Promising Role of Silk-Based Biomaterials for Ocular-Based Drug Delivery and Tissue Engineering
Abstract
1. Introduction
2. Silk Structure
3. Biocompatibility and Biodegradation of Regenerated Silk Fibroin
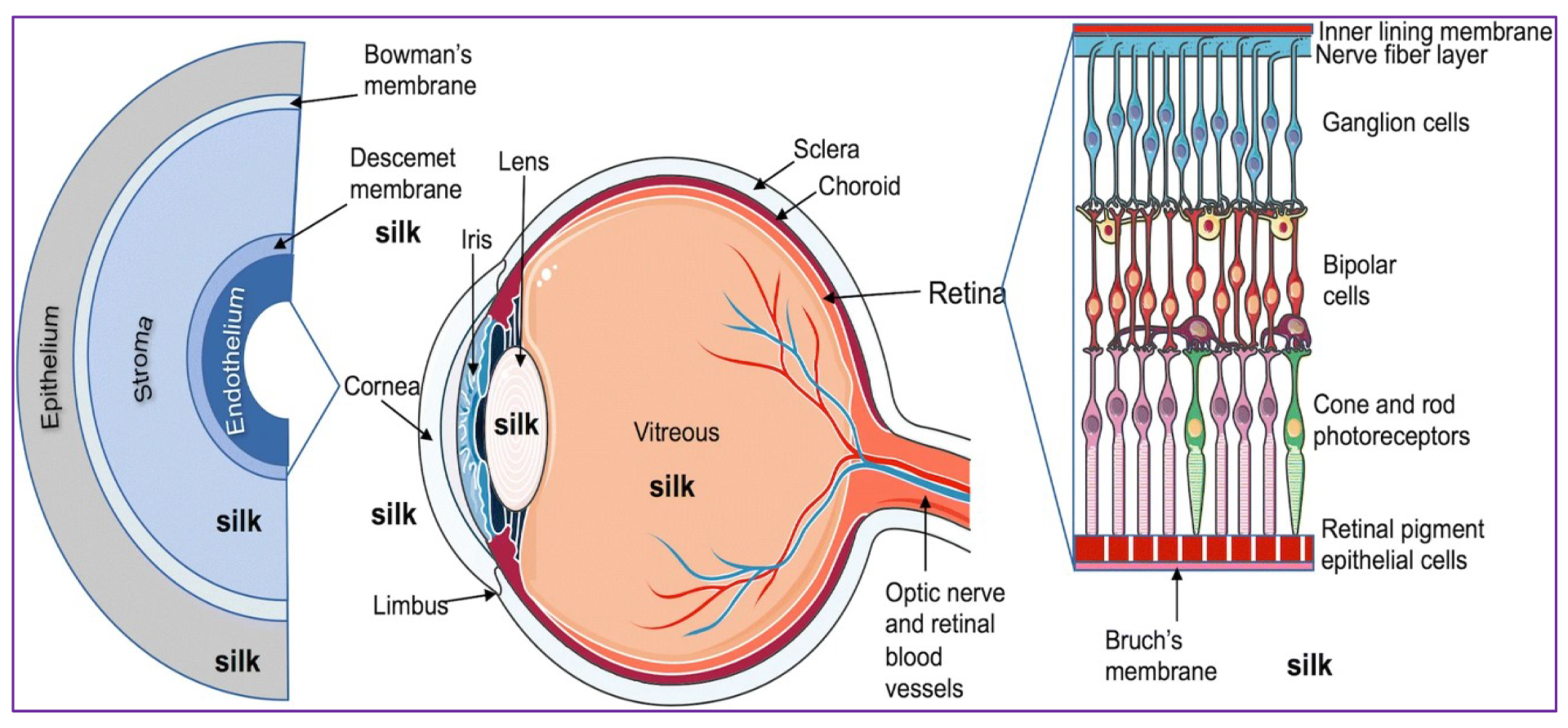
4. Ocular Wound Healing
5. Silk Fibroin-Based Ocular Drug Delivery
6. Conclusions
7. Possibilities for Improvement of Silk-Based Biomaterials for the Ocular Drug Delivery
8. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The biomedical use of silk: Past, present, future. Adv. Healthc. Mater. 2019, 10, E1800465. [Google Scholar] [CrossRef] [PubMed]
- Muffly, T.M.; Tizzano, A.P.; Walters, M.D. The history and evolution of sutures in pelvic surgery. J. Royal Soc. Med. 2011, 104, 107–112. [Google Scholar]
- Aigner, T.B.; DeSimone, E.; Scheibel, T. Biomedical applications of recombinant silk-based materials. Adv. Mater. 2018, 30, E1704636. [Google Scholar] [PubMed]
- Zhou, Z.; Zhang, S.; Cao, Y.; Marelli, B.; Xia, X.; Tao, T.H. Engineering the future of silk materials through advanced manufacturing. Adv. Mater. 2018, 30, E1706983. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S. Administering a subconjunctival injection. Commun. Eye Health 2009, 22, E15. [Google Scholar]
- Chong, R.S.; Su, D.H.; Tsai, A.; Jiang, Y.; Htoon, H.M.; Lamoureux, E.L.; Aung, T.; Wong, T.T. Patient acceptance and attitude toward an alternative method of subconjunctival injection for the medical treatment of glaucoma. J. Glaucoma 2013, 22, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Canavan, K.S.; Dark, A.; Garrioch, M.A. Sub-Tenon’s administration of local anaesthetic: A review of the technique. Br. J. Anaesth. 2003, 90, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; Cestari, D.M. Optic neuropathy following retrobulbar injection: A review. Semin. Ophthalmol. 2014, 29, 434–439. [Google Scholar] [CrossRef]
- Paolo, M.D.; Bugelli, V.; Figus, M.; Fornaro, S.; Guidi, B.; Giannarelli, C.; Tuccori, M. Acute myocardial infarction following off label retrobulbar injection of desmopressin for non-arteritic anterior ischemic optic neuropathy (NAION). Causal correlation or coincidence? Rom. J. Leg. Med. 2017, 25, 165–168. [Google Scholar] [CrossRef]
- Tran, S.H.; Wilson, C.G.; Seib, F.P. A review of the emerging role of silk for the treatment of the eye. Pharm. Res. 2018, 35, E248. [Google Scholar] [CrossRef] [PubMed]
- Lovett, M.L.; Wang, X.; Yucel, T.; York, L.; Keirstead, M.; Haggerty, L.; Kaplan, D.L. Silk hydrogels for sustained ocular delivery of anti-vascular endothelial growth factor (anti-VEGF) therapeutics. Eur. J. Pharm. Biopharm. 2015, 95, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Naby, W.; Cole, B.; Liu, A.; Liu, J.; Wan, P.; Schreiner, R.; Infanger, D.W.; Paulson, N.B.; Lawrence, B.D.; Rosenblatt, M.I. Treatment with solubilized silk-derived protein (SDP) enhances rabbit corneal epithelial wound healing. PLoS ONE 2017, 12, e0188154. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Dong, P.; Huang, D.; Mei, L.; Xia, Y.; Wang, Z.; Pan, X.; Li, G.; Wu, C. Fabrication and characterization of silk fibroin-coated liposomes for ocular drug delivery. Eur. J. Pharm. Biopharm. 2015, 91, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wang, L.; Dong, Y.; Pan, X.; Li, G.; Wu, C. A novel technology using transscleral ultrasound to deliver protein loaded nanoparticles. Eur. J. Pharm. Biopharm. 2014, 88, 104–115. [Google Scholar]
- Pianpian, Y.; Yixuan, D.; Di, H.; Chune, Z.; Hu, L.; Xin, P.; Chuanbin, W. Silk fibroin nanoparticles for enhanced bio-macromolecule delivery to the retina. Pharm. Dev. Technol. 2019, 24, 575–583. [Google Scholar]
