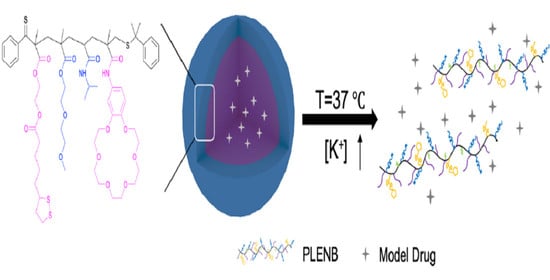
K+-Responsive Crown Ether-Based Amphiphilic Copolymer: Synthesis and Application in the Release of Drugs and Au Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
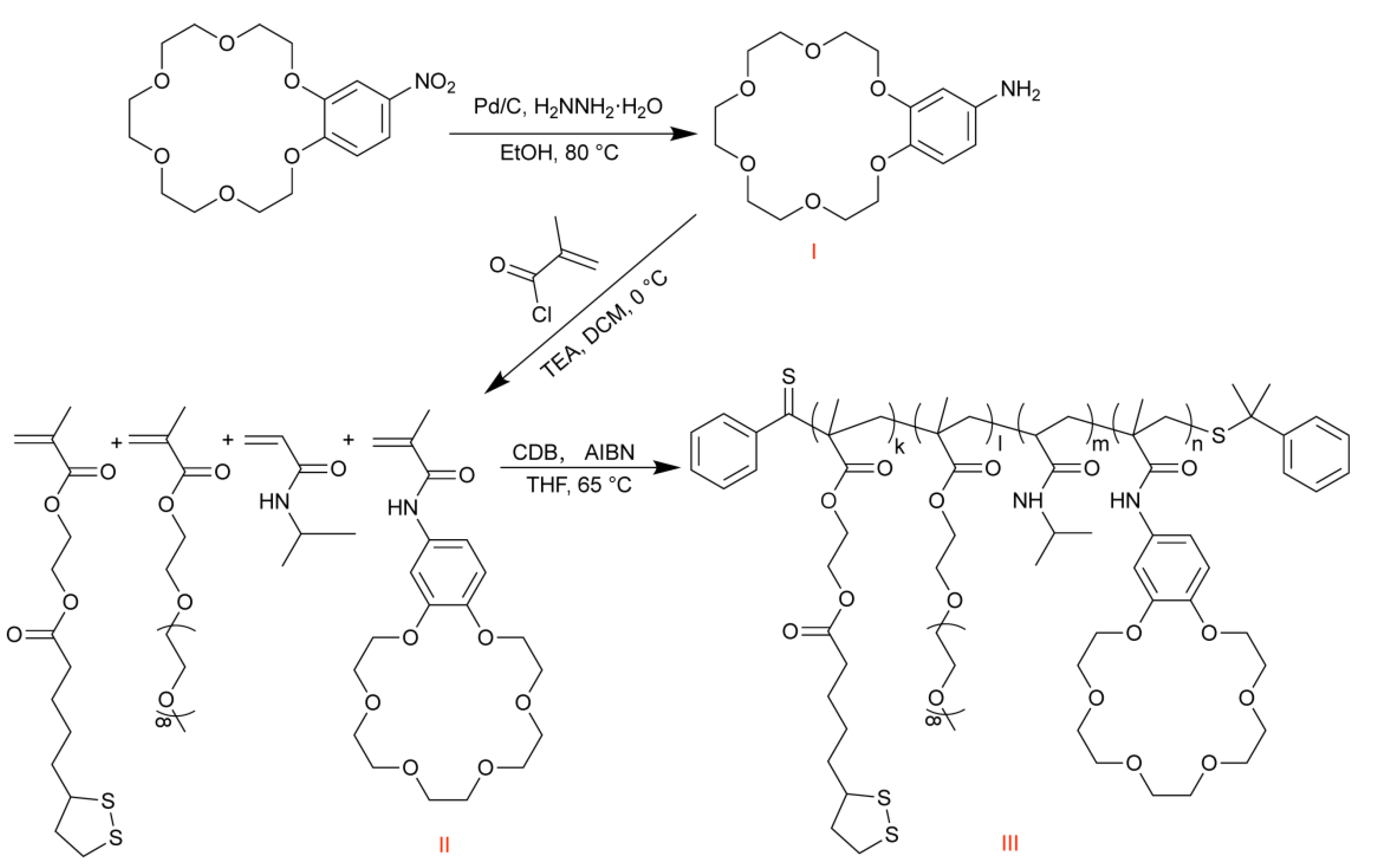
2.3. Synthesis of Monomer and Polymer
2.3.1. Synthesis of Monomer B18C6Am
2.3.2. Synthesis of Polymer PLENB
2.4. The Lowest Critical Solution Temperature LCST Detection
2.4.1. PLENB Solution without Additional Ions
2.4.2. PLENB Aqueous Solution with Additional Ions
2.5. The Self-Assembly of PLENB
2.6. Drug Release Behaviour of PLENB
2.7. Data Analysis
3. Results
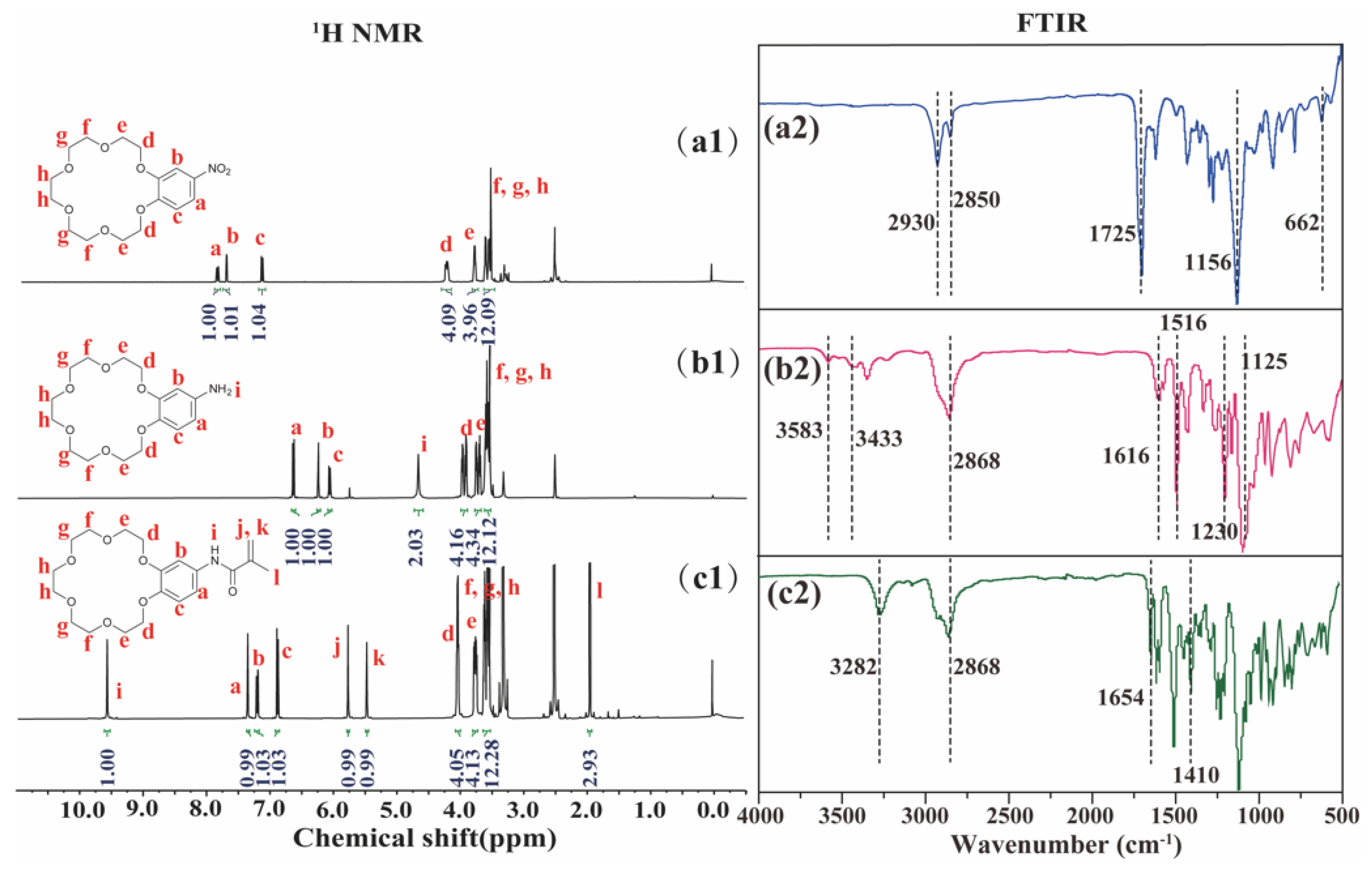
3.1. Characterization of Amphiphilic Polymer PLENB
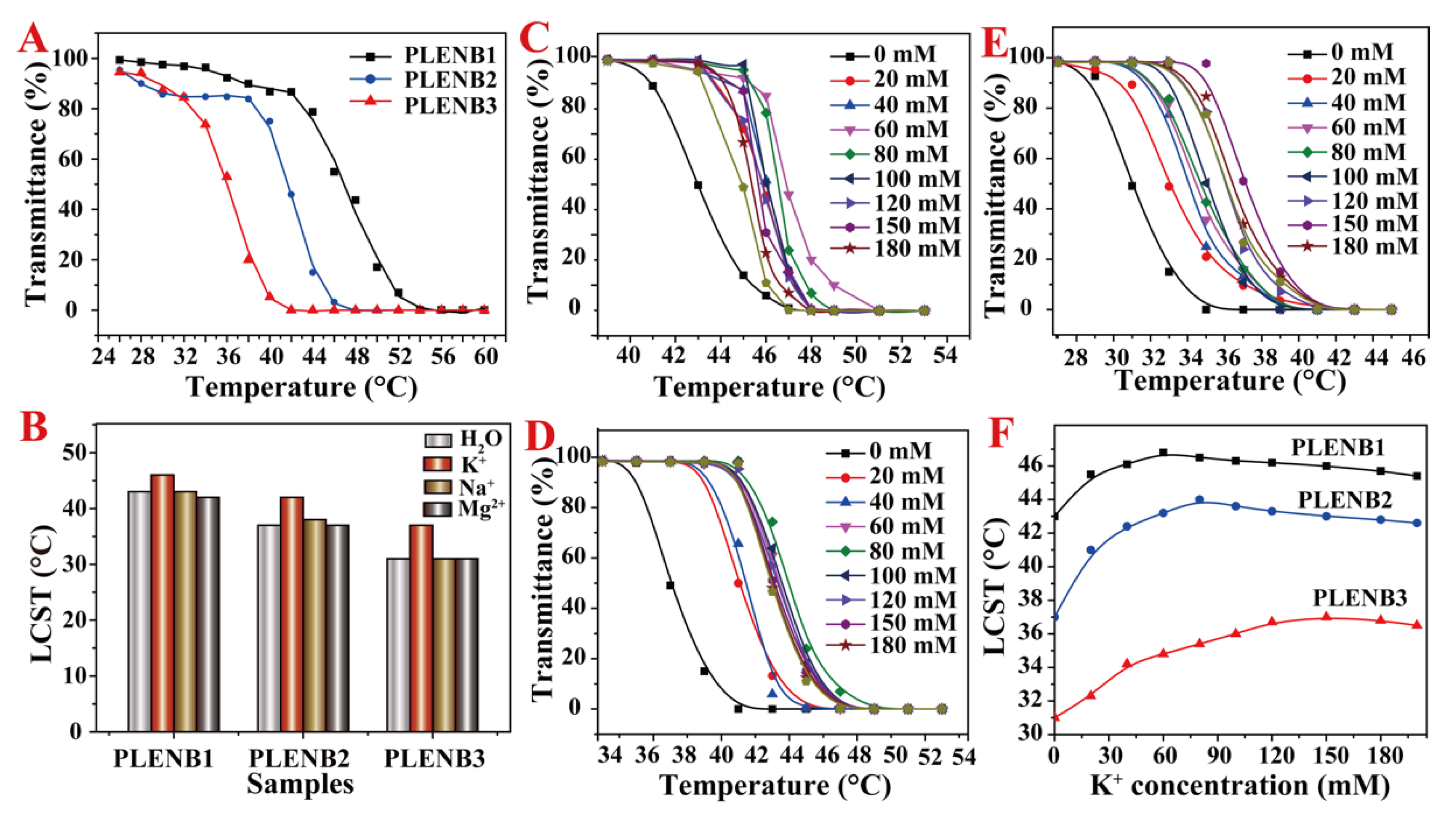
3.2. LCST of PLENB in Aqueous Solution with Ion
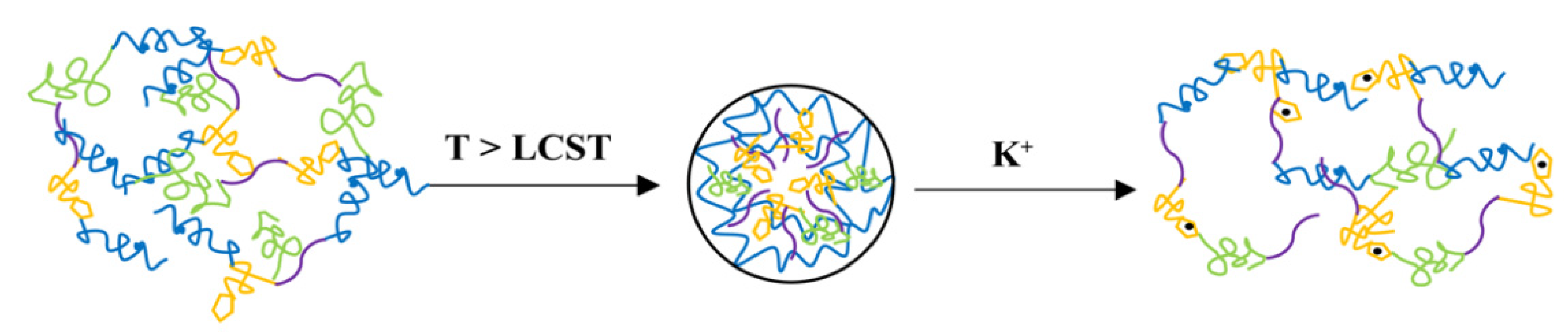
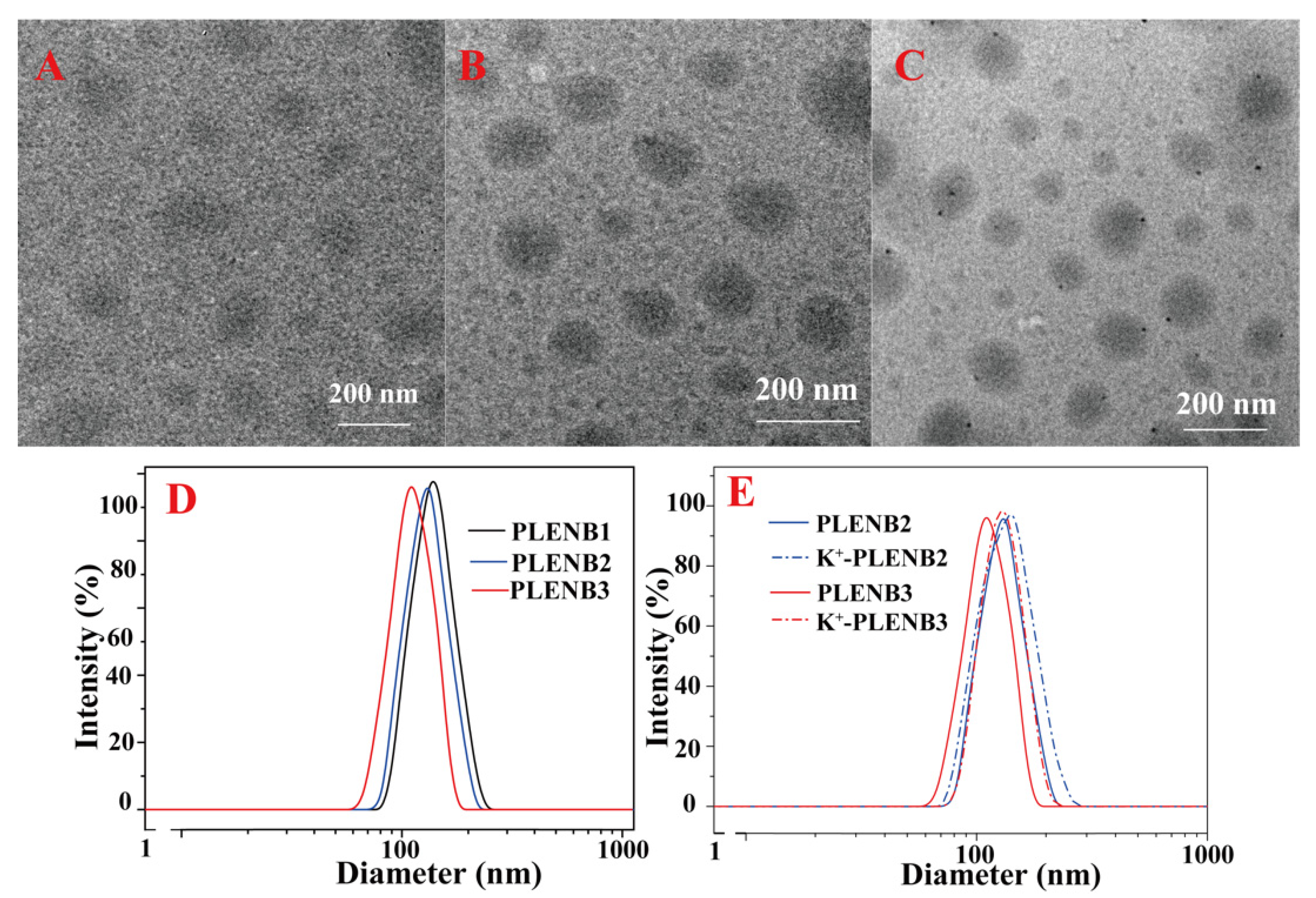
3.3. Self-Assembly of Amphiphilic Copolymer PLENB
3.4. K+-Triggered Disassembly of PLENB3-SH@Au Aggregates
3.5. K+-Triggered Release of DOX
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mamidi, N.; Velasco Delgadillo, R.M.; Barrera, E.V. Covalently Functionalized Carbon Nano-Onions Integrated Gelatin Methacryloyl Nanocomposite Hydrogel Containing γ-Cyclodextrin as Drug Carrier for High-Performance pH-Triggered Drug Release. Pharmaceuticals 2021, 14, 291. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, N.; Zuniga, A.E.; Villela-Castrejon, J. Engineering and evaluation of forcespun functionalized carbon nano-onions reinforced poly (ε-caprolactone) composite nanofibers for pH-responsive drug release. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110928. [Google Scholar] [CrossRef] [PubMed]
