From Strain Stiffening to Softening—Rheological Characterization of Keratins 8 and 18 Networks Crosslinked via Electron Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protein Preparation and Polymerization
2.2. Electron Irradiation
2.3. Shear Rheology
2.4. Keratin Gel Staining and Imaging
3. Results
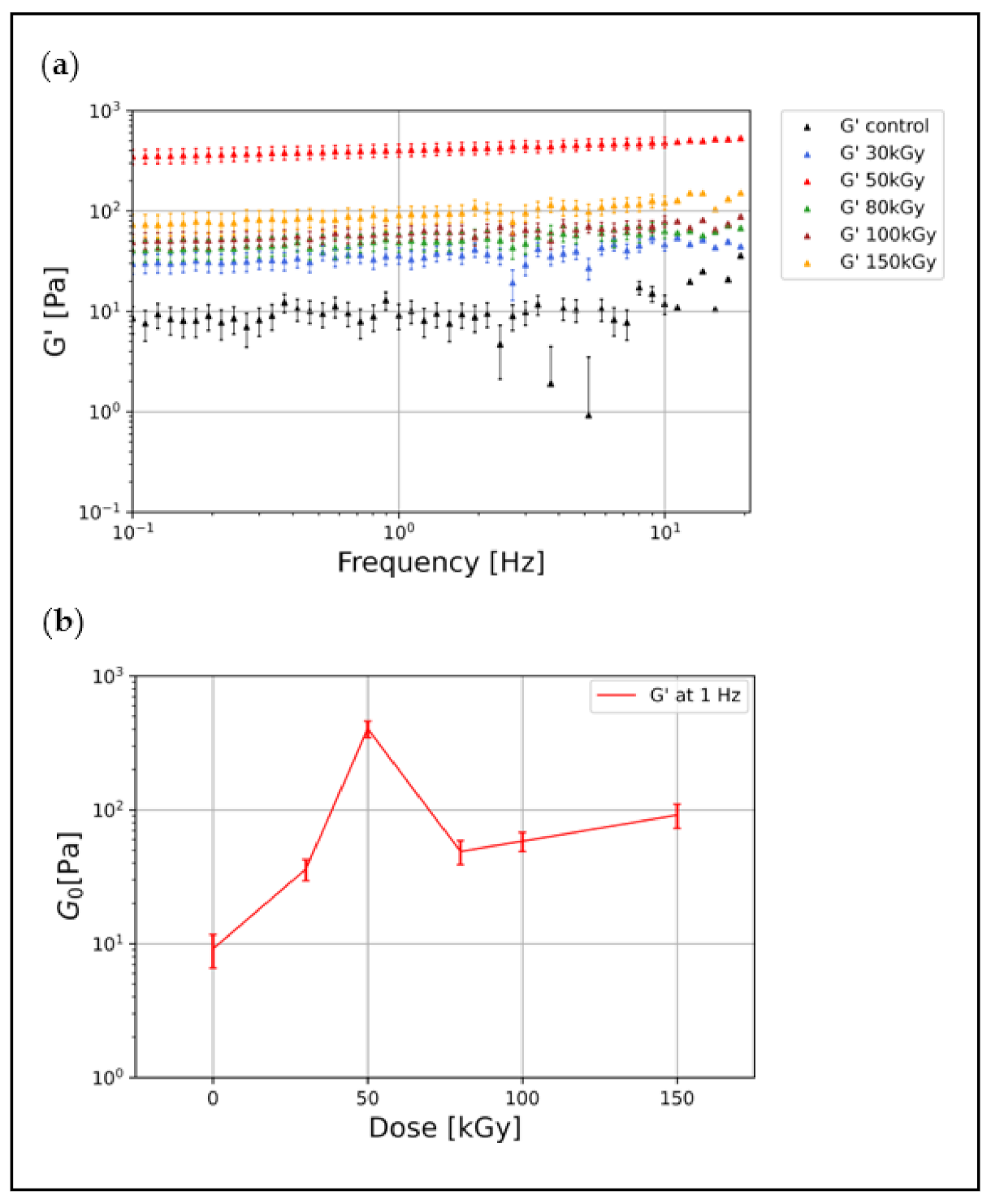
3.1. Irradiation Increases the Elastic Modulus of Keratin Gels
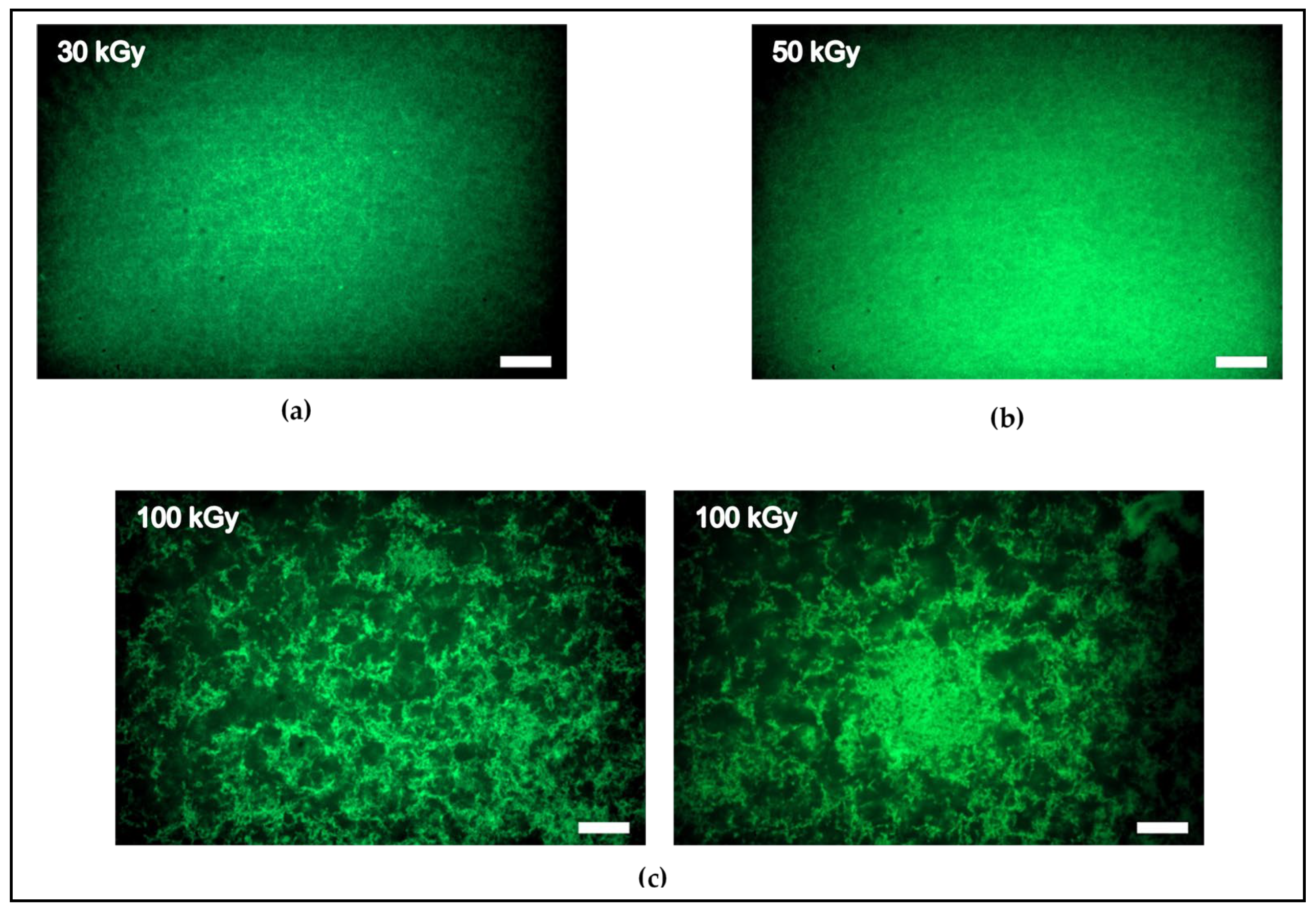
3.2. Networks Architectures
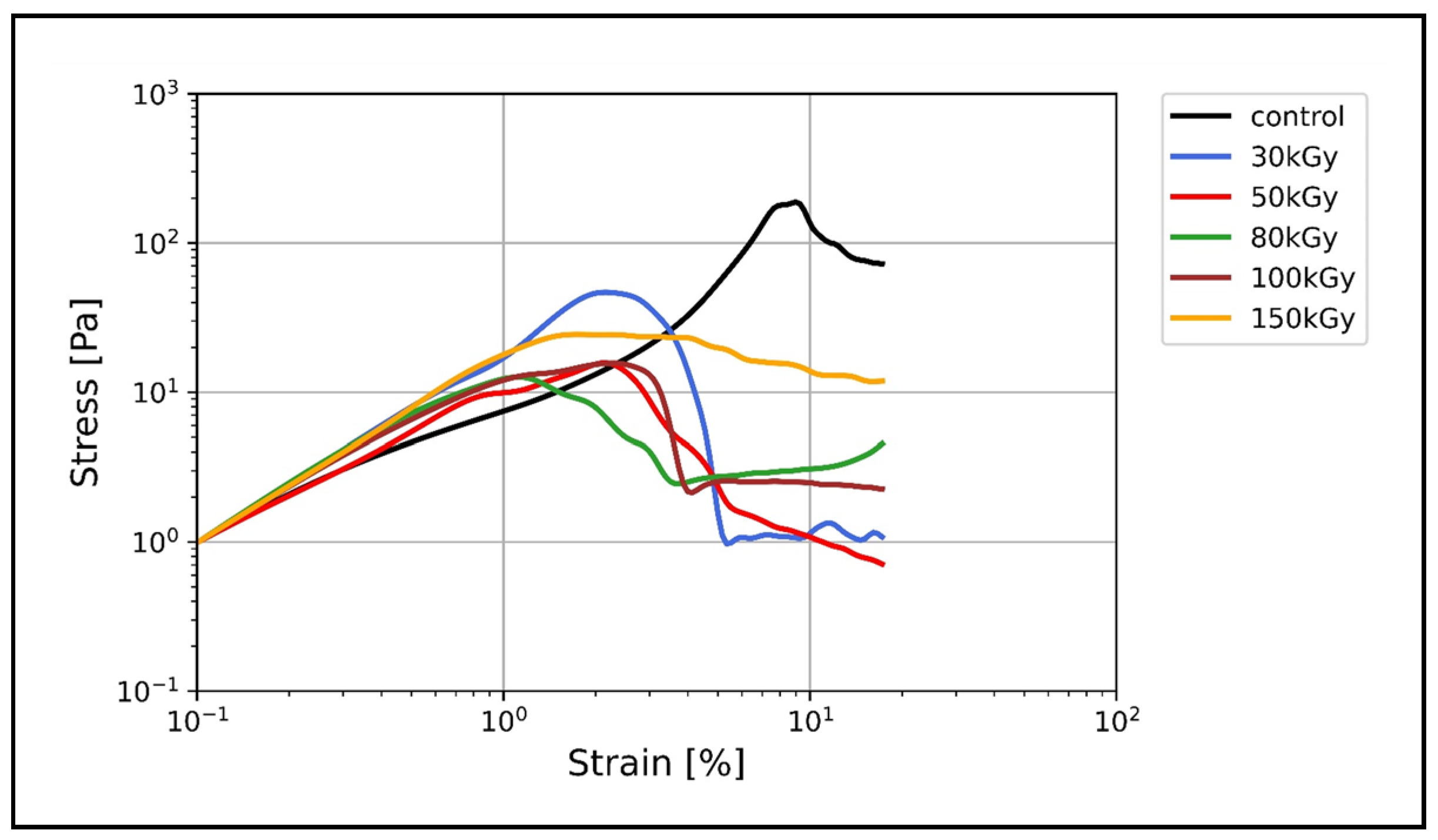
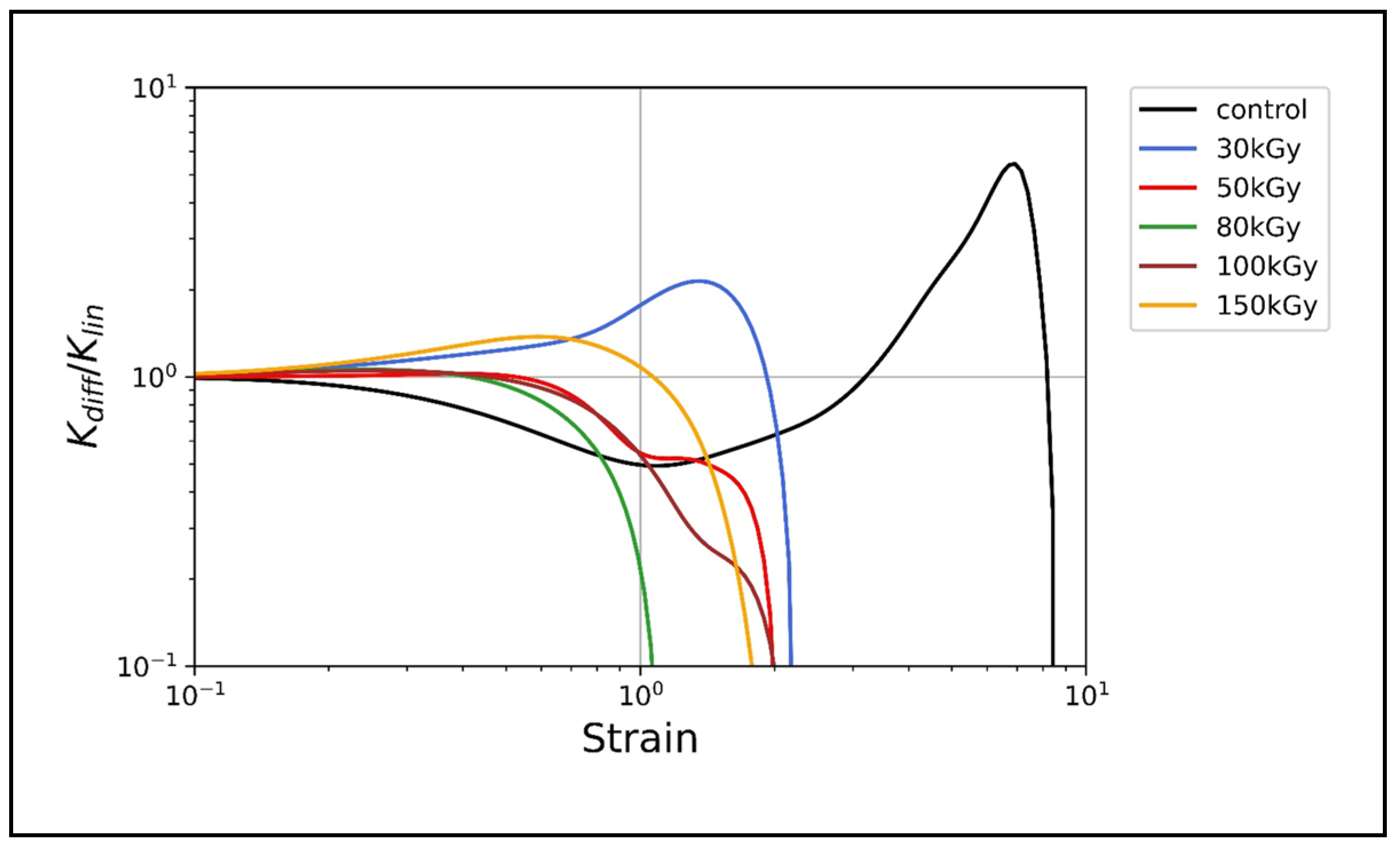
3.3. Nonlinear Rheology
Irradiation Suppresses the Nonlinear Strain Stiffening of Keratin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huber, F.; Schnauß, J.; Rönicke, S.; Rauch, P.; Müller, K.; Fütterer, C.; Käs, J. Emergent complexity of the cytoskeleton: From single filaments to tissue. Adv. Phys. 2013, 62, 1–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Gardel, M.L.; Kasza, K.E.; Brangwynne, C.P.; Liu, J.; Weitz, D.A. Chapter 19 Mechanical Response of Cytoskeletal Networks. Methods Cell Biol. 2008, 89, 487–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasza, K.E.; Rowat, A.C.; Liu, J.; Angelini, T.E.; Brangwynne, C.P.; Koenderink, G.H.; Weitz, D.A. The cell as a material. Curr. Opin. Cell Biol. 2007, 19, 101–107. [Google Scholar] [CrossRef]
- Bausch, A.R.; Kroy, K. A bottom-up approach to cell mechanics. Nat. Phys. 2006, 2, 231–238. [Google Scholar] [CrossRef]
- Burla, F.; Mulla, Y.; Vos, B.E.; Aufderhorst-Roberts, A.; Koenderink, G.H. From mechanical resilience to active material properties in biopolymer networks. Nat. Rev. Phys. 2019, 1, 249–263. [Google Scholar] [CrossRef]
- Bashirzadeh, Y.; Liu, A.P. Encapsulation of the cytoskeleton: Towards mimicking the mechanics of a cell. Soft Matter 2019, 15, 8425–8436. [Google Scholar] [CrossRef]
- Lorenz, J.S.; Schnauß, J.; Glaser, M.; Sajfutdinow, M.; Schuldt, C.; Käs, J.A.; Smith, D.M. Synthetic Transient Crosslinks Program the Mechanics of Soft, Biopolymer-Based Materials. Adv. Mater. 2018, 30, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Golde, T.; Glaser, M.; Tutmarc, C.; Elbalasy, I.; Huster, C.; Busteros, G.; Smith, D.M.; Herrmann, H.; Käs, J.A.; Schnauß, J. The role of stickiness in the rheology of semiflexible polymers. Soft Matter 2019, 15, 4865–4872. [Google Scholar] [CrossRef] [Green Version]
