Density Functional Theory Analysis of the Copolymerization of Cyclopropenone with Ethylene Using a Palladium Catalyst
Abstract
:1. Introduction
2. Computational Methods
3. Results and Discussion
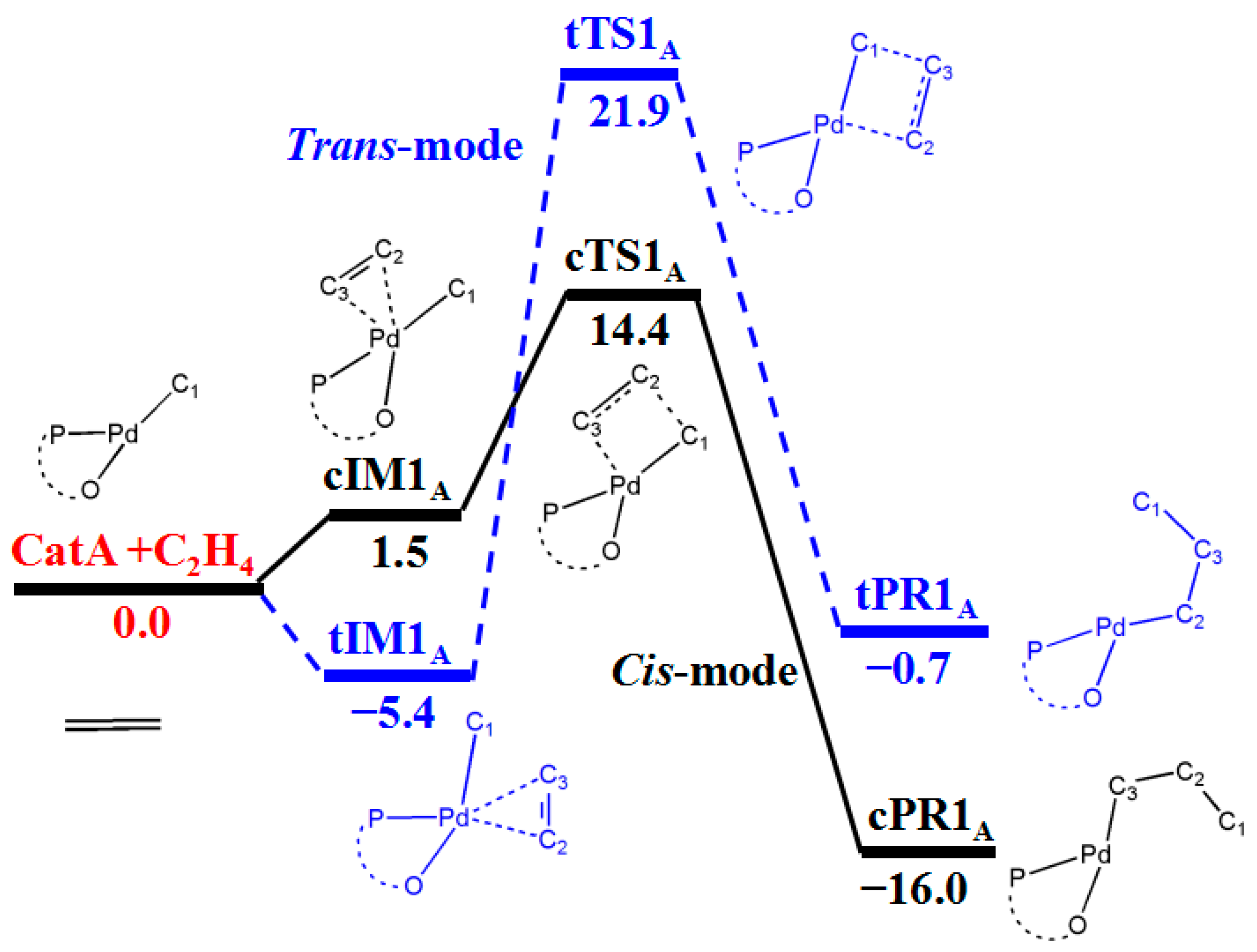
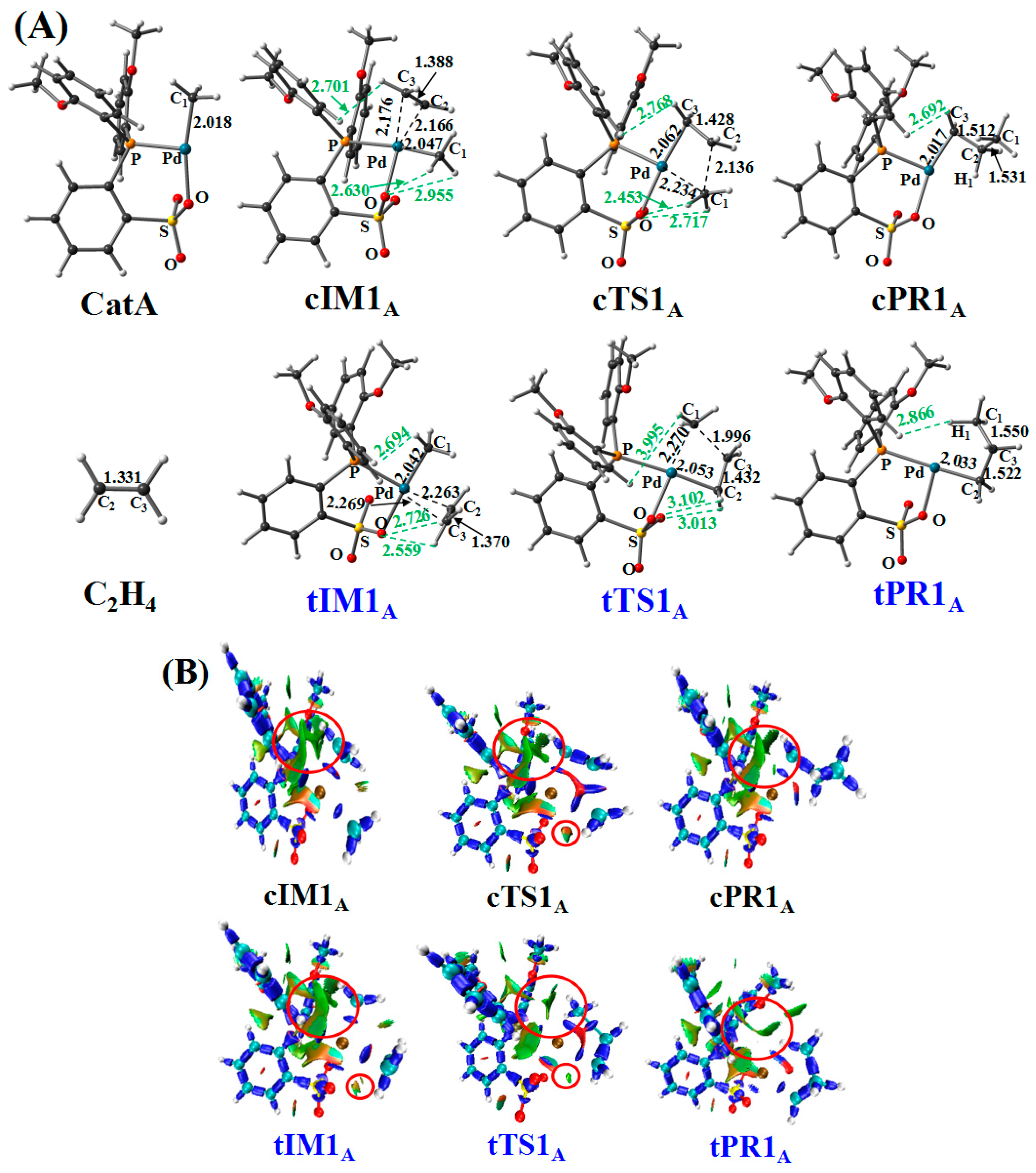
3.1. First Ethylene Insertion to Phosphine Sulfonate Pd Catalyst
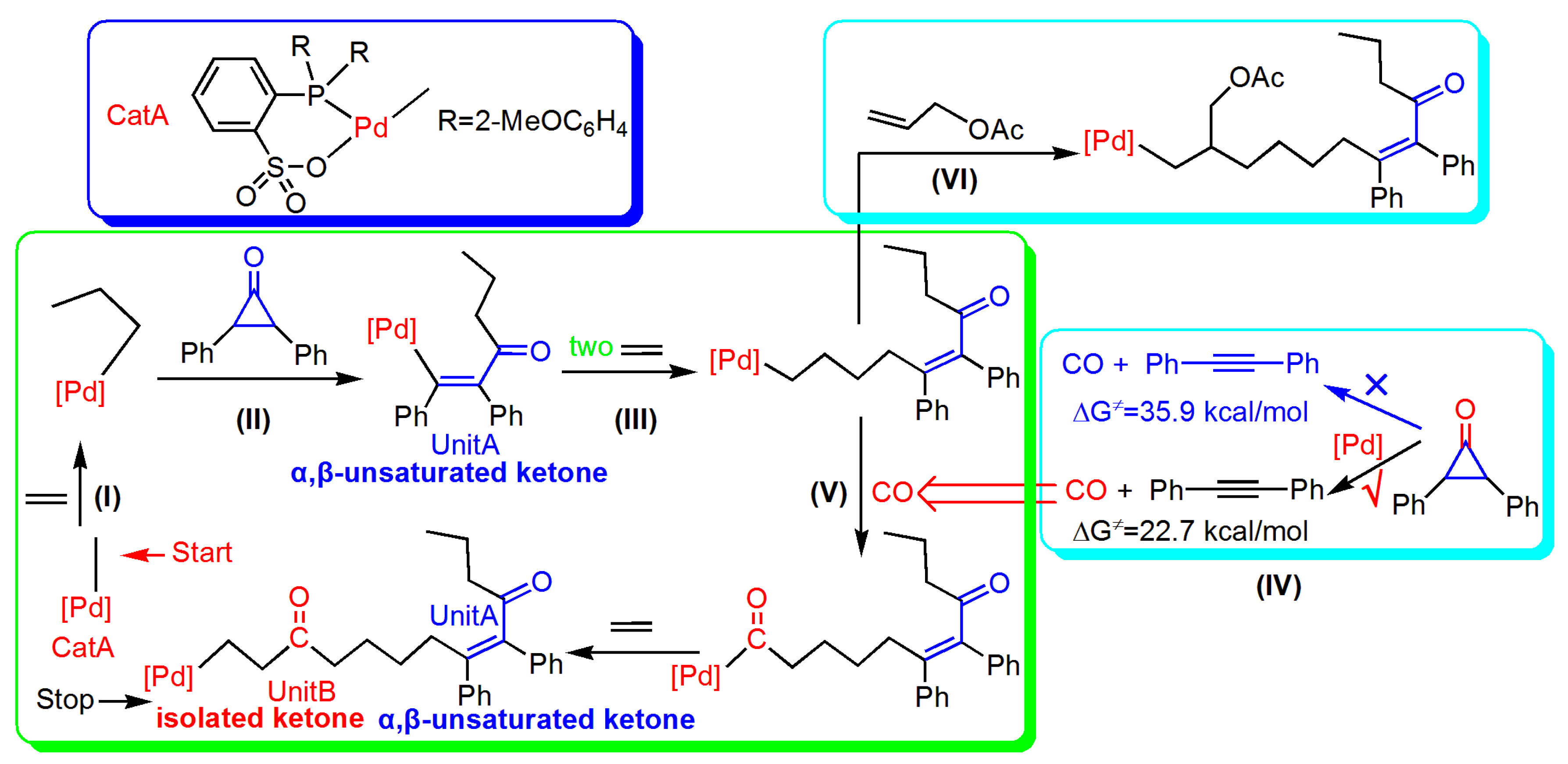
3.2. Reaction of Cyclopropenone
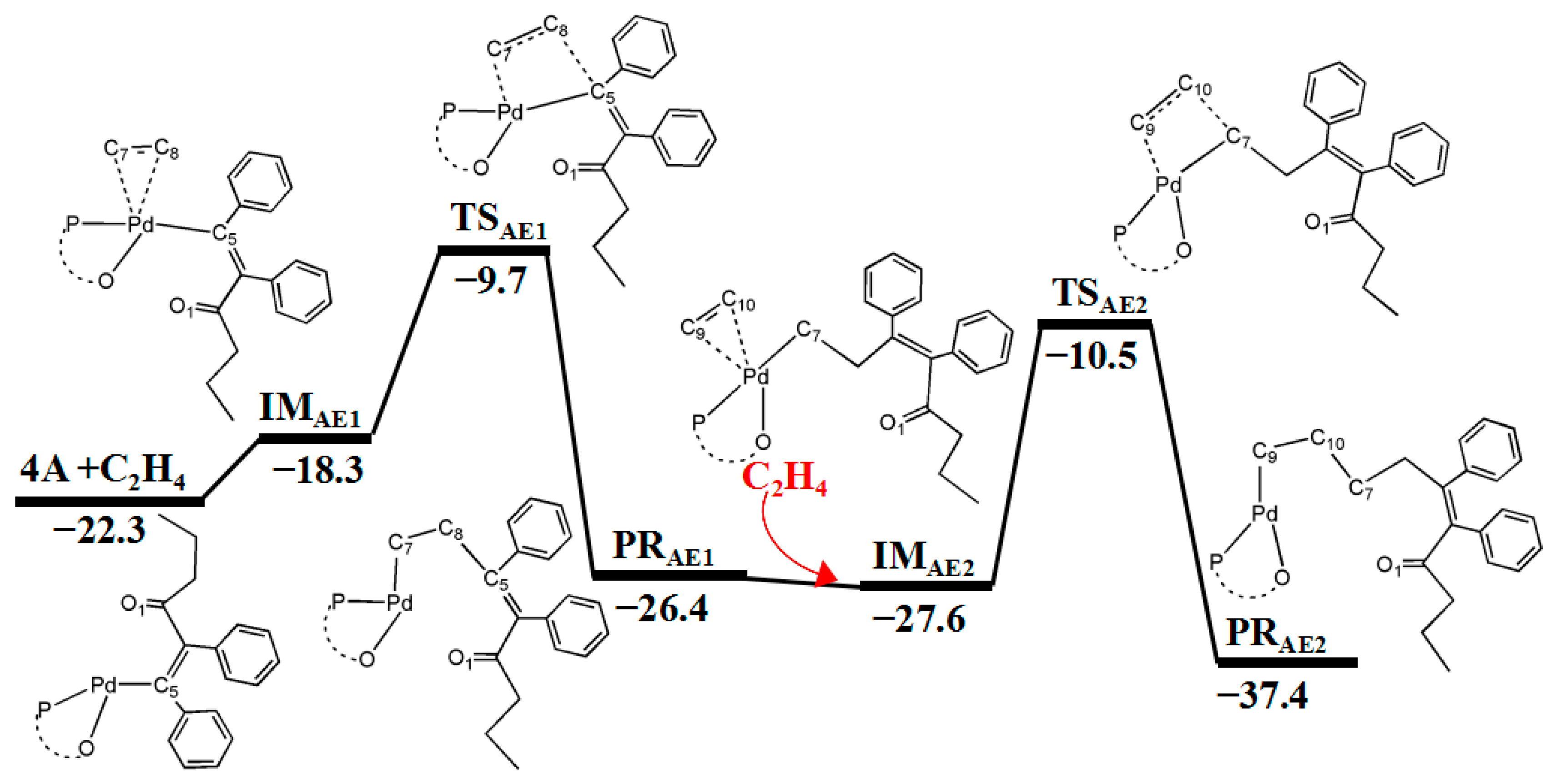
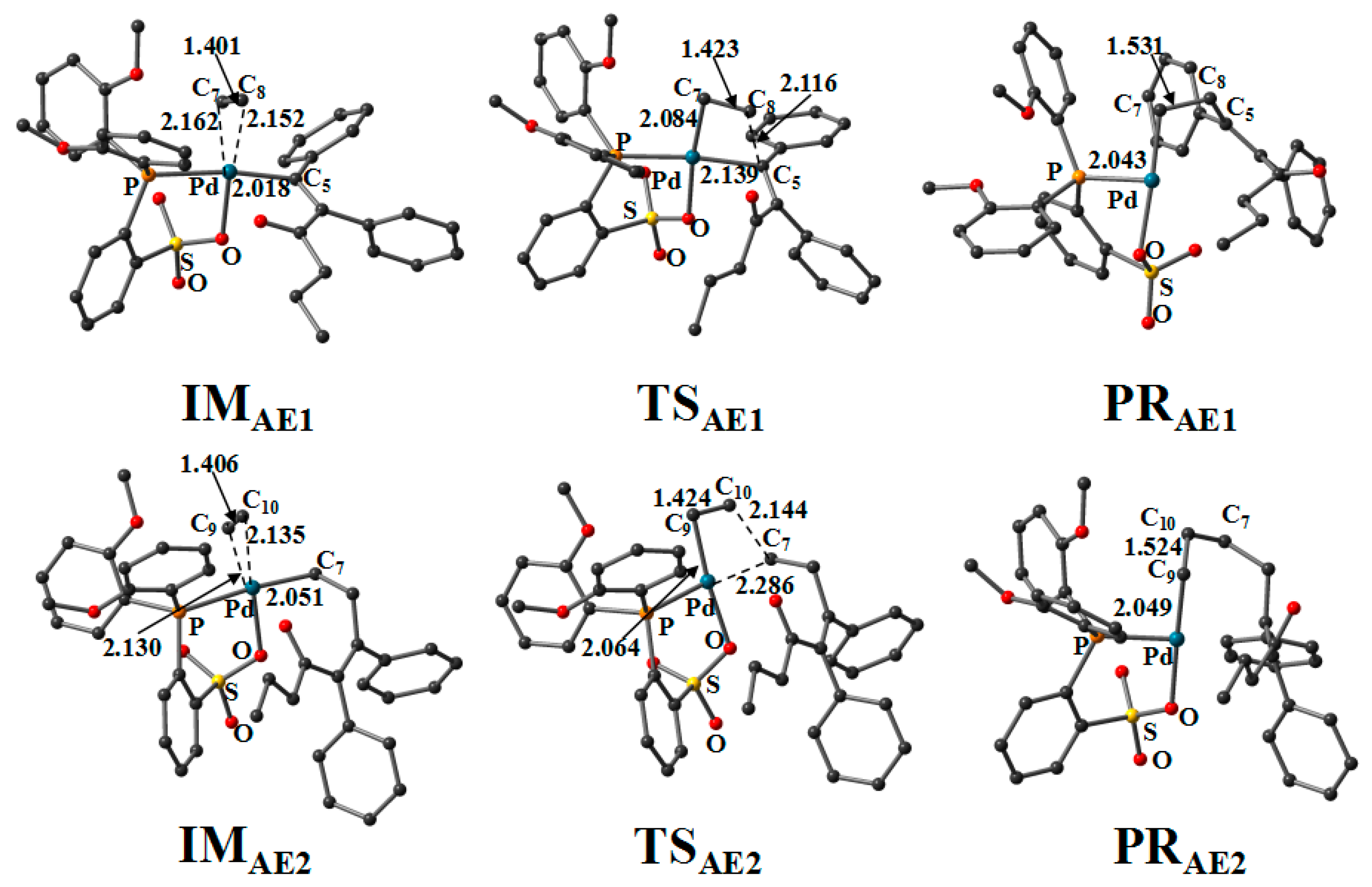
3.3. Second and Third Ethylene Insertion
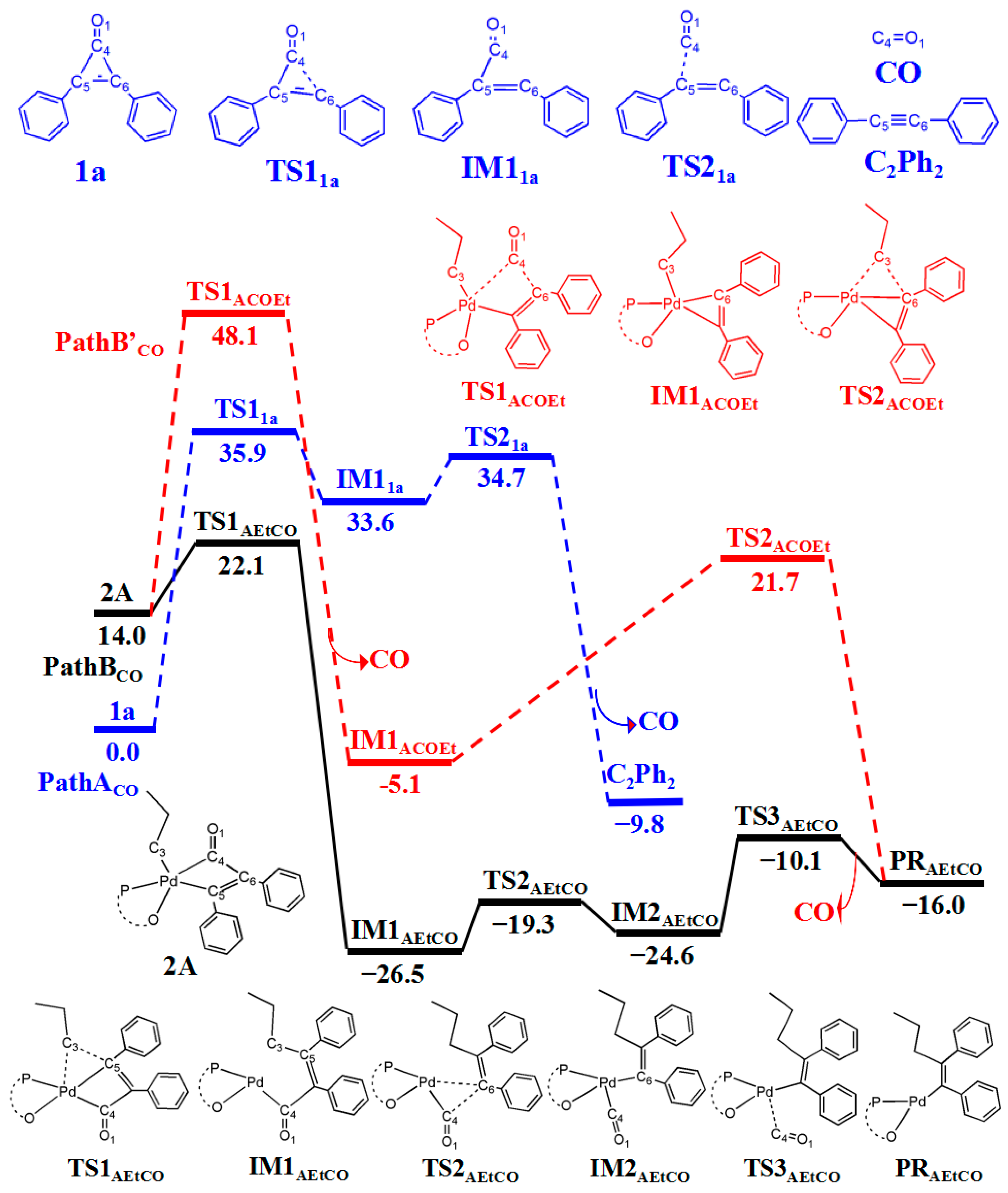
3.4. Generation of CO from Cyclopropenone
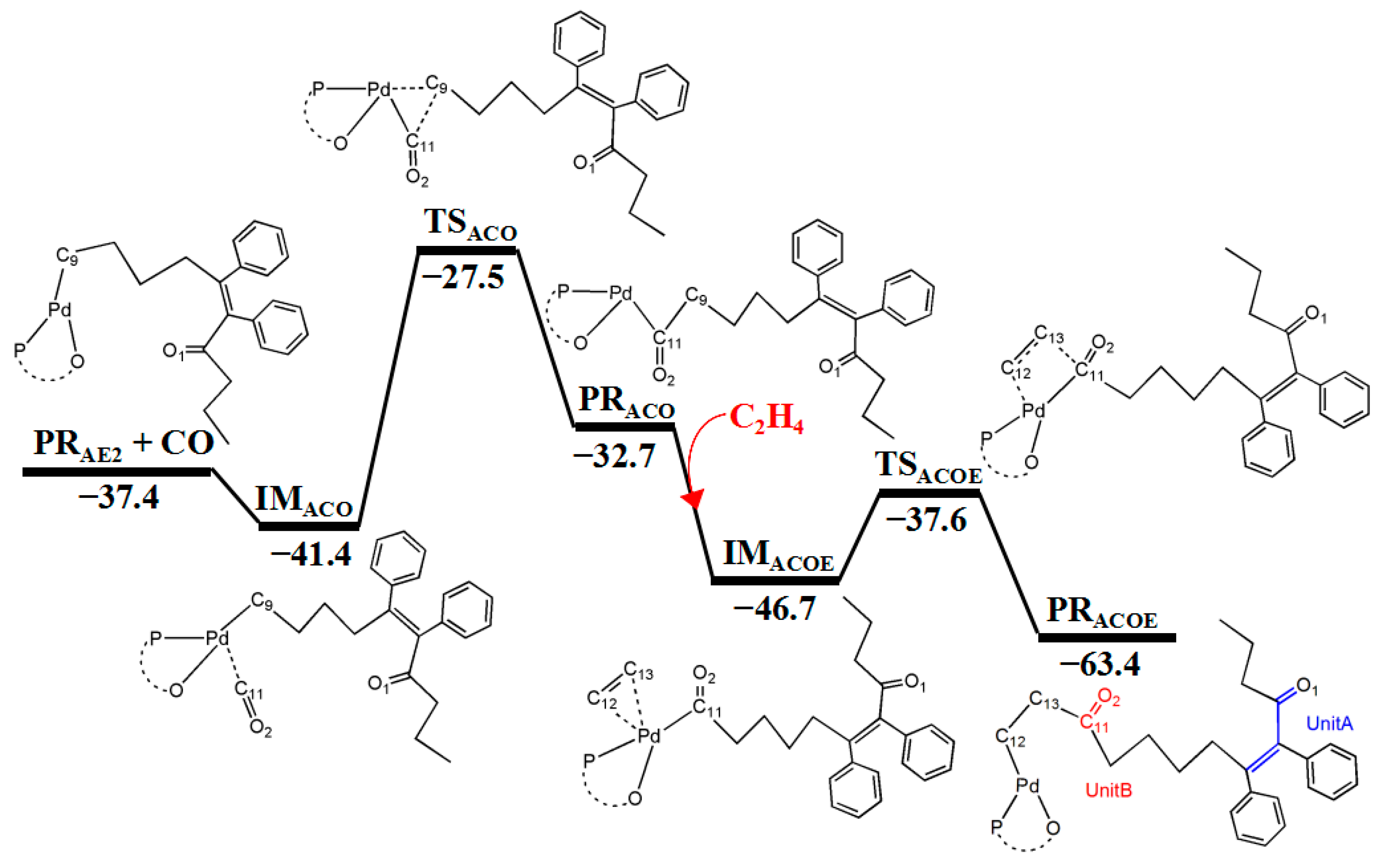
3.5. Reaction Pathway for the Generation of UnitB
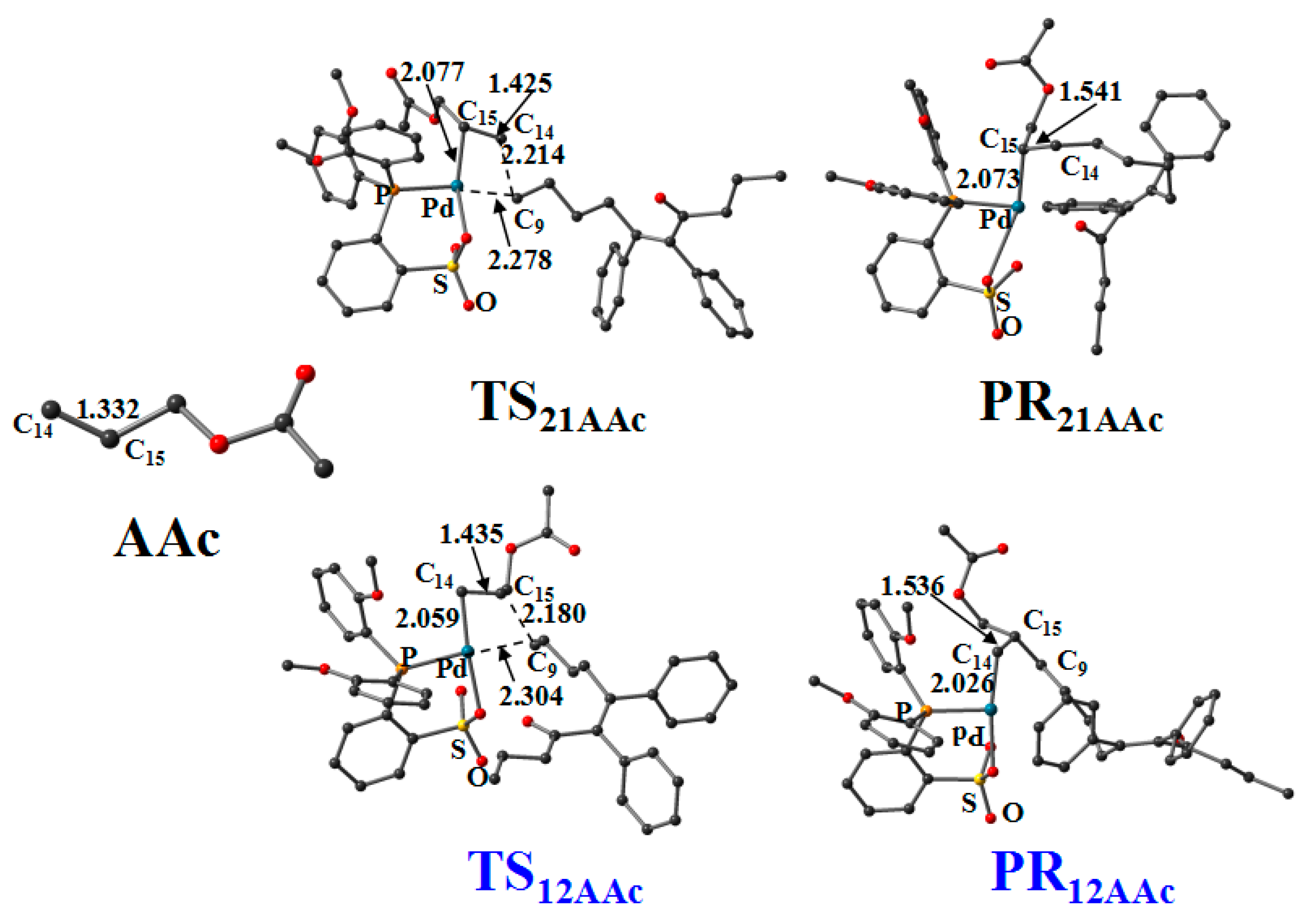
3.6. Reaction Pathway of the Allyl Acetate (AAc) Insertion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Chen, C. Transition metal-catalyzed copolymerization of olefins with polar functional monomers. In Comprehensive Organometallic Chemistry IV; Elsevier: Oxford, UK, 2022; pp. 404–430. [Google Scholar]
- Karimi, M.; Arabi, H.; Sadjadi, S. New advances in olefin homo and copolymerization using neutral, single component palladium/nickel complexes ligated by a phosphine-sulfonate. J. Catal. 2022, 412, 59–70. [Google Scholar] [CrossRef]
- Jian, Z. Synthesis of functionalized polyolefins: Design from catalysts to polar monomers. Acta Polym. Sin. 2018, 11, 1359–1371. [Google Scholar] [CrossRef]
- Johnson, L.K.; Killian, C.M.; Brookhart, M. New Pd(II)- and Ni(II)-based catalysts for polymerization of ethylene and α-Olefins. J. Am. Chem. Soc. 1995, 117, 6414–6415. [Google Scholar] [CrossRef]
- Drent, E.; van Dijk, R.; van Ginkel, R.; van Oort, B.; Pugh, R.I. Palladium catalysed copolymerisation of ethene with alkylacrylates: Polar comonomer built into the linear polymer chain. Chem. Comm. 2002, 7, 744–745. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Ito, S.; Nozaki, K. Coordination-insertion copolymerization of fundamental polar monomers. Chem. Rev. 2009, 109, 5215–5244. [Google Scholar] [CrossRef] [PubMed]
- Friedberger, T.; Wucher, P.; Mecking, S. Mechanistic insights into polar monomer insertion polymerization from acrylamides. J. Am. Chem. Soc. 2012, 134, 1010–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rünzi, T.; Fröhlich, D.; Mecking, S. Direct synthesis of ethylene-acrylic acid copolymers by insertion polymerization. J. Am. Chem. Soc. 2010, 132, 17690–17691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, S.; Munakata, K.; Nakamura, A.; Nozaki, K. Copolymerization of vinyl acetate with ethylene by palladium/alkylphosphine-sulfonate catalysts. J. Am. Chem. Soc. 2009, 131, 14606–14607. