Poly(glycidyl azide) as Photo-Crosslinker for Polymers
Abstract
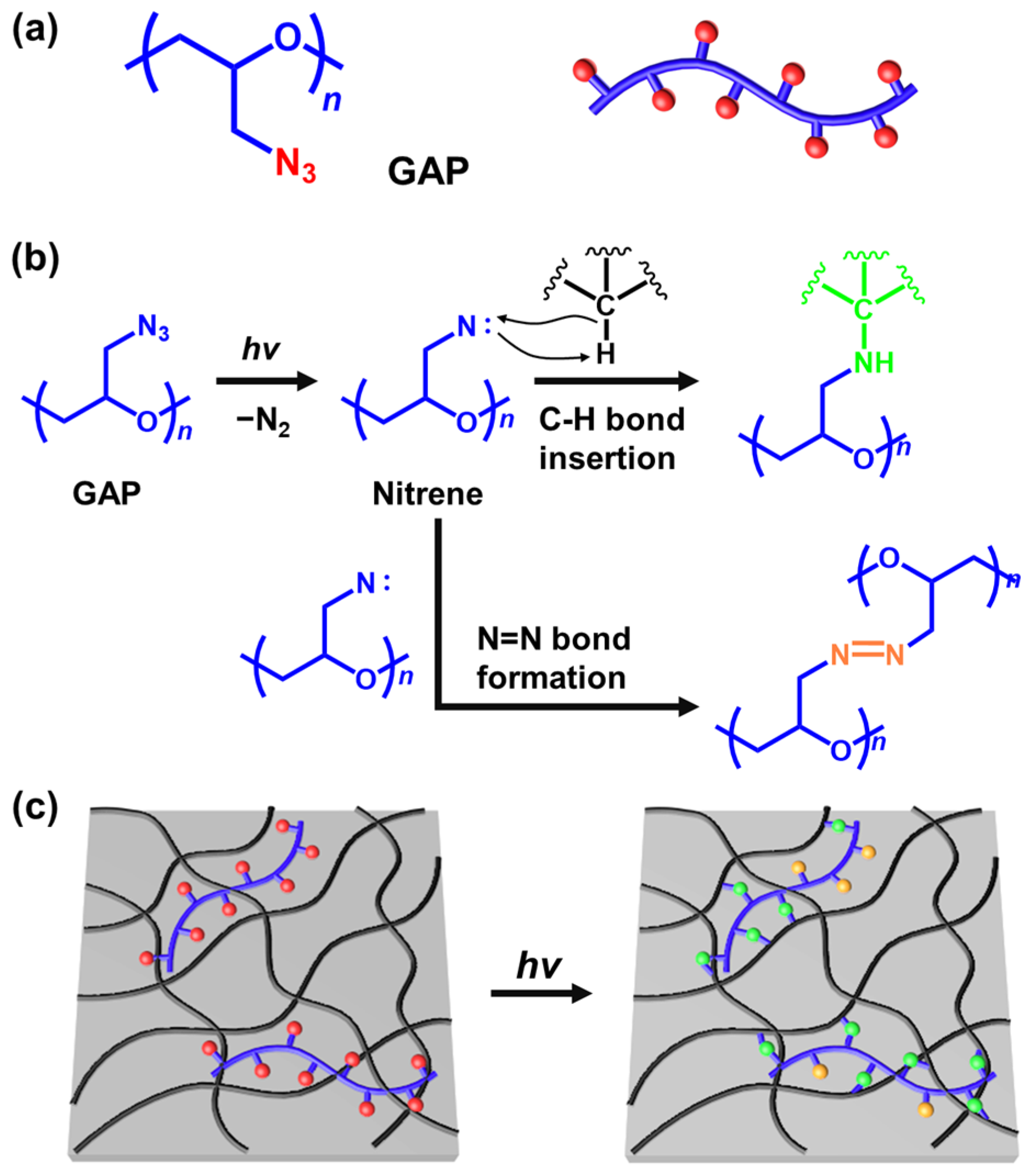
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Photo-Crosslinking of Polymer/GAP Mixture
2.2.2. Adhesion between Polymeric Plates with GAP
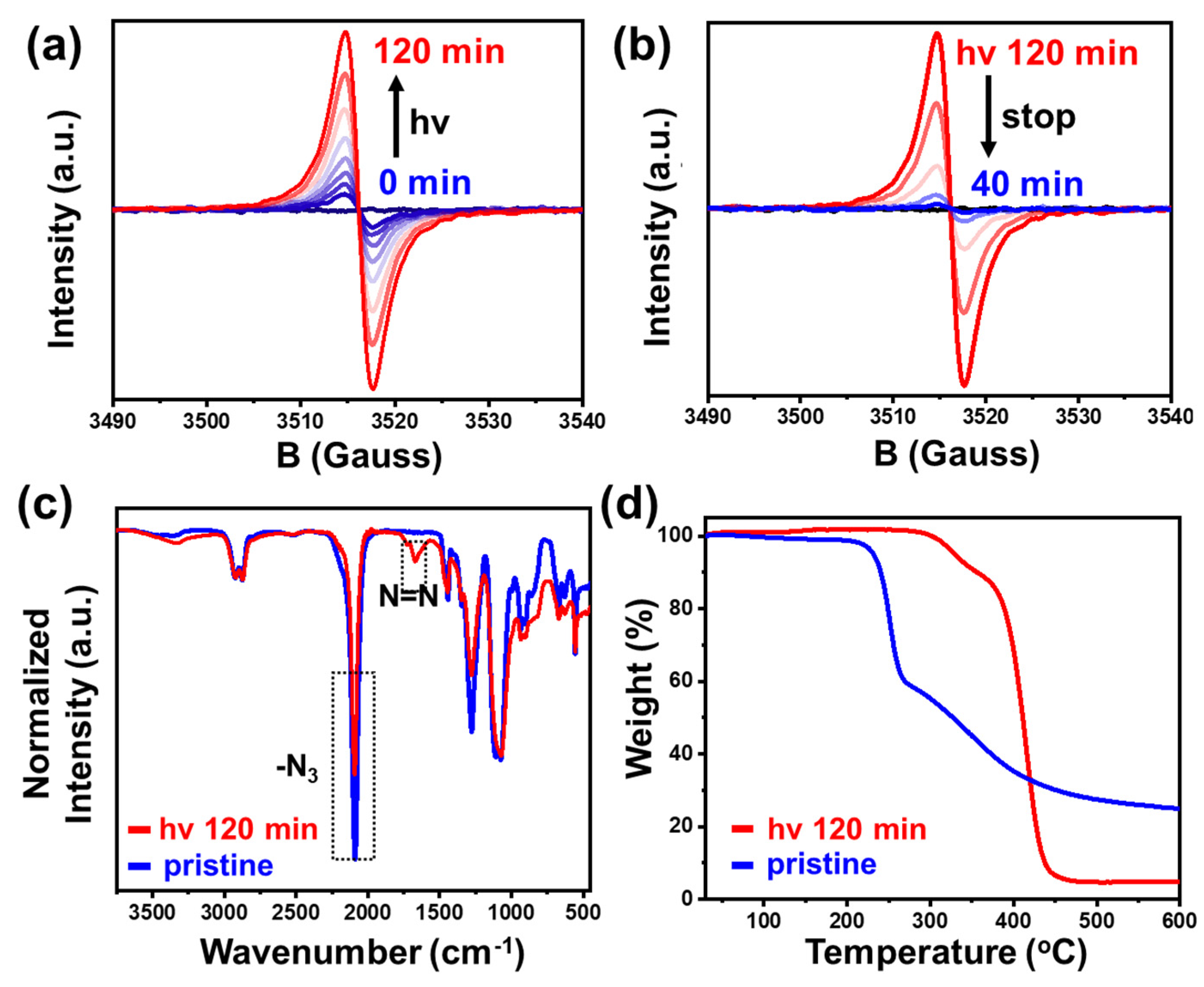
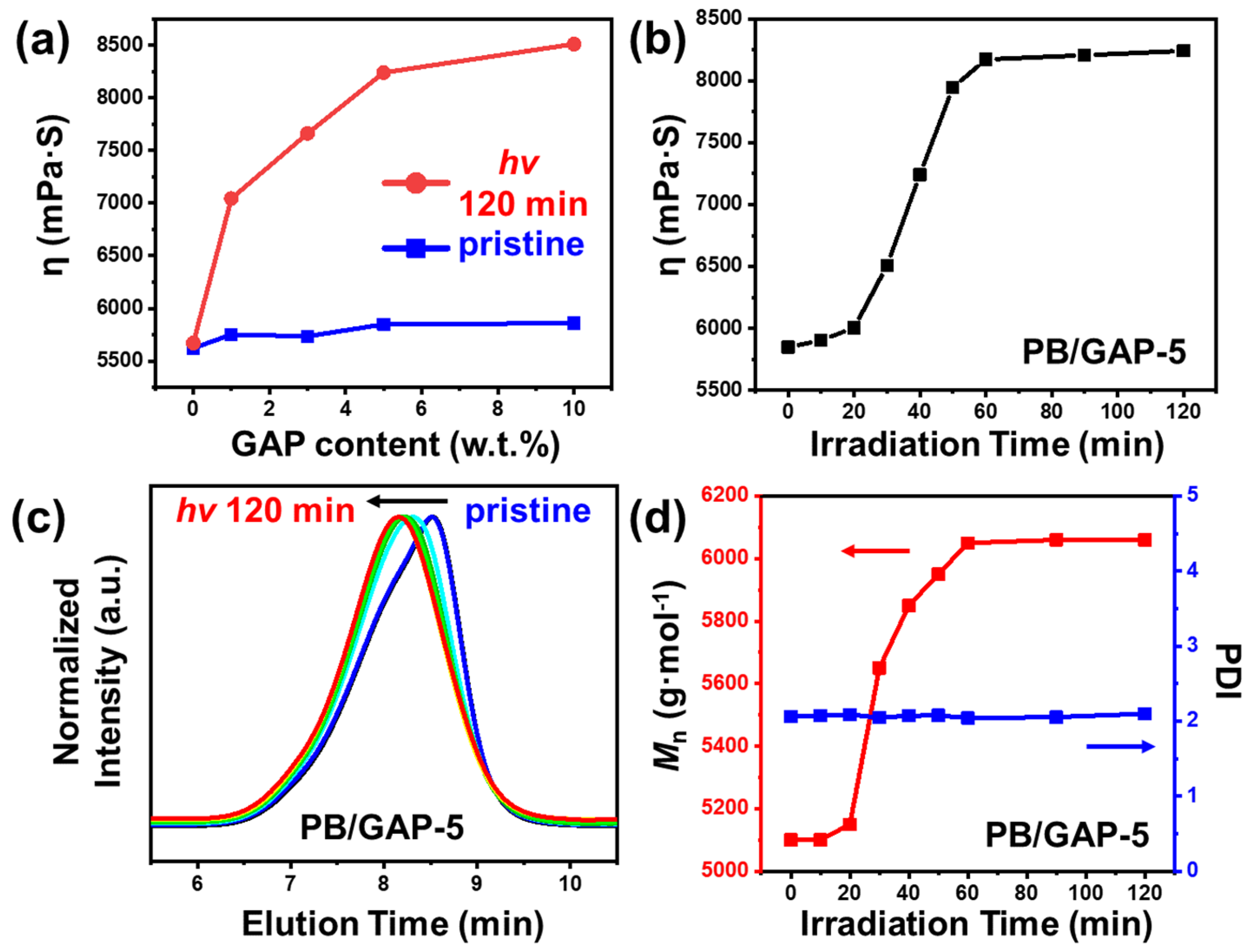
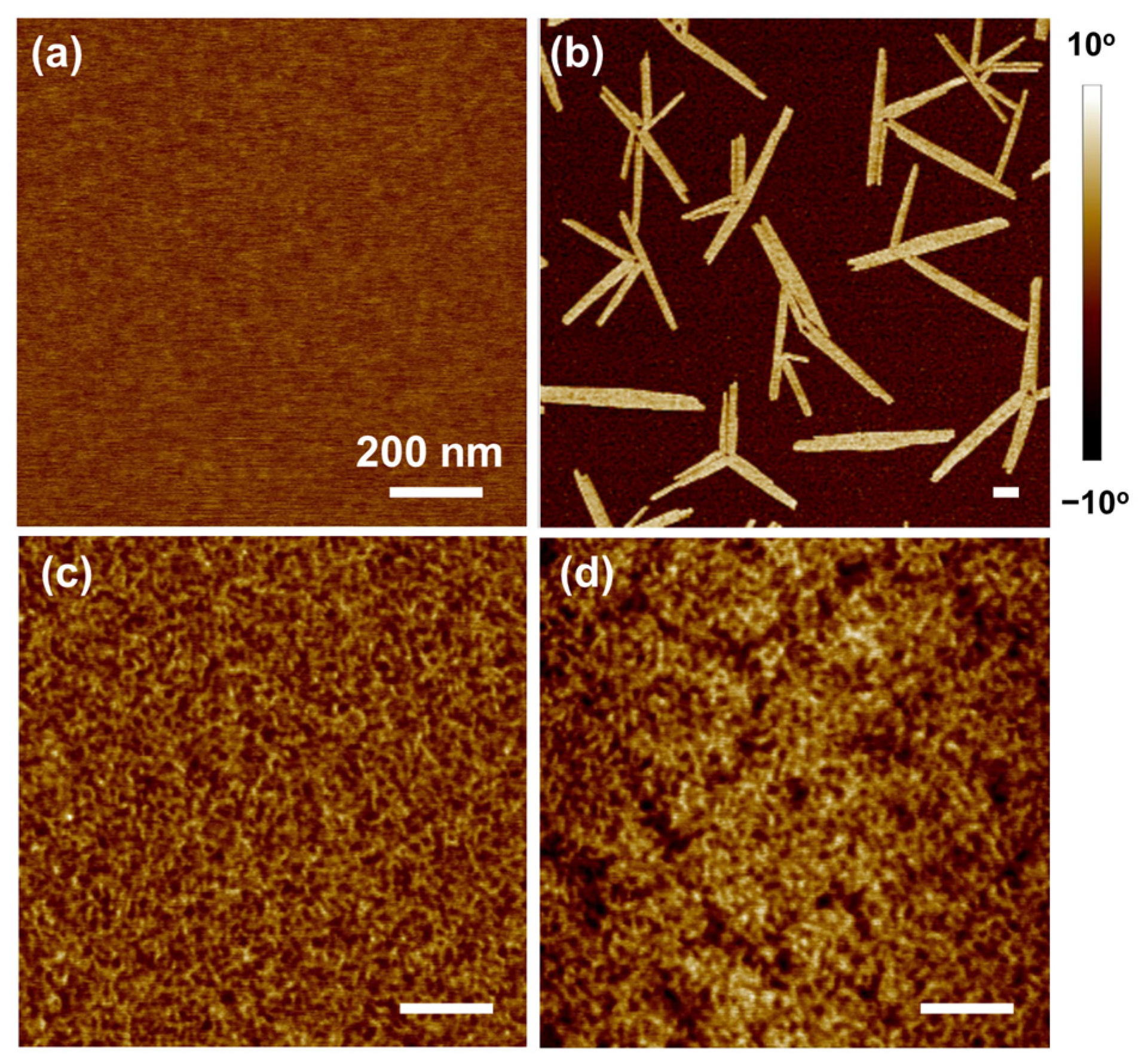
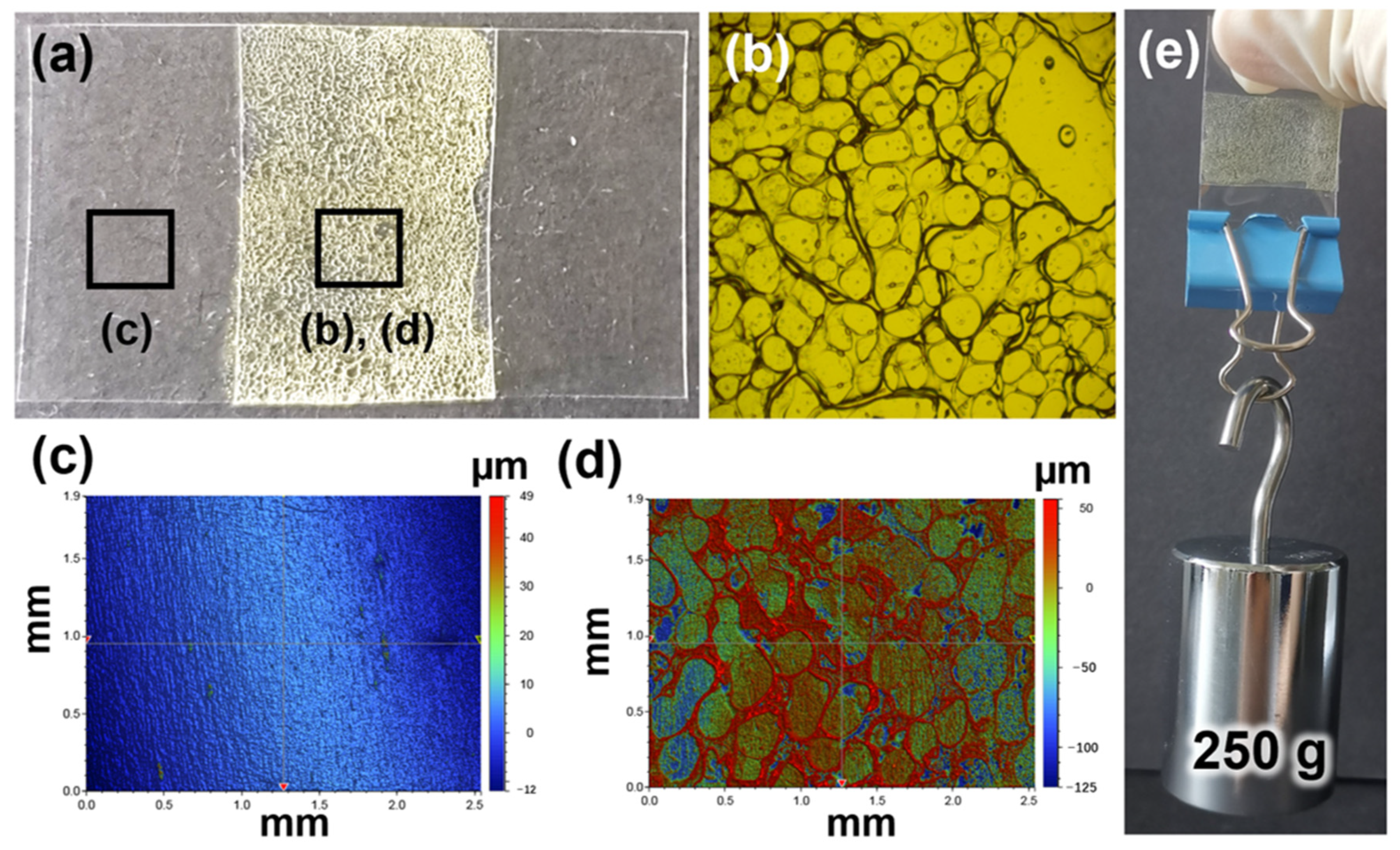
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stewart, K.A.; Shuster, D.; Leising, M.; Coolidge, I.; Lee, E.; Stevens, C.; Peloquin, A.J.; Kure, D.; Jennings, A.R.; Iacono, S.T. Synthesis, characterization, and thermal properties of Fluoropyridyl-Functionalized Siloxanes of diverse polymeric architectures. Macromolecules 2021, 54, 4871–4879. [Google Scholar] [CrossRef]
- Flory, P.J.; Volkenstein, M. Statistical mechanics of chain molecules. Biopolymers 1969, 8, 699–700. [Google Scholar] [CrossRef]
- Bates, F.S. Polymer-polymer phase behavior. Science 1991, 251, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Bates, C.M.; Bates, F.S. 50th Anniversary perspective: Block polymers-pure potential. Macromolecules 2017, 50, 3–22. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Z.; Chen, X.; Tan, R.; Li, G.; Gan, Z.; Shao, Y.; He, J.; Zhang, Z.; Li, W.; et al. Discrete giant polymeric chains based on nanosized monomers. J. Am. Chem. Soc. Au 2021, 1, 79–86. [Google Scholar] [CrossRef]
- Stubbs, C.J.; Worch, J.C.; Prydderch, H.; Wang, Z.; Mathers, R.T.; Dobrynin, A.V.; Becker, M.L.; Dove, A.P. Sugar-based polymers with stereochemistry-dependent degradability and mechanical properties. J. Am. Chem. Soc. 2022, 144, 1243–1250. [Google Scholar] [CrossRef]