- Jacob, J.T.; Coulombe, P.A.; Kwan, R.; Omary, M.B. Types I and II keratin intermediate filaments. Cold Spring Harb. Perspect. Biol. 2018, 10, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Honda, Y.; Koike, K.; Kubo, Y.; Masuko, S.; Arakawa, Y.; Ando, S. In vitro assembly properties of human type I and II hair keratins. Cell Struct. Funct. 2014, 39, 31–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbalasy, I.; Mollenkopf, P.; Tutmarc, C.; Herrmann, H.; Schnauß, J. Keratins determine network stress responsiveness in reconstituted actin-keratin filament systems. Soft Matter 2021, 17, 3954–3962. [Google Scholar] [CrossRef]
- Yamada, S.; Wirtz, D.; Coulombe, P. A Pairwise assembly determines the intrinsic potential for self-organization and mechanical properties of keratin filaments. Mol. Biol. Cell 2002, 13, 382–391. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Wirtz, D.; Coulombe, P.A. The mechanical properties of simple epithelial keratins 8 and 18: Discriminating between interfacial and bulk elasticities. J. Struct. Biol. 2003, 143, 45–55. [Google Scholar] [CrossRef]
- Ma, L.; Xu, J.; Coulombe, P.A.; Wirtz, D. Keratin Filaments Suspensions Show Unique Micromechanical Properties. J. Biol. Chem. 1999, 274, 19145–19151. [Google Scholar] [CrossRef] [Green Version]
- Pawelzyk, P.; Herrmann, H.; Willenbacher, N. Mechanics of intermediate filament networks assembled from keratins K8 and K18. Soft Matter 2013, 9, 8871–8880. [Google Scholar] [CrossRef] [Green Version]
- Pawelzyk, P.; Mücke, N.; Herrmann, H.; Willenbacher, N. Attractive interactions among intermediate filaments determine network mechanics in vitro. PLoS ONE 2014, 9, e93194. [Google Scholar] [CrossRef] [Green Version]
- Fudge, D.; Russell, D.; Beriault, D.; Moore, W.; Lane, E.B.; Vogl, A.W. The intermediate filament network in cultured human keratinocytes is remarkably extensible and resilient. PLoS ONE 2008, 3, e2327. [Google Scholar] [CrossRef] [Green Version]
