Modification of Physio-Mechanical Properties of Chitosan-Based Films via Physical Treatment Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Blending Solution
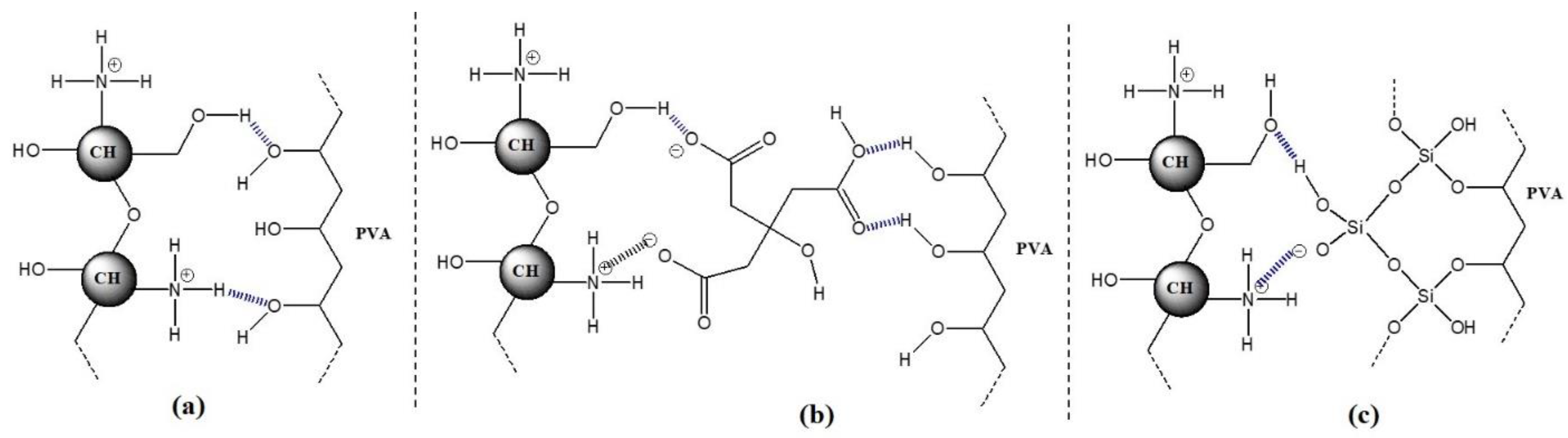
2.2.2. Physical Cross-Linking
2.2.3. Film Casting Procedure
2.3. Film Characterization
- Tensile strength (TS) and elongation at break (Eb) of the films were measured using a universal testing machine (Strograph, Toyo Seiki, Tokyo, Japan) with a preload cell of 0.1 MPa and a speed tensile modulus of 50 mm/min. The grip to grip separation at the start position was set at 50 mm. Rectangular specimens (8 cm × 1 cm × 0.05 cm) were produced with three samples, each measuring cut from the cast films. The TS (MPa) was calculated by dividing the maximum load on the film before failure by the cross-sectional area (m2) of the initial specimen. The samples were conditioned for at least 48 h in a drying chamber (25 °C and 50% RH).
- Surface wettability of the film was evaluated by the contact angle (CA) using a Drop Shape Analyzer (DSA 100; Kruss GmbH, Hamburg, Germany). The measurement was carried out through the static droplet method, in which one drop of water fell on the film’s surface. A video camera recorded the droplet’s shape to determine the contact angle.
- The chemical structure of the films was characterized using Fourier-transform infrared spectroscopy (FTIR), with a Nicolet iS5 spectrometer (Thermo, Waltham, MA, USA) at ambient temperature. Data were collected over 32 scans at a 4 cm−1 resolution, ranging from 400 to 4000 cm−1.
- Thermal properties were analyzed by Differential scanning calorimetry (DSC), (Q100, TA Instruments, New Castle, DE, USA). The sample film was placed in an aluminum crucible with less than 20 mg. DSC curves were obtained by heating the samples from 0 to 300 °C, with a heating rate of 10 °C/min, under a nitrogen gas flow of about 50 mL/min.
- The diffraction pattern of the sample films was recorded using X-Ray Diffraction (XRD), in a D8 Advance (Bruker, Billerica, MA, USA). The films were scanned at a scanning angle of 2θ from 5 to 45° with CuKa filter radiation, where θ was the incident angle of the X-ray beam on the sample.
- The cross-sectional image of the films was observed by scanning electron microscopy (SEM), (JEOL-2100F, JEOL Ltd., Tokyo, Japan). The films were frozen in liquid nitrogen and were cut later. The cross sections were sputter-coated with a gold layer about 10 nm thick to avoid charging under the electron beam.