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Vela, J.; Lief, G.R.; Jordan, R.F. Copolymerization of ethylene and alkyl vinyl ethers by a (phosphine-sulfonate)PdMe catalyst. J. Am. Chem. Soc. 2007, 129, 8946–8947. [Google Scholar] [CrossRef]
- Skupov, K.M.; Piche, L.; Claverie, J.P. Linear polyethylene with tunable surface properties by catalytic copolymerization of ethylene with N-vinyl-2-pyrrolidinone and N-isopropylacrylamide. Macromolecules 2008, 41, 2309–2310. [Google Scholar] [CrossRef]
- Daigle, J.-C.; Piche, L.; Claverie, J.P. Preparation of functional polyethylenes by catalytic copolymerization. Macromolecules 2011, 44, 1760–1762. [Google Scholar] [CrossRef]
- Guo, L.; Gao, H.; Guan, Q.; Hu, H.; Deng, J.; Liu, J.; Liu, F.; Wu, Q. Substituent effects of the backbone in α-diimine palladium catalysts on homo and copolymerization of ethylene with methyl acrylate. Organometallics 2012, 31, 6054–6062. [Google Scholar] [CrossRef]
- Carrow, B.P.; Nozaki, K. Synthesis of functional polyolefins using cationic bisphosphine monoxide-palladium complexes. J. Am. Chem. Soc. 2012, 134, 8802–8805. [Google Scholar] [CrossRef]
- Mitsushige, Y.; Carrow, B.P.; Ito, S.; Nozaki, K. Ligand-controlled insertion regioselectivity accelerates copolymerization of ethylene with methyl acrylate by cationic bisphosphine monoxide-palladium catalysts. Chem. Sci. 2016, 7, 737–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, K.E.; Campos, J.; Daugulis, O.; Brookhart, M. Living polymerization of ethylene and copolymerization of ethylene/methyl acrylate using “Sandwich” diimine palladium catalysts. ACS Catal. 2015, 5, 456–464. [Google Scholar] [CrossRef]
- Sui, X.; Dai, S.; Chen, C. Ethylene polymerization and copolymerization with polar monomers by cationic phosphine phosphonic amide palladium complexes. ACS Catal. 2015, 5, 5932–5937. [Google Scholar] [CrossRef]
- Nakano, R.; Nozaki, K. Copolymerization of propylene and polar monomers using Pd/IzQO catalysts. J. Am. Chem. Soc. 2015, 137, 10934–10937. [Google Scholar] [CrossRef]
- Yasuda, H.; Nakano, R.; Ito, S.; Nozaki, K. Palladium/IzQO-catalyzed coordination-insertion copolymerization of ethylene and 1,1-disubstituted ethylenes bearing a polar functional group. J. Am. Chem. Soc. 2018, 140, 1876–1883. [Google Scholar] [CrossRef]