- Storm, C.; Pastore, J.J.; MacKintosh, F.C.; Lubensky, T.C.; Janmey, P.A. Nonlinear elasticity in biological gels. Nature 2005, 435, 191–194. [Google Scholar] [CrossRef] [Green Version]
- Seltmann, K.; Fritsch, A.W.; Käs, J.A.; Magin, T.M. Keratins significantly contribute to cell stiffness and impact invasive behavior. Proc. Natl. Acad. Sci. USA 2013, 110, 18507–18512. [Google Scholar] [CrossRef] [Green Version]
- Ramms, L.; Fabris, G.; Windoffer, R.; Schwarz, N.; Springer, R.; Zhou, C.; Lazar, J.; Stiefel, S.; Hersch, N.; Schnakenberg, U.; et al. Keratins as the main component for the mechanical integrity of keratinocytes. Proc. Natl. Acad. Sci. USA 2013, 110, 18513–18518. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, Y.; Kasahara, K.; Inagaki, M. Intermediate filaments and IF-associated proteins: From cell architecture to cell proliferation. Proc. Japan Acad. B Phys. Biol. Sci. 2019, 95, 479–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitner, A.; Paust, T.; Marti, O.; Walther, P.; Herrmann, H.; Beil, M. Properties of intermediate filament networks assembled from keratin 8 and 18 in the presence of Mg2+. Biophys. J. 2012, 103, 195–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, I.; Moch, M.; Neckernuss, T.; Paschke, S.; Herrmann, H.; Marti, O. Both monovalent cations and plectin are potent modulators of mechanical properties of keratin K8/K18 networks. Soft Matter 2016, 12, 6964–6974. [Google Scholar] [CrossRef] [Green Version]
- Rouse, J.G.; Van Dyke, M.E. A review of keratin-based biomaterials for biomedical applications. Materials 2010, 3, 999–1014. [Google Scholar] [CrossRef] [Green Version]
- Donato, R.K.; Mija, A. Keratin associations with synthetic, biosynthetic and natural polymers: An extensive review. Polymers 2020, 12, 32. [Google Scholar] [CrossRef] [Green Version]
- Ham, T.R.; Lee, R.T.; Han, S.; Haque, S.; Vodovotz, Y.; Gu, J.; Burnett, L.R.; Tomblyn, S.; Saul, J.M. Tunable keratin hydrogels for controlled erosion and growth factor delivery. Biomacromolecules 2016, 17, 225–236. [Google Scholar] [CrossRef] [Green Version]
