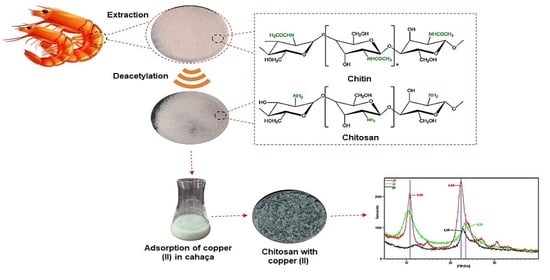
Adsorption of Cu (II) Ions Present in the Distilled Beverage (Sugar Cane Spirit) Using Chitosan Derived from the Shrimp Shell
Abstract
1. Introduction
2. Materials and Methods
2.1. The Samples, Reagents and Solutions
2.2. Chitosan Preparation
2.2.1. Precipitation of Chitin
2.2.2. Deacetylation of Chitin
2.3. Characterization of Chitosan
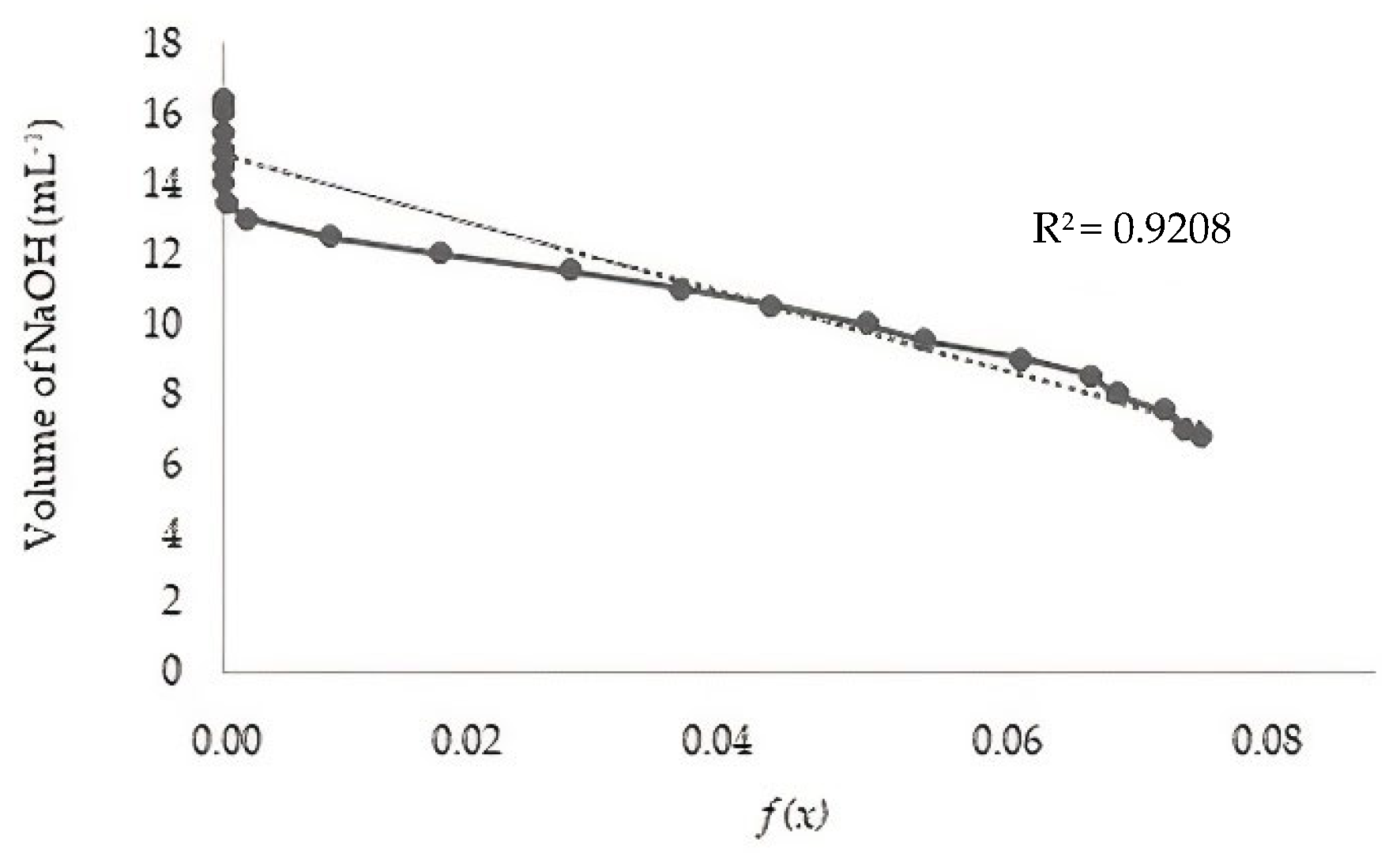
2.3.1. Potentiometric Titration and ATR-FTIR Analysis
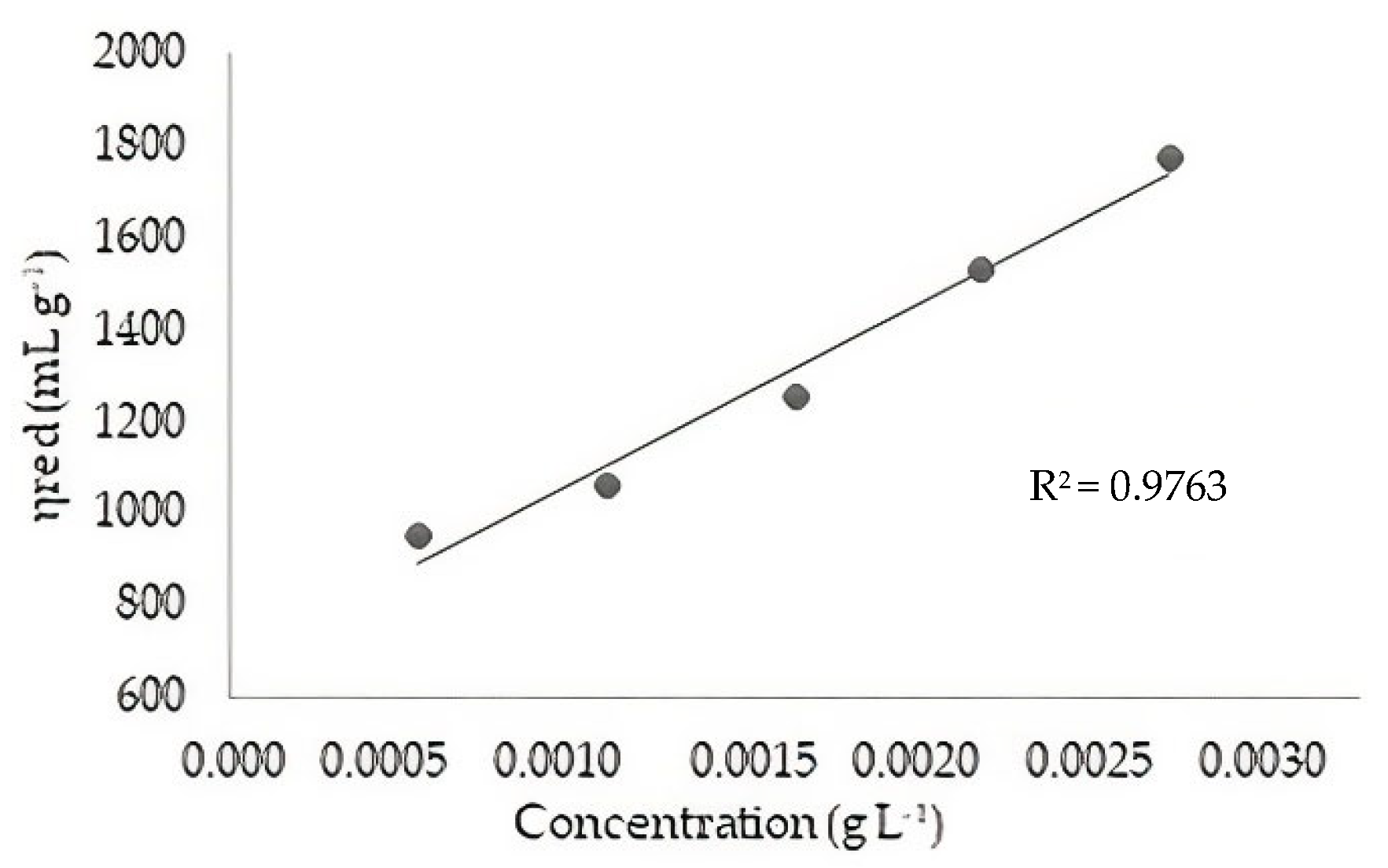