- Mohamady Hussein, M.A.; Guler, E.; Rayaman, E.; Cam, M.E.; Sahin, A.; Grinholc, M.; Sezgin Mansuroglu, D.; Sahin, Y.M.; Gunduz, O.; Muhammed, M.; et al. Dual-drug delivery of Ag-chitosan nanoparticles and phenytoin via core-shell PVA/PCL electrospun nanofibers. Carbohydr. Polym. 2021, 270, 118373. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, N.; Delgadillo, R.M.V. Design, fabrication and drug release potential of dual stimuli-responsive composite hydrogel nanoparticle interfaces. Colloids Surf. B Biointerfaces 2021, 204, 111819. [Google Scholar] [CrossRef]
- Kwak, H.W.; Woo, H.; Kim, I.-C.; Lee, K.H. Fish gelatin nanofibers prevent drug crystallization and enable ultrafast delivery. RSC Adv. 2017, 7, 40411–40417. [Google Scholar] [CrossRef] [Green Version]
- Mohammadinejad, R.; Madamsetty, V.S.; Kumar, A.; Varzandeh, M.; Dehshahri, A.; Zarrabi, A.; Sharififar, F.; Mohammadi, M.; Fahimipour, A.; Ramakrishna, S. Electrospun nanocarriers for delivering natural products for cancer therapy. Trends Food Sci. Technol. 2021, 118, 887–904. [Google Scholar] [CrossRef]
- Li, Z.; Tan, B.H. Towards the development of polycaprolactone based amphiphilic block copolymers: Molecular design, self-assembly and biomedical applications. Mater. Sci. Eng. C 2014, 45, 620–634. [Google Scholar] [CrossRef]