- Wisotzki, E.I.; Friedrich, R.P.; Weidt, A.; Alexiou, C.; Mayr, S.G.; Zink, M. Cellular Response to Reagent-Free Electron-Irradiated Gelatin Hydrogels. Macromol. Biosci. 2016, 16, 914–924. [Google Scholar] [CrossRef]
- Riedel, S.; Hietschold, P.; Krömmelbein, C.; Kunschmann, T.; Konieczny, R.; Knolle, W.; Mierke, C.T.; Zink, M.; Mayr, S.G. Design of biomimetic collagen matrices by reagent-free electron beam induced crosslinking: Structure-property relationships and cellular response. Mater. Des. 2019, 168, 107606. [Google Scholar] [CrossRef]
- Wilharm, N.; Fischer, T.; Ott, F.; Konieczny, R.; Zink, M.; Beck-Sickinger, A.G.; Mayr, S.G. Energetic electron assisted synthesis of highly tunable temperature-responsive collagen/elastin gels for cyclic actuation: Macroscopic switching and molecular origins. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Krömmelbein, C.; Mütze, M.; Konieczny, R.; Schönherr, N.; Griebel, J.; Gerdes, W.; Mayr, S.G.; Riedel, S. Impact of high-energy electron irradiation on mechanical, structural and chemical properties of agarose hydrogels. Carbohydr. Polym. 2021, 263, 117970. [Google Scholar] [CrossRef] [PubMed]
- Jauch, P.; Weidner, A.; Riedel, S.; Wilharm, N.; Dutz, S.; Mayr, S.G. Collagen-iron oxide nanoparticle based ferrogel: Large reversible magnetostrains with potential for bioactuation. Multifunct. Mater. 2020, 3, 035001. [Google Scholar] [CrossRef]
- Wisotzki, E.I.; Tempesti, P.; Mayr, S.G. Influence of high energy electron irradiation on the network structure of gelatin hydrogels as investigated by small-angle X-ray scattering (SAXS). Phys. Chem. Chem. Phys. 2017, 19, 12064–12074. [Google Scholar] [CrossRef] [PubMed]
- Porubská, M.; Hanzlíková, Z.; Braniša, J.; Kleinová, A.; Hybler, P.; Fülöp, M.; Ondruška, J.; Jomová, K. The effect of electron beam on sheep wool. Polym. Degrad. Stab. 2015, 111, 151–158. [Google Scholar] [CrossRef]
