Phase Change Material Evolution in Thermal Energy Storage Systems for the Building Sector, with a Focus on Ground-Coupled Heat Pumps
Abstract
:1. Introduction
2. PCMs’ Evolution in the Building Sector
2.1. The Early Stages: 2000–2010
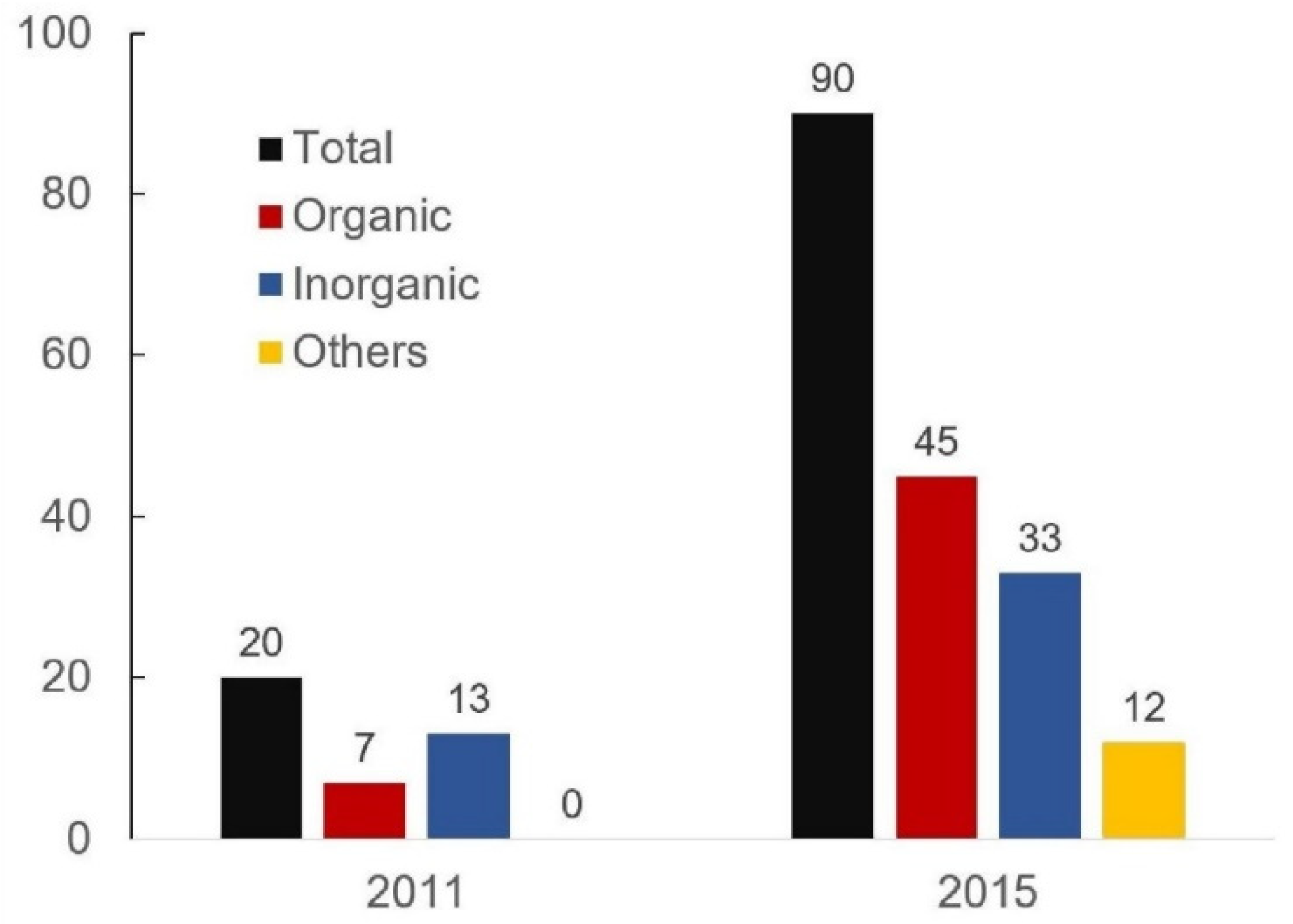
2.2. Further Material Evolution from 2011 to 2015
- -
- Direct incorporation: the PCM and the building material are physically mixed at the solid state.
- -
- Immersion: a building element, e.g., bricks or concrete, is dipped into a liquid PCM, which enters into the open pores thanks to capillary forces.
- -
- Macro-encapsulation: the PCM is encapsulated inside a container with a variable shape.
- -
- Micro-encapsulation: the PCM is encapsulated through a thin film of a polymeric material to form particles with low dimensions, with a diameter lower than 5 µm.
- -
- Shape stabilization: the formation of a composite material (SSPCM, shape-stabilized phase change material), where the PCM is the matrix, while another material acts as the reinforcement (e.g., HDPE).
2.3. The More Recent Evolution from 2016 to 2021
3. Future Challenges: PCM–GHE Coupling
| Reference | Study | Type | GHE | PCM | Melting Point (°C) | Latent Heat (kJ/kg) | PCM Employment |
|---|---|---|---|---|---|---|---|
| [124] | num | one-dimensional finite difference transient heat transport model | H | micro-encapsulated paraffin | 26 | 160 | backfilling soil |
| [126] | num | three-dimensional | V | decanoic acid and lauric acid mixture. | 25 | / | GHE’s borehole as grout. |
| [125] | num | two-dimensional | H | water, micro-encapsulated paraffin | 0, 26 | / | backfilling soil |
| [134] | num | modified composite model | V | hydrate sodium sulfate (type 47) | 8.3 | 95.4 | GCHP |
| [137] | num | computational fluid dynamics simulations | V | RT6 RT27 | 8.0–8.5 25.0–25.5 | 140 146 | GCHP |
| [131] | num | three-dimensional unsteady model | V | decanoic acid and lauric acid mixture | 20.15 | 128 | backfill material in a GCHP |
| [128] | num | three-dimensional finite element model | V | paraffin RT27. decanoic acid and lauric acid mixture (66:34) | 28–30 20.4 | 179 138.8 | GHE’s borehole grout. |
| [136] | exp | / | V | micro-encapsulated methyl stearate | 39.5 | 9.0–20.9 | HTF in a GCHP |
| [138] | num | finite element model | V | paraffin | / | 190 | GCHP borehole |
| [139] | num | three-dimensional unsteady model | V | micro-encapsulated paraffin. Shape-stabilized decanoic and lauric acid mixture | 23–27 19.9 | 150 109.2 | grout with a GCHP |
| [132] | exp & num | three-dimensional computational fluid dynamics | H | decyl acid and lauric acid (66:34) oleic acid | 20.55 8.11 | 133.65 94.51 | backfilling material of a GHE |
| [129] | num | three-dimensional computational fluid dynamics | H | salt hydrate (Infinite R, Insolcorp) | 23 | 200 | UTB with GCHP |
| [135] | num | Eulerian–Eulerian approach | H | micro-encapsulated n-octadecane | 28–30 | 167 | HTF in a GCHP |
| [130] | exp & num | computational fluid dynamics | V | RT6 RT27 | 8.0–8.5 25.0–25.5 | 140 146 | integrated with a GCHP |
| [133] | exp | / | H | n-octadecane paraffin A28 | 28 28 | 241 265 | backfilling material of a GCHP |
4. Conclusions
- -
- among different classes of PCM, paraffins are generally preferred for integration in building materials, due to their high performance/cost ratio, but more recent studies (2019–2021) also consider other organic PCMs, namely alcohols and fatty acids, as more specific applications need tailored materials.
- -
- microencapsulation and shape stabilization represent the main technologies actually used to incorporate PCMs into building materials, namely wallboards, mortars, and concrete, as they ensure better thermal properties, while macro-encapsulation is preferred for glazing elements, due to their higher chemical stability.