2.3.2. Determination of the Molecular Weight of Chitosan by Viscosimetry
2.4. Morphological Analysis of Chitosan
2.4.1. Scanning Electron Microscopy
2.4.2. X-ray Diffraction (XRD)
2.5. Copper Content in Cachaça
2.5.1. Spectrophotometric Titration with EDTA
2.5.2. Microwave-Induced Plasma Optical Emission Spectrometry (MIP OES)
2.6. Evaluation of Cu (II) Adsorption in the Chitosan Matrix
2.7. Kinetic Modeling
3. Results and Discussion
3.1. Degree of Deacetylation
3.2. Molecular Weight of Chitosan
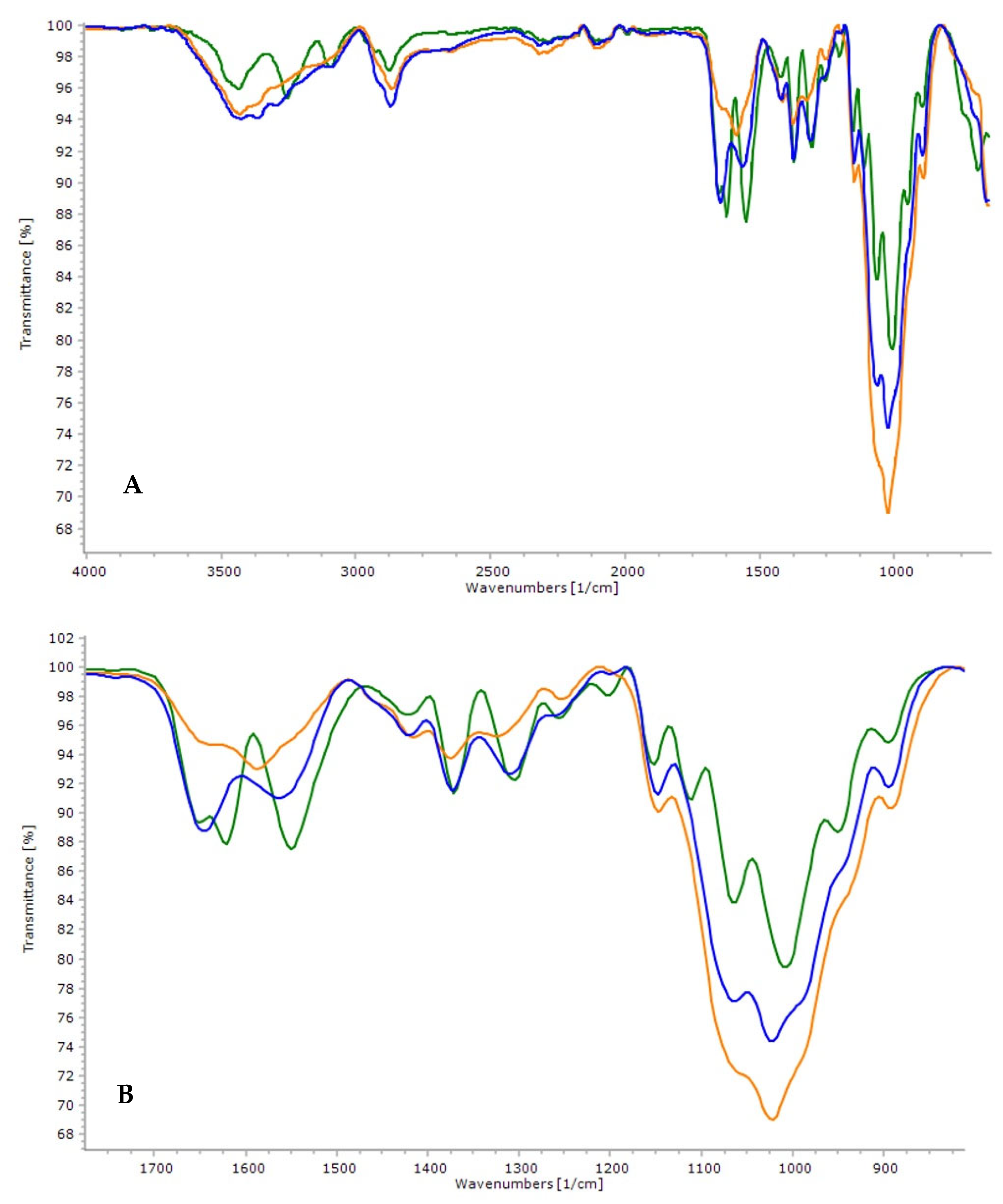
3.3. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
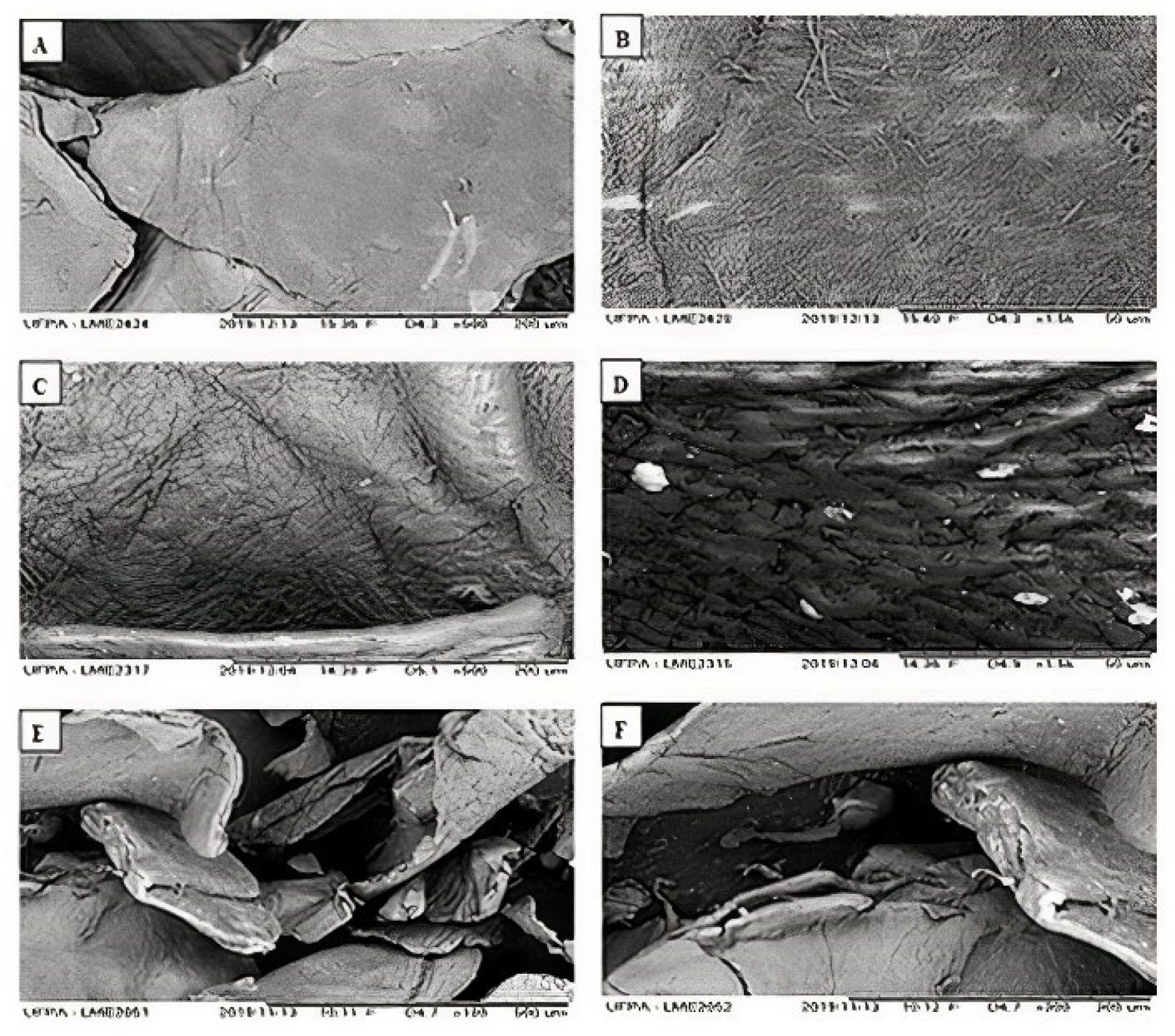
3.4. Scanning Electron Microscopy (SEM)
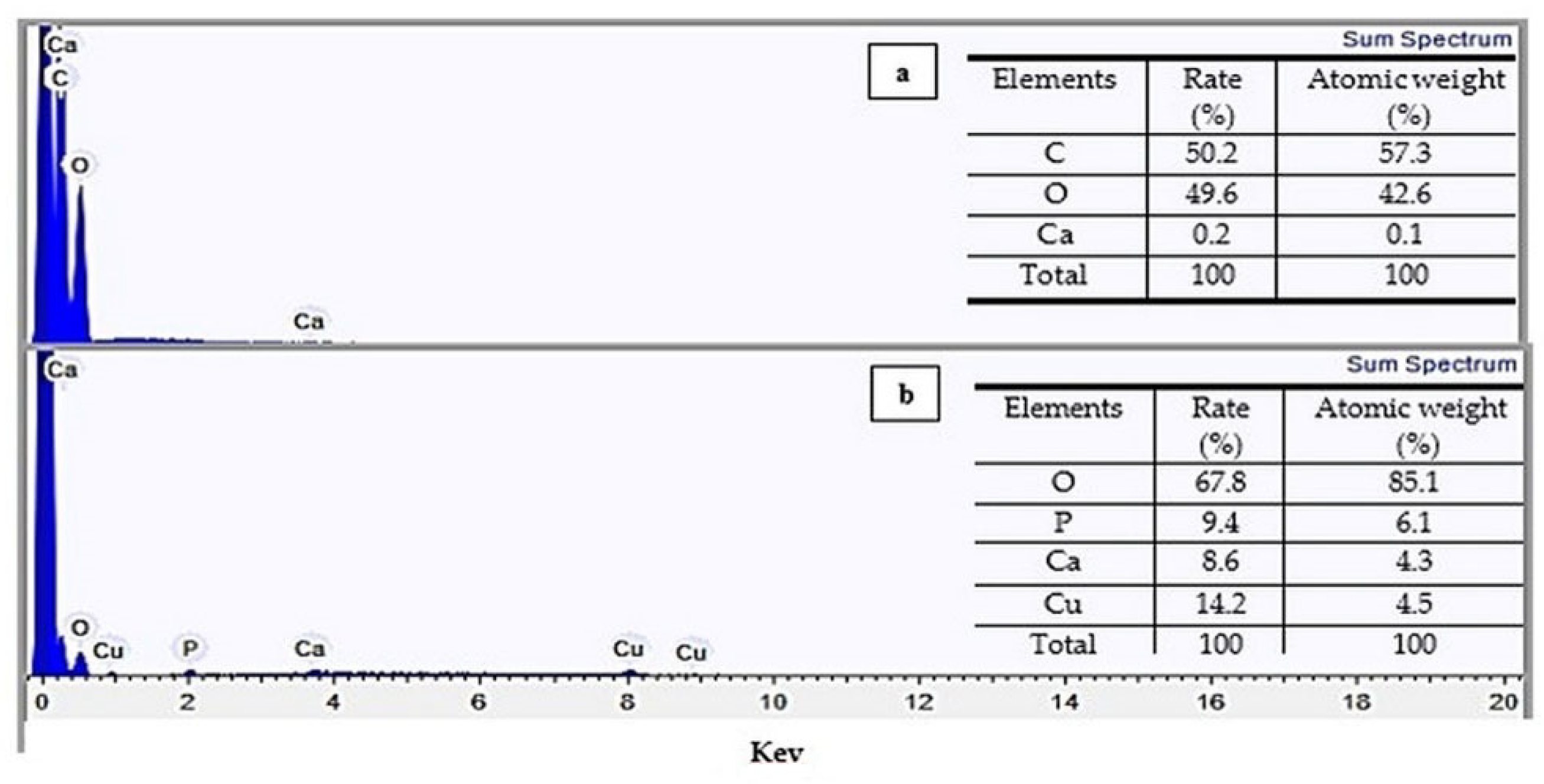
3.5. Energy Dispersion Spectroscopy (EDS)
3.6. Differentiation and Crystallinity by XRD
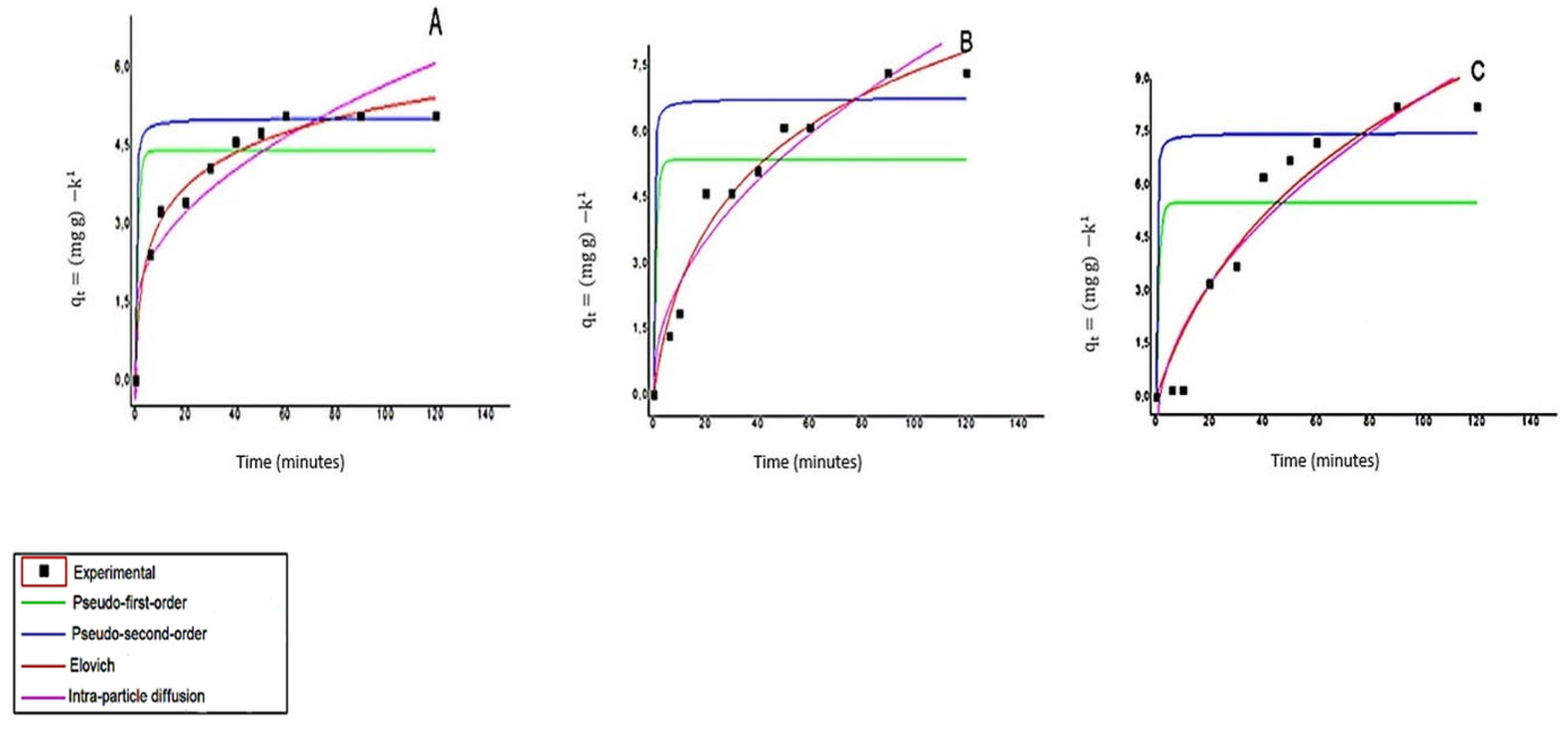
3.7. Adsorption Kinetics
Kinetic Profiles
3.8. Quantification of Copper by Microwave-Induced Plasma Optical Emission Spectrometry (MIP OES)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azevedo, S.M.; Cardoso, M.G.; Pereira, N.E.; Ribeiro, C.F.S.; Silva, V.F.S.; Aguiar, F.C. Levantamento da contaminação por cobre nas aguardentes de cana-de-açúcar produzidas em Minas Gerais. Ciênc. Agrotec. 2003, 27, 618. [Google Scholar] [CrossRef]
- Cantanhede, L.B.; Lima, J.B.; Lopes, G.S.; Farias, R.; Bezerra, C.W.B. Uso de sílica e sílica-titânia organofuncionalizadas para a remoção de Cu(II) em aguardentes. Ciênc. Tecnol. Aliment. 2005, 25, 500. [Google Scholar] [CrossRef][Green Version]
- Souza, L.M.; Alcarde, A.R.; Lima, F.V.; Bortoletto, A.M. Produção de Cachaça de Qualidade, 1st ed.; USP-ESALQ: Piracicaba, Brasil, 2013. [Google Scholar]
- Approves the technical regulation for the establishment of Identity and Quality Standards for sugar cane brandy and cachaça, Official Diary of the Union. In Normative Instruction N° 3; Ministry of Agriculture, Livestock and Supply: Brasilia, Brazil, 29 June 2005.