- Hanzlíková, Z.; Lawson, M.K.; Hybler, P.; Fülöp, M.; Porubská, M. Time-Dependent Variations in Structure of Sheep Wool Irradiated by Electron Beam. Adv. Mater. Sci. Eng. 2017, 2017, 3849648. [Google Scholar] [CrossRef] [Green Version]
- Hanzlíková, Z.; Braniša, J.; Hybler, P.; Šprinclová, I.; Jomová, K.; Porubská, M. Sorption properties of sheep wool irradiated by accelerated electron beam. Chem. Pap. 2016, 70, 1299–1308. [Google Scholar] [CrossRef]
- Hanzlíková, Z.; Braniša, J.; Jomová, K.; Fülöp, M.; Hybler, P.; Porubská, M. Electron beam irradiated sheep wool—Prospective sorbent for heavy metals in wastewater. Sep. Purif. Technol. 2018, 193, 345–350. [Google Scholar] [CrossRef]
- Porubská, M.; Kleinová, A.; Hybler, P.; Braniša, J. Why natural or electron irradiated sheep wool show anomalous sorption of higher concentrations of copper(II). Molecules 2018, 23, 3180. [Google Scholar] [CrossRef] [Green Version]
- Braniša, J.; Kleinová, A.; Jomová, K.; Malá, R.; Morgunov, V.; Porubská, M. Some properties of electron beam-irradiated sheep wool linked to Cr(III) sorption. Molecules 2019, 24, 4401. [Google Scholar] [CrossRef] [Green Version]
- Porubská, M.; Jomová, K.; Lapčík, L.; Braniša, J. Radiation-modified wool for adsorption of redox metals and potentially for nanoparticles. Nanotechnol. Rev. 2020, 9, 1017–1026. [Google Scholar] [CrossRef]
- Park, M.; Kim, B.S.; Shin, H.K.; Park, S.J.; Kim, H.Y. Preparation and characterization of keratin-based biocomposite hydrogels prepared by electron beam irradiation. Mater. Sci. Eng. C 2013, 33, 5051–5057. [Google Scholar] [CrossRef] [PubMed]
- Strnad, P.; Usachov, V.; Debes, C.; Grä, F.; Parry, D.A.D.; Omary Bishr, M. Unique amino acid signatures that are evolutionarily conserved distinguish simple-type, epidermal and hair keratins. J. Cell Sci. 2011, 124, 4221–4232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, H.; Kreplak, L.; Ueli, A. Isolation, characterization, and in vitro assembly of intermediate filaments. Methods Cell. Biol. 2004, 78, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, R.; Gruhn, T. Length dependence of crosslinker induced network formation of rods: A Monte Carlo study. Soft Matter 2012, 8, 11746–11754. [Google Scholar] [CrossRef]