- -
- Field- and laboratory-scale tests designed to evaluate the influence of PCMs on indoor thermal conditions are mainly focused on passive conditions and on avoiding active HVAC plants. Nevertheless, the generally inconstant performance of PCMs due to climate or seasonal conditions was determined. The use of active heating/cooling systems, based on PCM employment, is highly recommended, ensuring a constant performance of building elements during the same season or the whole year.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations Environment Programme (UNEP); International Energy Agency (IEA). Towards a Zero-Emission, Efficient, and Resilient Buildings and Construction Sector; International Energy Agency: Paris, France, 2017. [Google Scholar]
- International Energy Agency (IEA). Tracking Buildings 2020; International Energy Agency: Paris, France, 2020; pp. 1–11. [Google Scholar]
- European Parliament. Directive (EU) 2018/844 of the European Parliament and of the Council of 30 May 2018 amending. Off. J. Eur. Union 2018, 156, 75–91. [Google Scholar]
- European Parliament. Directive 2010/31/EU of the European Parliament and of the Council of 19 May 2010 on the Energy Performance of Buildings. Off. J. Eur. Union 2010, 153, 23. [Google Scholar]
- Stritih, U.; Tyagi, V.V.; Stropnik, R.; Paksoy, H.; Haghighat, F.; Joybari, M.M. Integration of passive PCM technologies for net-zero energy buildings. Sustain. Cities Soc. 2018, 41, 286–295. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications, Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Jemmal, Y.; Zari, N.; Maaroufi, M. Thermophysical and chemical analysis of gneiss rock as low cost candidate material for thermal energy storage in concentrated solar power plants. Sol. Energy Mater. Sol. Cells 2016, 157, 377–382. [Google Scholar] [CrossRef]
- Rathore, P.K.S.; Shukla, S.K. Potential of macroencapsulated PCM for thermal energy storage in buildings: A comprehensive review. Constr. Build. Mater. 2019, 225, 723–744. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Phase change materials for building applications: A state-of-the-art review. Energy Build. 2010, 42, 1361–1368. [Google Scholar] [CrossRef] [Green Version]
- Osterman, E.; Tyagi, V.V.; Butala, V.; Rahim, N.A.; Stritih, U. Review of PCM based cooling technologies for buildings. Energy Build. 2012, 49, 37–49. [Google Scholar] [CrossRef]
- Kenisarin, M.; Mahkamov, K. Salt hydrates as latent heat storage materials: Thermophysical properties and costs. Sol. Energy Mater. Sol. Cells 2015, 145, 1–32. [Google Scholar] [CrossRef]
- Wong-Pinto, L.-S.; Milian, Y.; Ushak, S. Progress on use of nanoparticles in salt hydrates as phase change materials. Renew. Sustain. Energy Rev. 2020, 122, 109727. [Google Scholar] [CrossRef]
- Lin, Y.; Alva, G.; Fang, G. Review on thermal performances and applications of thermal energy storage systems with inorganic phase change materials. Energy 2018, 165, 685–708. [Google Scholar] [CrossRef]
- Farid, M.M.; Khudhair, A.M.; Razack, S.A.K.; Al-Hallaj, S. A review on phase change energy storage: Materials and applications. Energy Convers. Manag. 2004, 45, 1597–1615. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Sulaiman, F.A.; Ibrahim, N.I.; Zahir, H.M.; Al-Ahmed, A.; Saidur, R.; Yılbaş, B.S.; Sahin, A.Z. A review on current status and challenges of inorganic phase change materials for thermal energy storage systems. Renew. Sustain. Energy Rev. 2017, 70, 1072–1089. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Bolivar Osorio, F.J.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Inamuddin; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Fallahi, A.; Guldentops, G.; Tao, M.; Granados-Focil, S.; Van Dessel, S. Review on solid-solid phase change materials for thermal energy storage: Molecular structure and thermal properties. Appl. Therm. Eng. 2017, 127, 1427–1441. [Google Scholar] [CrossRef]
- de Gracia, A.; Cabeza, L.F. Phase change materials and thermal energy storage for buildings. Energy Build. 2015, 103, 414–419. [Google Scholar] [CrossRef] [Green Version]
- Akeiber, H.; Nejat, P.; Majid, M.Z.A.; Wahid, M.A.; Jomehzadeh, F.; Zeynali Famileh, I.; Calautit, J.K.; Hughes, B.R.; Zaki, S.A. A review on phase change material (PCM) for sustainable passive cooling in building envelopes. Renew. Sustain. Energy Rev. 2016, 60, 1470–1497. [Google Scholar] [CrossRef]
- Iten, M.; Liu, S.; Shukla, A. A review on the air-PCM-TES application for free cooling and heating in the buildings. Renew. Sustain. Energy Rev. 2016, 61, 175–186. [Google Scholar] [CrossRef]
- Aranda-Usón, A.; Ferreira, G.; López-Sabirón, A.M.; Mainar-Toledo, M.D.; Zabalza Bribián, I. Phase change material applications in buildings: An environmental assessment for some Spanish climate severities. Sci. Total Environ. 2013, 444, 16–25. [Google Scholar] [CrossRef]
- de Gracia, A.; Rincón, L.; Castell, A.; Jimenez, M.; Boer, D.; Medrano, M.; Cabeza, L.F. Life Cycle Assessment of the inclusion of phase change materials (PCM) in experimental buildings. Energy Build. 2010, 42, 1517–1523. [Google Scholar] [CrossRef]
- Baldassarri, C.; Sala, S.; Caverzan, A.; Lomperti Tornaghi, M. Environmental and spatial assessment for the ecodesign of a cladding system with embedded Phase Change Materials. Energy Build. 2017, 156, 374–389. [Google Scholar] [CrossRef]
- Nienborg, B.; Gschwander, S.; Munz, G.; Fröhlich, D.; Helling, T.; Horn, R.; Weinläder, H.; Klinker, F.; Schossig, P. Life Cycle Assessment of thermal energy storage materials and components. Energy Procedia 2018, 155, 111–120. [Google Scholar] [CrossRef]
- Sutterlin, W.R. Phase Change Materials: A Brief Comparison of Ice Packs, Salts, Paraffins, and Vegetable-Derived Phase Change Materials—Pharmaceutical Outsourcing. Available online: https://www.pharmoutsourcing.com/Featured-Articles/37854-Phase-Change-Materials-A-Brief-Comparison-of-Ice-Packs-Salts-Paraffins-and-Vegetable-derived-Phase-Change-Materials/ (accessed on 25 January 2022).
- Zalba, B.; Marín, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Verma, P.; Varun; Singal, S. Review of mathematical modeling on latent heat thermal energy storage systems using phase-change material. Renew. Sustain. Energy Rev. 2008, 12, 999–1031. [Google Scholar] [CrossRef]
- Regin, A.F.; Solanki, S.; Saini, J. Heat transfer characteristics of thermal energy storage system using PCM capsules: A review. Renew. Sustain. Energy Rev. 2008, 12, 2438–2458. [Google Scholar] [CrossRef]
- Jegadheeswaran, S.; Pohekar, S.D. Performance enhancement in latent heat thermal storage system: A review. Renew. Sustain. Energy Rev. 2009, 13, 2225–2244. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Buddhi, D. PCM thermal storage in buildings: A state of art. Renew. Sustain. Energy Rev. 2007, 11, 1146–1166. [Google Scholar] [CrossRef]
- Chekhovskoi, V.Y. Thermal expansion and density of 80.5% LiF-19.5% CaF2 eutectic. High Temp. 2000, 38, 197–202. [Google Scholar] [CrossRef]
- Agyenim, F.; Hewitt, N.; Eames, P.; Smyth, M. A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS). Renew. Sustain. Energy Rev. 2010, 14, 615–628. [Google Scholar] [CrossRef]
- Raj, V.A.A.; Velraj, R. Review on free cooling of buildings using phase change materials. Renew. Sustain. Energy Rev. 2010, 14, 2819–2829. [Google Scholar] [CrossRef]
- Zalba, B.; Marín, J.M.; Cabeza, L.F.; Mehling, H. Free-cooling of buildings with phase change materials. Int. J. Refrig. 2004, 27, 839–849. [Google Scholar] [CrossRef]
- Zalba, B.C.L.; Marin, J.M.; Sanchez-Valverde, B. Free cooling: An application of PCMS in TES. In Proceedings of the 3rd Workshop IEA ECES IA (Annex 17), Tokyo, Japan, 1–2 October 2002. [Google Scholar]
- Lazaro, A.; Dolado, P.; Marín, J.M.; Zalba, B. PCM–air heat exchangers for free-cooling applications in buildings: Experimental results of two real-scale prototypes. Energy Convers. Manag. 2009, 50, 439–443. [Google Scholar] [CrossRef]
- Agyenim, F.; Eames, P.; Smyth, M. Heat transfer enhancement in medium temperature thermal energy storage system using a multitube heat transfer array. Renew. Energy 2010, 35, 198–207. [Google Scholar] [CrossRef]
- Papanicolaou, E.; Belessiotis, V. Transient natural convection in a cylindrical enclosure at high Rayleigh numbers. Int. J. Heat Mass Transf. 2002, 45, 1425–1444. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, Z.; Wang, R. An overview of phase change material slurries: MPCS and CHS. Renew. Sustain. Energy Rev. 2010, 14, 598–614. [Google Scholar] [CrossRef]
- Lacroix, M. Study of the heat transfer behavior of a latent heat thermal energy storage unit with a finned tube. Int. J. Heat Mass Transf. 1993, 36, 2083–2092. [Google Scholar] [CrossRef]
- Jegadheeswaran, S.; Pohekar, S.; Kousksou, T. Exergy based performance evaluation of latent heat thermal storage system: A review. Renew. Sustain. Energy Rev. 2010, 14, 2580–2595. [Google Scholar] [CrossRef]