- Yadav, M.; Gupta, R.; Arora, G.; Yadav, P.; Srivastava, A.; Sharma, R.K. Contaminants in Our Water: Identification and Remediation Methods; American Chemical Society: Washington, DC, USA, 2020; Chapter 3. [Google Scholar]
- Daraei, H.; Mittal, A.; Noorisepehr, M.; Daraei, F. Kinetic and equilibrium studiesof adsorptive removal of phenol onto eggshell waste. Environ. Sci. Pollut. Res. 2013, 20, 4603–4611. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Ahmad, R.; Hasan, I. Biosorption of Pb2+, Ni2+ and Cu2+ ions from aqueous solutions by L-cystein-modified montmorillonite-immobilized alginate nanocomposite. Desal. Water Treat. 2015, 57, 17790–17807. [Google Scholar] [CrossRef]
- Duarte, F.C.; Cardoso, M.G.; Magriotis, Z.M.; Santiago, W.D.; João Guilherme Pereira Mendonça, J.G.P.; Rodrigues, L.M.A. Removal of copper in cachaças using clays. Ciênc. Agrotec. 2014, 38, 382–389. [Google Scholar] [CrossRef][Green Version]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Zacaroni, L.M.; Magriotis, Z.M.; Cardoso, M.d.G.; Santiago, W.D.; Mendonça, J.G.; Vieira, S.S.; Nelson, D.L. Natural clay and commercial activated charcoal: Properties and application for the removal of copper from cachaça. Food Control 2015, 47, 536–544. [Google Scholar] [CrossRef]
- Ishchenko, V.; Asylkivskyi, I. Sustainable Production: Novel Trends in Energy, Environment and Material Systems; Springer Interational Publishing: Cham, Switzerland, 2020; Volume 198, pp. 161–175. [Google Scholar] [CrossRef]
- Vakili, M.; Deng, S.; Cagnetta, G.; Wang, W.; Meng, P.; Liu, D.; Yu, G. Regeneration of chitosan-based adsorbents used in heavy metal adsorption: A review. Sep. Purif. Technol. 2019, 224, 373–387. [Google Scholar] [CrossRef]
- Krstić, V.; Urošević, T.; Pešovski, B. A review on adsorbents for treatment of water and wastewaters containing copper ions. Chem. Eng. Sci. 2018, 192, 273–287. [Google Scholar] [CrossRef]
- Nitayaphat, W. Chitosan/coffee residue composite beads for removal of reactive dye. Mater. Today Proc. 2017, 4, 6274–6283. [Google Scholar] [CrossRef]
- Peng, W.; Xie, Z.; Cheng, G.; Shi, L.; Zhang, Y. Amino-functionalized adsorbent prepared by means of Cu (II) imprinted method and its selective removal of copper from aqueous solutions. J. Hazard. Mater. 2015, 294, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Marine Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Sila, A.; Nasri, M.; Bougatef, A. β-Chitin and chitosan from squid gladius: Biological activities of chitosan and its application as clarifying agent for apple juice. Int. J. Biol. Macromol. 2012, 51, 953. [Google Scholar] [CrossRef] [PubMed]
- Scurria, A.; Tixier, A.F.; Lino, C.; Pagliaro, M.; D’Agostino, F.; Avellone, G.; Farid Chemat, F.; Ciriminna, R. High yields of shrimp oil rich in Omega-3 and natural astaxanthin from shrimp waste. ACS Omega 2020, 28, 17500. [Google Scholar]
- Aktar, M.; Rashid, M.; Azam, M.G.; Howlider, M.A.R.; Hoque, M.A. Shrimp waste and marine waste as substitutes of fish meal in broiler diet. Bangladesh J. Anim. Sci. Bang 2011, 40, 18. [Google Scholar] [CrossRef]
- Królczyk, G.M.; Wzorek, M.; Król, A.; Kochan, O.; Su, J.; Kacprzyk, J. Sustainable Production: Novel Trends in Energy, Environment and Material Systems; Studies in Systems, Decision and Control; Springer: Cham, Switzerland, 2020; Volume 198, pp. 45–60. [Google Scholar]
- Samar, M.; El-Kalyoubi, M.; Khalaf, M.H.; El-Razik, M.M. Physicochemical, functional, antioxidant and antibacterial properties of chitosan extracted from shrimp wastes by microwave technique. Ann. Agric. Sci. 2013, 58, 33. [Google Scholar] [CrossRef]
- Tolaimate, A.; Desbrieres, J.; Rhazi, M.; Alagui, A. Contribution to the preparation of chitins and chitosans with controlled physico-chemical properties. Polymer 2003, 44, 7939–7952. [Google Scholar] [CrossRef]
- Sagheer, F.A.A.; Al-Sughayer, M.A.; Muslim, S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Carbohydr. Polym. 2009, 77, 410–419. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, L.; Zhong, W. A new linear potentiometric titration method for the determination of deacetylation degree of chitosan. Carbohydr. Polym. 2003, 54, 457–463. [Google Scholar] [CrossRef]
