Application of Gelatin in Food Packaging: A Review
Abstract
:1. Introduction
2. Types of Gelatin
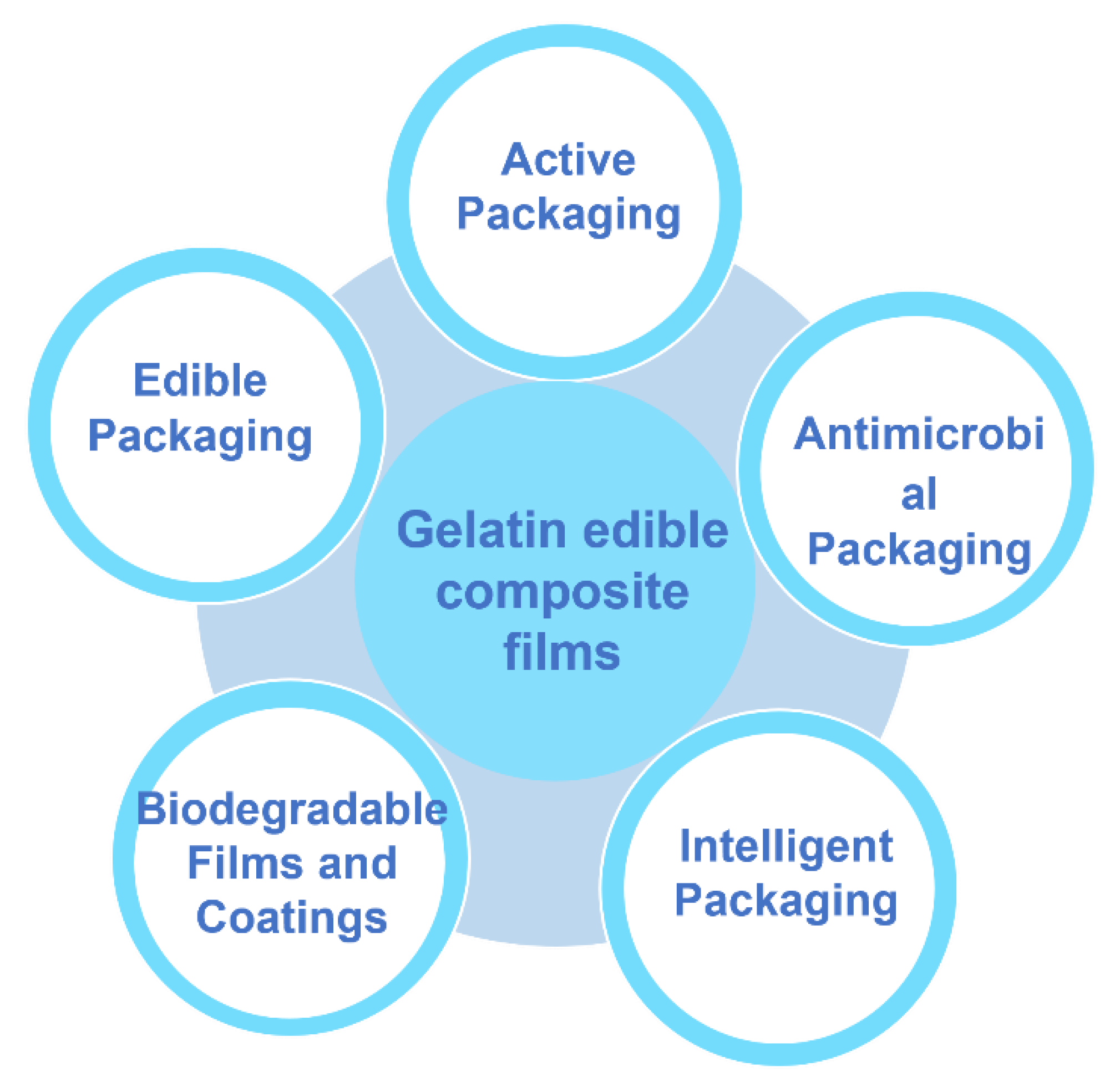
3. The Preparation Methods of Gelatin-Based Edible Composite Films and Coatings
3.1. Solution Casting
3.2. Extrusion
3.3. Coating
4. Modified with Other Polymers and Active Ingredients
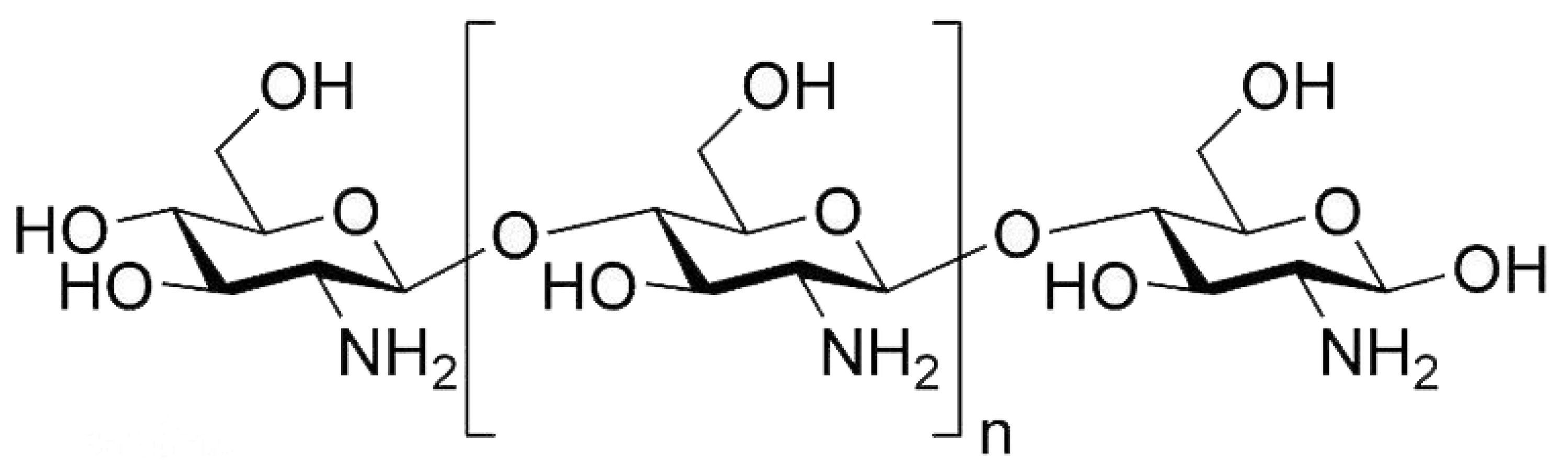
4.1. Gelatin Blended with Carbohydrate
4.2. Gelatin Modified with Enzymes and Proteins
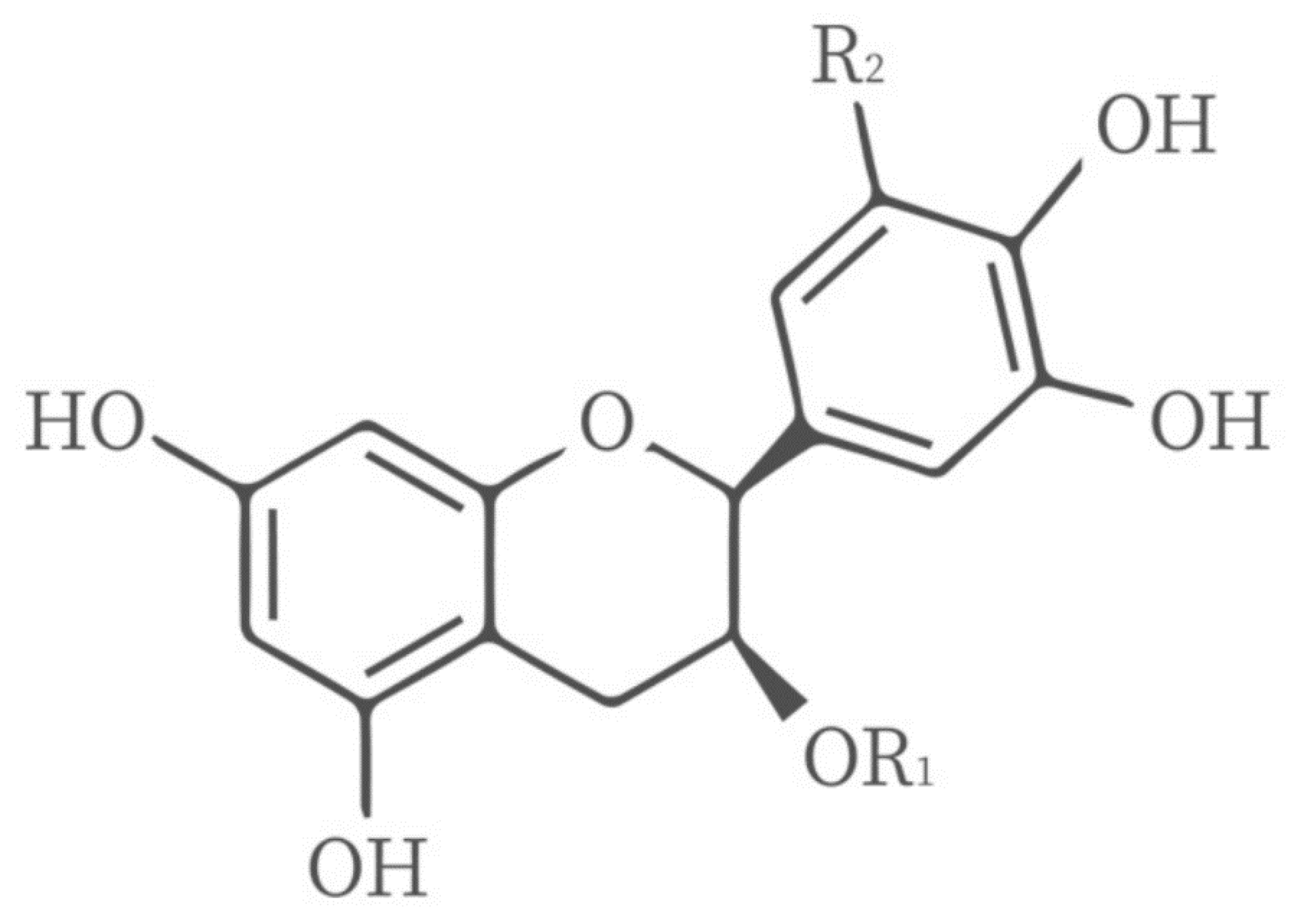
4.3. Gelatin Compounded with Polyphenols
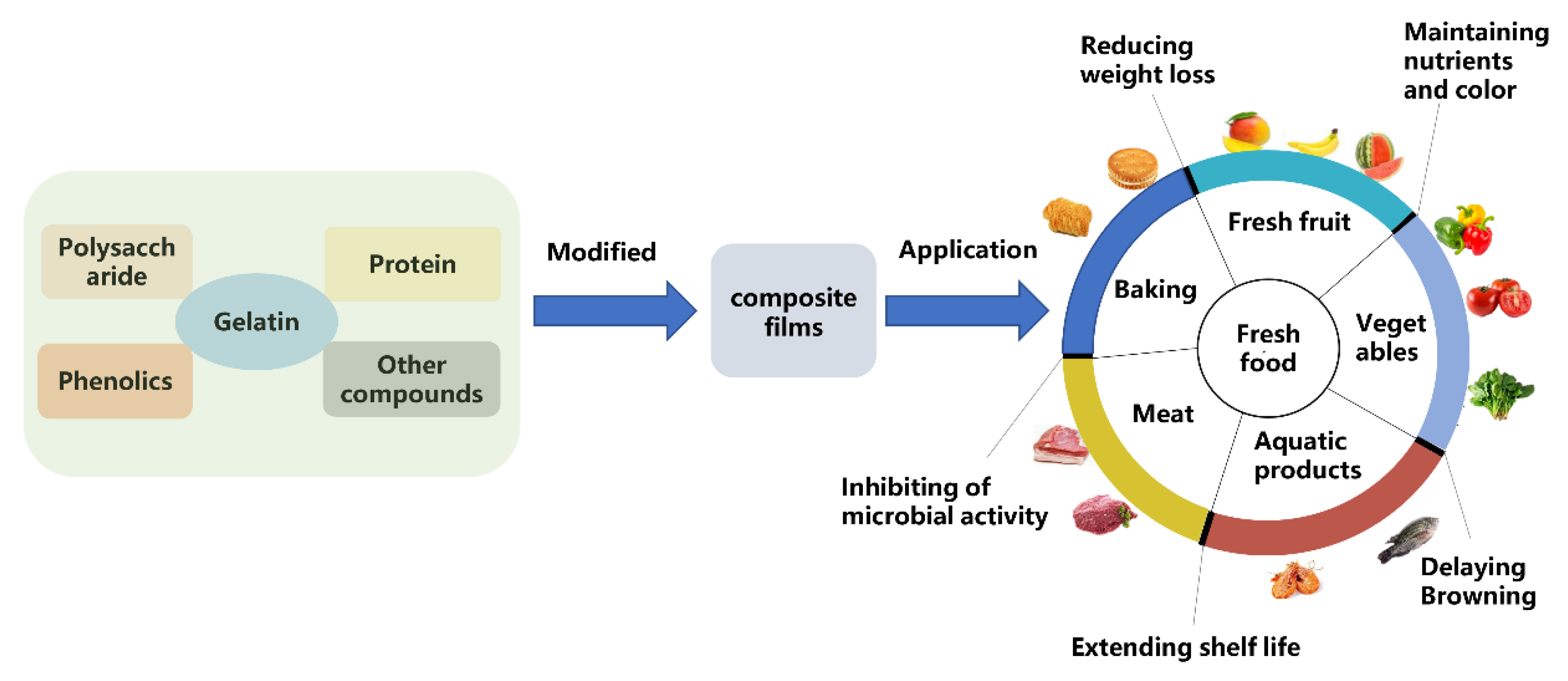
5. Application of Gelatin-Based Composite Edible Films and Coatings
5.1. Fresh Fruit
5.2. The Vegetables
5.3. Aquatic Products
5.4. Meat
5.5. Baking
6. Conclusions and Future Research Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elham, T.-K.; Hajar, S.; Mahdieh, M.-B. Development of edible films and coatings from alginates and carrageenans. Carbohyd. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef]
- Umaraw, P.; Munekata, P.E.S.; Verma, A.K.; Barba, F.J.; Singh, V.P.; Kumar, P.; Lorenzo, J.M. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Nafchi, A.M.; Salehabadi, A.; Oladzad-abbasabadi, N.; Jafari, S.M. Application of bio-nanocomposite films and edible coatings for extending the shelf life of fresh fruits and vegetables. Adv. Colloid Interface Sci. 2021, 291, 102405. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Ismail, A.; Ahmad, S.A.; Khalil, K.A.; Kumar, Y.; Adeyemi, K.D.; Sazili, A.Q. Recent advances on the role of process variables affecting gelatin yield and characteristics with special reference to enzymatic extraction: A review. Food Hydrocoll. 2017, 63, 85–96. [Google Scholar] [CrossRef]
- Xiaoqing, Z.; My Dieu, D.; Casey, P.; Sulistio, A.; Qiao, G.G.; Lundin, L.; Lillford, P.; Kosaraju, S. Chemical modification of gelatin by a natural phenolic cross-linker, tannic acid. J. Agric. Food Chem. 2010, 58, 6809–6815. [Google Scholar]
- Sazedul, H.; Soottawat, B.; Thummanoon, P. Effects of partial hydrolysis and plasticizer content on the properties of film from cuttlefish (Sepia pharaonis) skin gelatin. Food Hydrocolloid 2011, 25, 82–90. [Google Scholar]
- Rhim, J.W.; Park, H.M.; Ha, C.S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Estevez-Areco, S.; Guz, L.; Fama, L.; Candal, R.; Goyanes, S. Bioactive starch nanocomposite films with antioxidant activity and enhanced mechanical properties obtained by extrusion followed by thermo-compression. Food Hydrocoll. 2019, 96, 518–528. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, H.; Jia, R.; Dai, Y.; Dong, H.; Hou, H.; Guo, Q. High performance extrusion blown starch/polyvinyl alcohol/clay nanocomposite films. Food Hydrocoll. 2018, 79, 534–543. [Google Scholar] [CrossRef]
- Vargas, M.; Sánchez-González, L.; Cháfer, M.; Chiralt, A.; González-Martínez, C. 5-Edible chitosan coatings for fresh and minimally processed foods. In Emerging Food Packaging Technologies; Yam, K.L., Lee, D.S., Eds.; Woodhead Publishing: Thorston, UK, 2012; pp. 66–95. [Google Scholar]
- Guzmán, E.; Mateos-Maroto, A.; Ruano, M.; Ortega, F.; Rubio, R.G. Layer-by-Layer polyelectrolyte assemblies for encapsulation and release of active compounds. Adv. Colloid Interface Sci. 2017, 249, 290–307. [Google Scholar] [CrossRef]
- Pan, H.M.; Subramanian, A.; Ochs, C.J.; Dewavrin, J.-Y.; Beyer, S.; Trau, D.W. Edible polyelectrolyte microcapsules with water-soluble cargo assembled in organic phase. RSC Adv. 2014, 4, 35163–35166. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, J.; Zhang, Y.; Wang, X.; Lorenzo, J.M.; Zhong, J. Gelatins as emulsifiers for oil-in-water emulsions: Extraction, chemical composition, molecular structure, and molecular modification. Trends Food Sci. Technol. 2020, 106, 113–131. [Google Scholar] [CrossRef]