- Luckham, S.L.J.; Nozaki, K. Toward the copolymerization of propylene with polar comonomers. Acc. Chem. Res. 2021, 54, 344–355. [Google Scholar] [CrossRef]
- Ota, Y.; Ito, S.; Kobayashi, M.; Kitade, S.; Sakata, K.; Tayano, T.; Nozaki, K. Crystalline isotactic polar polypropylene from the palladium-catalyzed copolymerization of propylene and polar monomers. Angew. Chem. Int. Edit. 2016, 55, 7505–7509. [Google Scholar] [CrossRef]
- Zhang, W.; Waddell, P.M.; Tiedemann, M.A.; Padilla, C.E.; Mei, J.; Chen, L.; Carrow, B.P. Electron-rich metal cations enable synthesis of high molecular weight, linear functional polyethylenes. J. Am. Chem. Soc. 2018, 140, 8841–8850. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, C. A versatile ligand platform for palladium- and nickel-catalyzed ethylene copolymerization with polar monomers. Angew. Chem. Int. Edit. 2018, 57, 3094–3098. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Cai, W.; Hu, Y.; Chen, C. Improving the flame retardancy of polyethylenes through the palladium-catalyzed incorporation of polar comonomers. Polym. Chem. 2019, 10, 1416–1422. [Google Scholar] [CrossRef]
- Chen, M.; Chen, C. Direct and tandem routes for the copolymerization of ethylene with polar functionalized internal olefins. Angew. Chem. Int. Edit. 2019, 59, 1206–1210. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Chen, C. Polar-functionalized, crosslinkable, self-healing, and photoreponsive polyolefins. Angew. Chem. Int. Ed. 2019, 59, 395–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Nozaki, K. Selective chain-end functionalization of polar polyethylenes: Orthogonal reactivity of carbene and polar vinyl monomers in their copolymerization with ethylene. J. Am. Chem. Soc. 2018, 140, 15635–15640. [Google Scholar] [CrossRef]
- Matsuda, T.; Sakurai, Y. Palladium-catalyzed ring-opening alkynylation of cyclopropenones. Euro. J. Org. Chem. 2013, 20, 4219–4222. [Google Scholar] [CrossRef]
- Fumagalli, G.; Stanton, S.; Bower, J.F. Recent methodologies that exploit C-C single-bond cleavage of strained ring systems by transition metal complexes. Chem. Rev. 2017, 117, 9404–9432. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Seidel, F.W.; Nozaki, K. Synthesis of polyethylene with in-chain α,β-unsaturated ketone and isolated ketone units: Pd-catalyzed ring opening copolymerization of cyclopropenone with ethylene. Angew. Chem. Int. Ed. 2019, 58, 12955–12959. [Google Scholar] [CrossRef]