3. Results
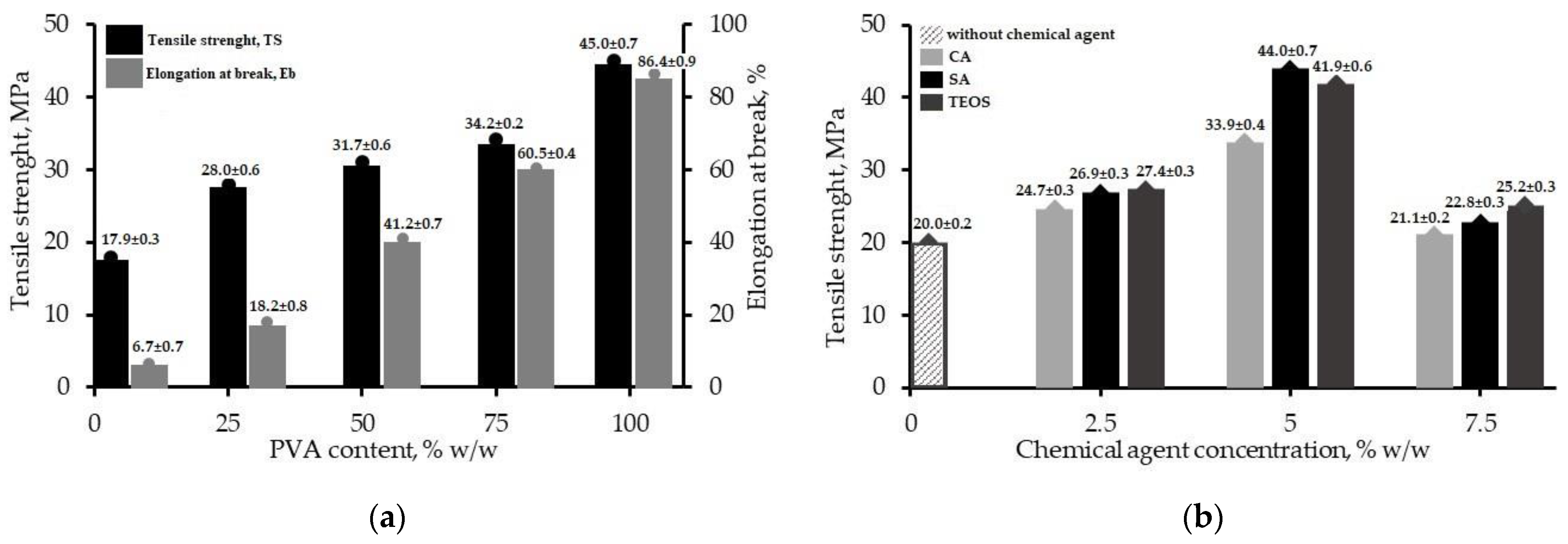
3.1. Mechanical Properties
3.2. Water Contact Angle
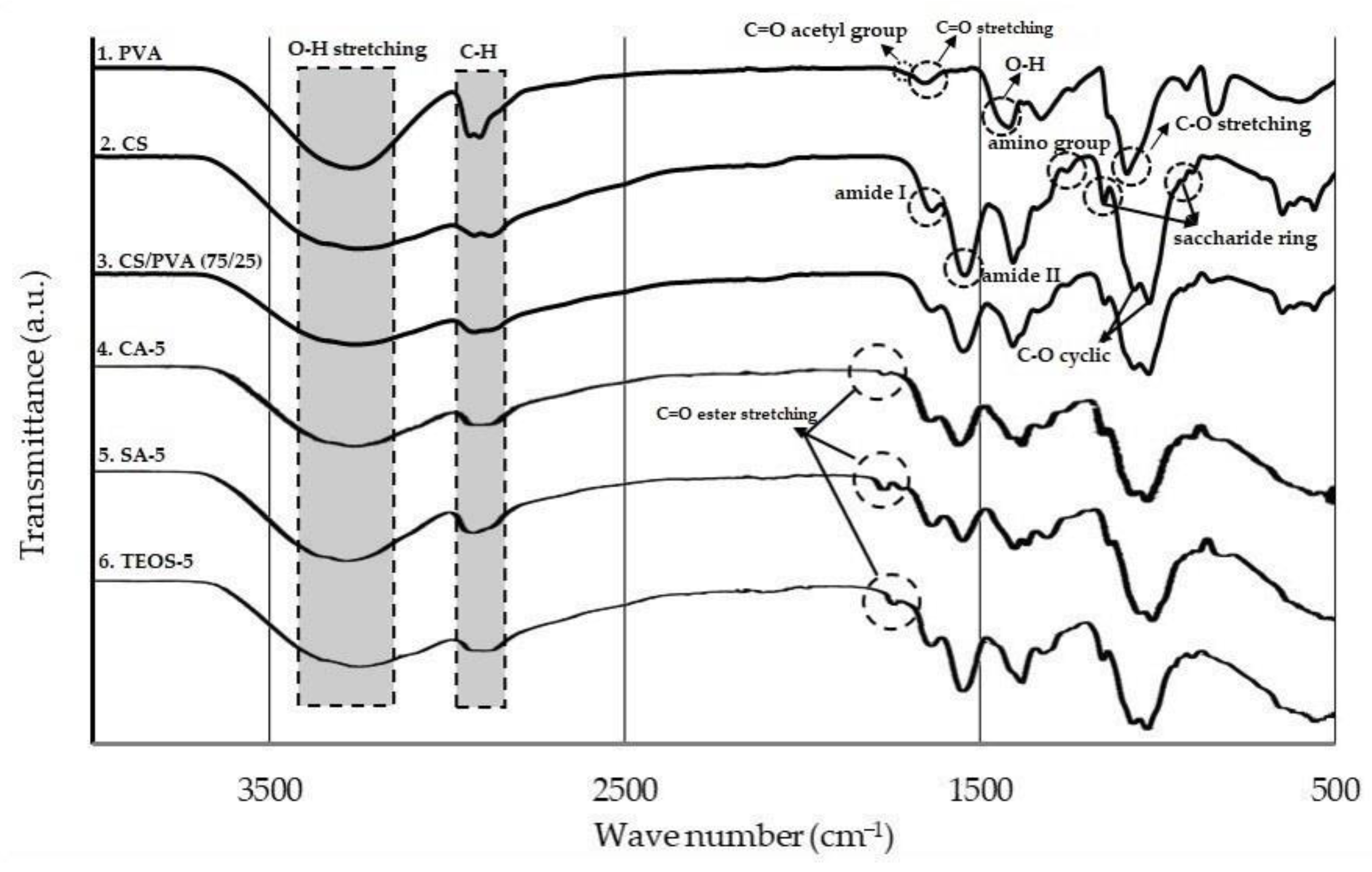
3.3. FTIR Analysis
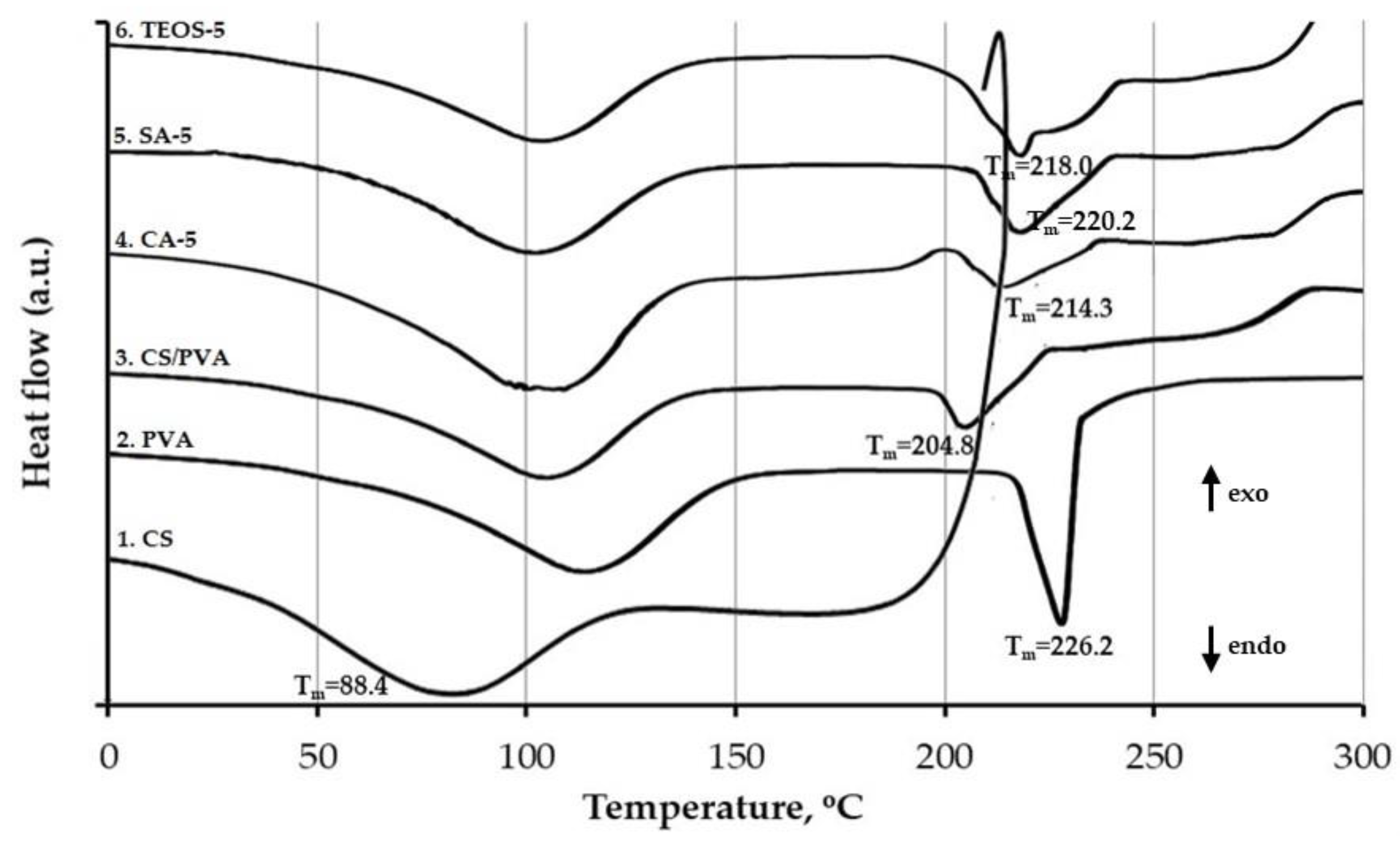
3.4. DSC Test
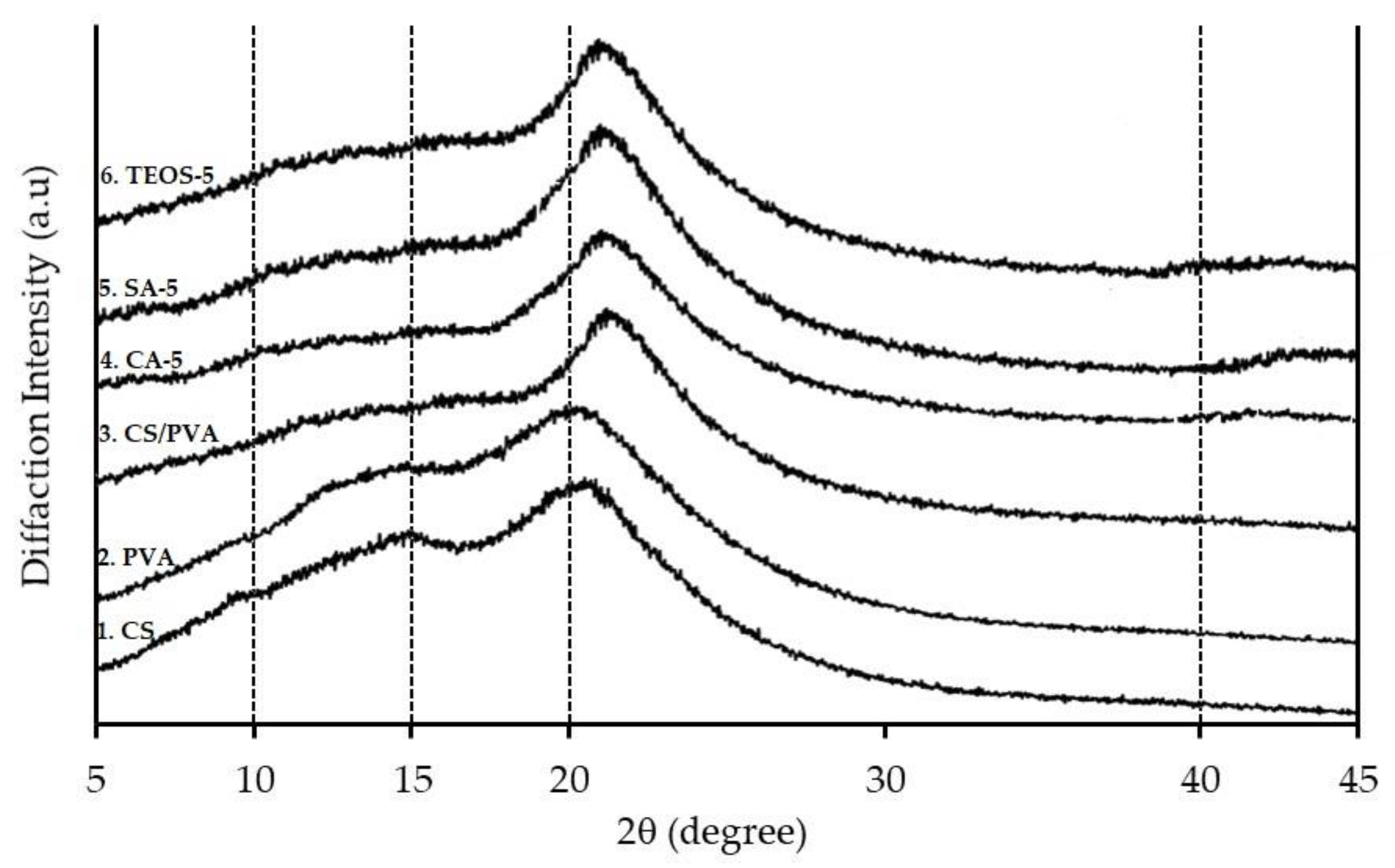
3.5. XRD Measurement
3.6. SEM Observation
4. Discussions
4.1. Quantitative Analysis
4.2. Qualitative Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Debeaufort, F.; Quezada-Gallo, J.-A.; Voilley, A. Mathematical Models for the Representation of Some Physiological and Quality Changes during Fruit Storage. Crit. Rev. Food Sci. 1998, 38, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Yahaya, S.M.; Mardiyya, A.Y. Review of post-harvest losses of fruits and vegetables. Biomed. J. Sci. Tech. Res. 2019, 13, 10192–10200. [Google Scholar]
- Atay, H.Y. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan; Springer: Singapore, 2019; pp. 457–489. [Google Scholar]
- Merzendorfer, H.; Cohen, E. Chitin/chitosan: Versatile ecological, industrial, and biomedical applications. In Extracellular Sugar-Based Biopolymers Matrices; Springer: Cham, Switzerland, 2019; pp. 541–624. [Google Scholar]
- Wardhono, E.Y.; Pinem, M.P.; Kustiningsih, I.; Effendy, M.; Clausse, D.; Saleh, K.; Guénin, E. Heterogeneous deacetylation reaction of chitin under low-frequency ultrasonic irradiation. Carbohydr. Polym. 2021, 267, 118180. [Google Scholar] [CrossRef] [PubMed]
- Basumatary, K.; Daimary, P.; Das, S.K.; Thapa, M.; Singh, M.; Mukherjee, A.; Kumar, S. Lagerstroemia speciosa fruit-mediated synthesis of silver nanoparticles and its application as filler in agar based nanocomposite films for antimicrobial food packaging. Food Packag. Shelf Life 2018, 17, 99–106. [Google Scholar] [CrossRef]
- Reddy, M.M.; Vivekanandhan, S.; Misra, M.; Bhatia, S.K.; Mohanty, A.K. Biobased plastics and bionanocomposites: Current status and future opportunities. Prog. Polym. Sci. 2013, 38, 1653–1689. [Google Scholar]
- Bonilla, J.; Fortunati, E.; Atarés, L.; Chiralt, A.; Kenny, J.M. Physical, structural and antimicrobial properties of poly vinyl alcohol–chitosan biodegradable films. Food Hydrocoll. 2014, 35, 463–470. [Google Scholar] [CrossRef]
- Santos, C.; Silva, C.J.; Büttel, Z.; Guimarães, R.; Pereira, S.B.; Tamagnini, P.; Zille, A. Preparation and characterization of polysaccharides/PVA blend nanofibrous membranes by electrospinning method. Carbohydr. Polym. 2014, 99, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Arefian, M.; Hojjati, M.; Tajzad, I.; Mokhtarzade, A.; Mazhar, M.; Jamavari, A. A review of Polyvinyl alcohol/Carboxymethyl cellulose (PVA/CMC) composites for various applications. J. Compos. Compd. 2020, 2, 69–76. [Google Scholar]