- Lock, G.S. Latent Heat Transfer; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Cabeza, L.F.; Castell, A.; Barreneche, C.; De Gracia, A.; Fernández, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, C.Y.; Tian, Y. Review on thermal energy storage with phase change materials (PCMs) in building applications. Appl. Energy 2012, 92, 593–605. [Google Scholar] [CrossRef] [Green Version]
- Kalnæs, S.E.; Jelle, B.P. Phase change materials and products for building applications: A state-of-the-art review and future research opportunities. Energy Build. 2015, 94, 150–176. [Google Scholar] [CrossRef] [Green Version]
- Rathod, M.K.; Banerjee, J. Thermal stability of phase change materials used in latent heat energy storage systems: A review. Renew. Sustain. Energy Rev. 2013, 18, 246–258. [Google Scholar] [CrossRef]
- Konuklu, Y.; Ostry, M.; Paksoy, H.O.; Charvat, P. Review on using microencapsulated phase change materials (PCM) in building applications. Energy Build. 2015, 106, 134–155. [Google Scholar] [CrossRef]
- Cao, L.; Su, D.; Tang, Y.; Fang, G.; Tang, F. Properties evaluation and applications of thermal energystorage materials in buildings. Renew. Sustain. Energy Rev. 2015, 48, 500–522. [Google Scholar] [CrossRef]
- Pomianowski, M.; Heiselberg, P.; Zhang, Y. Review of thermal energy storage technologies based on PCM application in buildings. Energy Build. 2013, 67, 56–69. [Google Scholar] [CrossRef]
- Soares, N.; Costa, J.J.; Gaspar, A.R.; Santos, P. Review of passive PCM latent heat thermal energy storage systems towards buildings’ energy efficiency. Energy Build. 2013, 59, 82–103. [Google Scholar] [CrossRef]
- Memon, S.A. Phase change materials integrated in building walls: A state of the art review. Renew. Sustain. Energy Rev. 2014, 31, 870–906. [Google Scholar] [CrossRef]
- Kuznik, F.; David, D.; Johannes, K.; Roux, J.-J. A review on phase change materials integrated in building walls. Renew. Sustain. Energy Rev. 2011, 15, 379–391. [Google Scholar] [CrossRef] [Green Version]
- Ling, T.C.; Poon, C.S. Use of phase change materials for thermal energy storage in concrete: An overview. Constr. Build. Mater. 2013, 46, 55–62. [Google Scholar] [CrossRef]
- Castell, A.; Menoufi, K.; de Gracia, A.; Rincón, L.; Boer, D.; Cabeza, L.F. Life Cycle Assessment of alveolar brick construction system incorporating phase change materials (PCMs). Appl. Energy 2013, 101, 600–608. [Google Scholar] [CrossRef]
- Menoufi, K.; Castell, A.; Farid, M.; Boer, D.; Cabeza, L.F. Life Cycle Assessment of experimental cubicles including PCM manufactured from natural resources (esters): A theoretical study. Renew. Energy 2013, 51, 398–403. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Yu, Z.; Yang, T.; Qin, D.; Li, S.; Zhang, G.; Haghighat, F.; Joybari, M.M. A review on macro-encapsulated phase change material for building envelope applications. Build. Environ. 2018, 144, 281–294. [Google Scholar] [CrossRef]
- Singh Rathore, P.K.; Shukla, S.K.; Gupta, N.K. Potential of microencapsulated PCM for energy savings in buildings: A critical review. Sustain. Cities Soc. 2020, 53, 101884. [Google Scholar] [CrossRef]
- da Cunha, S.R.L.; de Aguiar, J.L.B. Phase change materials and energy efficiency of buildings: A review of knowledge. J. Energy Storage 2020, 27, 101083. [Google Scholar] [CrossRef]
- Song, M.; Niu, F.; Mao, N.; Hu, Y.; Deng, S.S. Review on building energy performance improvement using phase change materials. Energy Build. 2018, 158, 776–793. [Google Scholar] [CrossRef]
- Zhu, N.; Li, S.; Hu, P.; Wei, S.; Deng, R.; Lei, F. A review on applications of shape-stabilized phase change materials embedded in building enclosure in recent ten years. Sustain. Cities Soc. 2018, 43, 251–264. [Google Scholar] [CrossRef]
- He, W.; Yu, C.; Yang, J.; Yu, B.; Hu, Z.; Shen, D.; Liu, X.; Qin, M.; Chen, H. Experimental study on the performance of a novel RC-PCM-wall. Energy Build. 2019, 199, 297–310. [Google Scholar] [CrossRef]
- Li, Y.; Darkwa, J.; Kokogiannakis, G.; Su, W. Phase change material blind system for double skin façade integration: System development and thermal performance evaluation. Appl. Energy 2019, 252, 113376. [Google Scholar] [CrossRef]
- Meng, E.; Wang, J.; Yu, H.; Cai, R.; Chen, Y.; Zhou, B. Experimental study of the thermal protection performance of the high reflectivity-phase change material (PCM) roof in summer. Build. Environ. 2019, 164, 106381. [Google Scholar] [CrossRef]
- Piselli, C.; Castaldo, V.L.; Pisello, A.L. How to enhance thermal energy storage effect of PCM in roofs with varying solar reflectance: Experimental and numerical assessment of a new roof system for passive cooling in different climate conditions. Sol. Energy 2019, 192, 106–119. [Google Scholar] [CrossRef]
- Qiao, Y.; Yang, L.; Bao, J.; Liu, Y.; Liu, J. Reduced-scale experiments on the thermal performance of phase change material wallboard in different climate conditions. Build. Environ. 2019, 160, 106191. [Google Scholar] [CrossRef]
- Valizadeh, S.; Ehsani, M.; Torabi Angji, M. Development and thermal performance of wood-HPDE- PCM nanocapsule floor for passive cooling in building. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 2114–2127. [Google Scholar] [CrossRef]
- Alqahtani, T.; Mellouli, S.; Bamasag, A.; Askri, F.; Phelan, P.E. Experimental and numerical assessment of using coconut oil as a phase-change material for unconditioned buildings. Int. J. Energy Res. 2020, 44, 5177–5196. [Google Scholar] [CrossRef]
- Maleki, B.; Khadang, A.; Maddah, H.; Alizadeh, M.; Kazemian, A.; Ali, H.M. Development and thermal performance of nanoencapsulated PCM/ plaster wallboard for thermal energy storage in buildings. J. Build. Eng. 2020, 32, 101727. [Google Scholar] [CrossRef]
- Musiał, M. Experimental and Numerical Analysis of the Energy Efficiency of Transparent Partitions with a Thermal Storage Unit. J. Ecol. Eng. 2020, 21, 201–211. [Google Scholar] [CrossRef]
- Sonnick, S.; Erlbeck, L.; Gaedtke, M.; Wunder, F.; Mayer, C.; Krause, M.J.; Nirschl, H.; Rädle, M. Passive room conditioning using phase change materials—Demonstration of a long-term real size experiment. Int. J. Energy Res. 2020, 44, 7047–7056. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Li, C.; Zhang, K.; Tang, Y.; Song, Y.; Wang, M. Preparation and thermophysical performance of diatomite-based composite PCM wallboard for thermal energy storage in buildings. J. Build. Eng. 2020, 32, 101753. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, X.; Medina, M.A. Experimental evaluation of structural insulated panels outfitted with phase change materials. Appl. Therm. Eng. 2020, 178, 115454. [Google Scholar] [CrossRef]
- Bolteya, A.M.; Elsayad, M.A.; Belal, A.M. Thermal efficiency of PCM filled double glazing units in Egypt. Ain Shams Eng. J. 2021, 12, 1523–1534. [Google Scholar] [CrossRef]
- Sinka, M.; Bajare, D.; Jakovics, A.; Ratnieks, J.; Gendelis, S.; Tihana, J. Experimental testing of phase change materials in a warm-summer humid continental climate. Energy Build. 2019, 195, 205–215. [Google Scholar] [CrossRef]
- Yun, B.Y.; Yang, S.; Cho, H.M.; Wi, S.; Kim, S. Thermal Storage Effect Analysis of Floor Heating Systems Using Latent Heat Storage Sheets. Int. J. Precis. Eng. Manuf. Green Technol. 2019, 6, 799–807. [Google Scholar] [CrossRef]
- Bogatu, D.I.; Kazanci, O.B.; Olesen, B.W. An experimental study of the active cooling performance of a novel radiant ceiling panel containing phase change material (PCM). Energy Build. 2021, 243, 110981. [Google Scholar] [CrossRef]
- Faraj, K.; Khaled, M.; Faraj, J.; Hachem, F.; Castelain, C. Experimental Study on the Use of Enhanced Coconut Oil and Paraffin Wax Phase Change Material in Active Heating Using Advanced Modular Prototype. J. Energy Storage 2021, 41, 102815. [Google Scholar] [CrossRef]
- Guo, J.; Dong, J.; Wang, H.; Jiang, Y.; Tao, J. On-site measurement of the thermal performance of a novel ventilated thermal storage heating floor in a nearly zero energy building. Build. Environ. 2021, 201, 107993. [Google Scholar] [CrossRef]
- Rao, V.V.; Parameshwaran, R.; Ram, V.V. PCM-mortar based construction materials for energy efficient buildings: A review on research trends. Energy Build. 2018, 158, 95–122. [Google Scholar] [CrossRef]
- Berardi, U.; Gallardo, A. Properties of concretes enhanced with phase change materials for building applications. Energy Build. 2019, 199, 402–414. [Google Scholar] [CrossRef]
- Cellat, K.; Tezcan, F.; Kardaş, G.; Paksoy, H. Comprehensive investigation of butyl stearate as a multifunctional smart concrete additive for energy-efficient buildings. Int. J. Energy Res. 2019, 43, 7146–7158. [Google Scholar] [CrossRef]