- Dolbier, W.R.; Battiste, M.A. Structure, synthesis, and chemical reactions of fluorinated cyclopropanes and cyclopropenes. Chem. Rev. 2003, 103, 1071–1098. [Google Scholar] [CrossRef]
- Luo, Y.; Shan, C.; Liu, S.; Zhang, T.; Zhu, L.; Zhong, K.; Bai, R.; Lan, Y. Oxidative addition promoted C-C bond cleavage in Rh-mediated cyclopropenone activation: A DFT study. ACS Catal. 2019, 9, 10876–10886. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.G.; Burns, L.A.; Patkowski, K.; Sherrill, C.D. Revised damping parameters for the D3 dispersion correction to density functional theory. J. Phys. Chem. Lett. 2016, 7, 2197–2203. [Google Scholar] [CrossRef] [PubMed]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results obtained with the correlation energy density functionals of Becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density functionals with broad applicability in chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Benchmark energetic data in a model system for grubbs II metathesis catalysis and their use for the development, assessment, and validation of electronic structure methods. J. Chem. Theory Comput. 2009, 5, 324–333. [Google Scholar] [CrossRef] [Green Version]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Dang, Y.; Qu, S.; Wang, Z.-X.; Wang, X. A computational mechanistic study of an unprecedented heck-type relay reaction: Insight into the origins of regio- and enantioselectivities. J. Am. Chem. Soc. 2014, 136, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Fu, Y.; Lee, S.Y.; Wang, C.; Liu, P.; Dong, G. Kinetic resolution via Rh-catalyzed C-C activation of cyclobutanones at room temperature. J. Am. Chem. Soc. 2019, 141, 16260–16265. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Qu, L.-B.; Houk, K.N.; Lan, Y. Origin of regiochemical control in Rh(III)/Rh(V)-catalyzed reactions of unsaturated oximes and alkenes to form pyrdines. ACS Catal. 2019, 9, 7154–7165. [Google Scholar] [CrossRef]
- Palani, V.; Hugelshofer, C.L.; Kevlishvili, I.; Liu, P.; Sarpong, R. A short synthesis of delavatine a unveils new insights into site-selective cross-coupling of 3,5-dibromo-2-pyrone. J. Am. Chem. Soc. 2019, 141, 2652–2660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, X.; Kohler, D.G.; Hull, K.L.; Liu, P. Energy decomposition analyses reveal the origins of catalyst and nucleophile effects on regioselectivity in nucleopalladation of alkenes. J. Am. Chem. Soc. 2019, 141, 11892–11904. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Chen, X.Y.; Wang, Z.X. Insight into the selective methylene oxidation catalyzed by Mn(CF3-PDP)(SbF6)2/H2O2/CH2ClCO2H) system: A DFT mechanistic study. Org. Lett. 2021, 23, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lu, Y.; Guo, J.; Hu, W.; Wang, Z.X. DFT mechanistic account for the site selectivity of electron-rich C(sp3)–H bond in the manganese-catalyzed aminations. Org. Lett. 2020, 22, 453–457. [Google Scholar] [CrossRef]