- Carruthers, J.; Fagien, S.; Dolman, P. Retro or peribulbar injection techniques to reverse visual loss after filler injections. Dermatol. Surg. 2015, 41, S354–S357. [Google Scholar] [CrossRef]
- Ozgun, C.O.; Syeda, R.B.; Muhammad, A.N. Self-assembled silk fibroin hydrogels: From preparation to biomedical application. Mater. Adv. 2022, 3, 6920–6949. [Google Scholar]
- Kunz, R.I.; Brancalhao, R.M.; Ribeiro, L.F.; Natali, M.R. Silkworm sericin: Properties and biomedical applications. Biomed. Res. Int. 2016, 2016, E8175701. [Google Scholar]
- Keten, S.; Xu, Z.; Ihle, B.; Buehler, M.J. Nanoconfinement controls stiffness, strength and mechanical toughness of beta-sheet crystals in silk. Nat. Mater. 2010, 9, 359–367. [Google Scholar] [PubMed]
- Asakura, T.; Okushita, K.; Williamson, M.P. Analysis of the structure of Bombyx mori silk fibroin by NMR. Macromolecules 2015, 48, 2345–2357. [Google Scholar]
- Wu, J.; Rnjak-Kovacina, J.; Du, Y.; Funderburgh, M.L.; Kaplan, D.L.; Funderburgh, J.L. Corneal stromal bioequivalents secreted on patterned silk substrates. Biomaterials 2014, 35, 3744–3755. [Google Scholar] [CrossRef] [PubMed]
- Motta, A.; Fambri, L.; Migliaresi, C. Regenerated silk fibroin films: Thermal and dynamic mechanical analysis. Macromol. Chem. Phys. 2002, 203, 1658–1665. [Google Scholar] [CrossRef]
- Asakura, T. Structure of Silk I (Bombyx mori Silk Fibroin before Spinning)-Type II β-Turn, Not α-Helix. Molecules 2021, 26, 3706. [Google Scholar] [CrossRef] [PubMed]
- Sashina, E.S.; Bochek, A.M.; Novoselov, N.P.; Kirichenko, D.A. Structure and solubility of natural silk fibroin. Russ. J. Appl. Chem. 2006, 79, 869–876. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Jewell, M.; Daunch, W.; Bengtson, B.; Mortarino, E. The development of SERI surgical scaffold, an engineered biological scaffold. Ann. N. Y. Acad. Sci. 2015, 1358, 44–55. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yucel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar]
- Holland, C.; Terry, A.E.; Porter, D.; Vollrath, F. Natural and unnatural silks. Polymer 2007, 48, 3388–3392. [Google Scholar] [CrossRef]
- Kim, H.H.; Song, D.W.; Kim, M.J.; Ryu, S.J.; UmI, C.; Ki, C.S.; Park, Y.H. Effect of silk fibroin molecular weight on physical property of silk hydrogel. Polymer 2016, 90, 26–33. [Google Scholar]
- Kim, H.J.; Kim, M.K.; Lee, K.H.; Nho, S.K.; Han, M.S.; Um, I.C. Effect of degumming methods on structural characteristics and properties of regenerated silk. Int. J. Biol. Macromol. 2017, 104, 294–302. [Google Scholar] [CrossRef]
- Wojcieszak, M.; Percot, A.; Colomban, P. Regenerated silk matrix composite materials reinforced by silk fibres: Relationship between processing and mechanical properties. J. Compos. Mater. 2017, 52, 2301–2311. [Google Scholar] [CrossRef]
- Seib, F.P. Reverse-engineered silk hydrogels for cell and drug delivery. Ther. Deliv. 2018, 9, 469–487. [Google Scholar] [CrossRef]
- Thurber, A.E.; Omenetto, F.G.; Kaplan, D.L. In vivo bioresponses to silk proteins. Biomaterials 2015, 71, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Shadforth, A.M.A.; Suzuki, S.; Theodoropoulos, C.; Richardson, N.A.; Chirila, T.V.; Harkin, D.G. A Bruch’s membrane substitute fabricated from silk fibroin supports the function of retinal pigment epithelial cells in vitro. J. Tissue Eng. Regen. Med. 2017, 11, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, N.; Rodriguez-Barrientos, C.A.; Aznar-Cervantes, S.D.; Chacon, M.; Cenis, J.L.; Riestra, A.C.; Sanchez-Avila, R.M.; Persinal, M.; Brea-Pastor, A.; Fernandez-Vega Cueto, L.; et al. Silk fibroin films for corneal endothelial regeneration: Transplant in a rabbit Descemet membrane endothelial keratoplasty. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3357–3365. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Song, Y.; Jin, Y.; Zhang, C.; Peng, D.; Chen, Z.; Chang, P.; Kundu, S.C.; Wang, G.; Wang, Z.; et al. In vivo characterizations of the immune properties of sericin: An ancient material with emerging value in biomedical applications. Macromol. Biosci. 2017, 17, E1700229. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, L.; Mari-Buye, N.; Barios, J.A.; Madurga, R.; Elices, M.; Perez-Rigueiro, J.; Ramos, M.; Guinea, G.V.; Gonzalez-Nieto, D. Safety and tolerability of silk fibroin hydrogels implanted into the mouse brain. Acta Biomater. 2016, 45, 262–275. [Google Scholar] [CrossRef]
- Maitz, M.F.; Sperling, C.; Wongpinyochit, T.; Herklotz, M.; Werner, C.; Seib, F.P. Biocompatibility assessment of silk nanoparticles: Hemocompatibility and internalization by Human corneal limbal epithelial cell response to varying silk film geometric topography in vitro blood cells. Nanomedicine 2017, 13, 2633–2642. [Google Scholar] [CrossRef]
- Totten, J.D.; Wongpinyochit, T.; Seib, F.P. Silk nanoparticles: Proof of lysosomotropic anticancer drug delivery at single-cell resolution. J. Drug Target. 2017, 25, 865–872. [Google Scholar] [CrossRef]
- Wongpinyochit, T.; Johnston, B.F.; Seib, F.P. Degradation behavior of silk nanoparticles—Enzyme responsiveness. ACS Biomater. Sci. 2018, 4, 942–951. [Google Scholar] [CrossRef]
- Wani, S.U.D.; Veerabhadrappa, G.H. Silk fibroin based drug delivery applications: Promises and challenges. Curr. Drug Targets 2018, 19, 1177–1190. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.U.D.; Gautam, S.P.; Qadrie, Z.L.; Gangadharappa, H.V. Silk fibroin as a natural polymeric based bio-material for tissue engineering and drug delivery systems-A review. Int. J. Biol. Macromol. 2020, 163, 2145–2161. [Google Scholar] [CrossRef]
- Moin, A.; Wani, S.U.D.; Osmani, R.A.; Lila, A.S.B.; Khafagy, E.; Arab, H.H.; Gangadharappa, H.V.; Allam, A.N. Formulation, characterization, and cellular toxicity assessment of tamoxifen-loaded silk fibroin nanoparticles in breast cancer. Drug Deliv. 2021, 28, 1626–1636. [Google Scholar] [CrossRef] [PubMed]
- Al Saqr, A.; Wani, S.U.D.; Gangadharappa, H.V.; Aldawsari, M.F.; Khafagy, E.; Lila, A.S.A. Enhanced cytotoxic activity of docetaxel-loaded silk fibroin nanoparticles against breast cancer cells. Polymers 2021, 13, 1416. [Google Scholar] [CrossRef] [PubMed]
- Adel-Naby, W.; Cole, B.; Liu, A.; Liu, J.; Wan, P.; Guaiquil, V.H.; Schreiner, R.; Infanger, D.; Lawrence, B.D.; Rosenblatt, M.I. Silk-derived protein enhances corneal epithelial migration, adhesion, and proliferation. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1425–1433. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Ho, C.S.; Deshmukh, R.; Said, D.G.; Dua, H.S. Infectious keratitis: An update on epidemiology, causative microorganisms, risk factors, and antimicrobial resistance. Eye 2021, 35, 1084–1101. [Google Scholar] [CrossRef]
- Kim, C.E.; Lee, J.H.; Yeon, Y.K.; Park, C.H.; Yang, J. Effects of silk fibroin in murine dry eye. Sci. Rep. 2017, 7, E44364. [Google Scholar] [CrossRef]
- Market Scope. Dry Eye Products Report: A Global Market Analysis for 2015 to 2021. Available online: https://market-scope.com (accessed on 9 March 2017).