- Rösler, A.; Vandermeulen, G.W.M.; Klok, H.-A. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv. Drug Deliv. Rev. 2012, 64, 270–279. [Google Scholar] [CrossRef]
- Mamidi, N.; Delgadillo, R.M.V.; Castrejón, J.V. Unconventional and facile production of a stimuli-responsive multifunctional system for simultaneous drug delivery and environmental remediation. Environ. Sci. Nano 2021, 8, 2081–2097. [Google Scholar] [CrossRef]
- Behroozi, F.; Abdkhodaie, M.J.; Abandansari, H.S.; Satarian, L.; Molazem, M.; Al-Jamal, K.T.; Baharvand, H. Engineering folate-targeting diselenide-containing triblock copolymer as a redox-responsive shell-sheddable micelle for antitumor therapy in vivo. Acta Biomater. 2018, 76, 239–256. [Google Scholar] [CrossRef] [Green Version]
- Klaikherd, A.; Nagamani, C.; Thayumanavan, S. Multi-stimuli sensitive amphiphilic block copolymer assemblies. J. Am. Chem. Soc. 2009, 131, 4830–4838. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Wei, Y.; Li, C.; Mao, S. Inner layer-embedded contact lenses for ion-triggered controlled drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 36–48. [Google Scholar] [CrossRef]
- Moczar, I.; Huszthy, P. Optically active crown ether-based fluorescent sensor molecules: A mini-review. Chirality 2019, 31, 97–109. [Google Scholar] [CrossRef]