- Ren, X.; Lu, Y.; Lu, G.; Wang, Z.X. Density functional theory mechanistic study of Ni-catalyzed reductive alkyne–alkyne cyclodimerization: Oxidative cyclization versus outer-sphere proton transfer. Org. Lett. 2020, 22, 2454–2459. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Chen, G.; Hong, X.; Yu, J.-Q.; Houk, K.N. The origins of dramatic differences in five-membered vs six-membered chelation of Pd(II) on efficiency of C(sp3)-H bond activation. J. Am. Chem. Soc. 2017, 139, 8514–8521. [Google Scholar] [CrossRef]
- Yu, S.-Y.; Peng, X.; Wang, F.; Cao, J.; Wang, F.; Zhang, C.-G. Density functional theory study of the regioselectivity in copolymerization of bis-styrenic molecules with propylene using zirconocene catalyst. Catalysts 2022, 12, 1039. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F.-W. Interaction region indicator (IRI): A simple real space function clearly revealing both chemical bonds and weak interactions. Chem.-Methods 2021, 1, 231–239. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q.X. Realization of conceptual density functional theory and information-theoretic approach in Multiwfn program. In Conceptual Density Functional Theory; WILEY-VCH GmbH: Weinheim, Germany, 2022; pp. 631–647. [Google Scholar]
- Parr, R.G.; Pearson, R.G. Absolute hardness-companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Parr, R.G.; Von Szentpaly, L.; Liu, S.B. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Domingo, L.R.; Chamorro, E.; Pérez, P. Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. A theoretical study. J. Org. Chem. 2008, 73, 4615–4624. [Google Scholar] [CrossRef] [PubMed]
- Parr, R.G.; Yang, W. Density functional approach to the frontier-electron theory of chemical reactivity. J. Am. Chem. Soc. 1984, 106, 4049–4050. [Google Scholar] [CrossRef]
- Fu, R.; Lu, T.; Chen, F.-W. Comparing methods for predicting the reactive site of electrophilic substitution. Acta Phys.-Chim. Sin. 2014, 30, 628–639. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F.-W. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graphics 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Rezabal, E.; Ugalde, J.M.; Frenking, G. The trans effect in palladium phosphine sulfonate complexes. J. Phys. Chem. A 2017, 121, 7709–7716. [Google Scholar] [CrossRef]