- Chile, L.E.; Mehrkhodavandi, P.; Hatzikiriakos, S.G. A comparison of the rheological and mechanical properties of isotactic, syndiotactic, and heterotactic poly(lactide). Macromolecules 2016, 49, 909–919. [Google Scholar] [CrossRef]
- Lee, S.W.; Park, J.W.; Park, C.H.; Kim, H.J.; Kim, E.A.; Woo, H.S. Optical properties and adhesion performance of optically clear acrylic pressure sensitive adhesives using chelate metal acetylacetonate. Int. J. Adhes. Adhes. 2013, 47, 21–25. [Google Scholar] [CrossRef]
- Cao, X.; Merlitz, H.; Wu, C. Mechanical strength management of polymer composites through tuning transient networks. J. Phys. Chem. Lett. 2020, 11, 710–715. [Google Scholar] [CrossRef]
- Guan, Q.; Han, Z.; Yang, K.; Yang, H.; Ling, Z.; Yin, C.; Yu, S. Sustainable double-network structural materials for electromagnetic shielding. Nano Lett. 2021, 21, 2532–2537. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Y.; Su, Q.; Huang, S.; Song, D.; Ma, R.; Zhu, C.; Lv, G.; Li, C. A flexible semi-interpenetrating network-enhanced lonogel polymer electrolyte for highly stable and safe lithium metal batteries. ACS Appl. Mater. Interfaces 2021, 13, 41946–41955. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Liu, H.; Li, H.; Cao, Y.; Hua, X.; Ge, S.; He, Y.; Jiang, C.; He, D. Cogel strategy for the preparation of a “Thorn”-like porous halloysite/gelatin composite aerogel with excellent mechanical properties and thermal insulation. ACS Appl. Mater. Interfaces 2022, 14, 17763–17773. [Google Scholar] [CrossRef] [PubMed]