- Lv, L.-C.; Huang, Q.-Y.; Ding, W.; Xiao, X.-H.; Zhang, H.-Y.; Xiong, L.-X. Fish gelatin: The novel potential applications. J. Funct. Foods 2019, 63, 103581. [Google Scholar] [CrossRef]
- Piao, Y.; You, H.; Xu, T.; Bei, H.-P.; Piwko, I.Z.; Kwan, Y.Y.; Zhao, X. Biomedical applications of gelatin methacryloyl hydrogels. Eng. Regen. 2021, 2, 47–56. [Google Scholar] [CrossRef]
- Mariod, A.A. Sorghum Bug (Agonoscelis pubescens) as a Source of Edible Oil, Protein, and Gelatin; Springer: Cham, Switzerland, 2020; pp. 149–158. [Google Scholar]
- Zhihua, P.; Deeth, H.; Hongshun, Y.; Sangeeta, P.; Nidhi, B. Evaluation of tilapia skin gelatin as a mammalian gelatin replacer in acid milk gels and low-fat stirred yogurt. J. Dairy Sci. 2017, 100, 3436–3447. [Google Scholar] [CrossRef] [Green Version]
- Mariod, A.A.; Adam, H.F. Review: Gelatin, source, extraction and industrial applications. Acta Sci. Pol.—Technol. Aliment. 2013, 12, 135–147. [Google Scholar]
- Stevens, J.R.; Newton, R.W.; Tlusty, M.; Little, D.C. The rise of aquaculture by-products: Increasing food production, value, and sustainability through strategic utilisation. Mar. Policy 2018, 90, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Pauly, D.; Zeller, D. Comments on FAOs State of World Fisheries and Aquaculture (SOFIA 2016). Mar. Policy 2017, 77, 176–181. [Google Scholar] [CrossRef]
- Luo, Q.; Hossen, M.A.; Zeng, Y.; Dai, J.; Li, S.; Qin, W.; Liu, Y. Gelatin-based composite films and their application in food packaging: A review. J. Food Eng. 2022, 313, 110762. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Carboxymethyl cellulose-based antioxidant and antimicrobial active packaging film incorporated with curcumin and zinc oxide. Int. J. Biol. Macromol. 2020, 148, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.-W. Fabrication of bioactive binary composite film based on gelatin/chitosan incorporated with cinnamon essential oil and rutin. Colloids Surf. B Biointerfaces 2021, 204, 111830. [Google Scholar] [CrossRef]
- Musso, Y.S.; Salgado, P.R.; Mauri, A.N. Smart edible films based on gelatin and curcumin. Food Hydrocoll. 2017, 66, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Wang, W.; Zhang, R.; Zhai, X.; Hou, H. Effect of gelatin bloom values on the physicochemical properties of starch/gelatin–beeswax composite films fabricated by extrusion blowing. Food Hydrocoll. 2021, 113, 106466. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Kingwascharapong, P.; Arisa, K.; Karnjanapratum, S.; Tanaka, F.; Tanaka, F. Effect of gelatin-based coating containing frog skin oil on the quality of persimmon and its characteristics. Sci. Hortic. 2020, 260, 108864. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Cui, Q.-L.; Wang, Y.; Shi, F.; Liu, Y.-P.; Liu, J.-L.; Nie, G.-W. Effect of carboxymethyl chitosan-gelatin-based edible coatings on the quality and antioxidant properties of sweet cherry during postharvest storage. Sci. Hortic. 2021, 289, 110462. [Google Scholar] [CrossRef]
- Sui Chin, S.; Han Lyn, F.; Nur Hanani, Z.A. Effect of Aloe vera (Aloe barbadensis Miller) gel on the physical and functional properties of fish gelatin films as active packaging. Food Packag. Shelf Life 2017, 12, 128–134. [Google Scholar] [CrossRef]
- Kulawik, P.; Jamróz, E.; Zając, M.; Guzik, P.; Tkaczewska, J. The effect of furcellaran-gelatin edible coatings with green and pu-erh tea extracts on the microbiological, physicochemical and sensory changes of salmon sushi stored at 4 °C. Food Control 2019, 100, 83–91. [Google Scholar] [CrossRef]