- Patel, H.A.; Selberg, J.; Salah, D.; Chen, H.; Liao, Y.; Mohan Nalluri, S.K.; Farha, O.K.; Snurr, R.Q.; Rolandi, M.; Stoddart, J.F. Proton Conduction in Tröger’s Base-Linked Poly (crown ether) s. ACS Appl. Mater. Interfaces 2018, 10, 25303–25310. [Google Scholar] [CrossRef]
- Yang, J.-S.; Hwang, C.-Y.; Chen, M.-Y. Bimodal fluorescence signaling based on control of the excited-state conformational twisting and the ground-state protonation processes. Tetrahedron Lett. 2007, 48, 3097–3102. [Google Scholar] [CrossRef]
- Esteves, C.I.C.; Batista, R.M.F.; Raposo, M.M.M.; Costa, S.P.G. Novel functionalised imidazo-benzocrown ethers bearing a thiophene spacer as fluorimetric chemosensors for metal ion detection. Dye. Pigment. 2016, 135, 134–142. [Google Scholar] [CrossRef]
- Chehardoli, G.; Bahmani, A. The role of crown ethers in drug delivery. Supramol. Chem. 2019, 31, 221–238. [Google Scholar] [CrossRef]
- Li, J.; Yim, D.; Jang, W.D.; Yoon, J. Recent progress in the design and applications of fluorescence probes containing crown ethers. Chem. Soc. Rev. 2017, 46, 2437–2458. [Google Scholar] [CrossRef]
- Sato, F.; Tsukano, M.; Sakamoto, K.; Umemoto, W.; Hashimoto, T.; Hayashita, T. Structural effect of amphiphilic crown ether azoprobes on alkali metal ion recognition and aggregation behavior in water. Bull. Chem. Soc. Jpn. 2008, 81, 1589–1594. [Google Scholar] [CrossRef]
- Yamauchi, A.; Hayashita, T.; Kato, A.; Teramae, N. Supramolecular crown ether probe/γ-cyclodextrin complex sensors for alkali metal ion recognition in water. Bull. Chem. Soc. Jpn. 2002, 75, 1527–1532. [Google Scholar] [CrossRef]