- Niedermeier, C.; Yamaura, J.I.; Wu, J.; He, X.; Katase, T.; Hosono, H.; Kamiya, T. Crystal structure built from a GeO6–GeO5 polyhedra network with high thermal stability: β–SrGe2O5. ACS Appl. Electron. Mater. 2019, 1, 1989–1993. [Google Scholar] [CrossRef]
- Katagiri, K.; Sakai, T.; Hishikawa, M.; Masu, H.; Tominaga, M.; Yamaguchi, K.; Azumaya, I. Synthesis, structure, and thermal stability of silver(I) coordination polymers with bis(pyridyl) ligands linked by an aromatic sulfonamide: One-dimensional-straight chain, one-dimensional-columnar with helical components, and two-dimensional-layer network structures. Cryst. Growth Des. 2014, 14, 199–206. [Google Scholar]
- Li, C.; Mu, C.; Lin, W.; Ngai, T. Gelatin effects on the physicochemical and hemocompatible properties of gelatin/PAAm/laponite nanocomposite hydrogels. ACS Appl. Mater. Interfaces 2015, 7, 18732–18741. [Google Scholar] [CrossRef]
- Tian, S.; Sun, J.; Jin, K.; Wang, J.; He, F.; Zheng, S.; Fang, Q. Postpolymerization of a fluorinated and reactive poly(aryl ether): An efficient way to balance the solubility and solvent resistance of the polymer. ACS Appl. Mater. Interfaces 2014, 6, 20437–20443. [Google Scholar] [CrossRef]
- Shi, Z.; Huang, J.; Liu, C.; Ding, B.; Kuga, S.; Cai, J.; Zhang, L. Three-dimensional nanoporous cellulose gels as a flexible reinforcement matrix for polymer nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 22990–22998. [Google Scholar] [CrossRef]
- Hamed, G.; Huang, J. Combining cobalt and resorcinolic bonding agents in brass-rubber adhesion. Rubber Chemisrty Technol. 1991, 64, 285–295. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, W.I.; Yoo, Y.E.; Kim, E.G. A study on fiber orientation in the compression molding of fiber reinforced polymer composite material. J. Mater. Process. Technol. 2001, 111, 233–239. [Google Scholar] [CrossRef]