- Cunha, S.; Leite, P.; Aguiar, J. Characterization of innovative mortars with direct incorporation of phase change materials. J. Energy Storage 2020, 30, 101439. [Google Scholar] [CrossRef]
- Parameshwaran, R.; Kumar, G.N.; Ram, V.V. Experimental analysis of hybrid nanocomposite-phase change material embedded cement mortar for thermal energy storage. J. Build. Eng. 2020, 30, 101297. [Google Scholar] [CrossRef]
- Frigione, M.; Lettieri, M.; Sarcinella, A.; De Aguiar, J.L.B. Applications of Sustainable Polymer-Based Phase Change Materials in Mortars Composed by Different Binders. Materials 2019, 12, 3502. [Google Scholar] [CrossRef] [Green Version]
- Giro-Paloma, J.; Barreneche, C.; Maldonado-Alameda, A.; Royo, M.; Formosa, J.; Fernandez, A.I.; Chimenos, J.M. Alkali-Activated Cements for TES Materials in Buildings’ Envelops Formulated With Glass Cullet Recycling Waste and Microencapsulated Phase Change Materials. Materials 2019, 12, 2144. [Google Scholar] [CrossRef] [Green Version]
- Hekimoğlu, G.; Nas, M.; Ouikhalfan, M.; Sarı, A.; Kurbetci, Ş.; Tyagi, V.V.; Sharma, R.K.; Saleh, T.A. Thermal management performance and mechanical properties of a novel cementitious composite containing fly ash/lauric acid-myristic acid as form-stable phase change material. Constr. Build. Mater. 2020, 274, 122105. [Google Scholar] [CrossRef]
- Hekimoğlu, G.; Nas, M.; Ouikhalfan, M.; Sarı, A.; Tyagi, V.V.; Sharma, R.K.; Kurbetci, Ş.; Saleh, T.A. Silica fume/capric acid-stearic acid PCM included-cementitious composite for thermal controlling of buildings: Thermal energy storage and mechanical properties. Energy 2020, 219, 119588. [Google Scholar] [CrossRef]
- Chang, H.; Jin, L. Preparation and Heat Transfer Performance of Steel Ball Phase Change Concrete. J. New Mater. Electrochem. Syst. 2020, 23, 204–212. [Google Scholar] [CrossRef]
- Shi, J.; Li, M. Lightweight mortar with paraffin/expanded vermiculite-diatomite composite phase change materials: Development, characterization and year-round thermoregulation performance. Sol. Energy 2021, 220, 331–342. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, M.; Liu, L.; Huan, C.; Zhao, Y.; Qi, C.; Song, K.-I. Experimental study on thermal and mechanical properties of cemented paste backfill with phase change material. J. Mater. Res. Technol. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Kheradmand, M.; Abdollahnejad, Z.; Pacheco-Torgal, F. Alkali-activated cement-based binder mortars containing phase change materials (PCMs): Mechanical properties and cost analysis. Eur. J. Environ. Civ. Eng. 2020, 24, 1068–1090. [Google Scholar] [CrossRef]
- Sarı, A.; Tyagi, V.V. Thermal energy storage properties and lab-scale thermal performance in cementitious plaster of composite phase change material for energy efficiency of buildings. Environ. Prog. Sustain. Energy 2020, 39, e13455. [Google Scholar] [CrossRef]
- Sarı, A.; Hekimoğlu, G.; Tyagi, V.; Sharma, R. Evaluation of pumice for development of low-cost and energy-efficient composite phase change materials and lab-scale thermoregulation performances of its cementitious plasters. Energy 2020, 207, 118242. [Google Scholar] [CrossRef]
- Valentini, F.; Morandini, F.; Bergamo, M.; Dorigato, A. Development of eco-sustainable plasters with thermal energy storage capability. J. Appl. Phys. 2020, 128, 1–12. [Google Scholar] [CrossRef]
- Hasanabadi, S.; Sadrameli, S.M.; Sami, S. Preparation, characterization and thermal properties of surface-modified expanded perlite/paraffin as a form-stable phase change composite in concrete. J. Therm. Anal. Calorim. 2021, 144, 61–69. [Google Scholar] [CrossRef]
- Illampas, R.; Rigopoulos, I.; Ioannou, I. Influence of microencapsulated Phase Change Materials (PCMs) on the properties of polymer modified cementitious repair mortar. J. Build. Eng. 2021, 40, 102328. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Pasupathy, K.; Sanjayan, J. Synthesis and properties of thermally enhanced aerated geopolymer concrete using form-stable phase change composite. J. Build. Eng. 2021, 40, 102756. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, S.; Zeng, C.; Zhang, Y.; Li, Y.; Han, X.; Yang, L.; Yang, X. Experimental thermal study of a new PCM-concrete thermal storage block (PCM-CTSB). Constr. Build. Mater. 2021, 293, 123540. [Google Scholar] [CrossRef]
- Wang, M.; Liu, L.; Zhang, X.-Y.; Chen, L.; Wang, S.-Q.; Jia, Y.-H. Experimental and numerical investigations of heat transfer and phase change characteristics of cemented paste backfill with PCM. Appl. Therm. Eng. 2019, 150, 121–131. [Google Scholar] [CrossRef]
- Dardir, M.; Panchabikesan, K.; Haghighat, F.; El Mankibi, M.; Yuan, Y. Opportunities and challenges of PCM-to-air heat exchangers (PAHXs) for building free cooling applications—A comprehensive review. J. Energy Storage 2019, 22, 157–175. [Google Scholar] [CrossRef]
- De Falco, M.; Capocelli, M.; Losito, G.; Piemonte, V. LCA perspective to assess the environmental impact of a novel PCM-based cold storage unit for the civil air conditioning. J. Clean. Prod. 2017, 165, 697–704. [Google Scholar] [CrossRef]
- Kylili, A.; Fokaides, P.A. Life Cycle Assessment (LCA) of Phase Change Materials (PCMs) for building applications: A review. J. Build. Eng. 2016, 6, 133–143. [Google Scholar] [CrossRef]
- Thaib, R.; Hamdani, H.; Amin, M. Utilization of Beeswax/Bentonite as energy storage material on building wall composite. J. Phys. Conf. Ser. 2019, 1402, 044038. [Google Scholar] [CrossRef]
- Hakim, I.I.; Putra, N.; Agustin, P.D. Measurement of PCM-concrete composites thermal properties for energy conservation in building material. AIP Conf. Proc. 2020, 2255, 030066. [Google Scholar] [CrossRef]
- Ji, R.; Zou, Z.; Liu, L.; Wei, S.; Qu, S. Development and energy evaluation of phase change material composite for building energy-saving. Int. J. Energy Res. 2019, 43, 8674–8683. [Google Scholar] [CrossRef]
- Montanari, C.; Li, Y.; Chen, H.; Yan, M.; Berglund, L.A. Transparent Wood for Thermal Energy Storage and Reversible Optical Transmittance. ACS Appl. Mater. Interfaces 2019, 11, 20465–20472. [Google Scholar] [CrossRef] [PubMed]
- Sarabandi, D.; Roudini, G.; Barahuie, F. Activated carbon derived from pine cone as a framework for the preparation of n-heptadecane nanocomposite for thermal energy storage. J. Energy Storage 2019, 24, 100795. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, K.; Lu, S.; Wang, C.; Li, X.; Yang, Y. Experimental research on an environment-friendly form-stable phase change material incorporating modified rice husk ash for thermal energy storage. J. Energy Storage 2020, 31, 101599. [Google Scholar] [CrossRef]
- Temiz, A.; Hekimoğlu, G.; Köse Demirel, G.; Sarı, A.; Mohamad Amini, M.H. Phase change material impregnated wood for passive thermal management of timber buildings. Int. J. Energy Res. 2020, 44, 10495–10505. [Google Scholar] [CrossRef]
- Xie, N.; Niu, J.; Zhong, Y.; Gao, X.; Zhang, Z.; Fang, Y. Development of polyurethane acrylate coated salt hydrate/diatomite form-stable phase change material with enhanced thermal stability for building energy storage. Constr. Build. Mater. 2020, 259, 119714. [Google Scholar] [CrossRef]
- Alassaad, F.; Touati, K.; Levacher, D.; Sebaibi, N. Impact of phase change materials on lightened earth hygroscopic, thermal and mechanical properties. J. Build. Eng. 2021, 41, 102417. [Google Scholar] [CrossRef]
- Biesuz, M.; Valentini, F.; Bortolotti, M.; Zambotti, A.; Cestari, F.; Bruni, A.; Sglavo, V.M.; Sorarù, G.D.; Dorigato, A.; Pegoretti, A. Biogenic architectures for green, cheap, and efficient thermal energy storage and management. Renew. Energy 2021, 178, 96–107. [Google Scholar] [CrossRef]
- Molinari, C.; Zanelli, C.; Laghi, L.; De Aloysio, G.; Santandrea, M.; Guarini, G.; Conte, S.; Dondi, M. Effect of scale-up on the properties of PCM-impregnated tiles containing glass scraps. Case Stud. Constr. Mater. 2021, 14, e00526. [Google Scholar] [CrossRef]
- Rathore, P.K.S.; Kumar Shukla, S. Improvement in thermal properties of PCM/Expanded vermiculite/expanded graphite shape stabilized composite PCM for building energy applications. Renew. Energy 2021, 176, 295–304. [Google Scholar] [CrossRef]
- Yousefi, A.; Tang, W.; Khavarian, M.; Fang, C. Development of novel form-stable phase change material (PCM) composite using recycled expanded glass for thermal energy storage in cementitious composite. Renew. Energy 2021, 175, 14–28. [Google Scholar] [CrossRef]
- RAL Gütesicherung. Phase Change Materials RAL-GZ 896; RAL German Institute for Quality Assurance and Certification: Sankt Augustin, Germany, 2018; p. 47. [Google Scholar]
- ASTM C1784-20; Standard Test Method for Using a Heat Flow Meter Apparatus for Measuring Thermal Storage Properties of Phase Change Materials and Products. ASTM International: West Conshohocken, PA, USA, 2020. Available online: https://www.astm.org/Standards/C1784.htm (accessed on 25 September 2020).