- Yu, H.R.; Hu, J.Q.; Liu, Z.; Ju, X.J.; Xie, R.; Wang, W.; Chu, L.Y. Ion-recognizable hydrogels for efficient removal of cesium ions from aqueous environment. J. Hazard. Mater. 2017, 323, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ju, X.J.; Xie, R.; Liu, Z.; Pi, S.W.; Chu, L.Y. Comprehensive effects of metal ions on responsive characteristics of P (NIPAM-co-B18C6Am). J. Phys. Chem. B 2012, 116, 5527–5536. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Ju, X.J.; Xin, Y.; Zheng, W.C.; Wang, W.; Wei, J.; Xie, R.; Liu, Z.; Chu, L.Y. A novel smart microsphere with magnetic core and ion-recognizable shell for Pb2+ adsorption and separation. ACS Appl. Mater. Interfaces 2014, 6, 9530–9542. [Google Scholar] [CrossRef] [PubMed]
- Mi, P.; Chu, L.-Y.; Ju, X.-J.; Niu, C.H. A smart polymer with ion-induced negative shift of the lower critical solution temperature for Phase Transition. Macromol. Rapid Commun. 2008, 29, 27–32. [Google Scholar] [CrossRef]
- Yin, J.; Li, C.; Wang, D.; Liu, S. Fret-derived ratiometric fluorescent K+ sensors fabricated from thermoresponsive poly (n-isopropylacrylamide) microgels labeled with crown ether moieties. J. Phys. Chem. B 2010, 114, 12213–12220. [Google Scholar] [CrossRef]
- Liang-Yin, C.T.Y.; Shin-ichi, N. A molecular-recognition microcapsule for environmental stimuli-responsive controlled release. Adv. Mater. 2002, 14, 386–389. [Google Scholar]