- Schuldt, C.; Schnauß, J.; Händler, T.; Glaser, M.; Lorenz, J.; Golde, T.; Käs, J.A.; Smith, D.M. Tuning Synthetic Semiflexible Networks by Bending Stiffness. Phys. Rev. Lett. 2016, 117, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisotzki, E.I.; Hennes, M.; Schuldt, C.; Engert, F.; Knolle, W.; Decker, U.; Käs, J.A.; Zink, M.; Mayr, S.G. Tailoring the material properties of gelatin hydrogels by high energy electron irradiation. J. Mater. Chem. B 2014, 2, 4297–4309. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Janmey, P.A. Polymer physics of the cytoskeleton. Curr. Opin. Solid State Mater. Sci. 2011, 15, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Karsch, S.; Büchau, F.; Magin, T.M.; Janshoff, A. An intact keratin network is crucial for mechanical integrity and barrier function in keratinocyte cell sheets. Cell. Mol. Life Sci. 2020, 77, 4397–4411. [Google Scholar] [CrossRef]
- MacKintosh, F.C.; Käs, J.; Janmey, P.A. Elasticity of semiflexible biopolymer networks. Phys. Rev. Lett. 1995, 75, 4425–4428. [Google Scholar] [CrossRef] [Green Version]
- Gardel, M.L.; Shin, J.H.; MacKintosh, F.C.; Mahadevan, L.; Matsudaira, P.; Weitz, D.A. Elastic behavior of cross-linked and bundled actin networks. Science 2004, 304, 1301–1305. [Google Scholar] [CrossRef] [Green Version]
- Žagar, G.; Onck, P.R.; Van Der Giessen, E. Two fundamental mechanisms govern the stiffening of cross-linked networks. Biophys. J. 2015, 108, 1470–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onck, P.R.; Koeman, T.; Van Dillen, T.; Van Der Giessen, E. Alternative explanation of stiffening in cross-linked semiflexible networks. Phys. Rev. Lett. 2005, 95, 178102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dillen, T.; Onck, P.R.; Van der Giessen, E. Models for stiffening in cross-linked biopolymer networks: A comparative study. J. Mech. Phys. Solids 2008, 56, 2240–2264. [Google Scholar] [CrossRef]
- Wagner, O.I.; Rammensee, S.; Korde, N.; Wen, Q.; Janmey, P.A. Softness, strength and self-repair in intermediate filament networks. Exp. Cell Res. 2007, 313, 2228–2235. [Google Scholar] [CrossRef] [Green Version]