- Farr, N.T.H.; Roman, S.; Schafer, J.; Quade, A.; Lester, D.; Hearnden, V.; MacNeil, S.; Rodenburg, C. A novel characterisation approach to reveal the mechano–chemical effects of oxidation and dynamic distension on polypropylene surgical mesh. RSC Adv. 2021, 11, 34710–34723. [Google Scholar] [CrossRef]
- Sawasaki, T.; Nojiri, A. Radiation crosslinking of polypropylene. Int. J. Radiat. Appl. Instrum. Part C Radiat. Phys. Chem. 1988, 31, 877–886. [Google Scholar] [CrossRef]
- Han, D.H.; Shin, S.H.; Petrov, S. Crosslinking and degradation of polypropylene by electron beam irradiation in the presence of trifunctional monomers. Radiat. Phys. Chem. 2004, 69, 239–244. [Google Scholar] [CrossRef]
- Gunasekaran, H.B.; Ponnan, S.; Thirunavukkarasu, N.; Wu, K.; Wu, L.; Wang, J. Investigation of in-situ chemical cross-linking during fused filament fabrication process on parts shrinkage reduction and interlayer adhesion. J. Mater. Res. Technol. 2021, 15, 2026–2035. [Google Scholar] [CrossRef]
- Bednarek, M.; Borska, K.; Kubisa, P. Crosslinking of polylactide by high energy irradiation and photo-curing. Molecules 2020, 25, 4919. [Google Scholar] [CrossRef]
- Du, N.; Dal-Cin, M.M.; Pinnau, I.; Nicalek, A.; Robertson, G.P.; Guiver, M.D. Azide-based cross-linking of polymers of intrinsic microporosity (PIMs) for condensable gas separation. Macromol. Rapid Commun. 2011, 32, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, C.; Jung, J.; Kang, H.; Kim, K.H.; Ma, B.W.; Kim, B.J. Facile photo-crosslinking of azide-containing hole-transporting polymers for highly efficient, solution-processed, multilayer organic light emitting devices. Adv. Funct. Mater. 2014, 24, 7588–7596. [Google Scholar] [CrossRef]