- Garry, J.H.; Hans, T.D.H.; Lester, P. α-Lipoic acid reduction by mammalian cells to the dithiol form, and release into the culture medium. Biochem. Pharmacol. 1994, 47, 1725–1730. [Google Scholar]
- Packer, L.; Witt, E.H.; Tritschler, H.J. Alpha-lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 1995, 19, 227–250. [Google Scholar] [CrossRef]
- Packer, L.; Kraemer, K.; Rimbach, G. Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition 2001, 17, 888–895. [Google Scholar] [CrossRef]
- Koh, E.H.; Lee, W.J.; Lee, S.A.; Kim, E.H.; Cho, E.H.; Jeong, E.; Kim, D.W.; Kim, M.S.; Park, J.Y.; Park, K.G.; et al. Effects of alpha-lipoic acid on body weight in obese subjects. Am. J. Med. 2011, 124, 85-e1. [Google Scholar] [CrossRef]
- Turcu, I.; Zarafu, I.; Popa, M.; Chifiriuc, M.C.; Bleotu, C.; Culita, D.; Ghica, C.; Ionita, P. Lipoic acid gold nanoparticles functionalized with organic compounds as bioactive materials. Nanomaterials 2017, 7, 43. [Google Scholar] [CrossRef] [Green Version]
- Kuo, T.Y.; Tseng, W.H.; Chen, C.H. Force Spectroscopy of Metal–Crown Ether Multivalency: Effect of Local Environments on Energy Landscape and Sensing Kinetics. Angew. Chem. Int. Ed. 2015, 54, 9213–9217. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, B.; Chen, T.; Liu, X.; Wu, T.; Shen, H.; Luo, W.; Yuan, C.; Xu, Y.; Chen, G.; et al. Polyethersulfone microfiltration membrane modified by an amphiphilic dithiolane-containing copolymer for improving anti-protein-fouling performance and rejection of nanoparticles. Polym. Adv. Technol. 2020, 31, 2816–2826. [Google Scholar] [CrossRef]