- International Energy Agency (IEA). Heat Pumps—Analysis; Tracking Report; International Energy Agency (IEA): Paris, France, 2020. [Google Scholar]
- Vocale, P.; Morini, G.L.; Spiga, M. Influence of Outdoor Air Conditions on the Air Source Heat Pumps Performance. Energy Procedia 2014, 45, 653–662. [Google Scholar] [CrossRef] [Green Version]
- Javadi, H.; Mousavi Ajarostaghi, S.S.; Rosen, M.A.; Pourfallah, M. Performance of ground heat exchangers: A comprehensive review of recent advances. Energy 2019, 178, 207–233. [Google Scholar] [CrossRef]
- You, T.; Wu, W.; Shi, W.; Wang, B.; Li, X. An overview of the problems and solutions of soil thermal imbalance of ground-coupled heat pumps in cold regions. Appl. Energy 2016, 177, 515–536. [Google Scholar] [CrossRef]
- Wu, Y.; Gan, G.; Gonzalez, R.G.; Verhoef, A.; Vidale, P.L. Prediction of the thermal performance of horizontal-coupled ground-source heat exchangers. Int. J. Low-Carbon Technol. 2011, 6, 261–269. [Google Scholar] [CrossRef]
- Habibi, M.; Hakkaki-Fard, A. Evaluation and improvement of the thermal performance of different types of horizontal ground heat exchangers based on techno-economic analysis. Energy Convers. Manag. 2018, 171, 1177–1192. [Google Scholar] [CrossRef]
- Dehdezi, P.K.; Hall, M.R.; Dawson, A.R. Enhancement of Soil Thermo-Physical Properties Using Microencapsulated Phase Change Materials for Ground Source Heat Pump Applications. Appl. Mech. Mater. 2012, 110–116, 1191–1198. [Google Scholar] [CrossRef]
- Bottarelli, M.; Bortoloni, M.; Su, Y.; Yousif, C.; Aydın, A.A.; Georgiev, A. Numerical analysis of a novel ground heat exchanger coupled with phase change materials. Appl. Therm. Eng. 2015, 88, 369–375. [Google Scholar] [CrossRef]
- Wang, J.L.; De Zhao, J.; Liu, N. Numerical Simulation of Borehole Heat Transfer with Phase Change Material as Grout. Appl. Mech. Mater. 2014, 577, 44–47. [Google Scholar] [CrossRef]
- Chen, F.; Mao, J.; Chen, S.; Li, C.; Hou, P.; Liao, L. Efficiency analysis of utilizing phase change materials as grout for a vertical U-tube heat exchanger coupled ground source heat pump system. Appl. Therm. Eng. 2018, 130, 698–709. [Google Scholar] [CrossRef]
- Qi, D.; Pu, L.; Sun, F.; Li, Y. Numerical investigation on thermal performance of ground heat exchangers using phase change materials as grout for ground source heat pump system. Appl. Therm. Eng. 2016, 106, 1023–1032. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, X.; Biswas, K.; Warner, J. A three-dimensional numerical investigation of a novel shallow bore ground heat exchanger integrated with phase change material. Appl. Therm. Eng. 2019, 162, 114297. [Google Scholar] [CrossRef]
- Bonamente, E.; Aquino, A. Environmental Performance of Innovative Ground-Source Heat Pumps with PCM Energy Storage. Energies 2020, 13, 117. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Tong, C.; Duanmu, L.; Liu, L. Research on U-tube Heat Exchanger with Shape-stabilized Phase Change Backfill Material. Procedia Eng. 2016, 146, 640–647. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Xu, R.; Yang, B.; Yang, J. Experimental and numerical investigations on the thermal performance of a borehole ground heat exchanger with PCM backfill. Energy 2019, 174, 216–235. [Google Scholar] [CrossRef]
- Barbi, S.; Barbieri, F.; Marinelli, S.; Rimini, B.; Merchiori, S.; Larwa, B.; Bottarelli, M.; Montorsi, M. Phase change material-sand mixtures for distributed latent heat thermal energy storage: Interaction and performance analysis. Renew. Energy 2021, 169, 1066–1076. [Google Scholar] [CrossRef]
- Zhu, N.; Hu, P.; Lei, Y.; Jiang, Z.; Lei, F. Numerical study on ground source heat pump integrated with phase change material cooling storage system in office building. Appl. Therm. Eng. 2015, 87, 615–623. [Google Scholar] [CrossRef]
- Pu, L.; Xu, L.; Zhang, S.; Li, Y. Optimization of ground heat exchanger using microencapsulated phase change material slurry based on tree-shaped structure. Appl. Energy 2019, 240, 860–869. [Google Scholar] [CrossRef]
- Kong, M.; Alvarado, J.L.; Thies, C.; Morefield, S.; Marsh, C.P. Field evaluation of microencapsulated phase change material slurry in ground source heat pump systems. Energy 2017, 122, 691–700. [Google Scholar] [CrossRef] [Green Version]
- Bonamente, E.; Aquino, A.; Cotana, F. A PCM Thermal Storage for Ground-source Heat Pumps: Simulating the System Performance via CFD Approach. Energy Procedia 2016, 101, 1079–1086. [Google Scholar] [CrossRef]
- Bayomy, A.M.; Nguyen, H.V.; Wang, J.; Dworkin, S.B. Performance analysis of a single underground thermal storage borehole using phase change material. In Proceedings of the International Ground Source Heat Pump Association (IGSHPA) Research Track Conference, Stockholm, Sweden, 18–20 September 2018; pp. 1–11. [Google Scholar] [CrossRef]
- Chen, F.; Mao, J.; Li, C.; Hou, P.; Li, Y.; Xing, Z.; Chen, S. Restoration performance and operation characteristics of a vertical U-tube ground source heat pump system with phase change grouts under different running modes. Appl. Therm. Eng. 2018, 141, 467–482. [Google Scholar] [CrossRef]
- Faizal, M.; Bouazza, A.; Singh, R.M. Heat transfer enhancement of geothermal energy piles. Renew. Sustain. Energy Rev. 2016, 57, 16–33. [Google Scholar] [CrossRef] [Green Version]
- Mohamad, Z.; Fardoun, F.; Meftah, F. A review on energy piles design, evaluation, and optimization. J. Clean. Prod. 2021, 292, 125802. [Google Scholar] [CrossRef]
- Han, C.; Yu, X. An innovative energy pile technology to expand the viability of geothermal bridge deck snow melting for different United States regions: Computational assisted feasibility analyses. Renew. Energy 2018, 123, 417–427. [Google Scholar] [CrossRef]
- Mousa, M.M.; Bayomy, A.M.; Saghir, M.Z. Experimental and Numerical Study on Energy Piles with Phase Change Materials. Energies 2020, 13, 4699. [Google Scholar] [CrossRef]
- Sommerfeldt, N.; Madani, H. In-depth techno-economic analysis of PV/Thermal plus ground source heat pump systems for multi-family houses in a heating dominated climate. Sol. Energy 2019, 190, 44–62. [Google Scholar] [CrossRef]
- Emmi, G.; Zarrella, A.; De Carli, M. A heat pump coupled with photovoltaic thermal hybrid solar collectors: A case study of a multi-source energy system. Energy Convers. Manag. 2017, 151, 386–399. [Google Scholar] [CrossRef]
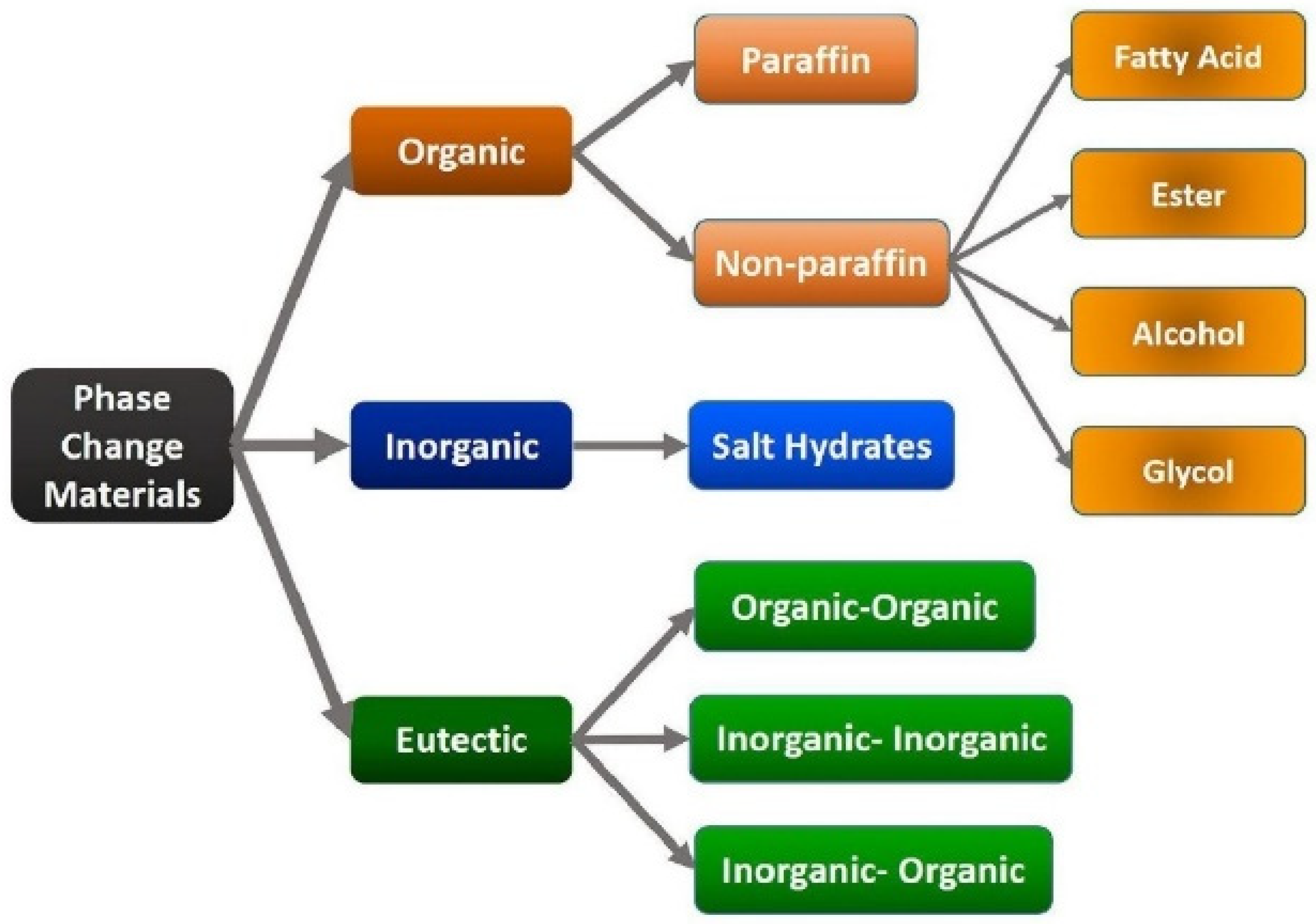

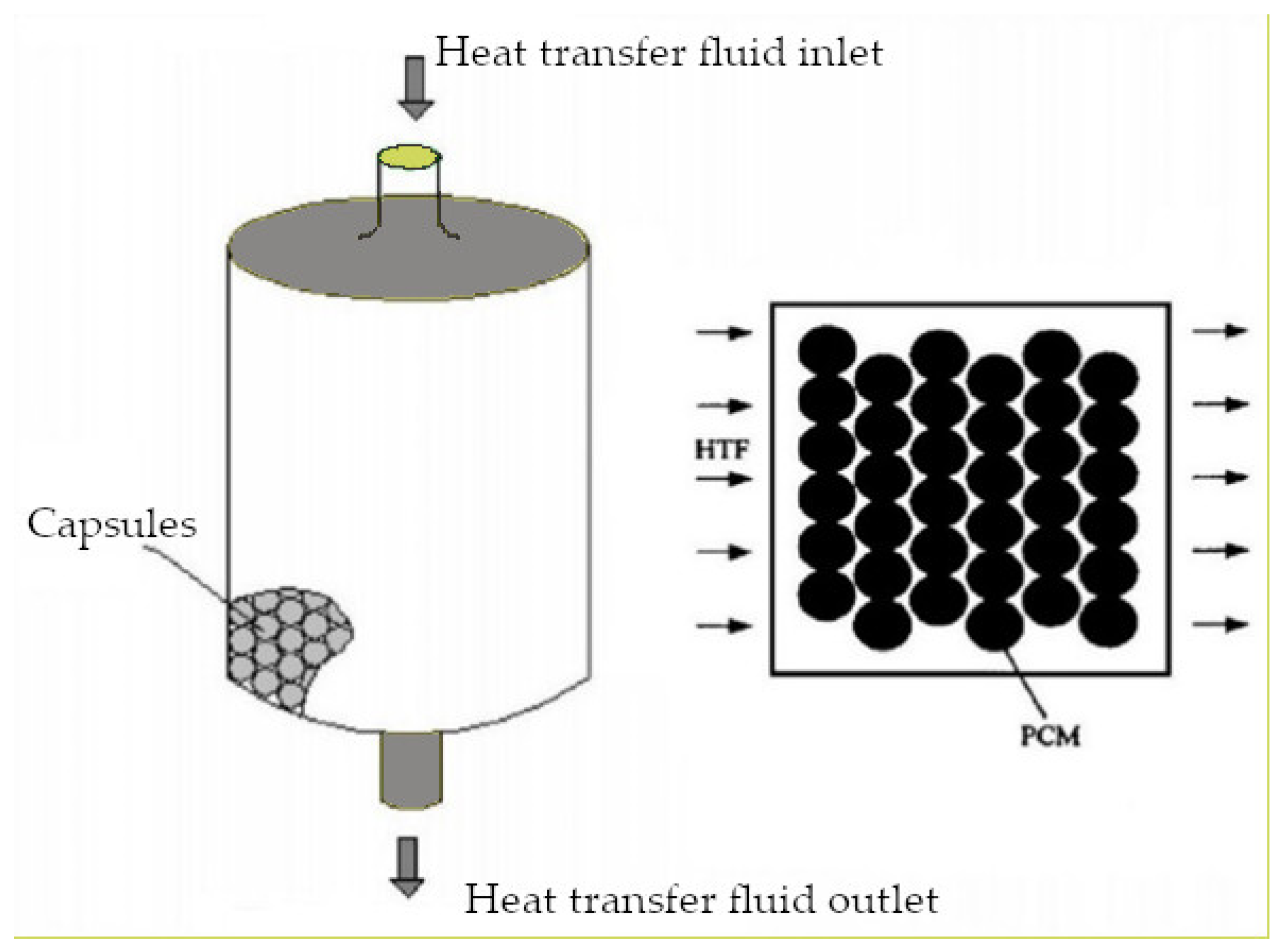
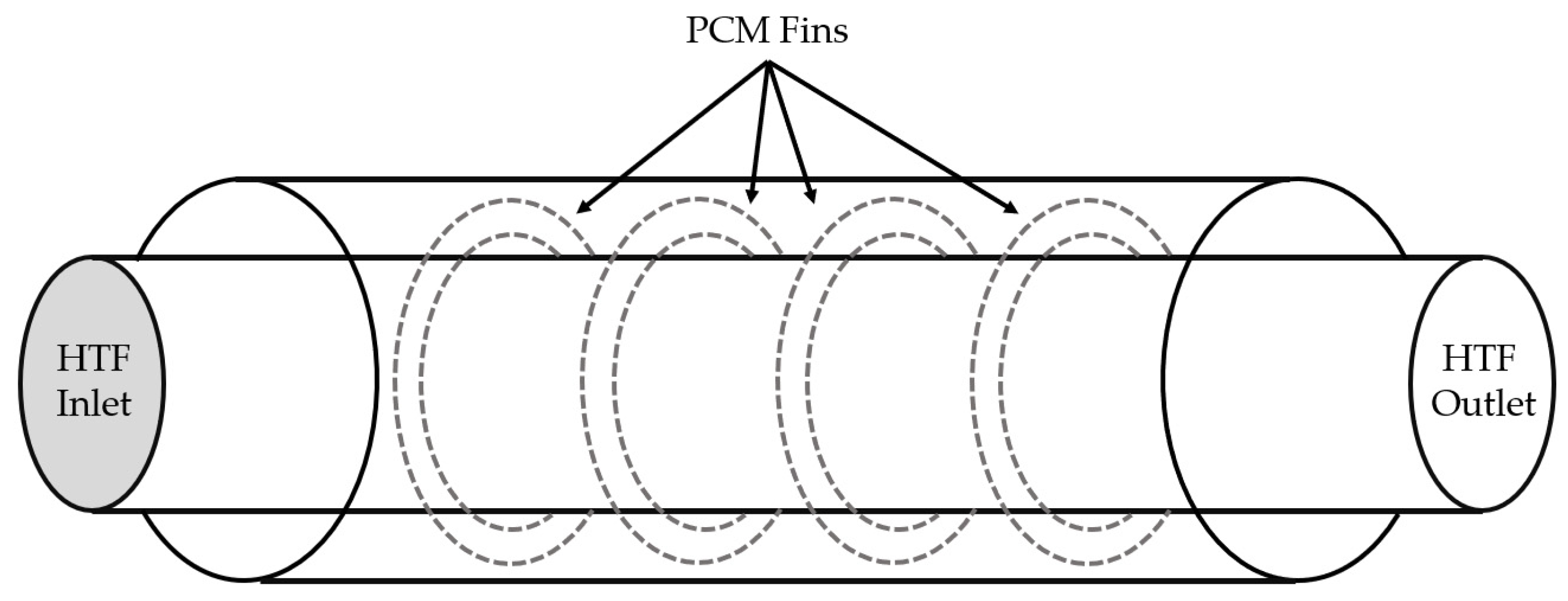

| Organics | Inorganics | Eutectics | |
|---|---|---|---|
| Advantages | Non-corrosive Low or no undercooling Chemical and thermal stability | Greater phase change enthalpy Greater density | Sharp melting point |
| Disadvantages | Lower phase change enthalpy Low thermal conductivity Flammability | Undercooling | Lack of data |