- Nolting, J.F.; Möbius, W.; Köster, S. Mechanics of individual keratin bundles in living cells. Biophys. J. 2014, 107, 2693–2699. [Google Scholar] [CrossRef] [Green Version]
- Fudge, D.S.; Gardner, K.H.; Forsyth, V.T.; Riekel, C.; Gosline, J.M. The mechanical properties of hydrated intermediate filaments: Insights from hagfish slime threads. Biophys. J. 2003, 85, 2015–2027. [Google Scholar] [CrossRef] [Green Version]
- Forsting, J.; Kraxner, J.; Witt, H.; Janshoff, A.; Köster, S. Vimentin Intermediate Filaments Undergo Irreversible Conformational Changes during Cyclic Loading. Nano Lett. 2019, 19, 7210–7216. [Google Scholar] [CrossRef]
- Qin, Z.; Kreplak, L.; Buehler, M.J. Hierarchical structure controls nanomechanical properties of vimentin intermediate filaments. PLoS ONE 2009, 4, e7294. [Google Scholar] [CrossRef]
- Haines, R.L.; Lane, E.B. Keratins and disease at a glance. J. Cell Sci. 2012, 125, 3923–3928. [Google Scholar] [CrossRef] [Green Version]
- Asquith, R.S.; Otterburn, M.S. 40—Self-cross-linking in heated keratin. J. Text. Inst. 1970, 61, 569–577. [Google Scholar] [CrossRef]
- Wilharm, N.; Bertmer, M.; Knolle, W.; Griebel, J.; Elsner, C.; Mayr, S.G. Biomimetic crosslinking of collagen gels by energetic electrons: The role of L-lysine. Acta Biomater. 2021, in press. [Google Scholar] [CrossRef]
- Eckert, R.L.; Kaartinen, M.T.; Nurminskaya, M.; Belkin, A.M.; Colak, G.; Johnson, G.V.W.; Mehta, K. Transglutaminase regulation of cell function. Physiol. Rev. 2014, 94, 383–417. [Google Scholar] [CrossRef] [Green Version]
- Snider, N.T.; Omary, M.B. Assays for Posttranslational Modifications of Intermediate Filament Proteins. Methods Enzymol. 2016, 568, 113–138. [Google Scholar] [CrossRef] [Green Version]
- Steinert, P.M. Structural-mechanical integration of keratin intermediate filaments with cell peripheral structures in the cornified epidermal keratinocyte. Biol. Bull. 1998, 194, 367–370. [Google Scholar] [CrossRef]