- Hu, H.; Xin, J.H.; Hu, H.; Chan, A.; He, L. Glutaraldehyde–chitosan and poly (vinyl alcohol) blends, and fluorescence of their nano-silica composite films. Carbohydr. Polym. 2013, 91, 305–313. [Google Scholar] [CrossRef]
- El-Hefian, E.A.; Nasef, M.M.; Yahaya, A.H. Mechanical, thermal and surface investigations of chitosan/agar/PVA ternary blended films. E-J. Chem. 2011, 8, S105–S112. [Google Scholar] [CrossRef]
- Lan, W.; Wang, S.; Chen, M.; Sameen, D.E.; Lee, K.; Liu, Y. Developing poly (vinyl alcohol)/chitosan films incorporate with d-limonene: Study of structural, antibacterial, and fruit preservation properties. Int. J. Biol. Macromol. 2020, 145, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Kadir, M.F.Z.; Majid, S.R.; Arof, A.K. Plasticized chitosan–PVA blend polymer electrolyte based proton battery. Electrochim. Acta 2010, 55, 1475–1482. [Google Scholar] [CrossRef]
- Abraham, A.; Soloman, P.A.; Rejini, V.O. Preparation of chitosan-polyvinyl alcohol blends and studies on thermal and mechanical properties. Procedia Technol. 2016, 24, 741–748. [Google Scholar] [CrossRef]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Physicochemical and bioactivity of cross-linked chitosan–PVA film for food packaging applications. Int. J. Biol. Macromol. 2009, 45, 372–376. [Google Scholar] [CrossRef]
- Utracki, L.A.; Mukhopadhyay, P.; Gupta, R.K. Polymer blends: Introduction. Polym. Blends Handb. 2014, 1, 3–170. [Google Scholar]
- Visakh, P.M.; Markovic, G.; Pasquini, D. Recent developments in polymer, macro micro and nano blends. In Recent Developments in Polymer Macro, Micro and Nano Blends; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Meng, F.; Zhang, Y.; Xiong, Z.; Wang, G.; Li, F.; Zhang, L. Mechanical, hydrophobic and thermal properties of an organic-inorganic hybrid carrageenan-polyvinyl alcohol composite film. Compos. Part B Eng. 2018, 143, 1–8. [Google Scholar] [CrossRef]
- Li, Z.; Lin, Z. Recent advances in polysaccharide-based hydrogels for synthesis and applications. Aggregate 2021, 2, e21. [Google Scholar] [CrossRef]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef]