- Rhee, D.; Pyfer, M. The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1999. [Google Scholar]
- Fraunfelder, F.W. Corneal toxicity from topical ocular and systemic medications. Cornea 2006, 25, 1133–1138. [Google Scholar] [CrossRef]
- Wilson, S.E.; Mohan, R.R.; Mohan, R.R.; Abrosio, R.; Hong, J.; Lee, J. The corneal wound healing response: Cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog. Rret. Eye Res. 2001, 20, 625–637. [Google Scholar]
- Agrawal, V.B.; Tsai, R.J. Corneal epithelial wound healing. Indian J. Ophthalmol. 2003, 51, 5–15. [Google Scholar]
- Suzuki, K.; Saito, J.; Yanai, R.; Yamada, N.; Chikama, T.; Seki, K.; Nishida, T. Cell-matrix and cell-cell interactions during corneal epithelial wound healing. Prog. Ret. Eye Res. 2003, 22, 113–133. [Google Scholar] [CrossRef] [PubMed]
- Cutler, T. Corneal epithelial disease. Vet. Clin. North Am. Equine Pract. 2004, 20, 319–343. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.; Infanger, D.W. Fibroin-Derived Protein Composition. US9394355, 7 April 2016. [Google Scholar]
- Wani, S.U.D.; Gangadharappa, H.V.; Ashish, N.P. Formulation, development and characterization of drug delivery systems based telmisartan encapsulated in silk fibroin nanosphere’s. Int. J. Appl. Pharm. 2019, 11, 247–254. [Google Scholar] [CrossRef]
- Phuagkhaopong, S.; Mendes, L.; Müller, K.; Wobus, M.; Bornhauser, M.; Carswell, H.V.O.; Duarte, I.F.; Seib, F.P. Silk hydrogel substrate stress relaxation primes mesenchymal stem cell behavior in 2D. ACS Appl. Mater. Interf. 2021, 13, 30420–30433. [Google Scholar] [CrossRef] [PubMed]
- Raia, N.R.; Jia, D.; Ghezzi, C.E.; Muthukumar, M.; Kaplan, D.L. Characterization of silk-hyaluronic acid composite hydrogels towards vitreous humor substitutes. Biomaterials 2020, 233, E119729. [Google Scholar] [CrossRef]
- Guziewicz, N.A.; Massetti, A.J.; Perez-Ramirez, B.J.; Kaplan, D.L. Mechanisms of monoclonal antibody stabilization and release from silk biomaterials. Biomaterials 2013, 34, 7766–7775. [Google Scholar] [CrossRef]
- Gaza-Bulseco, G.; Faldu, S.; Hurkmans, K.; Chumsae, C.; Liu, H. Effect of methionine oxidation of a recombinant monoclonal antibody on the binding affinity to protein a and protein G. J. Chromatogr. B. 2008, 870, 55–62. [Google Scholar] [CrossRef]