- Nur Hanani, Z.A.; Roos, Y.H.; Kerry, J.P. Use and application of gelatin as potential biodegradable packaging materials for food products. Int. J. Biol. Macromol. 2014, 71, 94–102. [Google Scholar] [CrossRef]
- Tongdeesoontorn, W.; Rawdkuen, S. Gelatin-Based Films and Coatings for Food Packaging Applications. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Liu, C.; Huang, J.; Zheng, X.; Liu, S.; Lu, K.; Tang, K.; Liu, J. Heat sealable soluble soybean polysaccharide/gelatin blend edible films for food packaging applications. Food Packag. Shelf Life 2020, 24, 100485. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Ghavi, F.F. Preparation and functional properties of fish gelatin–chitosan blend edible films. Food Chem. 2013, 136, 1490–1495. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Martelli, S.M.; Caon, T.; Velasco, J.I.; Mei, L.H.I. Edible films and coatings based on starch/gelatin: Film properties and effect of coatings on quality of refrigerated Red Crimson grapes. Postharvest Biol. Technol. 2015, 109, 57–64. [Google Scholar] [CrossRef]
- Cao, N.; Fu, Y.; He, J. Preparation and physical properties of soy protein isolate and gelatin composite films. Food Hydrocoll. 2007, 21, 1153–1162. [Google Scholar] [CrossRef]
- Nur Hazirah, M.A.S.P.; Isa, M.I.N.; Sarbon, N.M. Effect of xanthan gum on the physical and mechanical properties of gelatin-carboxymethyl cellulose film blends. Food Packag. Shelf Life 2016, 9, 55–63. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, H.; Julian McClements, D.; Chen, L.; Jiao, A.; Tian, Y.; Miao, M.; Jin, Z. Recent Advances in Intelligent Food Packaging Materials: Principles, Preparation and Applications. Food Chem. 2021, 375, 131738. [Google Scholar] [CrossRef]
- Kchaou, H.; Benbettaïeb, N.; Jridi, M.; Abdelhedi, O.; Karbowiak, T.; Brachais, C.-H.; Léonard, M.-L.; Debeaufort, F.; Nasri, M. Enhancement of structural, functional and antioxidant properties of fish gelatin films using Maillard reactions. Food Hydrocoll. 2018, 83, 326–339. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Balaguer, M.P.; López-Carballo, G.; Gavara, R.; Hernández-Muñoz, P. Improving antioxidant and antimicrobial properties of curcumin by means of encapsulation in gelatin through electrohydrodynamic atomization. Food Hydrocoll. 2017, 70, 313–320. [Google Scholar] [CrossRef]
- Zhong, C.; Hou, P.-F.; Li, Y.-X.; Yang, W.-Y.; Shu, M.; Wu, G.-P. Characterization, antioxidant and antibacterial activities of gelatin film incorporated with protocatechuic acid and its application on beef preservation. LWT 2021, 151, 112154. [Google Scholar] [CrossRef]
- Li, M.; Yu, H.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Fabrication of eugenol loaded gelatin nanofibers by electrospinning technique as active packaging material. LWT 2021, 139, 110800. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Ben Amara, C.; Oulahal, N.; Gharsallaoui, A.; Joly, C.; Tongdeesoontorn, W.; Rawdkuen, S.; Degraeve, P. Gelatin films with nisin and catechin for minced pork preservation. Food Packag. Shelf Life 2018, 18, 173–183. [Google Scholar] [CrossRef]
- Hajji, S.; Kchaou, H.; Bkhairia, I.; Ben Slama-Ben Salem, R.; Boufi, S.; Debeaufort, F.; Nasri, M. Conception of active food packaging films based on crab chitosan and gelatin enriched with crustacean protein hydrolysates with improved functional and biological properties. Food Hydrocoll. 2021, 116, 106639. [Google Scholar] [CrossRef]