| Corrosion | |||
| Phase separation | |||
| Phase segregation, lack of thermal stability |
| Cooling | Comfort Applications | Hot-Water Applications | High-Temperature Applications | |
|---|---|---|---|---|
| Temperature range | −30/+21 °C | +22/+28 °C | +29/+60 °C | +61/+120 °C |
| Total PCM number | 45 | 34 | 103 | 62 |
| Organic (paraffins, fatty acids, organic mixtures) (%) | 23 (51.1%) | 22 (64.7%) | 50 (48.5%) | 28 (45.2%) |
| Inorganic (salt, salt hydrates, metals, inorganic mixtures) (%) | 6 (13.3%) | 7 (20.6%) | 45 (43.7%) | 19 (30.6%) |
| Eutectics (%) | 14 (31.1%) | 5 (14.7%) | 8 (7.8%) | 15 (24.2%) |
| Cooling | Comfort Applications | Higher-Temperature Applications | |
|---|---|---|---|
| Temperature range | −33/+21 °C | +22/+28 °C | ≥29 °C |
| Total PCM number | 24 | 13 | 51 |
| Organic (paraffins) (%) | 2 (8.3%) | 5 (38.5%) | 29 (56.9%) |
| Inorganic (salt solutions, salt hydrates) (%) | 22 (91.7%) | 8 (61.5%) | 20 (39.2%) |
| Unclassified (%) | 0 (0.0%) | 0 (0.0%) | 2 (3.9%) |
| Category | Melting Point (°C) | Encapsulation Type | Shell Material | Reference | |
|---|---|---|---|---|---|
| RT 18 | Organic | 18 | Macro | Steel | [56] |
| Capric acid and lauric acid | Fatty acids | 20 | Macro | Stainless steel | [56,58] |
| RT 21 | Paraffin | 21 | Macro | EPDM and furnace dust | [19] |
| SP 22 | Inorganic | 21 | Macro | / | [19] |
| Hexadecane | Paraffin | 22 | Macro | Copper | [56] |
| Micronal DS-5008X | Organic | 23 | Micro | Acrylate polymer | [19,57,58] |
| Inertek | Organic | 23+27 | Micro | Polymer | [58] |
| PEG 600 | Polymer | 21–25 | Macro | PVC | [56,57,58] |
| Micronal DS 5001 X | Organic | 23–26 | Micro | Acrylate polymer | [57,58] |
| Capric acid and 1-dodecanol | Fatty acid and fatty alcohol | 26 | Macro | Aluminum | [19,56] |
| Dodecanol | Fatty alcohol | 26 | Micro | / | [57] |
| Capric acid and myristic acid | Fatty acids | 26 | Micro | Polystyrene | [57] |
| Capric acid and palmitic acid | Fatty acids | 26 | Macro | Gypsum wallboard | [19,56] |
| SP 25 | Salt hydrate | 26 | Macro | Aluminum | [56,58] |
| Calcium chloride hexahydrate | Salt hydrate | 25–27 | Macro | PVC | [19,56] |
| RT 27 | Organic | 28 | Macro Micro | Aluminum / | [56,57,58] |
| RT 28 | Organic | 28 | Micro | / | [57] |
| RT 28HC | Organic | 27–29 | Macro | Aluminum | [19,56,58] |
| MG29 | Paraffin | 27–29 | Macro | Glass | [19] |
| Octadecane | Paraffin | 28–29 | Micro Micro | CaCl2 PMMA/TiO2 | [57] |
| Eicosane | Paraffin | 30 | Micro Macro | Brookite (TiO2) Galvanized steel | [57,58] |
| Capric Acid | Fatty acid | 30 | Macro | Aluminum | [19,56] |
| Salt hydrate | Salt hydrate | 31 | Macro | Polymer | [56] |
| RT 35 | Organic | 28–35 | Macro | Aluminum | [19,56] |
| Tetradecanol and myristic acid | Fatty alcohol and fatty acid | 29–32 | Macro | PE-RT | [56] |
| MPCM37-D | Paraffin | 37 | Micro | Polymer | [57] |
| RT 42 | Organic | 38–43 | Macro | Stainless steel | [56] |
| Reference | Test Scale | PCM | PCM Form | Tmelt (°C) | Building Material | Position in Building | Remarks and Results |
|---|---|---|---|---|---|---|---|
| [61] | field test | RT28HC | macro | 28 | aluminum panel | wall | The PCM decreased the cooling load, peak, and average temperature in the room by 0.8 °C when coupled with the radiative panel. |
| [62] | field test | PX 35 | micro | 35 | aluminum panel | blind in a double-skin façade | The PCM blind was used to reduce the overheating problem typical of double skin facades in summer, stabilizing the internal air temperature between the two glass layers. |
| [63] | field test | paraffin | SSPCM (paraffin + graphite) | 25.5 | concrete panel | roof | The PCM roof allowed a reduction of indoor air temperature fluctuations from 7% to 15%. Combined with a high-reflectivity roof, it reduced the indoor air temperature fluctuation from 8.5% to 17.0%, while the inner surface temperature of the roof was reduced by 2.2 °C. |
| [64] | lab test | paraffins | SSPCM | 25, 31 and 44 | polyurethane membrane | roof | The integration of PCMs reduced both indoor and materials’ temperature. Cool roof materials benefit from lower phase change temperatures (25–35 °C), while common dark membranes show a better performance with higher temperatures (31–45 °C). |
| [65] | lab test | CaCl2 ∗ 6H2O | macro (Polyvinyl chloride) | 26 | wallboard | Wall | PCM reduced the indoor average temperature and its fluctuations, but this was highly dependent on climate conditions. |
| [66] | lab and field test | paraffin | nano | 27.4 | wood fiber-polymer composite | floor | Tensile strength of the wood fiber composite was reduced up to 58%, and flexural strength up to 68% with 40 wt% of PCM. Use of PCM coupled with natural night ventilation help to reduce the overheating period (temperatures above 23 °C) to about 50%. |
| [67] | field test | coconut oil | macro | 22.6 | pouches | walls, windows | The PCM in south-facing walls and window allowed a higher reduction of indoor temperatures (up to 7.2 °C) If the PCM is applied only on the wall, the reduction is equal to 5.2 °C. |
| [68] | field test | n-octadecane | nano | 23.3 | plaster wallboard | walls, ceiling | The PCM (30 wt%) helped to stabilize the indoor temperature but not enough to reach the comfort range (18–23 °C), so natural night-time ventilation is required during summer. |
| [69] | field test | RT28 | macro | 27.5 | integration of separate TES in the window | window | The PCM enhanced the heat balance of the window by 10% during the heating season but suffered from overheating during summer. |
| [70] | field test | CaCl2 ∗ 6H2O and MgCl ∗ 6H2O mixture | macro | 21 | polyethylene containers | wall | The PCM integration in the wood walls showed a reduction in overall temperature fluctuations equal to 57%, while the day/night fluctuations were reduced by 62% with respect to the reference room. |
| [71] | field test | 1-tetradecanol 1-hexadecanol | SSPCM (PCM + diatomite) | 33.8 (tetradecanol) 41.8 (hexadecanol) | plaster wallboard | wall | Tetradecanol attenuated better than hexadecanol heat waves at the west wall. However, the reference board performed better on both PCM-enhanced boards when placed on the east wall. This suggests that the suitable phase change temperature depends also on the orientation of the elements of the building. |
| [72] | lab test | n-octadecane (RT26) | macro | 32.2 | copper or PVC tubes embebbed in insulated panels | wall | Depending on the encapsulating material and on the orientation of capsules (vertical/horizontal), the PCM (12–15%) reduced the peak heat flux in the wall between 12% and 33% with respect to a reference insulation panel. Copper was shown to be more efficient than PVC as encapsulating material. |