- de Oliveira, A.C.S.; Ugucioni, J.C.; Borges, S.V. Effect of glutaraldehyde/glycerol ratios on the properties of chitosan films. J. Food Process. Preserv. 2021, 45, e15060. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Genipin-Crosslinked Gelatin/Chitosan-Based Functional Films Incorporated with Rosemary Essential Oil and Quercetin. Materials 2022, 15, 3769. [Google Scholar] [CrossRef]
- Ojogbo, E.; Ogunsona, E.O.; Mekonnen, T.H. Chemical and physical modifications of starch for renewable polymeric materials. Mater. Today Sustain. 2020, 7, 100028. [Google Scholar]
- MWihodo, M.; Moraru, C.I. Physical and chemical methods used to enhance the structure and mechanical properties of protein films: A review. J. Food Eng. 2013, 114, 292–302. [Google Scholar]
- Olewnik-Kruszkowska, E.; Gierszewska, M.; Jakubowska, E.; Tarach, I.; Sedlarik, V.; Pummerova, M. Antibacterial Films Based on PVA and PVA–Chitosan Modified with Poly(Hexamethylene Guanidine). Polymers 2019, 11, 2093. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, S.B.; Kordestani, S.S.; Mirzadeh, H.; Mansouri, P. Poly (vinyl alcohol)-chitosan blends: Preparation, mechanical and physical properties. Iran. J. Polym. Sci. Technol. 2003, 12, 139–146. [Google Scholar]
- Nugraheni, A.D.; Purnawati, D.; Kusumaatmaja, A. Physical Evaluation of PVA/Chitosan Film Blends with Glycerine and Calcium Chloride. J. Phys. Conf. Ser. 2018, 1011, 012052. [Google Scholar] [CrossRef]
- Nishi, T.; Wang, T.T. Melting point depression and kinetic effects of cooling on crystallization in poly (vinylidene fluoride)-poly (methyl methacrylate) mixtures. Macromolecules 1975, 8, 909–915. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, J. The role of hydrogen-bonding interaction in poly (vinyl alcohol)/poly (acrylic acid) blending solutions and their films. Chin. J. Polym. Sci. 2010, 28, 903–911. [Google Scholar] [CrossRef]
- Kumar, S.; Krishnakumar, B.; Sobral, A.J.; Koh, J. Bio-based (chitosan/PVA/ZnO) nanocomposites film: Thermally stable and photoluminescence material for removal of organic dye. Carbohydr. Polym. 2019, 205, 559–564. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, X.; Cao, W.; Ren, X.; Ren, G.; Liu, P.; Chen, J. Effects of Different Drying Methods on the Characterization, Dissolution Rate and Antioxidant Activity of Ursolic Acid-Loaded Chitosan Nanoparticles. Foods 2021, 10, 2470. [Google Scholar] [CrossRef]
- Guirguis, O.W.; Moselhey, M.T.H. Thermal and structural studies of poly (vinyl alcohol) and hydroxypropyl cellulose blends. Nat. Sci. 2011, 4, 57–67. [Google Scholar] [CrossRef]