- Barragán, C.R.; Fornué, E.D.; Ortega, J.A. Determinación del grado de desacetilación de quitosana mediante titulación potenciométrica, FTIR y Raman. J. CIM 2016, 4, 769–776. [Google Scholar]
- Roberts, G.A.F.; Domszy, J.G. Determination of the viscometric constants for chitosan. J. Biol. Macromol. 1982, 4, 374–377. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar Annamareddy, S.H.; Abanti, S.; Kumar Rath, P. Physicochemical properties and characterization of chitosan synthesized from fish scales, crab and shrimp shells. Int. J. Biol. Macromol. 2017, 104, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Abdou, E.S.; Nagy, K.S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from local sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef]
- Maachou, H.; Bal, Y.; Chagnes, A.; Cote, G. Copper sorption on chitin and acid-washed shrimp shells from Palinurus elephas: Isotherm and kinetic studies. Int. J. Environ. Sci. Technol. 2019, 16, 5049–5054. [Google Scholar] [CrossRef]
- Labidi, A.; Salaberria, A.M.; Fernandes, S.C.M.; Labidi, J.; Abderrabba, M. Adsorption of copper on chitin-based materials: Kinetic and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 2016, 65, 140–148. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie der Sogenannten Adsorption Geloster Stoffe. Vetenskapsakad. Handl. 1898, 24, 1. [Google Scholar]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modi fi ed chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Ho, Y.S.G.M. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Pérez-Marín, A.B.; Zapata, V.M.; Ortuño, J.F.; Aguilar, M.; Sáez, J.; Lloréns, M. Removal of cadmium from aqueous solutions by adsorption onto orange waste. J. Hazard. Mater. 2007, 139, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Khor, E.; Wan, A.C.A. Chitin: Fulfilling a Biomaterials Promise, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Tabasso, S.; Carnaroglio, D.; Gaudinob, C.; Cravotto, E. Microwave, ultrasound and ball mill procedures for bio-waste valorisation. Green Chem. 2015, 17, 684. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioprocess Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Goy, R.C.; De Britto, D.; Assis, O.B.G. A Review of the Antimicrobial Activity of Chitosan. Polímeros Ciência Tecnologia 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, W.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Mendoza, L.J.; Argüelles Monal, W.M.; Goycoolea Valencia, F.M. Chemical Characteristics and Functional Properties of Chitosan. Chitosan in the Preservation of Agricultural Commodities; Elsevier Inc.: Cambridge, UK, 2016. [Google Scholar]
- Anbinder, P.S.; Macchi, C.; Amalvy, J.; Somoza, A. A study of the structural changes in a chitosan matrix produced by the adsorption of copper and chromium ions. Carbohydr. Polym. 2019, 222, 14987. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, Y.; Yu, L.; Zhang, C.; Xu, X.; Xue, Y.; Xue, C. Crystalline structure and thermal property characterization of chitin from Antarctic krill (Euphausia superba). Carbohydr. Polym. 2013, 92, 90–97. [Google Scholar] [CrossRef]
- Daraghmeh, N.H.; Chowdhry, B.Z.; Leharne, S.A.; Al Omari, M.M.; Badwan, A.; Chitin, A. Profiles of Drug Substances. Excipients and Related Methodology; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Chapter 2; pp. 35–102. [Google Scholar] [CrossRef]
- El Knidri, H.; El Khalfaouy, R.; Laajeb, A.; Addaou, A.; Lahsini, A. Eco-friendly extraction and characterization of chitin and chitosan from the shrimp shell waste via microwave irradiation. Process Saf. Environ. Prot. 2016, 104, 395. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A Review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Rocchetti, R. Enhanced capacity of chitosan for transition-metal ions in sulphate-sulphuric acid solutions. Talanta 1974, 21, 1137–1143. [Google Scholar] [CrossRef]