| [73] | field test | RT28HC | macro | 28 | glass | window | The internal temperature of the window decreased by 7.6 °C when filled with PCM instead of air, and the CFD model suggested that PCM thickness should not exceed 30 mm. |
| Reference | Test Scale | PCM | PCM Form | Tmelt (°C) | Building Material | Position in Building | Remarks and Results |
|---|---|---|---|---|---|---|---|
| [74] | field test | paraffin (DuPont Energain). Soy and palm oil (BioPCM Q25 M51) | SSPCM (DuPont Energain) macro (BioPCM) | 21.6 (Energain) 25 (BioPCM) | wallboard laminated with aluminum (Energain) pouches (BioPCM) | walls, ceiling | BioPCM was not efficient for cooling during summer, but it was found to be effective in winter, to increase the thermal inertia of buildings. Energain reduced the peak temperature by 3–4 °C and the daily temperature fluctuations to 1–2 °C. |
| [75] | lab test | paraffin | SSPCM | 17.2 | resin sheet | floor | The PCM increased maximum floor temperatures during heating and cooling respectively by 5.04 °C and 1.08 °C. The cooling delay time of the floor was increased up to 3.6 h. |
| [76] | lab test | paraffin | macro | 24 | Panel steel | ceiling | Macroencapsulated PCM showed higher cooling power with respect to the one with microencapsulated PCM but lower respect to the one without PCM. This panel also showed greater flexibility in shifting the cooling load to off-peak hours. |
| [77] | field test | coconut oil | macro | 25 | steel | floor, wall, ceiling | The inclusion of PCM was useful to shift the heating load to low-peak periods. Inclusion of PCM in the ceiling was less efficient than inclusion in floor and walls. |
| [78] | field test | not specified | micro | 27.8 | steel | floor | The PCM increased the thermal storage capacity of the floor by 77.36% and stabilized indoor thermal stability. The heat gained from solar radiation and stored in the PCM floor can increase indoor air temperature by 3 °C if coupled with the ventilation system. |
| Reference | PCM | PCM Form | PCM Tmelt (°C) | Supporting Material | Building Material | Incorporation in the Concrete/Mortar | PCM Latent Heat Capacity (kJ/kg) | Results |
|---|---|---|---|---|---|---|---|---|
| [81] | Butyl stearate | bulk | 23.4 | none | concrete | direct | 134.2 | The PCM prevented a concrete temperature rise, improved its workability, and reduced the corrosive damages on steel embedded in concrete. |
| [84] | PEG 1000 | SSPCM | 37–40 | Lecce stone | Hydraulic lime, gypsum, cement mortars | impregnated aggregates | 129 | The PCM lowered both the phase change temperatures (from 37–40 °C to 13–17 °C) and the phase change enthalpy (from 129 kJ/kg to 7–9 kJ/kg). Both flexural and compressive strength showed a considerable decrease for all the binders with a water increment of 15%. |
| [85] | Micronal 5008 (Octadecane) | micro | 23 | acrylic polymer | Alkali-activated cements | microcapsules | 100 | Microencapsulated PCM (up to 20%) enhanced the heat storage capacity of the cement but decreased its compressive strength from 43% to 50%. |
| [90] | Capric acid and myristyl alcohol (weight ratio = 9:1) | SSPCM | 17–32 | expanded perlite | cement | impregnated aggregates | 167.2 | The addition of composite PCM to the cement led to a significant decrease in its compressive strength (from 54% to 82% decrease for 10 wt% to 30 wt% of composite addition). Indoor temperature fluctuations were reduced, and no leakage was shown. |
| [88] | Butyl stearate | macro | 19 | steel balls | concrete | blending of macrocapsules and concrete | 107.3 | The use of steel balls (30%) prevented leakage problems but reduced the concrete’s compressive strength by 18%. A total of 5% of the cement mass was replaced with slag and fly ash to solve this problem. Coarse aggregates substitution (10% in volume) with steel balls greatly enhanced the heat transfer efficiency without lowering too much the mechanical properties. |
| [82] | paraffin | bulk | 20–23 | none | concrete | direct | 107.3 | PCM addition to concrete reduced compressive strength from 18.5 MPa (0% PCM) to 14.9 MPa (20% PCM). The addition of PCM up to 10% does not cause significant changes in flexural and compressive strength. The direct incorporation of PCM increased the liquid/binder ratio and decreased the water absorption of concrete. |
| [91] | BSF26 | micro | 26 | not specified | Alkali-activated cements | blending of microcapsules and cement | 110 | The addition of PCM from 0% to 30% caused a serious reduction in mechanical properties (compressive and flexural strength). No more than 20% of PCM should be added. |
| [83] | 1-dodecanol | bulk | 22 | none | Portland cement | direct | 195 | The mortar was enriched with 6 wt% of PCM, enhanced with copper oxide and titania, generating a 10% decrease in the compressive strength |
| [92] | octadecane (75%) and eicosane (25%) eutectic mixture | SSPCM | 20.4 | pumice | cement plaster | impregnated aggregates | 232.7 | SSPCM (Pumice + 34 wt% of PCM) showed good compatibility, no leakage, and good thermal stability. Cement mortar was formed with 30 wt% of the SSPCM achieving sufficient thermal regulation properties. |
| [93] | capric acid and polyethylene glycol (PEG 600) | SSPCM | 31.3 (Capric acid) 9.9 (PEG) | pumice | Portland cement | impregnated aggregates | 190.2 (Capric acid) 148.5 (PEG) | The SSPCMs (62 wt% of capric acid and 56 wt% of PEG) showed good thermal stability. Plaster enriched with SSPCM (20 wt%) for thermal regulation in buildings. |
| [94] | PureTemp 23 | 23 | expanded perlite, hydrated lime | hydraulic lime and Portland cement plaster | impregnated materials | 227 | A plaster with 6 wt% PCM was tested, showing no leakage problems and compressive strength, similar to a commercial plaster taken as reference. | |
| [95] | n-nonacosane | SSPCM | 64 | expanded perlite | Portland cement concrete | impregnated aggregates | 195 | No leakage was detected and the time delay in temperature rising was verified with respect to concrete without PCM. Compressive strength decreased by 40% (from 37.5 MPa to 22.5 MPa). |
| [86] | Lauric acid (66 wt%) and myristic acid (34 wt%) eutectic mixture | SSPCM | 32.2 | fly ash | Portland cement mortar | impregnated fly ash | 177 | The addition of 20 wt% of SSPCM (37% of PCM) to the cement mortar reduced the compressive strength by 54% (from 45.12 MPa to 20.21 MPa) and flexural strength by 67% (from 5.25 MPa to 1.74 MPa). |
| [87] | Capric acid (82 wt%) and stearic acid (18 wt%) eutectic mixture | SSPCM | 24.7 | silica fume | Portland cement mortar | impregnated silica fume | 178 | The addition of 20% of SSPCM (27% of PCM) to the mortar showed good temperature regulation properties. Its compressive and flexural strength were respectively decreased by 37% and 36.57% compared with the reference mortar. |