- Sun, J.; Chen, M.; Luo, G.; Chen, C.; Luo, Y. Diphosphazane-monoxide and phosphine-sulfonate palladium catalyzed ethylene copolymerization with polar monomers: A computational study. Organometallics 2019, 38, 638–646. [Google Scholar] [CrossRef]
- Noda, S.; Nakamura, A.; Kochi, T.; Chung, L.W.; Morokuma, K.; Nozaki, K. Mechanistic studies on the formation of linear polyethylene chain catalyzed by palladium phosphine-sulfonate complexes: Experiment and theoretical studies. J. Am. Chem. Soc. 2009, 131, 14088–14100. [Google Scholar] [CrossRef] [PubMed]
- Kuzmanich, G.; Gard, M.N.; Garcia-Garibay, M.A. Photonic amplification by a singlet-state quantum chain reaction in the photodecarbonylation of crystalline diarylcyclopropenones. J. Am. Chem. Soc. 2009, 131, 11606–11614. [Google Scholar] [CrossRef] [PubMed]
- Poloukhtine, A.; Popik, V.V. Mechanism of the cyclopropenone decarbonylation reaction. A density functional theory and transient spectroscopy study. J. Phys. Chem. A 2006, 110, 1749–1757. [Google Scholar] [CrossRef] [PubMed]





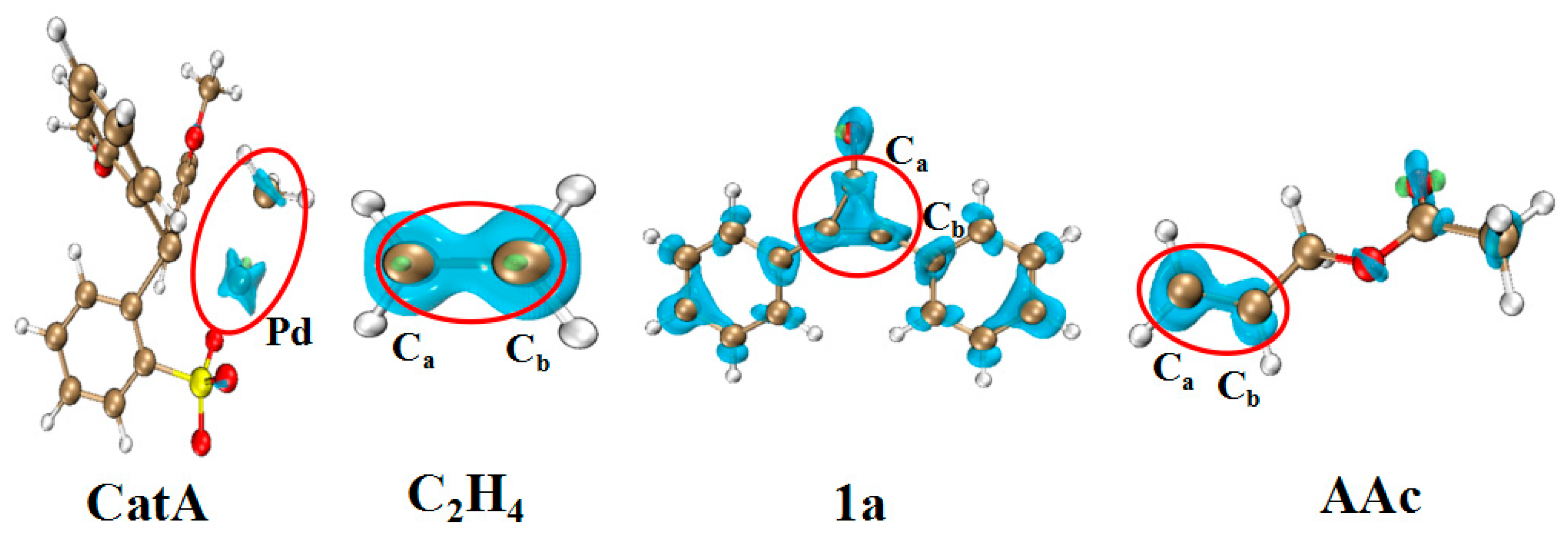
| WBI | QNBO (e) | |||||
|---|---|---|---|---|---|---|
| B (Pd-C1) | B (C2-C3) | B (Pd-C3) | B (C1-C2) | Pd | C1 | |
| CatA + C2H4 | 0.704 | 2.039 | 0.318 | −0.819 | ||
| cIM1A | 0.718 | 1.550 | 0.408 | 0.243 | −0.768 | |
| cTS1A | 0.406 | 1.310 | 0.553 | 0.538 | 0.161 | −0.739 |
| cPR1A | 0.010 | 1.069 | 0.672 | 1.108 | 0.219 | −0.666 |
| B (Pd-C1) | B (C2-C3) | B (Pd-C2) | B (C1-C3) | Pd | C1 | |
| tIM1A | 0.743 | 1.680 | 0.297 | 0.194 | −0.782 | |
| tTS1A | 0.365 | 1.296 | 0.577 | 0.618 | 0.210 | −0.769 |
| tPR1A | 0.035 | 0.727 | 1.007 | 0.316 | −0.666 | |
| WBI | QNBO (e) | |||||||
|---|---|---|---|---|---|---|---|---|
| Path A1a | B (Pd-C3) | B (Pd-C4) | B (Pd-C5) | B (C4-C5) | B (C3-C4) | Pd | C4 | C5 |
| cPR1A + 1a | 0.672 | 1.078 | 0.219 | 0.521 | −0.016 | |||
| TS1A | 0.691 | 0.181 | 0.298 | 0.730 | 0.025 | 0.185 | 0.567 | −0.051 |
| 2A | 0.629 | 0.577 | 0.564 | 0.137 | 0.092 | 0.166 | 0.587 | 0.021 |
| TS2A | 0.329 | 0.263 | 0.667 | 0.085 | 0.554 | 0.200 | 0.584 | 0.018 |
| 4A | 0.017 | 0.685 | 0.061 | 1.008 | 0.370 | 0.583 | 0.022 | |
| Path B1a | B (Pd-C3) | B (Pd-O1) | B (C4-O1) | B (C4-C5) | B (Pd-C5) | Pd | C4 | O1 |
| cPR1A + 1a | 0.672 | 1.682 | 1.078 | 0.219 | 0.521 | −0.594 | ||