- Schoneich, C. Methionine oxidation by reactive oxygen species: Reaction mechanisms and relevance to Alzheimer’s disease. Biochim. Biophys. Acta 2005, 1703, 111–119. [Google Scholar] [CrossRef]
- Torosantucci, R.; Schoneich, C.; Jiskoot, W. Oxidation of therapeutic proteins and peptides: Structural and biological consequences. Pharm. Res. 2014, 31, 541–553. [Google Scholar] [CrossRef]
- Meisner, D.; Mezei, M. Liposome ocular delivery systems. Adv. Drug Deliv. Rev. 1995, 16, 75–93. [Google Scholar] [CrossRef]
- Shen, Y.; Tu, J. Preparation and ocular pharmacokinetics of ganciclovir liposomes. AAPS J. 2007, 9, E371–E377. [Google Scholar] [PubMed]
- Budai, L.; Hajdú, M.; Budai, M.; Gróf, P.; Béni, S.; Noszál, B.; Klebovich, I.; Antal, I. Gels and liposomes in optimized ocular drug delivery: Studies on ciprofloxacin formulations. Int. J. Pharm. 2007, 343, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Nagarsenker, M.S.; Londhe, V.Y.; Nadkarni, G.D. Preparation and evaluation of liposomal formulations of tropicamide for ocular delivery. Int. J. Pharm. 1999, 190, 63–71. [Google Scholar] [PubMed]
- Gobin, A.S.; Rhea, R.; Newman, R.A.; Mathur, A.B. Silk-fibroin-coated liposomes for long-term and targeted drug delivery. Int. J. Nanomed. 2006, 1, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Chung, Y.-I.; Kim, Y.H.; Tae, G.; Kundu, S.C. Silk fibroin nanoparticles for cellular uptake and control release. Int. J. Pharm. 2010, 388, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Gordois, A.; Cutler, H.; Pezzullo, L.; Gordon, K.; Cruess, A.; Winyard, S.; Hamilton, W.; Chua, K. An estimation of the worldwide economic and health burden of visual impairment. Glob. Public Health 2012, 7, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Gehrs, K.M.; Anderson, D.H.; Johnson, L.V.; Hageman, G.S. Age-related macular degeneration-emerging pathogenetic and therapeutic concepts. Ann. Med. 2006, 38, 450–471. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y.; Group, M.S. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef]
- Avery, R.L.; Pieramici, D.J.; Rabena, M.D.; Castellarin, A.A.; Nasir, M.A.; Giust, M.J. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology 2006, 113, 363–372. [Google Scholar] [CrossRef]