- Nur Amila Najwa, I.S.; Mat Yusoff, M.; Nur Hanani, Z.A. Potential of Silver-Kaolin in Gelatin Composite Films as Active Food Packaging Materials. Food Packag. Shelf Life 2020, 26, 100564. [Google Scholar] [CrossRef]
- Wang, L.; Lin, L.; Guo, Y.; Long, J.; Mu, R.-J.; Pang, J. Enhanced functional properties of nanocomposite film incorporated with EGCG-loaded dialdehyde glucomannan/gelatin matrix for food packaging. Food Hydrocoll. 2020, 108, 105863. [Google Scholar] [CrossRef]
- Wu, J.; Sun, X.; Guo, X.; Ge, S.; Zhang, Q. Physicochemical properties, antimicrobial activity and oil release of fish gelatin films incorporated with cinnamon essential oil. Aquac. Fish. 2017, 2, 185–192. [Google Scholar] [CrossRef]
- Zhang, Z.; Hao, G.; Liu, C.; Fu, J.; Hu, D.; Rong, J.; Yang, X. Recent progress in the preparation, chemical interactions and applications of biocompatible polysaccharide-protein nanogel carriers. Food Res. Int. 2021, 147, 110564. [Google Scholar] [CrossRef] [PubMed]
- Khatri, D.; Panigrahi, J.; Prajapati, A.; Bariya, H. Attributes of Aloe vera gel and chitosan treatments on the quality and biochemical traits of post-harvest tomatoes. Sci. Hortic. 2020, 259, 108837. [Google Scholar] [CrossRef]
- Uranga, J.; Puertas, A.I.; Etxabide, A.; Dueñas, M.T.; Guerrero, P.; de la Caba, K. Citric acid-incorporated fish gelatin/chitosan composite films. Food Hydrocoll. 2019, 86, 95–103. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, M.; Warner, R.D.; Fang, Z. Incorporating nisin and grape seed extract in chitosan-gelatine edible coating and its effect on cold storage of fresh pork. Food Control 2020, 110, 107018. [Google Scholar] [CrossRef]
- Ahammed, S.; Liu, F.; Wu, J.; Khin, M.N.; Yokoyama, W.H.; Zhong, F. Effect of transglutaminase crosslinking on solubility property and mechanical strength of gelatin-zein composite films. Food Hydrocoll. 2021, 116, 106649. [Google Scholar] [CrossRef]
- Weng, W.; Zheng, H. Effect of transglutaminase on properties of tilapia scale gelatin films incorporated with soy protein isolate. Food Chem. 2015, 169, 255–260. [Google Scholar] [CrossRef]
- Khedri, S.; Sadeghi, E.; Rouhi, M.; Delshadian, Z.; Mortazavian, A.M.; de Toledo Guimarães, J.; Fallah, M.; Mohammadi, R. Bioactive edible films: Development and characterization of gelatin edible films incorporated with casein phosphopeptides. LWT 2021, 138, 110649. [Google Scholar] [CrossRef]
- Pinto, T.; Aires, A.; Cosme, F.; Bacelar, E.; Morais, M.C.; Oliveira, I.; Ferreira-Cardoso, J.; Anjos, R.; Vilela, A.; Gonçalves, B. Bioactive (Poly)phenols, Volatile Compounds from Vegetables, Medicinal and Aromatic Plants. Foods 2021, 10, 106. [Google Scholar] [CrossRef]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical properties and antioxidant activity of gelatin-sodium alginate edible films with tea polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhou, C.; Fu, F.; Chen, Z.; Wu, Q. Effect of high-pressure homogenization on particle size and film properties of soy protein isolate. Ind. Crops Prod. 2013, 43, 538–544. [Google Scholar] [CrossRef]
- Bitencourt, C.M.; Fávaro-Trindade, C.S.; Sobral, P.J.A.; Carvalho, R.A. Gelatin-based films additivated with curcuma ethanol extract: Antioxidant activity and physical properties of films. Food Hydrocoll. 2014, 40, 145–152. [Google Scholar] [CrossRef]
- Tan, Y.M.; Lim, S.H.; Tay, B.Y.; Lee, M.W.; Thian, E.S. Functional chitosan-based grapefruit seed extract composite films for applications in food packaging technology. Mater. Res. Bull. 2015, 69, 142–146. [Google Scholar] [CrossRef]
