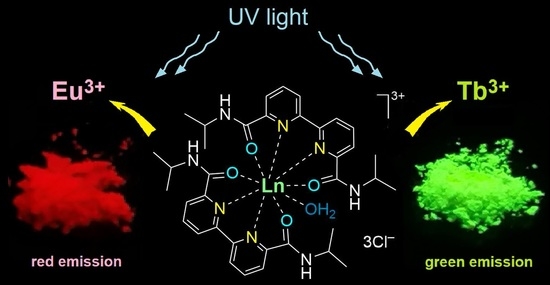
Structural Features of Eu3+ and Tb3+-Bipyridinedicarboxamide Complexes
Abstract
1. Introduction
2. Results
2.1. Synthesis of Lanthanide Complexes
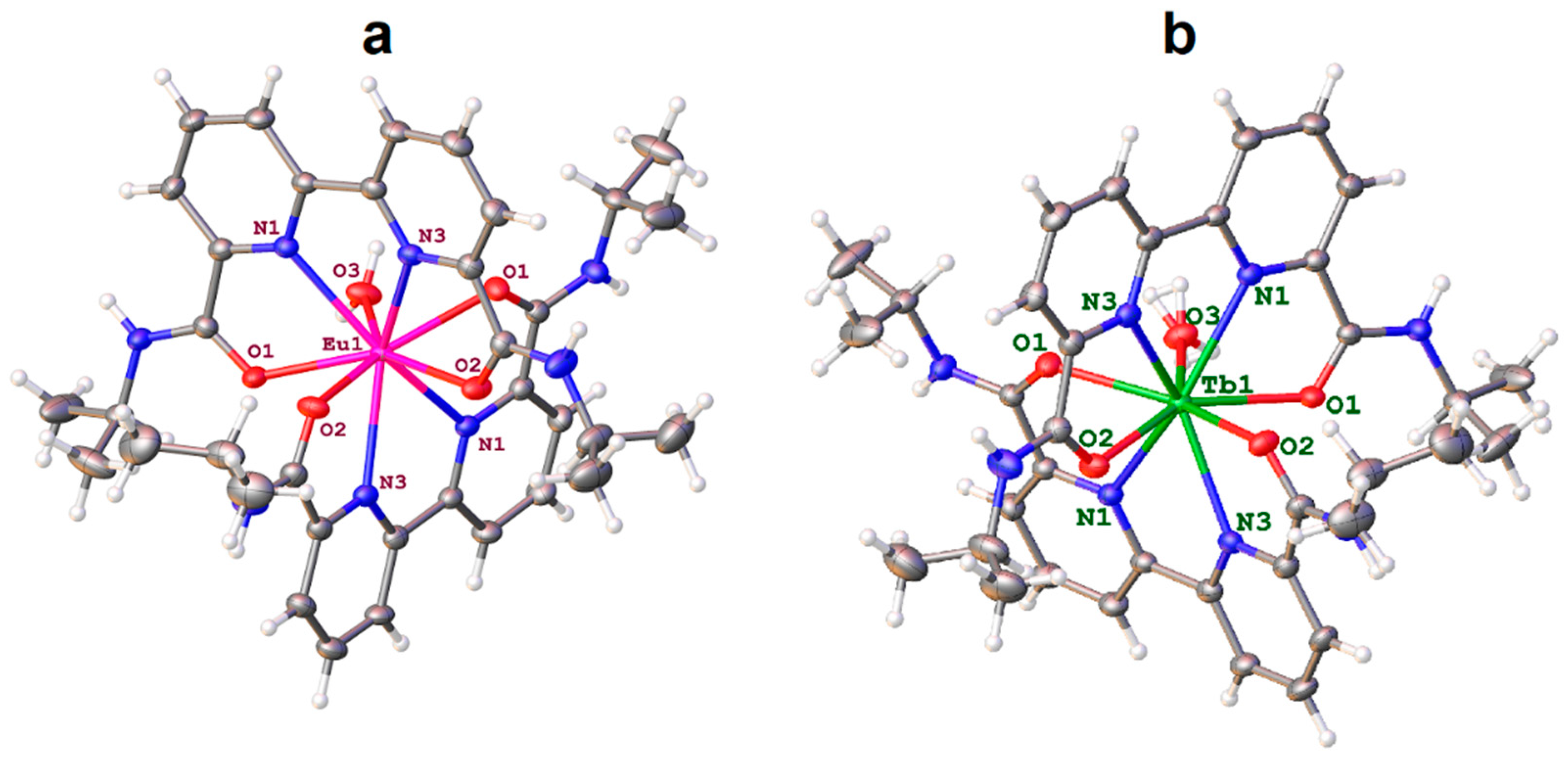
2.2. Structure of Lanthanide Complexes
2.3. Theoretical Calculations of HOMO–LUMO Energy Gaps
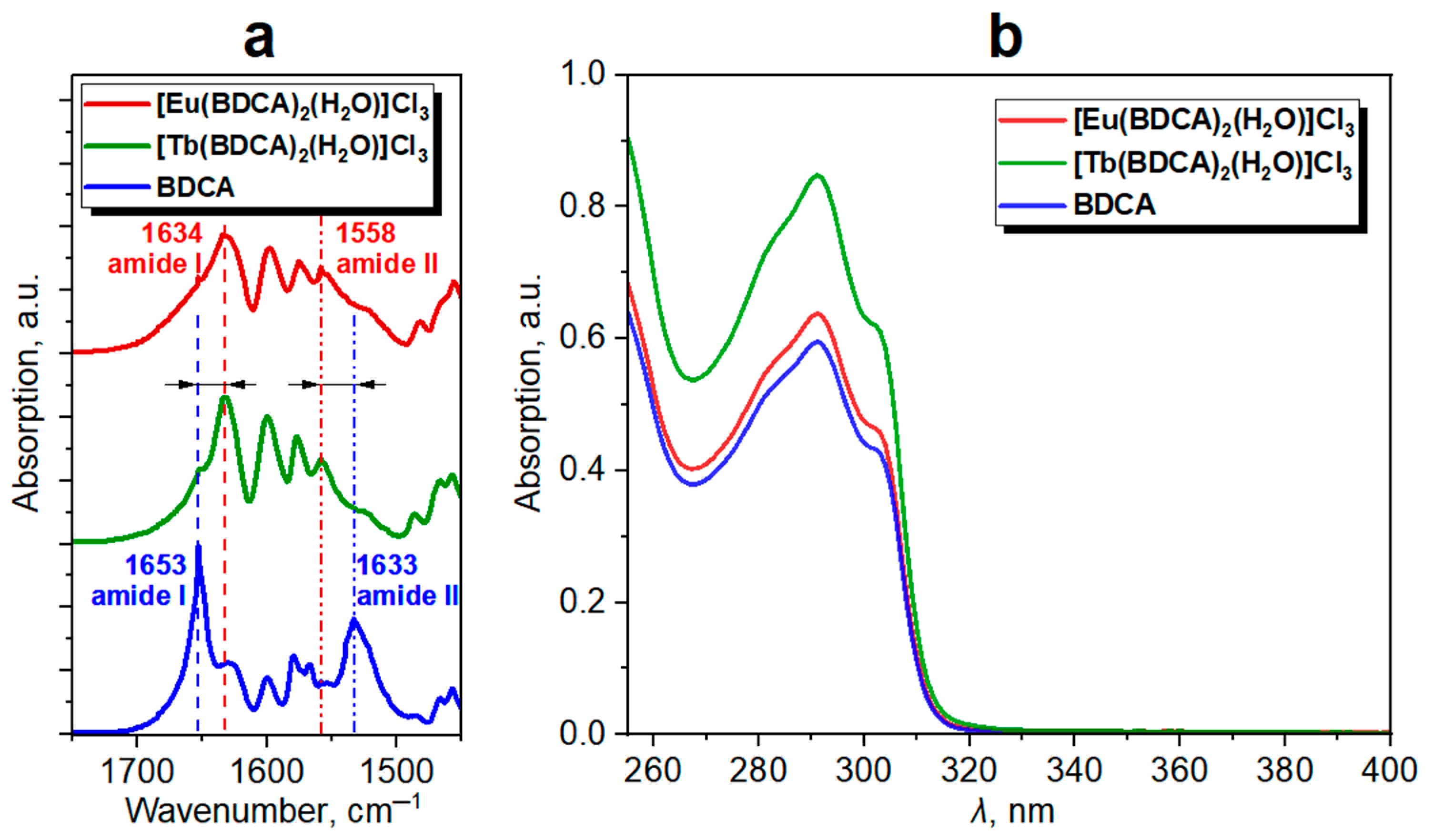
2.4. Band Gap Estimation by UV-Vis Absorption Study
2.5. Luminescent Properties
2.6. Thermal Stability
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. Synthetic Procedures
3.3.1. [2,2′-Bipyridine]-6,6′-Dicarbonyl Dichloride
3.3.2. N6,N6’-Diisopropyl-[2,2′-Bipyridine]-6,6′-Dicarboxamide (BDCA)
3.3.3. [Eu(BDCA)2(H2O)]Cl3
3.3.4. [Tb(BDCA)2(H2O)]Cl3
3.4. Encapsulation of Lanthanide Complexes in a PEG Matrix
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, H.; Chen, R.; Sun, Q.; Lai, W.; Su, Q.; Huang, W.; Liu, X. Recent Progress in Metal–Organic Complexes for Optoelectronic Applications. Chem. Soc. Rev. 2014, 43, 3259–3302. [Google Scholar] [CrossRef]
- Bedi, M.; Punia, M.; Taxak, V.B.; Khatkar, S.P.; Doon, P.B. Investigations into Spectroscopic and Optoelectronic Behaviour of Furoic Acid-based Eu(III) Complexes for Advanced Photonic Applications. Luminescence 2022, 37, 1098–1108. [Google Scholar] [CrossRef]
- Rocha, J.; Carlos, L.D.; Paz, F.A.A.; Ananias, D. Luminescent Multifunctional Lanthanides-Based Metal–Organic Frameworks. Chem. Soc. Rev. 2011, 40, 926–940. [Google Scholar] [CrossRef]
- Shinoda, S.; Tsukube, H. Luminescent Lanthanide Complexes as Analytical Tools in Anion Sensing, PH Indication and Protein Recognition. Analyst 2011, 136, 431–435. [Google Scholar] [CrossRef]