- Kwon, H.J.; Tang, X.; Shin, S.; Hong, J.; Jeong, W.; Jo, Y.; An, T.K.; Lee, J.; Kim, S.H. Facile photo-cross-linking system for polymeric gate dielectric materials toward solution-processed organic field-effect transistors: Role of a cross-linker in various polymer types. ACS Appl. Mater. Interfaces 2020, 12, 30600–30615. [Google Scholar] [CrossRef]
- Smolinsky, G. Electrophilic substitution at a saturated carbon by electron deficient nitrogen. J. Am. Chem. Soc. 1960, 82, 4717–4719. [Google Scholar] [CrossRef]
- Anastassiou, A.G.; Simmons, H.E.; Marsh, F.D. Cyanonitrene. Reaction with saturated hydrocarbons. J. Am. Chem. Soc. 1965, 87, 2296–2297. [Google Scholar] [CrossRef]
- Breslow, D.S.; Sloan, M.F.; Newburg, N.R.; Renfrow, W.B. Thermal reactions of sulfonyl azides. J. Am. Chem. Soc. 1969, 91, 2273–2279. [Google Scholar] [CrossRef]
- Fairchild, P.; Smith, G.P.; Crosley, D.; Jeffries, J.B. Lifetimes and transition probabilities for NH(A3Πi-X3Σ−). Chem. Phys. Lett. 1984, 107, 181–186. [Google Scholar] [CrossRef]
- Dequirez, G.; Pons, V.; Dauban, P. Nitrene chemistry in organic synthesis: Still in its infancy? Angew. Chem. Int. Ed. 2012, 51, 7384–7395. [Google Scholar] [CrossRef] [PubMed]
- Frankel, M.B.; Grant, L.R.; Flanagan, J.E. Historical development of glycidyl azide polymer. J. Propul. Power 1992, 8, 560–563. [Google Scholar] [CrossRef]