| TS3A | 0.337 | 0.507 | 1.176 | 1.034 | 0.340 | 0.364 | −0.596 | |
| 3A | 0.015 | 0.588 | 0.958 | 0.921 | 0.020 | 0.415 | 0.271 | −0.686 |
| TS4A | 0.017 | 0.557 | 1.078 | 0.744 | 0.148 | 0.414 | 0.320 | −0.641 |
| 4A | 0.017 | 0.394 | 1.449 | 0.061 | 0.685 | 0.370 | 0.583 | −0.556 |
| WBI | QNBO (e) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Path ACO | B (C4-O1) | B (C4-C5) | B (C4-C6) | B (C5-C6) | C4 | C5 | C6 | O1 | ||
| 1a | 1.682 | 1.078 | 1.078 | 1.478 | 0.521 | −0.016 | −0.016 | −0.594 | ||
| TS11a | 1.887 | 1.269 | 0.566 | 1.505 | 0.656 | −0.221 | 0.060 | −0.476 | ||
| IM11a | 2.025 | 1.189 | 0.415 | 1.585 | 0.687 | −0.314 | 0.090 | −0.405 | ||
| TS21a | 2.063 | 0.806 | 0.391 | 1.964 | 0.557 | −0.222 | 0.102 | −0.442 | ||
| CO + C2Ph2 | 2.250 | 2.660 | 0.506 | 0.006 | 0.006 | −0.506 | ||||
| Path BCO | B (Pd-C3) | B (Pd-C5) | B (C3-C5) | B (Pd-C6) | B (Pd-C4) | B (C4-C6) | Pd | C4 | C6 | O1 |
| 2A | 0.629 | 0.564 | 0.136 | 0.080 | 0.577 | 1.109 | 0.166 | 0.587 | −0.184 | −0.499 |
| TS1AEtCO | 0.316 | 0.429 | 0.536 | 0.117 | 0.633 | 1.544 | 0.136 | 0.557 | −0.146 | −0.504 |
| IM1AEtCO | 0.017 | 0.206 | 1.009 | 0.206 | 0.650 | 1.046 | 0.215 | 0.352 | −0.136 | 0.016 |
| TS2AEtCO | 0.012 | 0.034 | 1.003 | 0.404 | 0.834 | 0.500 | 0.089 | 0.604 | −0.171 | −0.454 |
| IM2AEtCO | 0.020 | 0.031 | 0.996 | 0.605 | 0.908 | 0.131 | 0.079 | 0.669 | −0.091 | −0.424 |
| TS3AEtCO | 0.014 | 0.051 | 0.998 | 0.644 | 0.467 | 0.018 | 0.128 | 0.616 | −0.031 | −0.475 |
| PRAEtCO | 0.010 | 0.050 | 0.995 | 0.562 | 0.273 | 0.506 | −0.051 | −0.506 | ||
| Path B’CO | B (Pd-C3) | B (Pd-C4) | B (Pd-C5) | B (C4-C6) | B (C5-C6) | B (C3-C5) | Pd | C4 | C6 | O1 |
| 2A | 0.629 | 0.577 | 0.564 | 1.109 | 1.548 | 0.136 | 0.166 | 0.587 | −0.184 | −0.499 |
| TS1ACOEt | 0.675 | 0.115 | 0.368 | 0.619 | 1.815 | 0.022 | 0.155 | 0.549 | −0.023 | −0.441 |
| IM1ACOEt | 0.701 | 0.200 | 2.431 | 0.026 | 0.205 | 0.506 | −0.024 | 0.506 | ||
| TS2ACOEt | 0.368 | 0.291 | 1.872 | 0.567 | 0.180 | 0.006 | ||||
| PRAEtCO | 0.010 | 0.050 | 1.233 | 1.245 | 0.273 | −0.051 | ||||
| WBI | QNBO (e) | |||||||
|---|---|---|---|---|---|---|---|---|
| CO Insertion | B (Pd-C11) | B (Pd-C9) | B (C9-C11) | B (C11-O2) | Pd | C11 | O2 | |
| PRAE2 + CO | 0.706 | 1.138 | 0.232 | 0.506 | −0.506 | |||
| IMACO | 0.815 | 0.701 | 0.076 | 2.224 | 0.026 | 0.665 | −0.415 | |
| TSACO | 0.798 | 0.306 | 0.668 | 2.001 | 0.216 | 0.571 | −0.455 | |
| PRACO | 0.748 | 0.158 | 0.919 | 1.921 | 0.258 | 0.554 | −0.459 | |