- Chang, M.; Juang, R. Equilibrium and kinetic studies on the adsorption of surfactant, organic acids and dyes from water onto natural biopolymers. Colloids Surf. A Physicochem. 2005, 269, 35–46. [Google Scholar] [CrossRef]
- De Cantão, F.O.; de Melo, W.C.; das Cardoso, M.G.; dos Anjos, P.J.; de Oliveira, L.C.A. Evaluation and removal of sugar cane brandy using aluminossilicates: Zeolite and bentonite. Ciênc. Agrotec. 2010, 34, 1109. [Google Scholar] [CrossRef]
- Ozbek, N.; Akman, S. Method development for the determination of calcium, copper, magnesium, manganese, iron, potassium, phosphorus and zinc in different types of breads by microwave induced plasma-atomic emission spectrometry. Food Chem. 2016, 200, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.S.; Nóbrega, C.; Morte, J.; Santos, E.; Korn, D.M. Microwave induced plasma optical emission spectrometry for multielement determination in instant soups. J. Food Compos. Anal. 2019, 86, 103376. [Google Scholar] [CrossRef]
- Gonçalves, D.A.; McSweeney, T.; Santos, M.C.; Jones, B.T.; Donati, G.L. Standard Dilution Analysis of Beverages by Microwave-Induced Plasma Optical Emission Spectrometry. Anal. Chim. Acta 2016, 909, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Espinoza Cruz, T.L.; Guerrero Esperanza, M.; Wrobel, K.; Yañez Barrientos, E.; Acevedo Aguilar, F.J.; Wrobel, K. Determination of major and minor elements in Mexican red wines by microwave-induced plasma optical emission spectrometry, evaluating different calibration methods and exploring potential of the obtained data in the assessment of wine provenance. Spectrochim. Acta B Atomic Spectrosc. 2020, 164, 105754. [Google Scholar] [CrossRef]

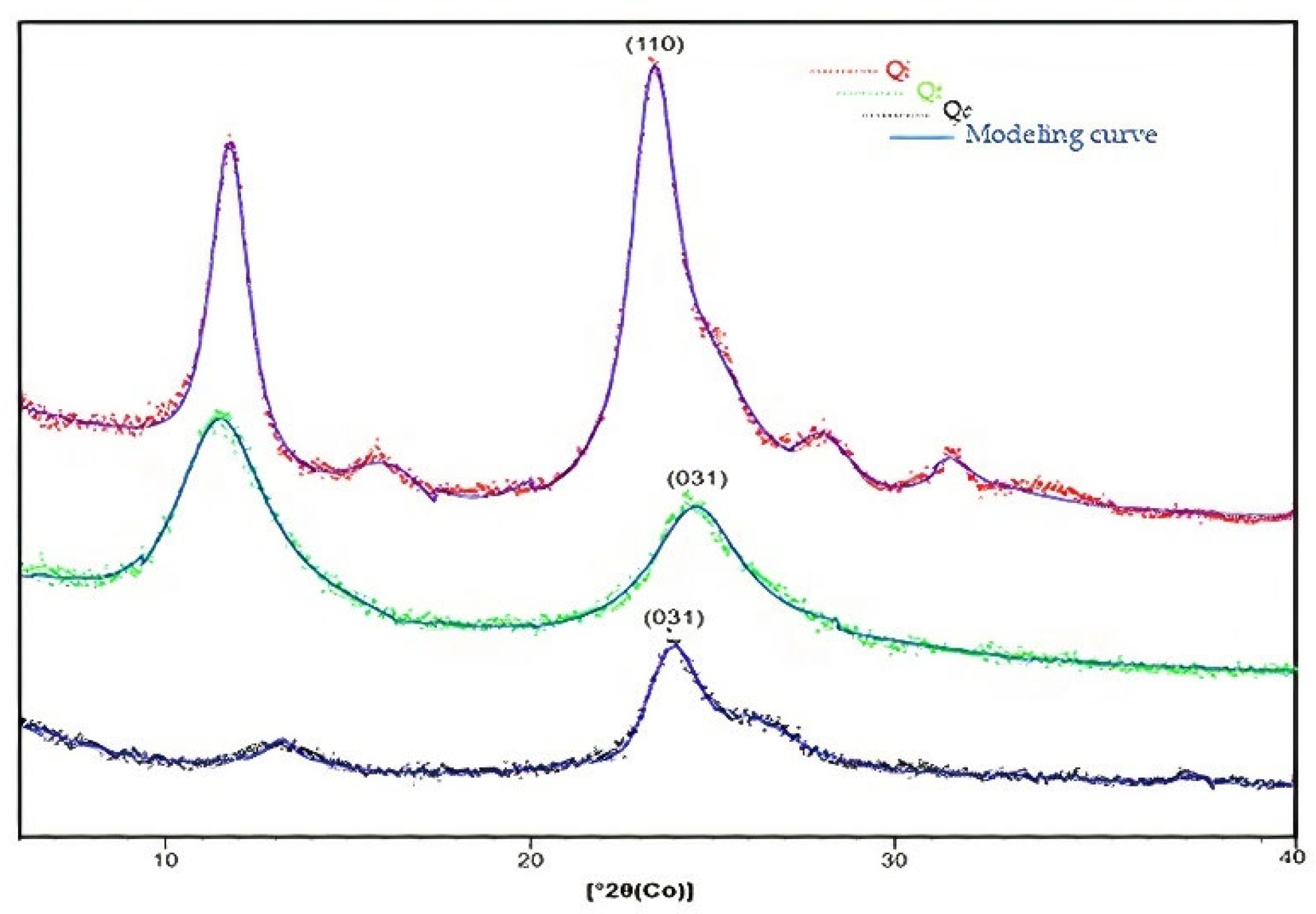
| Sample | Characteristic Peak | dhkl * (Å) | Imax ** | I0 *** | Relative Crystallinity (%) |
|---|---|---|---|---|---|
| Chitin | 2θ = 22.4° (h k l = 110) | 4.6 | 2451 | 368 | 84.9 |
| Chitosan | 2θ = 23.5° (h k l = 031) | 4.3 | 1086 | 479 | 54.2 |
| Chitosan adsorbed with Cu (II) | 2θ = 22.9° (h k l = 031) | 4.5 | 924 | 377 | 59.2 |
| Models | Parameters | 6 mg mL−1 | 4 mg mL−1 | 2 mg mL−1 |
|---|---|---|---|---|
| Pseudo-first-order | K1 (min−1) | 1 | 1 | 1 |
| Qe (mg g−1) | 4.3883 | 5.3617 | 5.4830 | |
| R2 | 0.6192 | 0.2639 | 0.0744 | |
| Pseudo-second-order | K2 (g mg−1 min−1) | 1 | 1 | 1 |
| Qe (mg g−1) | 5.0079 | 6.7516 | 7.4483 | |
| R2 | 0.4036 | −21485 | −50261 | |
| Elovich | α (mg g−1 min−1) | 2.1716 | 0.4205 | 0.2215 |
| β (g mg −1) | 1.0359 | 0.3857 | 0.1985 | |
| R2 | 0.9780 | 0.9665 | 0.9179 | |
| Intra-particle-diffusion | Kp (mg g−1 min−1/2) | 0.4413 | 0.7449 | 0.9591 |
| C (mg g−1) | 1.2383 | 0.1832 | −1.1005 | |
| R2 | 0.8139 | 0.9291 | 0.8858 |
| Sample | Copper (372.395) *,ª |
|---|---|
| Cachaça | 3.4 ± 0.003 |
| Cachaça with copper | 48.4 ± 0.52 |
| Cachaça after adsorption | 7.7 ± 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, L.N.; Santos, A.S.; das Graças Fernandes Dantas, K.; Ferreira, N.R. Adsorption of Cu (II) Ions Present in the Distilled Beverage (Sugar Cane Spirit) Using Chitosan Derived from the Shrimp Shell. Polymers 2022, 14, 573. https://doi.org/10.3390/polym14030573
dos Santos LN, Santos AS, das Graças Fernandes Dantas K, Ferreira NR. Adsorption of Cu (II) Ions Present in the Distilled Beverage (Sugar Cane Spirit) Using Chitosan Derived from the Shrimp Shell. Polymers. 2022; 14(3):573. https://doi.org/10.3390/polym14030573
Chicago/Turabian Styledos Santos, Lucely Nogueira, Alberdan Silva Santos, Kelly das Graças Fernandes Dantas, and Nelson Rosa Ferreira. 2022. "Adsorption of Cu (II) Ions Present in the Distilled Beverage (Sugar Cane Spirit) Using Chitosan Derived from the Shrimp Shell" Polymers 14, no. 3: 573. https://doi.org/10.3390/polym14030573
APA Styledos Santos, L. N., Santos, A. S., das Graças Fernandes Dantas, K., & Ferreira, N. R. (2022). Adsorption of Cu (II) Ions Present in the Distilled Beverage (Sugar Cane Spirit) Using Chitosan Derived from the Shrimp Shell. Polymers, 14(3), 573. https://doi.org/10.3390/polym14030573