- Page, S.M.; Parelkar, S.; Gerasimenko, A.; Shin, D.Y.; Peyton, S.R.; Emrick, T. Promoting cell adhesion on slippery phosphorylcholine hydrogel surfaces. J. Mater. Chem. B 2014, 2, 620–624. [Google Scholar] [CrossRef]
- Ungaro, R.; El Haj, B.; Smid, J. Substituent effects on the stability of cation complexes of 4’-substituted monobenzo crown ethers. J. Am. Chem. Soc. 1976, 98, 5198–5202. [Google Scholar] [CrossRef]
- Koji, Y.; Maria, C.S.J.A.R. Cation binding properties of polymethacrylamide derivatives of crown ethersa. Makromol. Chem. 1980, 1, 263–268. [Google Scholar]
- Kumari, A.; Yadav, S.K.; Pakade, Y.B.; Singh, B.; Yadav, S.C. Development of biodegradable nanoparticles for delivery of quercetin. Colloids Surf. B Biointerfaces 2010, 80, 184–192. [Google Scholar] [CrossRef]
- Kalyanasundaram, K.; Thomas, J.K. Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J. Am. Chem. Soc. 1977, 99, 2039–2044. [Google Scholar] [CrossRef]
- Lim, I.I.S.; Chandrachud, U.; Wang, L.; Gal, S.; Zhong, C.J. Assembly− Disassembly of DNAs and Gold Nanoparticles: A Strategy of Intervention Based on Oligonucleotides and Restriction Enzymes. Anal. Chem. 2008, 80, 6038–6044. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, D.Q.; Zheng, X.P.; Wang, Z.; Zhu, D.B. Thymine-Protected Gold Nanoparticles: Assembly and Disassembly of Gold Nanoparticles in the Presence of Hg2+. J. Nanosci. Nanotechnol. 2009, 9, 3975–3981. [Google Scholar] [CrossRef]