- Gragoudas, E.S.; Adamis, A.P.; Chunnigham, E.T., Jr.; Feinsod, M.; Guyer, D.R. Pegaptanib for neovascular age-related macular degeneration. N. Engl. J. Med. 2004, 351, 2805–2816. [Google Scholar] [CrossRef]
- Marsh, D.A. Selection of drug delivery approaches for the back of the eye: Opportunities and unmet needs. Drug Prod. Dev. Back Eye 2011, 2, 1–20. [Google Scholar]
- Diebold, Y.; Calonge, M. Applications of nanoparticles in ophthalmology. Progr. Ret. Eye Res. 2010, 29, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Janoria, K.G.; Gunda, S.; Boddu, S.H.S.; Mitra, A.K. Novel approaches to retinal drug delivery. Expert Opin. Drug Deliv. 2007, 4, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Jager, R.D.; Aiello, L.P.; Patel, S.C.; Chunnigham, E.T., Jr. Risks of intravitreous injection: A comprehensive review. Retina 2004, 24, 676–698. [Google Scholar] [CrossRef]
- Nomoto, H.; Shiraga, F.; Kuno, N.; Kimura, E.; Fujji, S.; Shinomiya, K.; Nugent, A.K.; Hirooka, K.; Baba, T. Pharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbits. Invest. Ophthalmol. Vis. Sci. 2009, 50, 4807–4813. [Google Scholar] [CrossRef]
- Wiendl, H.; Gold, R.; Berger, T.; Derfuss, T.; Linker, R.; Mäurer, M.; Aktas, O.; Baum, K.; Berghoff, M.; Bittner, S.; et al. Multiple Sclerosis Therapy Consensus Group (MSTCG): Position statement on disease-modifying therapies for multiple sclerosis (white paper). Ther. Adv. Neurol. Disord. 2021, 14, 17562864211039648. [Google Scholar] [CrossRef]
- Geroski, D.H.; Edelhauser, H.F. Transscleral drug delivery for posterior segment disease. Adv. Drug Deliv. Rev. 2001, 52, 37–48. [Google Scholar] [CrossRef]
- Kim, S.H.; Lutz, R.J.; Wang, N.S.; Robinson, M.R. Transport barriers in transscleral drug delivery for retinal diseases. Ophthalmic Res. 2007, 39, 244–254. [Google Scholar] [CrossRef]
- El Sanharawi, M.; Kowalczuk, L.; Touchard, E.; Omri, S.; de Kozak, Y.; Behar-Cohen, F. Protein delivery for retinal diseases: From basic considerations to clinical applications. Prog. Ret. Eye Res. 2010, 29, 443–465. [Google Scholar] [CrossRef]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef]
- Murali, K.K.; Pinnamaneni, S.; Das, N.G.; Das, S.K. Microparticles and nanoparticles in ocular drug delivery. Drugs Pharm. Sci. 2003, 130, 437–466. [Google Scholar]