| [96] | Nextek 37D | micro | 37 | not specified | polymer modified cement mortar | mechanical blending | 190 | Up to 20 wt% of PCM was added to the mortar. The compressive strength decreased from 64 MPa to 14 MPa, while the flexural strength decreased from 8.6 MPa to 4.8 MPa. |
| [97] | RT27 (paraffin) | SSPCM | 26.5 | expanded perlite | geopolymer concrete, geopolymer foam concrete | impregnated materials | 189 | The addition of 15 wt% and 30 wt% of SSPCM decreased the compressive strength of the geopolymer concrete, respectively, by 35% and 64%. However, the addition of 30 wt% SSPCM to the geopolymer foam concrete enhanced its compressive strength by 87% and its thermal storage capacity by 181%. |
| [98] | paraffin | SSPCM | 58.1 | clasting light shale ceramsite | Portland cement concrete | impregnated aggregates | 178.3 | Up to 6 wt% of PCM was added to the concrete. The compressive strength decreased by 76.5% compared with the reference concrete, while specific heat capacity increased by 41.2%. |
| [89] | paraffin | SSPCM | 25.2 | expanded vermiculite and diatomite | Portland cement | impregnated aggregates | 175.6 | The use of diatomite increased the thermal storage capacity of the SSPCM (52 wt% of PCM) by 15.6% and enhanced both its strength and long-term stability. The mortar with diatomite-based SSPCM had a compressive strength 25% higher with respect to the mortar enriched only with vermiculite-based PCM (45 wt%). |
| PCM | Name in the EI99 | Impact kg/Material [EI99 Pt] |
|---|---|---|
| Paraffin | Paraffin, at plant, RER | 0.208 |
| Salts hydrates | Calcium chloride, CaCl2, at regional storage, CH | 0.058 |
| Disposal, paraffin | (assumption) | 0.015 |
| Disposal, salts hydrates | (assumption) | 0.008 |
| Reference | PCM | PCM Tmelt (°C) | Supporting Material | Type of Incorporation | Composite Latent Heat Capacity (kJ/kg) | Remarks and Results |
|---|---|---|---|---|---|---|
| [105] | capric acid, lauric acid | 25.5 | fly ash | vacuum adsorption | 45.38 | Fly ash was obtained from a power plant. |
| [106] | PEG 1000 | 38 | transparent (delignified) wood and polymethyl methacrylate | vacuum impregnation | 76 | The composite material showed good transmittance up to 84% by decreasing thickness (up to 0.5 mm) of the composite material. No changes in elastic modulus were observed, except a reduction in flexural strength (70.5 MPa instead of 129.6 MPa) due to the inclusion of PCM. |
| [107] | n-heptadecane | 25.1 | activated carbon from pine cones | one-step impregnation | 138.2 | Heptadecane 62 wt% was found to be the optimum content, to avoid leakage and enhance thermal conductivity. |
| [108] | paraffin | 52.1 | rice husk ash | mechanical mixing and impregnation | 68.1 | A paraffin/rice husk ratio equal to 50% prevented leakage problems. PCM and rice husk ash showed good compatibility and thermal stability. |
| [109] | capric acid (83 wt%) and stearic acid (17 wt%) eutectic mixture | 24.7 | Scots pine sapwood | vacuum impregnation | 94 | The composite showed good chemical and thermal performance stability after 600 phase change cycles. The presence of PCMs decreased the water absorption from 80% to 20%, enhancing wood’s hydrophobicity and anti-swelling efficiency. The mechanical properties of wood were also enhanced: modulus of rupture (+22.3%), modulus of elasticity (25.3%), and compression strength parallel to grain (24.5%). |
| [110] | NaHPO4 ∗ 12H2O (58 wt%) and Na2CO3 ∗ 10H2O (42 wt%) eutectic mixture | 25 | diatomite, polyurethane acrylate | impregnation, coating, UV curing | 102.6 | The composite material, with 40% of diatomite, was coated with a polymer to avoid leakage problems. Supercooling was almost eliminated (0.5 °C) performance stability confirmed up to 300 phase change cycles. |
| [111] | Nextek 24D (paraffin and polymeric shell) | 22.4 | silty-clay soil and reed fiber | mechanical mixing | Not specified | A microencapsulated PCM was integrated (up to 20 wt%) in a soil and reed fiber mixture. The thermal conductivity decreased by up to 14%. Water vapor permeability’s decrease was 20%. The compressive strength was not affected by the addition of PCM; however, the soil-fiber mixture itself showed low values of compressive strength. |
| [112] | PureTemp 23 | 23 | cuttlebone pomelo peel | one-step impregnation | 145 for both composites | PCM and supporting materials are both biodegradable and obtained from renewable sources. Good chemical compatibility and limited leakage was demonstrated with a thermal storage efficiency equal to 70% of the pure PCM. The performance stability was confirmed up to 100 cycles. |
| [113] | organic | 29.9 | porcelain stoneware and soda-lime glass | vacuum impregnation | Not specified | The PCM impregnation efficiency in the glass-ceramic foam was between 24% and 39%. Thermal properties still need to be measured and leakage problems need to be addressed. |
| [114] | OM37 (inorganic) | 39.1 | expanded graphite and expanded vermiculite | ultrasonication and vacuum impregnation | 99.3 | The addition of expanded graphite up to 7% led to a decrease in latent heat storage capacity, while thermal conductivity increased by 114% and no leakage was detected. |
| [115] | PureTemp 23 | 201 | expanded glass aggregates and fly ash (coating) | vacuum impregnation | 92.7 | Glass aggregates absorbed up to 80% of PCM and, when coated with fly ash, showed no leakage problems. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbi, S.; Barbieri, F.; Marinelli, S.; Rimini, B.; Merchiori, S.; Bottarelli, M.; Montorsi, M. Phase Change Material Evolution in Thermal Energy Storage Systems for the Building Sector, with a Focus on Ground-Coupled Heat Pumps. Polymers 2022, 14, 620. https://doi.org/10.3390/polym14030620
Barbi S, Barbieri F, Marinelli S, Rimini B, Merchiori S, Bottarelli M, Montorsi M. Phase Change Material Evolution in Thermal Energy Storage Systems for the Building Sector, with a Focus on Ground-Coupled Heat Pumps. Polymers. 2022; 14(3):620. https://doi.org/10.3390/polym14030620
Chicago/Turabian StyleBarbi, Silvia, Francesco Barbieri, Simona Marinelli, Bianca Rimini, Sebastiano Merchiori, Michele Bottarelli, and Monia Montorsi. 2022. "Phase Change Material Evolution in Thermal Energy Storage Systems for the Building Sector, with a Focus on Ground-Coupled Heat Pumps" Polymers 14, no. 3: 620. https://doi.org/10.3390/polym14030620
APA StyleBarbi, S., Barbieri, F., Marinelli, S., Rimini, B., Merchiori, S., Bottarelli, M., & Montorsi, M. (2022). Phase Change Material Evolution in Thermal Energy Storage Systems for the Building Sector, with a Focus on Ground-Coupled Heat Pumps. Polymers, 14(3), 620. https://doi.org/10.3390/polym14030620