- Fu, S.; Wu, C.; Wu, T.; Yu, H.; Yang, S.; Hu, Y. Preparation and characterisation of Chlorogenic acid-gelatin: A type of biologically active film for coating preservation. Food Chem. 2017, 221, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Temiz, N.N.; Zdemir, K.S. Microbiological and physicochemical quality of strawberries (Fragaria × ananassa) coated with Lactobacillus rhamnosus and inulin enriched gelatin films. Postharvest Biol. Technol. 2020, 173, 111433. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Z.; Shi, J.; Zou, X.; Zhai, X.; Huang, X.; Li, Z.; Holmes, M.; Daglia, M.; Xiao, J. Physical properties and bioactivities of chitosan/gelatin-based films loaded with tannic acid and its application on the preservation of fresh-cut apples—ScienceDirect. LWT 2021, 144, 111223. [Google Scholar] [CrossRef]
- Soradech, S.; Nunthanid, J.; Limmatvapirat, S.; Luangtana-Anan, M. Utilization of shellac and gelatin composite film for coating to extend the shelf life of banana. Food Control 2017, 73, 1310–1317. [Google Scholar] [CrossRef]
- Singh Sooch, B.; Kaur Mann, M. Nanoreinforced biodegradable gelatin based active food packaging film for the enhancement of shelf life of tomatoes (Solanum lycopersicum L.). Food Control 2021, 130, 108322. [Google Scholar] [CrossRef]
- Ruoyi, H.; Yang, L.; Liming, S.; Lining, X.; Hui, J.; Qi, L.; Jinfeng, P. Sodium alginate coating with plant extract affected microbial communities, biogenic amine formation and quality properties of abalone (Haliotis discus hannai Ino) during chill storage. LWT—Food Sci. Technol. 2017, 81, 1–9. [Google Scholar] [CrossRef]
- Xiao, F.; Nidhi, B.; Hongshun, Y. Fish gelatin combined with chitosan coating inhibits myofibril degradation of golden pomfret (Trachinotus blochii) fillet during cold storage. Food Chem. 2016, 200, 283–292. [Google Scholar] [CrossRef]
- Hu, H.; Yao, X.; Qin, Y.; Yong, H.; Liu, J. Development of multifunctional food packaging by incorporating betalains from vegetable amaranth (Amaranthus tricolor L.) into quaternary ammonium chitosan/fish gelatin blend films. Int. J. Biol. Macromol. 2020, 159, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Tkk, A.; Hiy, A.; Sj, B.; Ybk, A.; Ysc, A. Effects of replacing pork fat with grape seed oil and gelatine/alginate for meat emulsions—ScienceDirect. Meat Sci. 2020, 163, 108079. [Google Scholar]
- Jridi, M.; Mora, L.; Souissi, N.; Aristoy, M.C.; Nasri, M.; Toldrá, F. Effects of active gelatin coated with henna (L. inermis) extract on beef meat quality during chilled storage. Food Control 2018, 84, 238–245. [Google Scholar] [CrossRef]
- Oliveira, M.A.; Gonzaga, M.L.C.; Bastos, M.S.R.; Magalhães, H.C.R.; Benevides, S.D.; Furtado, R.F.; Zambelli, R.A.; Garruti, D.S. Packaging with cashew gum/gelatin/essential oil for bread: Release potential of the citral. Food Packag. Shelf Life 2020, 23, 100431. [Google Scholar] [CrossRef]
- Abdollahzadeh, E.; Nematollahi, A.; Hosseini, H. Composition of antimicrobial edible films and methods for assessing their antimicrobial activity: A review. Trends Food Sci. Technol. 2021, 110, 291–303. [Google Scholar] [CrossRef]
- Pella, M.C.G.; Silva, O.A.; Pella, M.G.; Beneton, A.G.; Caetano, J.; Simoes, M.R.; Dragunski, D.C. Effect of gelatin and casein additions on starch edible biodegradable films for fruit surface coating. Food Chem. 2020, 309, 125764. [Google Scholar] [CrossRef]