| C2H4 insertion | B (Pd-C11) | B (Pd-C12) | B (C12-C13) | B (C11-C13) | Pd | C11 | C12 | C13 |
| PRACO | 0.748 | 2.039 | 0.258 | 0.554 | −0.427 | −0.427 | ||
| IMACOE | 0.650 | 0.411 | 1.522 | 0.051 | 0.187 | 0.554 | −0.490 | −0.441 |
| TSACOE | 0.259 | 0.598 | 1.206 | 0.618 | 0.167 | 0.548 | −0.505 | −0.546 |
| PRACOE | 0.022 | 0.705 | 1.010 | 1.026 | 0.240 | 0.652 | −0.589 | −0.564 |
| WBI | QNBO (e) | |||||||
|---|---|---|---|---|---|---|---|---|
| B (Pd-C9) | B (Pd-C15) | B (C14-C15) | B (C9-C14) | Pd | C9 | C15 | C14 | |
| PRAE2 + AAc | 0.706 | 2.039 | 0.232 | −0.517 | −0.427 | −0.427 | ||
| TS21AAc | 0.406 | 0.518 | 1.302 | 0.487 | 0.168 | −0.465 | −0.339 | −0.445 |
| PR21AAc | 0.066 | 0.663 | 1.014 | 1.004 | 0.244 | −0.440 | −0.345 | −0.489 |
| B (Pd-C9) | B (Pd-C14) | B (C14-C15) | B (C9-C15) | Pd | C9 | C14 | C15 | |
| PRAE2 + AAc | 0.706 | 2.039 | 0.232 | −0.517 | −0.427 | −0.427 | ||
| TS12AAc | 0.382 | 0.547 | 1.302 | 0.486 | 0.145 | −0.473 | −0.511 | −0.270 |
| PR12AAc | 0.016 | 0.689 | 1.010 | 0.988 | 0.222 | −0.465 | −0.544 | −0.305 |
| η a | μ b | ω c | NNu d | f+e | f−f | ||
|---|---|---|---|---|---|---|---|
| Pd | Ca | Cb | |||||
| CatA | 6.534 | −4.175 | 1.473 | 3.105 | 0.228 | ||
| C2H4 | 13.705 | −3.509 | 0.592 | 1.865 | 0.313 | 0.313 | |
| 1a | 7.360 | −4.108 | 1.303 | 3.283 | 0.059 | 0.057 | |
| AAc | 11.579 | −3.524 | 0.771 | 1.972 | 0.167 | 0.116 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Yu, S.; Wang, F.; Wang, F.; Cao, J.; Zheng, H.; Chen, X.; Ren, A. Density Functional Theory Analysis of the Copolymerization of Cyclopropenone with Ethylene Using a Palladium Catalyst. Polymers 2022, 14, 5273. https://doi.org/10.3390/polym14235273
Zhang C, Yu S, Wang F, Wang F, Cao J, Zheng H, Chen X, Ren A. Density Functional Theory Analysis of the Copolymerization of Cyclopropenone with Ethylene Using a Palladium Catalyst. Polymers. 2022; 14(23):5273. https://doi.org/10.3390/polym14235273
Chicago/Turabian StyleZhang, Chenggen, Shuyuan Yu, Fei Wang, Fuping Wang, Jian Cao, Huimin Zheng, Xiaoyu Chen, and Aijin Ren. 2022. "Density Functional Theory Analysis of the Copolymerization of Cyclopropenone with Ethylene Using a Palladium Catalyst" Polymers 14, no. 23: 5273. https://doi.org/10.3390/polym14235273
APA StyleZhang, C., Yu, S., Wang, F., Wang, F., Cao, J., Zheng, H., Chen, X., & Ren, A. (2022). Density Functional Theory Analysis of the Copolymerization of Cyclopropenone with Ethylene Using a Palladium Catalyst. Polymers, 14(23), 5273. https://doi.org/10.3390/polym14235273