- Runt, J.; Miley, D.M.; Zhang, X.; Gallagher, K.P.; McFeaters, K.; Fishburn, J. Crystallization of poly(butylene terephthalate) and its blends with polyarylate. Macromolecules 1992, 25, 1929–1934. [Google Scholar] [CrossRef]
- Kumar, H.N.; Prabhakar, M.; Prasad, C.V.; Rao, K.M.; Reddy, T.A.K.; Subha, M. Compatibility studies of chitosan/PVA blend in 2% aqueous acetic acid solution at 30 °C. Carbohydr. Polym. 2010, 82, 251–255. [Google Scholar] [CrossRef]
- Wang, S.-F.; Shen, L.; Zhang, W.-D.; Tong, Y.-J. Preparation and Mechanical Properties of Chitosan/Carbon Nanotubes Composites. Biomacromolecules 2005, 6, 3067–3072. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Cui, S.; Wang, S.-Y. Preparation and crystalline analysis of high-grade bamboo dissolving pulp for cellulose acetate. J. Appl. Polym. Sci. 2008, 107, 1029–1038. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S. Totally and partially biodegradable polymer blends based on natural and synthetic macromolecules: Preparation, physical properties, and potential as food packaging materials. J. Macromol. Sci. Part C 1999, 39, 205–271. [Google Scholar] [CrossRef]
- Ricciardi, R.; Auriemma, F.; de Rosa, C.; Lauprêtre, F. X-ray diffraction analysis of poly (vinyl alcohol) hydrogels, obtained by freezing and thawing techniques. Macromolecules 2004, 37, 1921–1927. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.Y.; Lee, Y.M.; Kim, K.Y. Properties and swelling characteristics of cross-linked poly (vinyl alcohol)/chitosan blend membrane. J. Appl. Polym. Sci. 1992, 45, 1711–1717. [Google Scholar] [CrossRef]
- Urbina, L.; Guaresti, O.; Requies, J.; Gabilondo, N.; Eceiza, A.; Corcuera, M.A.; Retegi, A. Design of reusable novel membranes based on bacterial cellulose and chitosan for the filtration of copper in wastewaters. Carbohydr. Polym. 2018, 193, 362–372. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Rana, S.; Cho, J.W.; Li, L.; Chan, S.H. Polymer nanocomposites based on functionalized carbon nanotubes. Prog. Polym. Sci. 2010, 35, 837–867. [Google Scholar] [CrossRef]
- Ren, J.; Yu, D. Effects of enhanced hydrogen bonding on the mechanical properties of poly (vinyl alcohol)/carbon nanotubes nanocomposites. Compos. Interfaces 2018, 25, 205–219. [Google Scholar] [CrossRef]