- Otoni, C.G.; Avena-Bustillos, R.J.; Azeredo, H.M.C.; Lorevice, M.V.; Moura, M.R.; Mattoso, L.H.C.; Mchugh, T.H. Recent Advances on Edible Films Based on Fruits and Vegetables—A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Bai, M.; Rashed, M.M.A.; Lin, L. The antibacterial activity of clove oil/chitosan nanoparticles embedded gelatin nanofibers against Escherichia coli O157:H7 biofilms on cucumber. Int. J. Food Microbiol. 2018, 266, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Ghaly, A.E.; Dave, D.; Budge, S.; Brooks, M.S. Fish Spoilage Mechanisms and Preservation Techniques: Review. Am. J. Appl. Sci. 2010, 7, 859–877. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Zhao, Y.; Warner, R.D.; Johnson, S.K. Active and intelligent packaging in meat industry. Trends Food Sci. Technol. 2017, 61, 60–71. [Google Scholar] [CrossRef]
- Yu, D.; Regenstein, J.M.; Xia, W. Bio-based edible coatings for the preservation of fishery products: A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2481–2493. [Google Scholar] [CrossRef]
- Yun, X.; Kamboj, M.; Ajlouni, S.; Zhongxiang, F. Incorporation of salmon bone gelatine with chitosan, gallic acid and clove oil as edible coating for the cold storage of fresh salmon fillet. Food Control 2021, 125, 107994. [Google Scholar] [CrossRef]
- Fengping, W.; Huijun, Z.; Wengang, J.; Lirong, L. Effects of tartary buckwheat polysaccharide combined with nisin edible coating on the storage quality of tilapia (Oreochromis niloticus) fillets. J. Sci. Food Agric. 2018, 98, 2880–2888. [Google Scholar] [CrossRef]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010, 120, 193–198. [Google Scholar] [CrossRef]
- He, F.; Kong, Q.; Jin, Z.; Mou, H. Developing a unidirectionally permeable edible film based on -carrageenan and gelatin for visually detecting the freshness of grass carp fillets. Carbohyd. Polym. 2020, 241, 116336. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.E.-D.A.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Hematizad, I.; Khanjari, A.; Basti, A.A.; Karabagias, I.K.; Noori, N.; Ghadami, F.; Gholami, F.; Teimourifard, R. In vitro antibacterial activity of gelatin-nanochitosan films incorporated with Zataria multiflora Boiss essential oil and its influence on microbial, chemical, and sensorial properties of chicken breast meat during refrigerated storage. Food Packag. Shelf Life 2021, 30, 100751. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Fernández-Prior, Á.; Fernández-Bolaños, J. Effect of edible pectin-fish gelatin films containing the olive antioxidants hydroxytyrosol and 3,4-dihydroxyphenylglycol on beef meat during refrigerated storage. Meat Sci. 2019, 148, 213–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.; Xu, D.; Zhang, H.; Guo, L.; Hong, T.; Zhang, W.; Jin, Y.; Xu, X. Effect of pigskin gelatin on baking, structural and thermal properties of frozen dough: Comprehensive studies on alteration of gluten network. Food Hydrocoll. 2020, 102, 105591. [Google Scholar] [CrossRef]
- Yu, W.; Xu, D.; Li, D.; Guo, L.; Su, X.; Zhang, Y.; Wu, F.; Xu, X. Effect of pigskin-originated gelatin on properties of wheat flour dough and bread. Food Hydrocoll. 2019, 94, 183–190. [Google Scholar] [CrossRef]
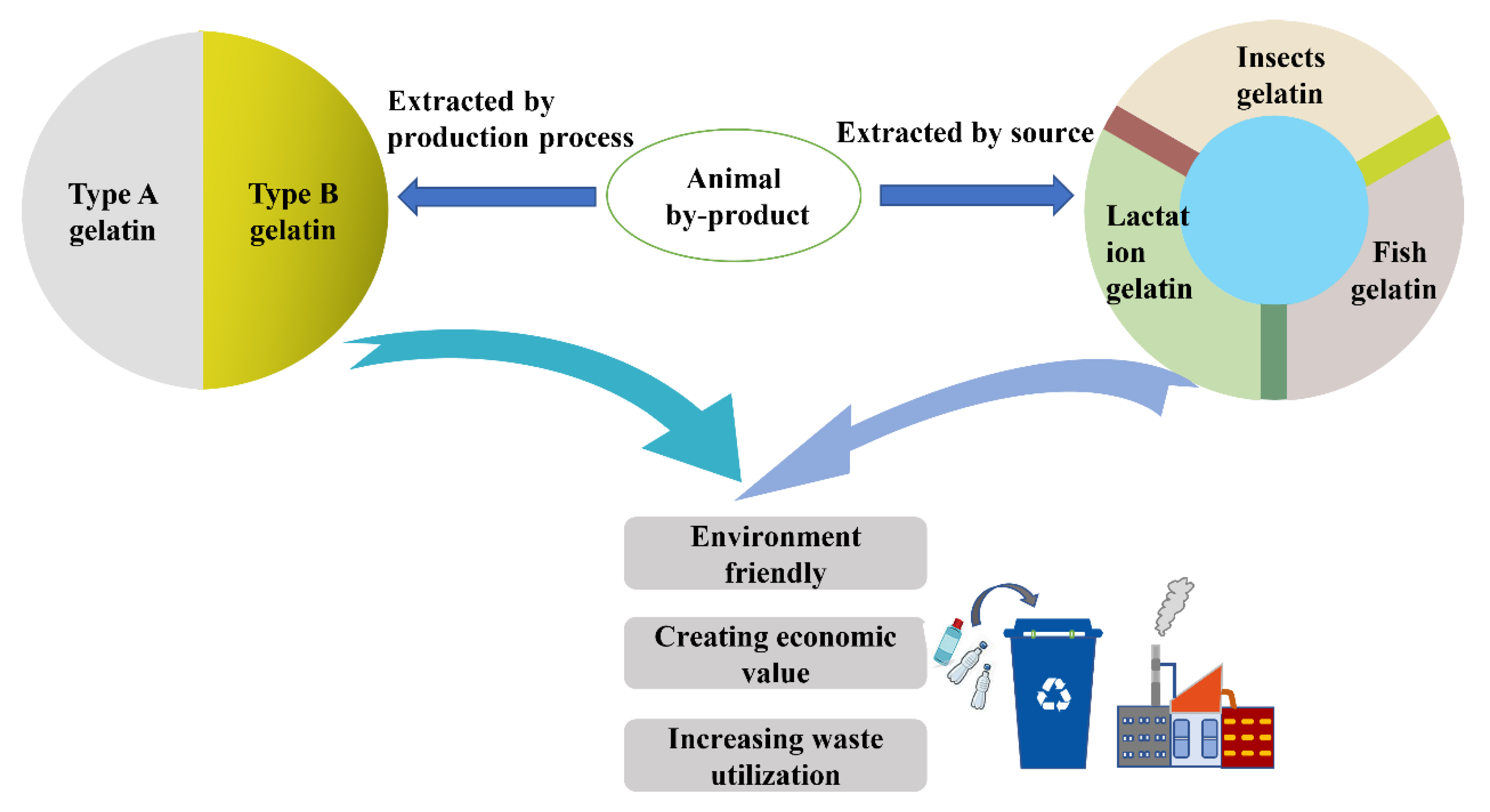
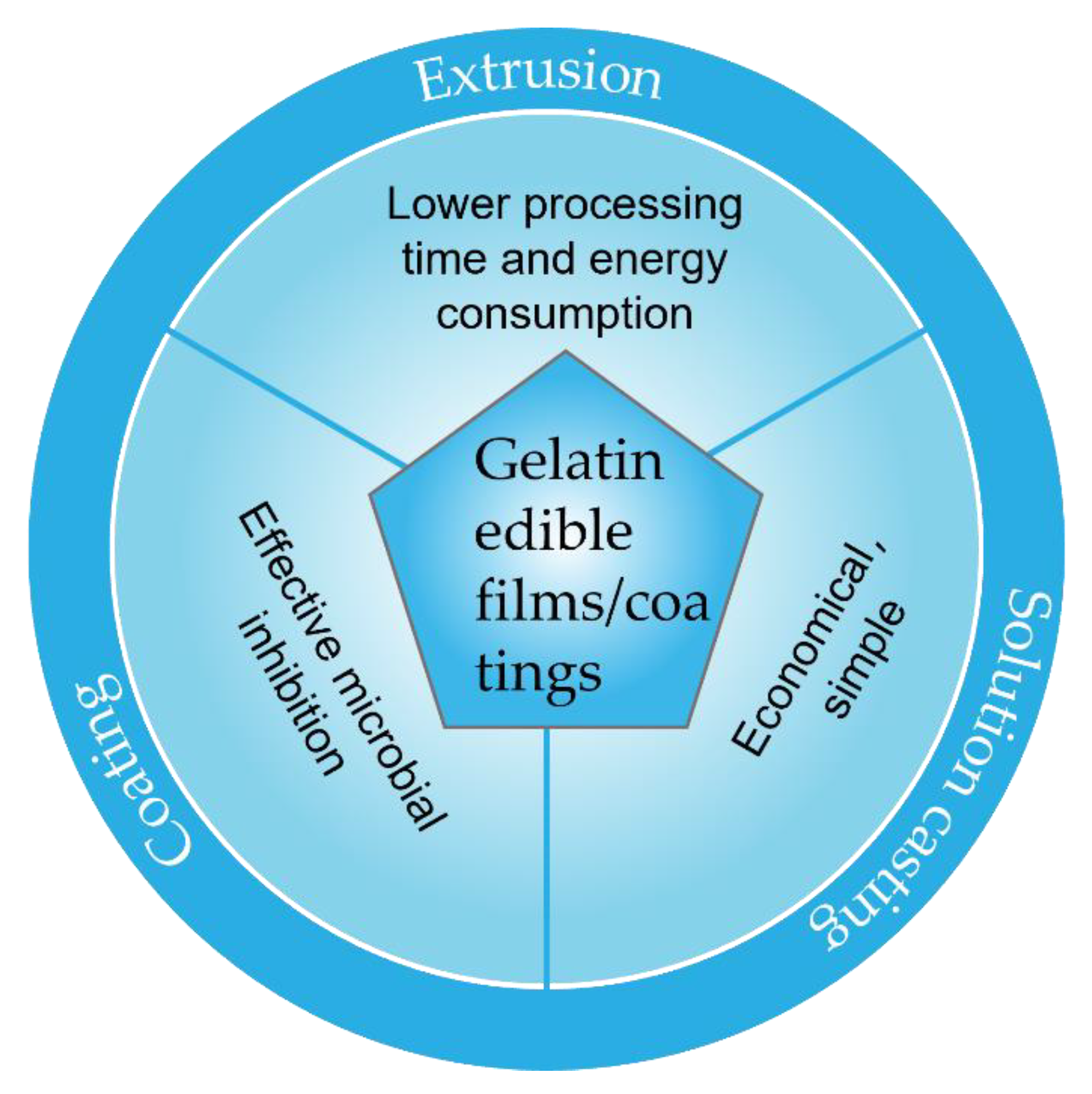

| Composite Materials | Physical Properties | Biological Properties | Reference |
|---|---|---|---|
| Carbohydrate | |||
| Aloe vera gel | —Reduced water solubility and tensile strength —No significant effect on thickness, water vapor permeability (WVP) or color —The mechanical properties of aloe gel were decreased with the increase of aloe gel concentration —The plasticity was increased significantly | —Increased antioxidant properties | [30] |
| RNA | —Shielded ultraviolet ray (UV) radiation to the highest degree —Increased color change and browning index | —Increased antibacterial activity and antioxidant activity | [39] |