- Candi, E.; Tarcsa, E.; Digiovanna, J.J.; Compton, J.G.; Elias, P.M.; Marekov, L.N.; Steinert, P.M. A highly conserved lysine residue on the head domain of type II keratins is essential for the attachment of keratin intermediate filaments to the cornified cell envelope through isopeptide crosslinking by transglutaminases. Proc. Natl. Acad. Sci. USA 1998, 95, 2067–2072. [Google Scholar] [CrossRef] [Green Version]
- Kwan, R.; Hanada, S.; Harada, M.; Strnad, P.; Li, D.H.; Omary, M.B. Keratin 8 phosphorylation regulates its transamidation and hepatocyte Mallory-Denk body formation. FASEB J. 2012, 26, 2318–2326. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, V.; Klingstedt, T.; Simon, R.; Nilsson, K.P.R.; Thueringer, A.; Kashofer, K.; Haybaeck, J.; Denk, H.; Abuja, P.M.; Zatloukal, K. Cross β-sheet conformation of keratin 8 is a specific feature of MalloryDenk bodies compared with other hepatocyte inclusions. Gastroenterology 2011, 141, 1080–1090.e7. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbalasy, I.; Wilharm, N.; Herchenhahn, E.; Konieczny, R.; Mayr, S.G.; Schnauß, J. From Strain Stiffening to Softening—Rheological Characterization of Keratins 8 and 18 Networks Crosslinked via Electron Irradiation. Polymers 2022, 14, 614. https://doi.org/10.3390/polym14030614
Elbalasy I, Wilharm N, Herchenhahn E, Konieczny R, Mayr SG, Schnauß J. From Strain Stiffening to Softening—Rheological Characterization of Keratins 8 and 18 Networks Crosslinked via Electron Irradiation. Polymers. 2022; 14(3):614. https://doi.org/10.3390/polym14030614
Chicago/Turabian StyleElbalasy, Iman, Nils Wilharm, Erik Herchenhahn, Robert Konieczny, Stefan G. Mayr, and Jörg Schnauß. 2022. "From Strain Stiffening to Softening—Rheological Characterization of Keratins 8 and 18 Networks Crosslinked via Electron Irradiation" Polymers 14, no. 3: 614. https://doi.org/10.3390/polym14030614
APA StyleElbalasy, I., Wilharm, N., Herchenhahn, E., Konieczny, R., Mayr, S. G., & Schnauß, J. (2022). From Strain Stiffening to Softening—Rheological Characterization of Keratins 8 and 18 Networks Crosslinked via Electron Irradiation. Polymers, 14(3), 614. https://doi.org/10.3390/polym14030614