- Nicolle, L.; Journot, C.M.; Gerber-Lemaire, S. Chitosan functionalization: Covalent and non-covalent interactions and their characterization. Polymers 2021, 13, 4118. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Mayer, J.; Felt, O.; Peppas, N.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2003, 57, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, M.S.; Desoky, W.M.; Hussein, E.M.; El-Shaer, N.H.; Gomaa, M.; Gamal, A.A.; Esawy, M.A.; Guirguis, O.W. Biological applications study of bio-nanocomposites based on chitosan/TiO2 nanoparticles polymeric films modified by oleic acid. J. Biomed. Mater. Res. Part A 2021, 109, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Elsabahy, M.; Hamad, M.A. Design and preclinical evaluation of chitosan/kaolin nanocomposites with enhanced hemostatic efficiency. Mar. Drugs 2021, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Q.; Wang, X.; Kang, I.-S. Ester crosslinking of cotton fabric by polymeric carboxylic acids and citric acid. Text. Res. J. 1997, 67, 334–342. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef]
- Ubaid, M.; Murtaza, G. Fabrication and characterization of genipin cross-linked chitosan/gelatin hydrogel for pH-sensitive, oral delivery of metformin with an application of response surface methodology. Int. J. Biol. Macromol. 2018, 114, 1174–1185. [Google Scholar] [CrossRef]
- Benítez-Martínez, J.A.; Garnica-Palafox, I.M.; Vázquez-Victorio, G.; Hautefeuille, M.; Sánchez-Arévalo, F.M. Semi-interpenetrating polymeric networks based on poly (dimethylsiloxane)-chitosan-poly (vinyl alcohol) crosslinked with genipin with possible use in biomedical applications. J. Mater. Sci. 2021, 56, 5936–5955. [Google Scholar] [CrossRef]
- Prabu, S.; Nasar, A.S.; Sivakumar, C. Morphological and antimicrobial studies of chitosan/poly (vinyl alcohol)/acyl chloride terminated hyperbranched polyester chemical crosslinked blends. J. Coat. Technol. Res. 2022, 19, 1357–1364. [Google Scholar] [CrossRef]
- ECosta-Júnior, S.; Barbosa-Stancioli, E.F.; Mansur, A.A.; Vasconcelos, W.L.; Mansur, H.S. Preparation and characterization of chitosan/poly (vinyl alcohol) chemically crosslinked blends for biomedical applications. Carbohydr. Polym. 2009, 76, 472–481. [Google Scholar] [CrossRef]
- Mallakpour, S.; Rashidimoghadam, S. Preparation, characterization, and in vitro bioactivity study of glutaraldehyde crosslinked chitosan/poly (vinyl alcohol)/ascorbic acid-MWCNTs bionanocomposites. Int. J. Biol. Macromol. 2020, 144, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Lusiana, R.A.; Siswanta, D.; Mudasir, M. Preparation of citric acid crosslinked chitosan/poly (vinyl alcohol) blend membranes for creatinine transport. Indones. J. Chem. 2016, 16, 144–150. [Google Scholar] [CrossRef]
