
|---|---|---|---|---|---|---|---|---|
| PEF | Transesterification | 77–80 | 214 | 398 | 2.45 | 35 | 22.4–83 | [10,33] |
| PEF | Direct esterification | 80–89 | 210.4 | 407 | 2.1 | 66.7 | 105.3 | [11,35] |
| PEF | ROP | 80 | 215 | - | - | - | - | [32] |
| PPF | Direct esterification | 53–57.9 | 180 | 396 | 1.6 | 68.2 | 13.9–60.2 | [11,41] |
| PBF | Direct esterification | 30.5–40 | 171 | 392–428 | 0.7–1.1 | 5.5–32.9 | 8.0–23.2 | [11,21] |
| PHF | Direct esterification | 28.1 | 148.2 | 389 | 0.5 | 35.5 | 32.1 | [11] |
| POF | Direct esterification | 21.8 | 148.6 | 391 | 0.3 | 20.3 | 20.7 | [11] |
| POF | Transesterification | −5 | 140 | - | 0.3 | 26.5 | 34.6 | [42] |
| Poly(nonylene 2,5-furanoate) (PNF) | Transesterification | −30 | 69 | - | 0.2 | 21 | 40.0 | [42] |
| Poly(decylene 2,5-furanoate) (PDeF) | Transesterification | −8 | 116 | - | 0.2 | 11 | 36.7 | [42] |
| Poly(dodecylene 2,5-furanoate) (PDoF) | Transesterification | −22 | 111 | - | 0.2 | 11 | 39.4 | [42] |
| Polyester | Synthetic Method | Tg /°C | Tm /°C | Td5% /°C | Tdmax /°C | Tensile Modulus (MPa) | Tensile Strength (MPa) | Mn (103 g/mol) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PDASF | Solution polycondensation | 180 | - | - | 450 | - | - | 13.8 | [10] |
| PDAIF | Solution polycondensation | 140 | - | - | 396 | - | - | 5.7 | [10] |
| PIsF | Transesterification | 157 | - | - | 421 | - | - | - | [58] |
| PDIsFs | Transesterification | −1–21 | 64–111 | 405–413 | 439–444 | 14–559 | 0.7–20 | 11.5–25.4 | [59] |
| PBIF | Transesterification | 45–105 | 160 | 339–357 | 365–384 | - | - | 24.4–31.0 | [60] |
| PISBF | Transesterification | 55–151 | - | 370–376 | 405–417 | 1470 | 63 | 9.3–19.1 | [61] |
| PCIsFs | Transesterification | 75–103 | 220–257 | 363–375 | 402–409 | - | - | - | [62] |
| PIsI | Transesterification | 73 | - | 274 | 310/382 | - | - | 2.6 | [63] |
| PBIS | Direct esterification | −28–−11 | 89–109 | 64–87 | 17–23 | 45.7–53.5 | [64] | ||
| PXIIF | Transesterification/SSPC | 94 | 250 | 411 | - | - | 30.3 | [65] | |
| PCF | Transesterification | 71–87 | 219–291 | 377–403 | 1.69–1.82 | 44–52 | 8.3–26.2 | [23,66] | |
| PCdF | Transesterification | 175 | - | 380 | - | - | 6.5 | [66] |
| Polyester | Synthetic Method | Tg /°C | Tm /°C | Td5% /°C | Tdmax /°C | Tensile Modulus (GPa) | Tensile Strength (MPa) | Mn (103 g/mol) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PECF | Transesterification | 80–85 | 206–225 | 365–404 | 393–417 | 2.3–3.2 | 60–72 | 27.6–32.0 | [76,77] |
| PPCF | Transesterification | 59–73 | 193–232 | 378–382 | 412–421 | 1.9–2.1 | 79–88 | 35–41 | [80] |
| PETF | Transesterification | 90.9–91.1 | - | 368–369 | 400–403 | 3.1–3.3 | 97–98 | (Mv = 51–56) | [82] |
| PEFC | Direct esterification | 32.3–76.1 | - | 365–390 | 394–421 | 0.8–1.8 | 18–49 | 18.8–22.7 | [78] |
| PEFEG | Direct esterification | 78.5–84.9 | 177–209 | 345–360 | - | - | 11–27 | 34.0–46.8 | [83] |
| PEICF | Direct esterification | 90–119 | - | - | - | - | - | 16.4–19.6 | [81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fei, X.; Wang, J.; Zhang, X.; Jia, Z.; Jiang, Y.; Liu, X. Recent Progress on Bio-Based Polyesters Derived from 2,5-Furandicarbonxylic Acid (FDCA). Polymers 2022, 14, 625. https://doi.org/10.3390/polym14030625
Fei X, Wang J, Zhang X, Jia Z, Jiang Y, Liu X. Recent Progress on Bio-Based Polyesters Derived from 2,5-Furandicarbonxylic Acid (FDCA). Polymers. 2022; 14(3):625. https://doi.org/10.3390/polym14030625
Chicago/Turabian StyleFei, Xuan, Jinggang Wang, Xiaoqin Zhang, Zhen Jia, Yanhua Jiang, and Xiaoqing Liu. 2022. "Recent Progress on Bio-Based Polyesters Derived from 2,5-Furandicarbonxylic Acid (FDCA)" Polymers 14, no. 3: 625. https://doi.org/10.3390/polym14030625
APA StyleFei, X., Wang, J., Zhang, X., Jia, Z., Jiang, Y., & Liu, X. (2022). Recent Progress on Bio-Based Polyesters Derived from 2,5-Furandicarbonxylic Acid (FDCA). Polymers, 14(3), 625. https://doi.org/10.3390/polym14030625