- Kaur, I.P.; Kanwar, M. Ocular preparations: The formulation approach. Drug Dev. Ind. Pharm. 2002, 28, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.P.; Garg, A.; Singla, A.K.; Aggarwal, D. Vesicular systems in ocular drug delivery: An overview. Int. J. Pharam. 2004, 269, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Kadam, R.S.; Cheruvu, N.P.S.; Kompella, U.B. Hydrophilic prodrug approach for reduced pigment binding and enhanced transscleral retinal delivery of celecoxib. Mol. Pharm. 2012, 9, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Eljarrat-Binstock, E.; Orucov, F.; Aldouby, Y.; Frucht-Pery, J.; Domb, A.J. Charged nanoparticles delivery to the eye using hydrogel iontophoresis. J. Control. Release 2008, 126, 156–161. [Google Scholar] [CrossRef]
- Cheung, A.C.Y. Ultrasound-enhanced intrascleral delivery of protein. Int. J. Pharm. 2010, 401, 16–24. [Google Scholar] [CrossRef]
- Jiang, J.; Moore, J.S.; Edelhauser, H.F.; Prausnitz, M.R. Intrascleral drug delivery to the eye using hollow microneedles. Pharm. Res. 2009, 26, 395–403. [Google Scholar] [CrossRef]
- Kunou, N.; Ogura, Y.; Yasukawa, T.; Kimura, H.; Miyamoto, H.; Honda, Y.; Ikada, Y. Long-term sustained release of ganciclovir from biodegradable scleral implant for the treatment of cytomegalovirus retinitis. J. Control. Release 2000, 68, 263–271. [Google Scholar] [CrossRef]
- Shimazaki, H.; Hironaka, K.; Fujisawa, T.; Tsuruma, K.; Tozuka, Y.; Shimazawa, M.; Takeuchi, H.; Hara, H. Edaravone-loaded liposome eyedrops protect against light-induced retinal damage in mice. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7289–7297. [Google Scholar] [CrossRef]
- Yanlei, H.; Yujun, Z.; Yue, M.; Juan, Y.; Liang, L.; Meijuan, C.; Shengjie, L.; Yimin, F. Formulation of Silk Fibroin Nanobrush-Stabilized Biocompatible Pickering Emulsions. Langmuir 2022, 38, 14302–14312. [Google Scholar] [CrossRef]
- Kim, E.S.; Durairaj, C.; Kadam, R.S.; Lee, S.J.; Mo, Y.; Geroski, D.H.; Kompella, U.B.; Edelhauser, H.F. Human scleral diffusion of anticancer drugs from solution and nanoparticle formulation. Pharm. Res. 2009, 26, 1155–1161. [Google Scholar] [CrossRef] [PubMed]


| S. No | Formulation | Outcome | Released Agents | Ref |
|---|---|---|---|---|