| Glucose | —Increased tensile strength, elongation at break and glass transition temperature —Increased UV resistance and water resistance (water solubility and water contact angle) | —Increased the antioxidant capacity significantly with the increase of glucose content —The antioxidant activity was increased to a lesser extent with increasing reaction time | [40] |
| Polyphenols | |||
| Curcumin | —Increased the solubility of curcumin | —Increased antioxidant activity and inhibited the growth of pathogenic bacteria, Streptococcus enterococcu Staphylococcus aureus, Listeria monocytogenes, and E. coli | [41] |
| Protocatechuic acid (PCA) | —Increased thickness —Improved transparency and obtained fine appearance —Reduced light transmittance significantly —Reduced tensile strength and increased elongation at break | —High free radical scavenging activity of DPPH —Improved film stability and biological activity —Obtained good bacteriostatic effect with the increase of concentration of PCA | [42] |
| Polylactic acid, eugenol | —The internal fibers are uniform in shape —Enhanced surface water resistance and hydrophobicity —Improved encapsulation efficiency and load capacity | —Antioxidant and antibacterial activities were enhanced | [43] |
| Enzymes and proteins | |||
| Microbial transglutaminase, gelatin-streptococcus, lactin/catechin | —Increased mechanical strength —Improved water vapor and UV resistance —Reduced the solubility and fluidity of films —Increased the viscosity of fish glue | —Increased the antibacterial and antioxidant activity to pathogenic bacteria —Prevented microbial growth and lipid oxidation of pork mince | [44] |
| Egg white protein | —Improved melting point and gel strength —Tensile strength, mechanical strength and deformation were significantly reduced —Improved UV barrier performance —Contact angle was significantly reduced, and surface wettability was increased | —Improved antibacterial and antioxidant properties | [45] |
| Other polymers | |||
| Silver-kaolin | —Improved the surface morphology and structure —Increased the waterproof performance significantly —Increased the thickness and opacity —Reduced the flexibility and tensile strength —Lowed ultraviolet transmittance | —It showed significant inhibition against gram-negative bacteria and gram-positive bacteria (E. coli, Staphylococcus aureus, Listeria monocytogenes and Salmonella typhimurium) | [46] |
| Rutin functionalized cellulose nanocrystal (RCNC) | —Improved thermal stability, dispersion and compatibility —The highest UV-visible light and water vapor resistance —Improved tensile strength and antibacterial performance significantly with the addition of RCNC | —The antibacterial performance of Staphylococcus aureus and E. coli was improved | [47] |
| Cinnamon essential oil (CEO) | —The tensile strength, elongation at break and water content of gelatin-based films was decreased with the increase of CEO concentration, but water vapor permeability was increased. —Improved the light resistance —Improved the UV resistance | —Showed strong inhibition to various microbial pathogens —Enhanced the antibacterial and antioxidant properties of active films | [48] |
| Foods | Composite Materials | Effects | Forming Method | Reference |
|---|---|---|---|---|
| Fresh fruit | ||||
| Strawberry | Probiotics, inulin | —Significantly reduced weight loss, water loss, respiration rate and delays decay —Slowed down pH change —Delayed titratable acidity (TA) content change —Significantly decreased the increase of total soluble solid (TSS) value —The total phenol (TPC) content and antioxidant activity were retained effectively —Inhibited the growth of yeast and mold | Coating | [62] |