- Ren, S.; Jiang, W.; Wang, Q.; Li, Z.; Qiao, Y.; Che, G. Synthesis, Structures and Properties of Six Lanthanide Complexes Based on a 2-(2-Carboxyphenyl)Imidazo(4,5-f)-(1,10)Phenanthroline Ligand. RSC Adv. 2019, 9, 3102–3112. [Google Scholar] [CrossRef] [PubMed]
- Manzani, D.; Nigoghossian, K.; Iastrensk, M.F.; Coelho, G.R.; dos Santos, M.V.; Maia, L.J.Q.; Ribeiro, S.J.L.; Segatelli, M.G. Luminescent Silicone Materials Containing Eu3+ -Complexes for Photonic Applications. J. Mater. Chem. C 2018, 6, 8258–8265. [Google Scholar] [CrossRef]
- Ranjan, S.; Jayakumar, M.K.G.; Zhang, Y. Luminescent Lanthanide Nanomaterials: An Emerging Tool for Theranostic Applications. Nanomedicine 2015, 10, 1477–1491. [Google Scholar] [CrossRef]
- Bui, A.T.; Grichine, A.; Duperray, A.; Lidon, P.; Riobé, F.; Andraud, C.; Maury, O. Terbium(III) Luminescent Complexes as Millisecond-Scale Viscosity Probes for Lifetime Imaging. J. Am. Chem. Soc. 2017, 139, 7693–7696. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Eliseeva, S.V. Intriguing Aspects of Lanthanide Luminescence. Chem. Sci. 2013, 4, 1939. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ohmagari, H.; Tanaka, H.; Machida, K. Luminescence of Lanthanide Complexes: From Fundamental to Prospective Approaches Related to Water- and Molecular-Stimuli. J. Photochem. Photobiol. C Photochem. Rev. 2022, 50, 100484. [Google Scholar] [CrossRef]
- Miroshnichenko, A.S.; Deriabin, K.V.; Baranov, A.I.; Neplokh, V.; Mitin, D.M.; Kolesnikov, I.E.; Dobrynin, M.V.; Parshina, E.K.; Mukhin, I.S.; Islamova, R.M. Lanthanide(III)-Incorporating Polysiloxanes as Materials for Light-Emitting Devices. ACS Appl. Polym. Mater. 2022, 4, 2683–2690. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. Benefiting from the Unique Properties of Lanthanide Ions. Acc. Chem. Res. 2006, 39, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Nehra, K.; Dalal, A.; Hooda, A.; Kumar, P.; Singh, D.; Kumar, S.; Malik, R.S.; Kumar, P. Luminous Terbium and Samarium Complexes with Diacetylmethane and Substituted 1,10-Phenanthroline Derivatives for Display Applications: Preparation and Optoelectronic Investigations. J. Lumin. 2022, 249, 119032. [Google Scholar] [CrossRef]
- Dalal, A.; Nehra, K.; Hooda, A.; Saini, R.K.; Singh, D.; Kumar, S.; Malik, R.S. Preparation and Optoelectronic Enhancement of Trivalent Terbium Complexes with Fluorinated β-Diketone and Bidentate Ancillary Ligands. J. Mater. Sci. Mater. Electron. 2022, 33, 12984–12996. [Google Scholar] [CrossRef]
- Dalal, A.; Nehra, K.; Hooda, A.; Singh, S.; Singh, D.; Kumar, S. Synthesis, Optoelectronic and Photoluminescent Characterizations of Green Luminous Heteroleptic Ternary Terbium Complexes. J. Fluoresc. 2022, 32, 1019–1029. [Google Scholar] [CrossRef]
- Hooda, P.; Lather, V.; Malik, R.K.; Taxak, V.B.; Khatkar, S.P.; Khatri, S.; Ahlawat, P.; Kumar, R. Achieving Crimson Red Emission of Europium (III) Complexes with β-Keto Acids and Ancillary Ligands for Their Applications in Optoelectronic Devices and Biomedical Domain. Optik 2022, 264, 169389. [Google Scholar] [CrossRef]
- Okayasu, Y.; Yuasa, J. Structure Determination of Europium Complexes in Solution Using Crystal-Field Splitting of the Narrow f–f Emission Lines. J. Phys. Chem. Lett. 2021, 12, 6867–6874. [Google Scholar] [CrossRef]
- Hooda, A.; Nehra, K.; Dalal, A.; Bhagwan, S.; Gupta, I.; Singh, D.; Kumar, S. Luminescent Features of Ternary Europium Complexes: Photophysical and Optoelectronic Evaluation. J. Fluoresc. 2022, 32, 1529–1541. [Google Scholar] [CrossRef]
- Chen, F.-F.; Bian, Z.-Q.; Liu, Z.-W.; Nie, D.-B.; Chen, Z.-Q.; Huang, C.-H. Highly Efficient Sensitized Red Emission from Europium (III) in Ir−Eu Bimetallic Complexes by 3MLCT Energy Transfer. Inorg. Chem. 2008, 47, 2507–2513. [Google Scholar] [CrossRef]