| 01 | Hydrogels | Sustained ocular delivery entails releasing the product over a period of 90 days. Biodegradation begins three months after intravitreal injection. | Bevacizumab | [11] |
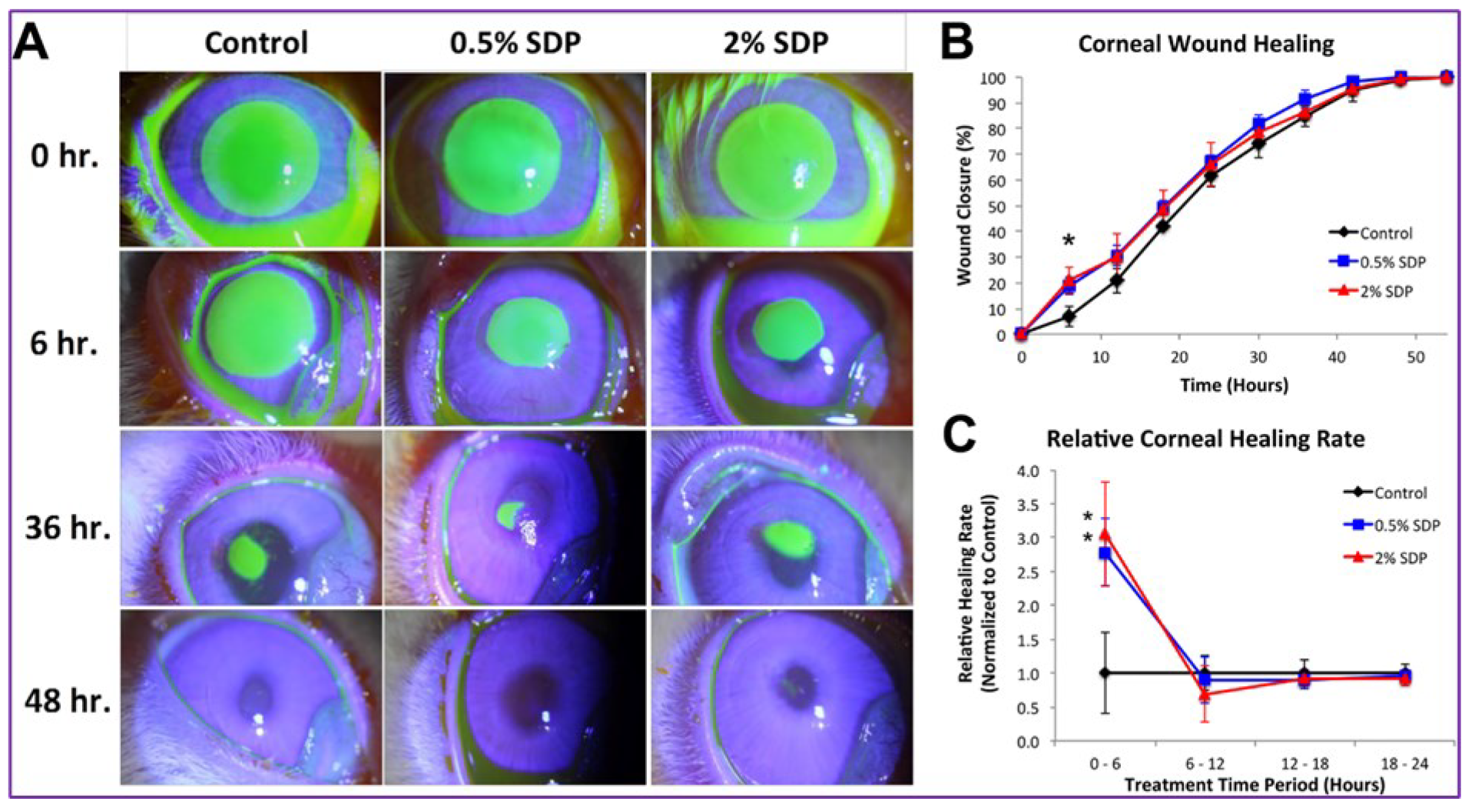
| 02 | Silk-Derived Protein (SDP) | Improves the healing of rabbit corneal epithelial wounds. | Phosphate buffered saline (PBS) | [12] |
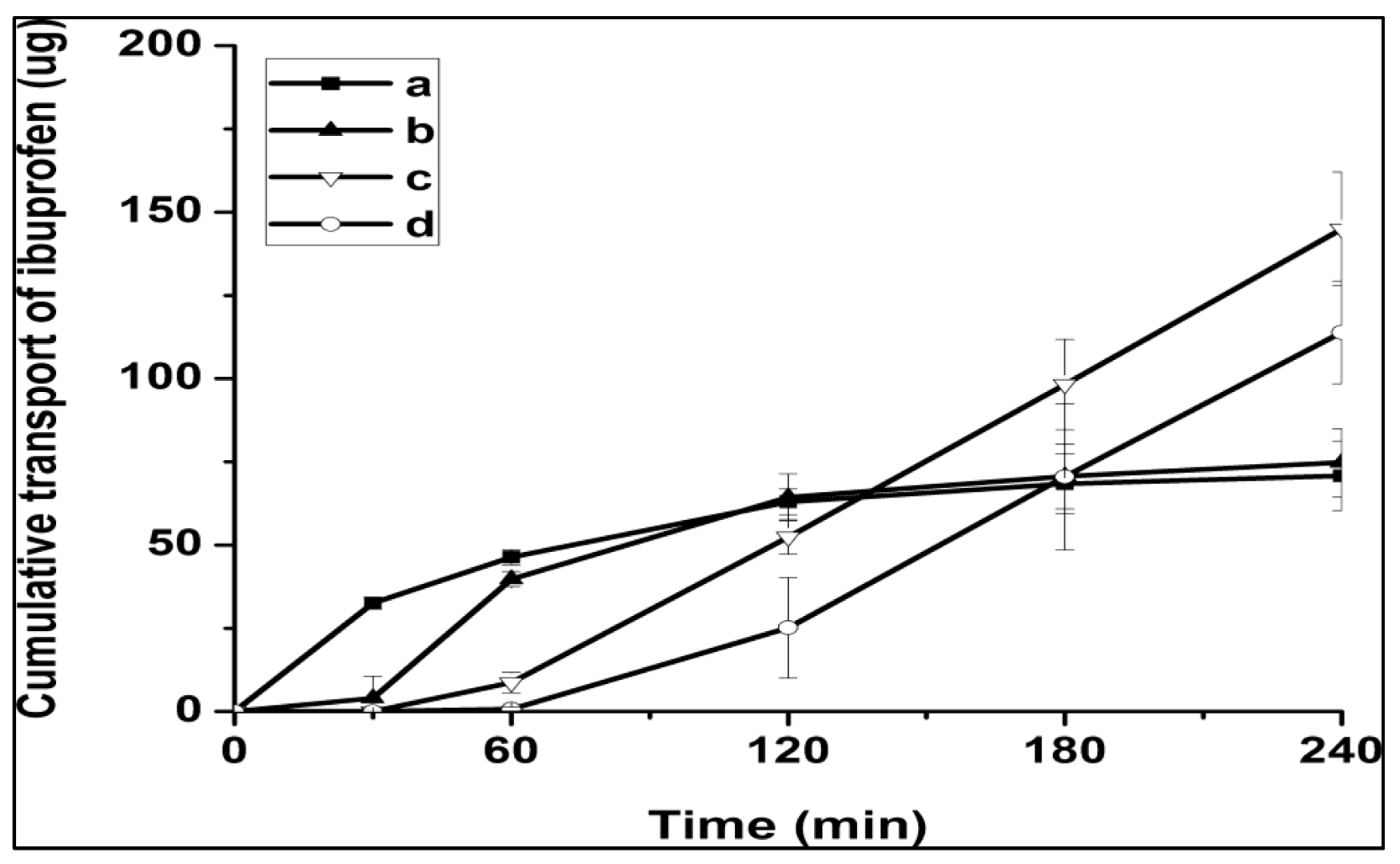
| 03 | Silk fibroin-coated liposomes (SLs) | Continuous and rapid adhesion and uptake of SF and SLs in cornea cells has been reported, with no detectable cytotoxicity during the experimental course. | Ibuprofen | [13] |
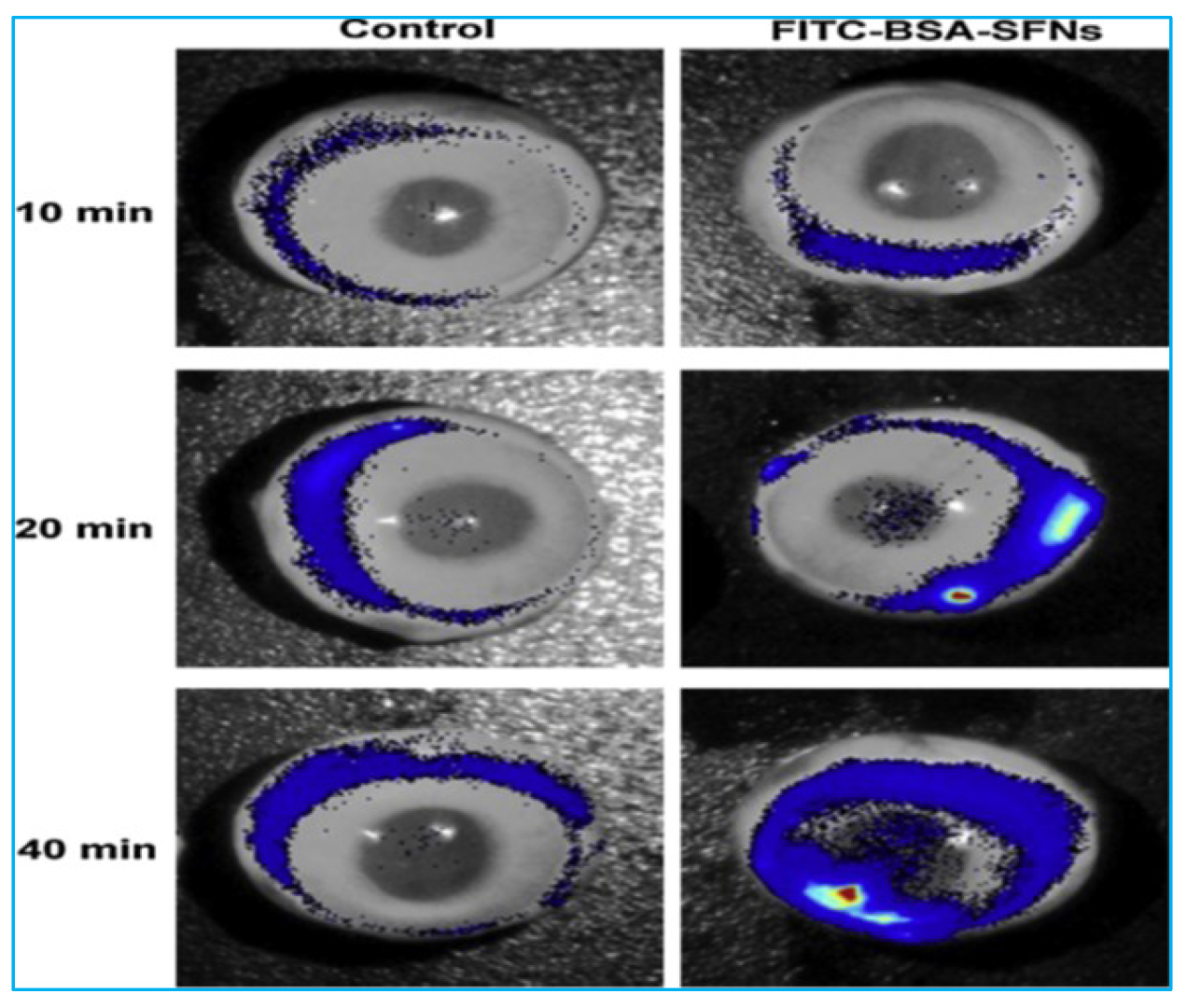
| 04 | Silk fibroin nanoparticles (SFNPs) | SFNP carriers for noninvasive transscleral administration of macromolecular protein drugs | Fluorescein isothiocyanate labelled bovine serum albumin | [14] |
| 05 | Silk fibroin nanoparticles | Bio-macromolecule delivery to the retina is improved. | Fluorescein isothiocyanate labelled bovine serum albumin (FITC-BSA) | [15] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wani, S.U.D.; Masoodi, M.H.; Gautam, S.P.; Shivakumar, H.G.; Alshehri, S.; Ghoneim, M.M.; Alam, P.; Shakeel, F. Promising Role of Silk-Based Biomaterials for Ocular-Based Drug Delivery and Tissue Engineering. Polymers 2022, 14, 5475. https://doi.org/10.3390/polym14245475
Wani SUD, Masoodi MH, Gautam SP, Shivakumar HG, Alshehri S, Ghoneim MM, Alam P, Shakeel F. Promising Role of Silk-Based Biomaterials for Ocular-Based Drug Delivery and Tissue Engineering. Polymers. 2022; 14(24):5475. https://doi.org/10.3390/polym14245475
Chicago/Turabian StyleWani, Shahid Ud Din, Mubashir Hussain Masoodi, Surya Prakash Gautam, H. G. Shivakumar, Sultan Alshehri, Mohammed M. Ghoneim, Prawez Alam, and Faiyaz Shakeel. 2022. "Promising Role of Silk-Based Biomaterials for Ocular-Based Drug Delivery and Tissue Engineering" Polymers 14, no. 24: 5475. https://doi.org/10.3390/polym14245475
APA StyleWani, S. U. D., Masoodi, M. H., Gautam, S. P., Shivakumar, H. G., Alshehri, S., Ghoneim, M. M., Alam, P., & Shakeel, F. (2022). Promising Role of Silk-Based Biomaterials for Ocular-Based Drug Delivery and Tissue Engineering. Polymers, 14(24), 5475. https://doi.org/10.3390/polym14245475