- Han, X.; Huo, P.; Ding, Z.; Kumar, P.; Liu, B. Preparation of lutein-loaded PVA/sodium alginate nanofibers and investigation of its release behavior. Pharmaceutics 2019, 11, 449. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Yaqoob, Z.; Ansari, M.N.M.; Razak, S.I.A.; Raza, M.A.; Sajjad, A.; Haider, S.; Busra, F.M. Chitosan/poly vinyl alcohol/graphene oxide based pH-responsive composite hydrogel films: Drug release, anti-microbial and cell viability studies. Polymers 2021, 13, 3124. [Google Scholar] [CrossRef]
- Ali, I.; Raza, M.A.; Mehmood, R.; Islam, A.; Sabir, A.; Gull, N.; Haider, B.; Park, S.H.; Khan, R.U. Novel Maleic Acid, Crosslinked, Nanofibrous Chitosan/Poly (Vinylpyrrolidone) Membranes for Reverse Osmosis Desalination. Int. J. Mol. Sci. 2020, 21, 7338. [Google Scholar] [CrossRef]
- Heydari, M.; Moheb, A.; Ghiaci, M.; Masoomi, M. Effect of cross-linking time on the thermal and mechanical properties and pervaporation performance of poly (vinyl alcohol) membrane cross-linked with fumaric acid used for dehydration of isopropanol. J. Appl. Polym. Sci. 2013, 128, 1640–1651. [Google Scholar] [CrossRef]
- Abdelrazek, E.M.; Elashmawi, I.S.; Labeeb, S. Chitosan filler effects on the experimental characterization, spectroscopic investigation and thermal studies of PVA/PVP blend films. Phys. B Condens. Matter 2010, 405, 2021–2027. [Google Scholar] [CrossRef]
- de Souza Costa-Júnior, E.; Pereira, M.M.; Mansur, H.S. Properties and biocompatibility of chitosan films modified by blending with PVA and chemically crosslinked. J. Mater. Sci. Mater. Med. 2009, 20, 553–561. [Google Scholar] [CrossRef]
- Buraidah, M.H.; Arof, A.K. Characterization of chitosan/PVA blended electrolyte doped with NH4I. J. Non-Cryst. Solids 2011, 357, 3261–3266. [Google Scholar] [CrossRef]
- Pereira, V.A., Jr.; de Arruda, I.N.Q.; Stefani, R. Active chitosan/PVA films with anthocyanins from Brassica oleraceae (Red Cabbage) as Time–Temperature Indicators for application in intelligent food packaging. Food Hydrocoll. 2015, 43, 180–188. [Google Scholar] [CrossRef]
- Mao, H.; Wei, C.; Gong, Y.; Wang, S.; Ding, W. Mechanical and Water-Resistant Properties of Eco-Friendly Chitosan Membrane Reinforced with Cellulose Nanocrystals. Polymers 2019, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Vinod, A.; Sanjay, M.R.; Suchart, S.; Jyotishkumar, P. Renewable and sustainable biobased materials: An assessment on biofibers, biofilms, biopolymers and biocomposites. J. Clean. Prod. 2020, 258, 120978. [Google Scholar]