- Wang, X.; Bodunov, E.N.; Nau, W.M. Fluorescence Quenching Kinetics in Short Polymer Chains: Dependence on Chain Length. Opt. Spectrosc. 2003, 95, 560–570. [Google Scholar] [CrossRef]
- Deriabin, K.V.; Ignatova, N.A.; Kirichenko, S.O.; Novikov, A.S.; Kryukova, M.A.; Kukushkin, V.Y.; Islamova, R.M. Structural Features of Polymer Ligand Environments Dramatically Affect the Mechanical and Room-Temperature Self-Healing Properties of Cobalt(II)-Incorporating Polysiloxanes. Organometallics 2021, 40, 2750–2760. [Google Scholar] [CrossRef]
- Deriabin, K.V.; Ignatova, N.A.; Kirichenko, S.O.; Novikov, A.S.; Islamova, R.M. Nickel(II)-Pyridinedicarboxamide-Co-Polydimethylsiloxane Complexes as Elastic Self-Healing Silicone Materials with Reversible Coordination. Polymer 2021, 212, 123119. [Google Scholar] [CrossRef]
- Li, C.-H.; Wang, C.; Keplinger, C.; Zuo, J.-L.; Jin, L.; Sun, Y.; Zheng, P.; Cao, Y.; Lissel, F.; Linder, C.; et al. A Highly Stretchable Autonomous Self-Healing Elastomer. Nat. Chem. 2016, 8, 618–624. [Google Scholar] [CrossRef]
- Rao, N.S.; Rajesh, M.; Prasad, K.; Reddy, G.R.; Raju, B.D.P.; Dhanapandian, S. Study of Trivalent Samarium Ion Embedded Lithium-based Borate Glass for High-density Optical Memory Devices. Luminescence 2020, 35, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Khatkar, A.; Taxak, V.B.; Dalal, M.; Chahar, S.; Dalal, J.; Khatkar, S.P. Optical Properties of Trivalent Samarium-Doped Ba5Zn4Y8O21 Nanodiametric Rods Excitable by NUV Light. J. Alloys Compd. 2018, 767, 409–418. [Google Scholar] [CrossRef]