- Sahu, S.K.; Panda, S.P.; Sadafule, D.S.; Kumbhar, C.G.; Kulkarni, S.G.; Thakur, J.V. Thermal and photodegradation of glycidyl azide polymers. Polym. Degrad. Stab. 1998, 62, 495–500. [Google Scholar] [CrossRef]
- Liu, D.; Geng, D.; Yang, K.; Lu, J.; Chan, S.H.Y.; Chen, C.; Hng, H.H.; Chen, L. Decomposition and energy-enhancement mechanism of the energetic binder glycidyl azide polymer at explosive detonation temperatures. J. Phys. Chem. 2020, 124, 5542–5554. [Google Scholar] [CrossRef] [PubMed]
- Warminski, M.; Kowalska, J.; Jemielity, J. Solid-phase synthesis of RNA 5′-Azides and their application for labeling, ligation, and cyclization via click chemistry. Curr. Protoc. Nucleic Acid Chem. 2020, 82, e112. [Google Scholar] [CrossRef] [PubMed]
- Horak, J.; Hofer, S.; Lindner, W. Optimization of a ligand immobilization and azide group endcapping concept via “Click-Chemistry” for the preparation of adsorbents for antibody purification. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2010, 878, 3382–3394. [Google Scholar] [CrossRef]
- Presolski, S.I.; Hong, V.P.; Finn, M.G. Copper-catalyzed azide–alkyne click chemistry for bioconjugation. Curr. Protoc. Chem. Biol. 2011, 3, 153–162. [Google Scholar] [CrossRef]
- Joralemon, M.J.; O’Reilly, R.K.; Hawker, C.J.; Wooley, K.L. Shell click-crosslinked (SCC) nanoparticles: A new methodology for synthesis and orthogonal functionalization. J. Am. Chem. Soc. 2005, 127, 16892–16899. [Google Scholar] [CrossRef]
- Breugst, M.; Reissig, H.U. The Huisgen Reaction: Milestones of the 1,3-Dipolar cycloaddition. Angew. Chem. Int. Ed. 2020, 59, 12293–12307. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Liu, H.Y.; Hudson, B.N.; Lin, C.C. Enzymatic cross-linking of dynamic thiol-norbornene click hydrogels. ACS Biomater. Sci. Eng. 2019, 5, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Yordanov, N.D.; Mladenova, R. EPR study of free radicals in bread. Spectrochimi. Acta Part A 2004, 60, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers, P.F.; Ivar, J.; Schneider, S.; Bruin, B.D. Nitrene radical intermediates in catalytic synthesis. Chem. Eur. J. 2017, 23, 13819–13829. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, Z.; Fang, W.; Zhu, Y.; Zhang, Y.; Jin, J. Polyamide thin films grown on PD/SWCNT-interlayered-PTFE microfiltration membranes for high-permeance organic solvent nanofiltration. Ind. Eng. Chem. Res. 2020, 59, 22533–22540. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, G.; Cao, K.; Guo, D.; Serrano, J.; Esker, A.; Liu, G. Solvent-resistant self-crosslinked poly(ether imide). Macromolecules 2021, 54, 3405–3412. [Google Scholar] [CrossRef]
- Zhang, Z.; He, Z.; Bi, S.; Asare-Yeboah, K. Phase segregation controlled semiconductor crystallization for organic thin film transistors. J. Sci-adv. Mater. Dev. 2020, 5, 151–163. [Google Scholar] [CrossRef]
- Li, X.; Wolanin, P.J.; MacFarlane, L.R.; Harniman, R.L.; Qian, J.; Gould, O.E.C.; Dane, T.G.; Rudin, J.; Cryan, M.J.; Schmaltz, T.; et al. Uniform electroactive fibre-like micelle nanowires for organic electronics. Nat. Commun. 2017, 8, 15909. [Google Scholar] [CrossRef]
- Simhadri, C.; Bi, L.; Lepage, M.L.; Takaffoli, M.; Pei, Z.; Musolino, S.F.; Milani, A.S.; DiLabio, G.A.; Wulff, J.E. Flexible polyfluorinated bis-diazirines as molecular adhesives. Chem. Sci. 2021, 12, 4147–4153. [Google Scholar] [CrossRef]