| Chemical Reagent | Ratio % | |||||
|---|---|---|---|---|---|---|
| Mass | Molar | Mass | Molar | Mass | Molar | |
| CA | 2.5 | 10.6 | 5.0 | 19.3 | 7.5 | 26.4 |
| SA | 2.5 | 16.2 | 5.0 | 28.0 | 7.5 | 36.8 |
| TEOS | 2.5 | 9.9 | 5.0 | 18.0 | 7.5 | 24.8 |
| Component | Wave Number, cm−1 | Assignment |
|---|---|---|
| PVA | 1108 | C-O stretching |
| 1430 | O-H bending | |
| 1758 | C=O acetyl group | |
| 2930 | C-H methylene group | |
| 3450 | O-H stretching | |
| CS | 890 & 1150 | Saccharide structure |
| 1250 | Amino group | |
| 1558 | Amide II | |
| 1658 | Amide I | |
| 3430 | O-H stretching | |
| CS/PVA | 1075 | O-H group of PVA |
| 3380 | O-H group of Chitosan | |
| CA-5; SA-5; TEOS-5 | 1720 | C=O ester stretching |
| Ratio CS/PVA (wt%) | Chemical Agent | Tensile Strength, MPa | |
|---|---|---|---|
| Exp. Results | Reference | ||
| 75/25 | Citric acid | 33.9 | - |
| 75/25 | Succinic acid | 44.0 | - |
| 75/25 | TEOS | 41.9 | - |
| 50/50 | Citric acid | 29.7 [56] | |
| 50/50 | Poly(Hexamethylene Guanidine) | 62.0 [26] | |
| 75/25 | Calcium chloride | 33.53 [28] | |
| 75/25 | Glutaraldehyde | 25.3 [54] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wardhono, E.Y.; Pinem, M.P.; Susilo, S.; Siom, B.J.; Sudrajad, A.; Pramono, A.; Meliana, Y.; Guénin, E. Modification of Physio-Mechanical Properties of Chitosan-Based Films via Physical Treatment Approach. Polymers 2022, 14, 5216. https://doi.org/10.3390/polym14235216
Wardhono EY, Pinem MP, Susilo S, Siom BJ, Sudrajad A, Pramono A, Meliana Y, Guénin E. Modification of Physio-Mechanical Properties of Chitosan-Based Films via Physical Treatment Approach. Polymers. 2022; 14(23):5216. https://doi.org/10.3390/polym14235216
Chicago/Turabian StyleWardhono, Endarto Yudo, Mekro Permana Pinem, Sidik Susilo, Bintang Junita Siom, Agung Sudrajad, Agus Pramono, Yenny Meliana, and Erwann Guénin. 2022. "Modification of Physio-Mechanical Properties of Chitosan-Based Films via Physical Treatment Approach" Polymers 14, no. 23: 5216. https://doi.org/10.3390/polym14235216
APA StyleWardhono, E. Y., Pinem, M. P., Susilo, S., Siom, B. J., Sudrajad, A., Pramono, A., Meliana, Y., & Guénin, E. (2022). Modification of Physio-Mechanical Properties of Chitosan-Based Films via Physical Treatment Approach. Polymers, 14(23), 5216. https://doi.org/10.3390/polym14235216