| Sample | LAMA:PEGMA:NIPAM:B18C6Am | Molecular Weight (g/mol) | |
|---|---|---|---|
| The Ratio of Monomers in 1H NMR | The Polymerization Degree | ||
| PLENB1 | 1.5:6.78:10.28:1 | 6:27:41:4 | 2.11 × 104 |
| PLENB2 | 1.0:4.5:7.0:1.5 | 6:28:42:9 | 2.35 × 104 |
| PLENB3 | 0.3:1.35:2.1:1.0 | 6:27:42:20 | 2.74 × 104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zheng, X.; Liu, X.; Zeng, B.; Xu, Y.; Yuan, C.; Dai, L. K+-Responsive Crown Ether-Based Amphiphilic Copolymer: Synthesis and Application in the Release of Drugs and Au Nanoparticles. Polymers 2022, 14, 406. https://doi.org/10.3390/polym14030406
Wang X, Zheng X, Liu X, Zeng B, Xu Y, Yuan C, Dai L. K+-Responsive Crown Ether-Based Amphiphilic Copolymer: Synthesis and Application in the Release of Drugs and Au Nanoparticles. Polymers. 2022; 14(3):406. https://doi.org/10.3390/polym14030406
Chicago/Turabian StyleWang, Xiao, Xianghong Zheng, Xinyu Liu, Birong Zeng, Yiting Xu, Conghui Yuan, and Lizong Dai. 2022. "K+-Responsive Crown Ether-Based Amphiphilic Copolymer: Synthesis and Application in the Release of Drugs and Au Nanoparticles" Polymers 14, no. 3: 406. https://doi.org/10.3390/polym14030406
APA StyleWang, X., Zheng, X., Liu, X., Zeng, B., Xu, Y., Yuan, C., & Dai, L. (2022). K+-Responsive Crown Ether-Based Amphiphilic Copolymer: Synthesis and Application in the Release of Drugs and Au Nanoparticles. Polymers, 14(3), 406. https://doi.org/10.3390/polym14030406