- Yuk, H.; Varela, C.E.; Nabzdyk, C.S.; Mao, X.; Padera, R.F.; Roche, E.T.; Zhao, X. Dry double-sided tape for adhesion of wet tissues and devices. Nature 2019, 575, 169–174. [Google Scholar] [CrossRef]
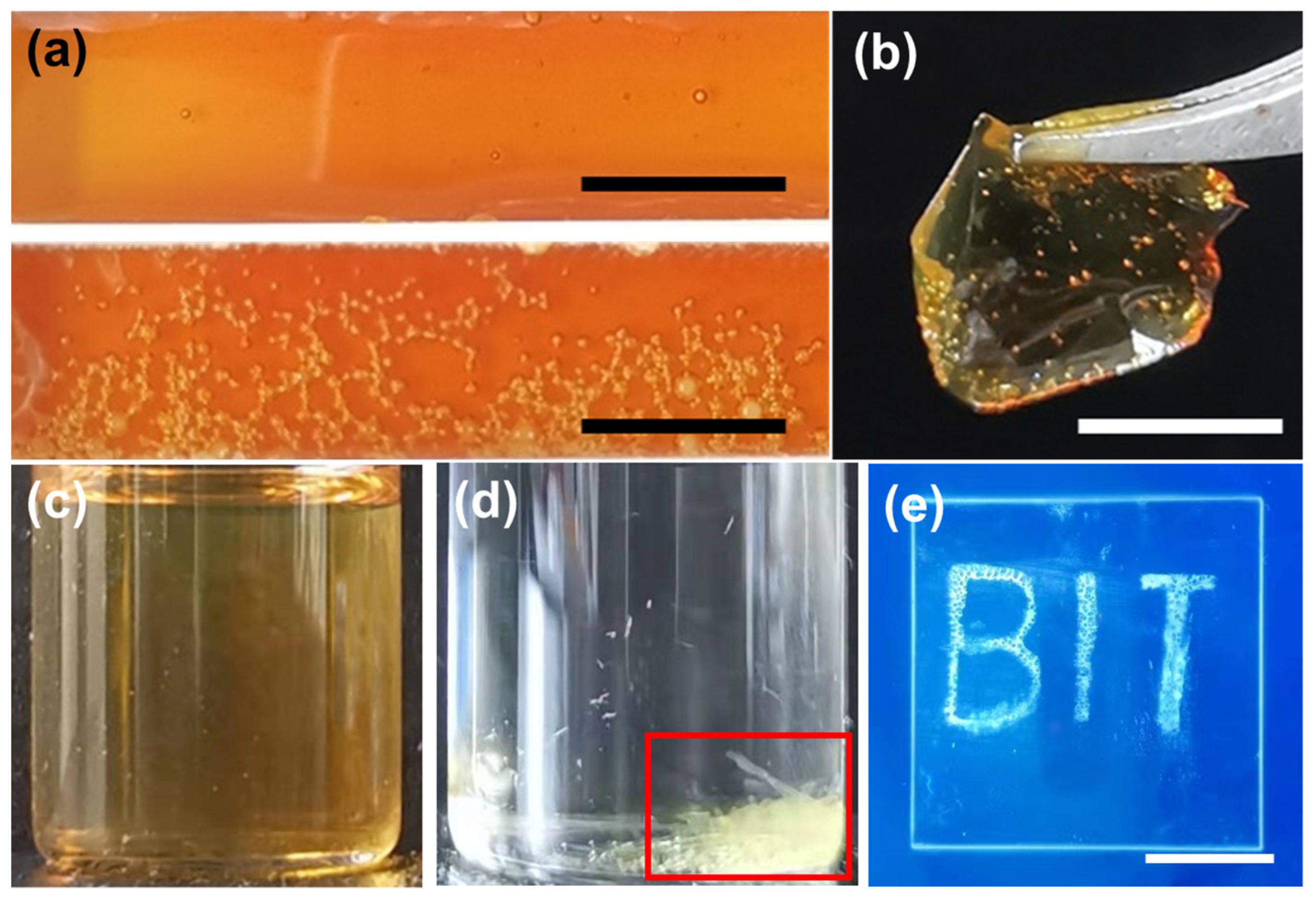

| Sample | Solution in 10 min | mpristine (mg) | minsoluble (mg) | Insoluble Content (wt.%) |
|---|---|---|---|---|
| PEG2k/GAP-1 | Yes | 50.2 | 0.0 | 0.0 |
| PEG2k/GAP-3 | No | 49.8 | 13.2 | 26.5 |
| PEG2k/GAP-5 | No | 50.4 | 18.6 | 36.9 |
| PEG4k/GAP-5 | No | 49.8 | 12.4 | 24.9 |
| PEG20k/GAP-5 | No | 50.5 | 8.6 | 17.0 |
| Sample | Melting Enthalpy (J/g) | Tm (°C) | Tg (°C) |
|---|---|---|---|
| PEG2k | −159.4 | 55.0 | N.A. |
| PEG2k UV | −169.7 | 54.8 | N.A. |
| PEG2k/GAP-5 UV | −157.8 | 54.4 | N.A. |
| PB | N.A. | N.A. | −27.8 |
| PB UV | N.A. | N.A. | −29.2 |
| PB/GAP-5 UV | N.A. | N.A. | −27.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Wei, W.; Hou, X.; Tang, G.; Luo, Y.; Li, X. Poly(glycidyl azide) as Photo-Crosslinker for Polymers. Polymers 2022, 14, 5451. https://doi.org/10.3390/polym14245451
Zhou X, Wei W, Hou X, Tang G, Luo Y, Li X. Poly(glycidyl azide) as Photo-Crosslinker for Polymers. Polymers. 2022; 14(24):5451. https://doi.org/10.3390/polym14245451
Chicago/Turabian StyleZhou, Xinyan, Wei Wei, Xiaojian Hou, Gang Tang, Yunjun Luo, and Xiaoyu Li. 2022. "Poly(glycidyl azide) as Photo-Crosslinker for Polymers" Polymers 14, no. 24: 5451. https://doi.org/10.3390/polym14245451
APA StyleZhou, X., Wei, W., Hou, X., Tang, G., Luo, Y., & Li, X. (2022). Poly(glycidyl azide) as Photo-Crosslinker for Polymers. Polymers, 14(24), 5451. https://doi.org/10.3390/polym14245451