- Khanagwal, J.; Khatkar, S.P.; Dhankhar, P.; Bala, M.; Kumar, R.; Boora, P.; Taxak, V.B. Synthesis and Photoluminescence Analysis of Europium(III) Complexes with Pyrazole Acid and Nitrogen Containing Auxiliary Ligands. Spectrosc. Lett. 2020, 53, 625–647. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Bredas, J.-L. Mind the Gap! Mater. Horiz. 2014, 1, 17–19. [Google Scholar] [CrossRef]
- Wahsner, J.; Seitz, M. Perdeuterated 2,2′-Bipyridine-6,6′-Dicarboxylate: An Extremely Efficient Sensitizer for Thulium Luminescence in Solution. Inorg. Chem. 2013, 52, 13301–13303. [Google Scholar] [CrossRef]
- de Bettencourt-Dias, A. (Ed.) Luminescence of Lanthanide Ions in Coordination Compounds and Nanomaterials; John Wiley & Sons Ltd.: Chichester, UK, 2014; ISBN 978-1-118-68276-0. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miroshnichenko, A.S.; Deriabin, K.V.; Rashevskii, A.A.; Suslonov, V.V.; Novikov, A.S.; Mukhin, I.S.; Islamova, R.M. Structural Features of Eu3+ and Tb3+-Bipyridinedicarboxamide Complexes. Polymers 2022, 14, 5540. https://doi.org/10.3390/polym14245540
Miroshnichenko AS, Deriabin KV, Rashevskii AA, Suslonov VV, Novikov AS, Mukhin IS, Islamova RM. Structural Features of Eu3+ and Tb3+-Bipyridinedicarboxamide Complexes. Polymers. 2022; 14(24):5540. https://doi.org/10.3390/polym14245540
Chicago/Turabian StyleMiroshnichenko, Anna S., Konstantin V. Deriabin, Artem A. Rashevskii, Vitalii V. Suslonov, Alexander S. Novikov, Ivan S. Mukhin, and Regina M. Islamova. 2022. "Structural Features of Eu3+ and Tb3+-Bipyridinedicarboxamide Complexes" Polymers 14, no. 24: 5540. https://doi.org/10.3390/polym14245540
APA StyleMiroshnichenko, A. S., Deriabin, K. V., Rashevskii, A. A., Suslonov, V. V., Novikov, A. S., Mukhin, I. S., & Islamova, R. M. (2022). Structural Features of Eu3+ and Tb3+-Bipyridinedicarboxamide Complexes. Polymers, 14(24), 5540. https://doi.org/10.3390/polym14245540