| Fresh cut apple | Chitosan, tannin | —Reduced weight loss and malondialdehyde content —Delayed Browning —Inhibition of lipid oxidase activity —Improved oxidation resistance and barrier performance —Improved appearance quality | Mix dry | [63] |
| Banana | Lac | —Slowed chlorophyll degradation and aging, with slight color changes —Reduced titratable acidity, total soluble sugar content and weight loss —Significant increase in hardness —The total number of banana mold/yeast was increased slowly —Extended shelf life and maintained quality | Coating | [64] |
| Fresh vegetable | ||||
| Tomatoes | Titanium (Ti), nanoparticles (CuO) | —Greatly increased shelf life (up to 18 days at 40 ± 3 °C) —It had a higher antibacterial effect on gram-negative cells than gram-positive cells —The film was clean, transparent, shiny and flexible —Helped to maintain flavor, nutrition and color —Film biodegradability was enhanced | Casting | [65] |
| Aquatic products | ||||
| Abalone | Sodium alginate, plant extract (bamboo leaf extract, rosemary extract five) | —Kept good sensory characteristics —Inhibited microbial reproduction and endogenous enzyme activity —Extended the shelf life of abalone —Biogenic amine content was decreased —Decreased microbial population —Delayed pH to drop | Coating | [66] |
| Golden pompano piece | Chitosan | —Prevented myosin and myoglobin from degrading —Effectively controlled the weight loss, economic loss, retained the nutrition and original color —Significantly inhibited amino acid degradation and biogenic amine production —pH was more stable —Better anti-corrosion ability, antibacterial ability | Coating | [67] |
| Shrimp | Amaranth extract, quaternary ammonium chitosan | —Improved light blocking ability —Increased flexibility and oxidation resistance —Reduced stability and improved permeability —It had antibacterial and antioxidant capacity | Casting | [68] |
| Meat | ||||
| Meat emulsion | Grape seed oil, alginate | —Reduced the value of fat content, pH, firmness, chewiness, toughness, and lipid oxidation of the meat emulsion —Had a substantial effect on the physico-chemical properties of meat emulsion | Emulsion | [69] |
| Beef | Aqueous extracts of henna | —Preserved color properties significantly —Decreased the rate of proteolysis process —Decreased the lipid oxidation and microorganisms’ counts —Decrease weight loss and pH —Improve meat preservation | Coating | [70] |
| Baking | ||||
| Bread | Cashew gum, essential oil, ferulic acid | —Maintained bread quality characteristics —Delayed moisture loss and fungus growth —Storage period promoted six days | Casting | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Luo, Q.; Chu, Y.; Tao, N.; Deng, S.; Wang, L.; Li, L. Application of Gelatin in Food Packaging: A Review. Polymers 2022, 14, 436. https://doi.org/10.3390/polym14030436
Lu Y, Luo Q, Chu Y, Tao N, Deng S, Wang L, Li L. Application of Gelatin in Food Packaging: A Review. Polymers. 2022; 14(3):436. https://doi.org/10.3390/polym14030436
Chicago/Turabian StyleLu, Yanan, Qijun Luo, Yuchan Chu, Ningping Tao, Shanggui Deng, Li Wang, and Li Li. 2022. "Application of Gelatin in Food Packaging: A Review" Polymers 14, no. 3: 436. https://doi.org/10.3390/polym14030436
APA StyleLu, Y., Luo, Q., Chu, Y., Tao, N., Deng, S., Wang, L., & Li, L. (2022). Application of Gelatin in Food Packaging: A Review. Polymers, 14(3), 436. https://doi.org/10.3390/polym14030436






