Impact of the Polymer Backbone Structure on the Separation Properties of New Stationary Phases Based on Tricyclononenes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
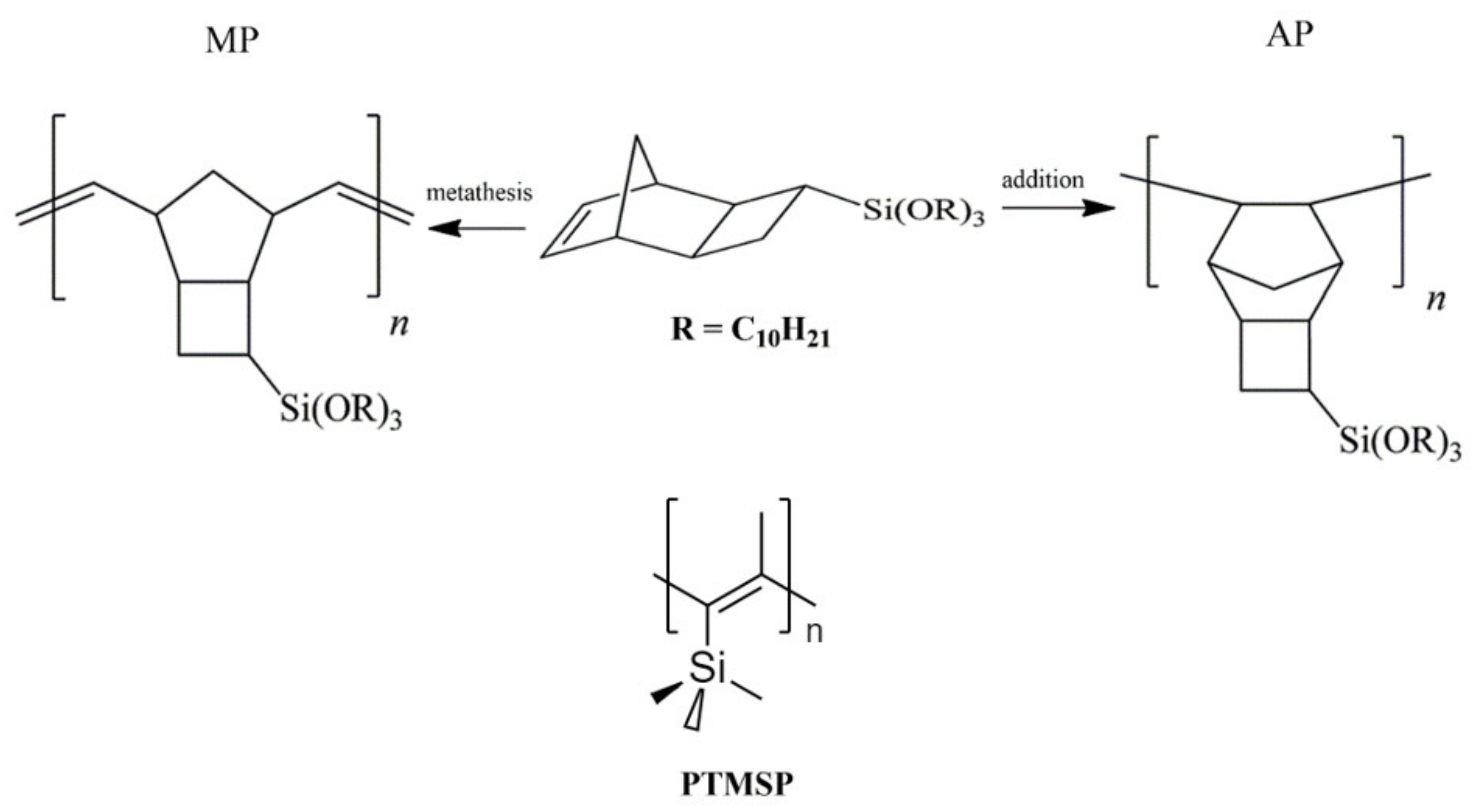
2.2. Synthesis of Polymers
2.3. Column Preparation
2.4. Chromatographic Measurements
2.5. Thermogravimetric and Calorimetric Measurements
2.6. Testing Columns for Physical Ageing
3. Results and Discussion
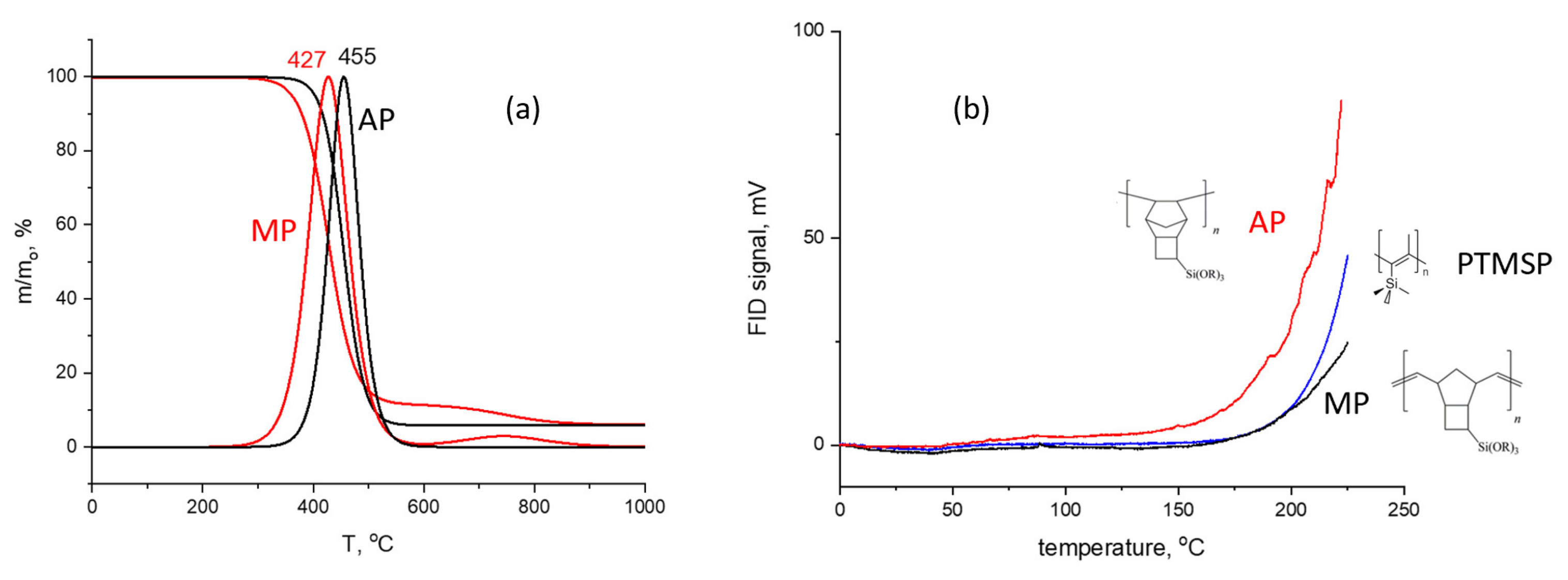
3.1. Synthesis and Thermal Stability of Polytricyclononenes
3.2. Chromatographic Performance of Tricyclononene Stationary Phases
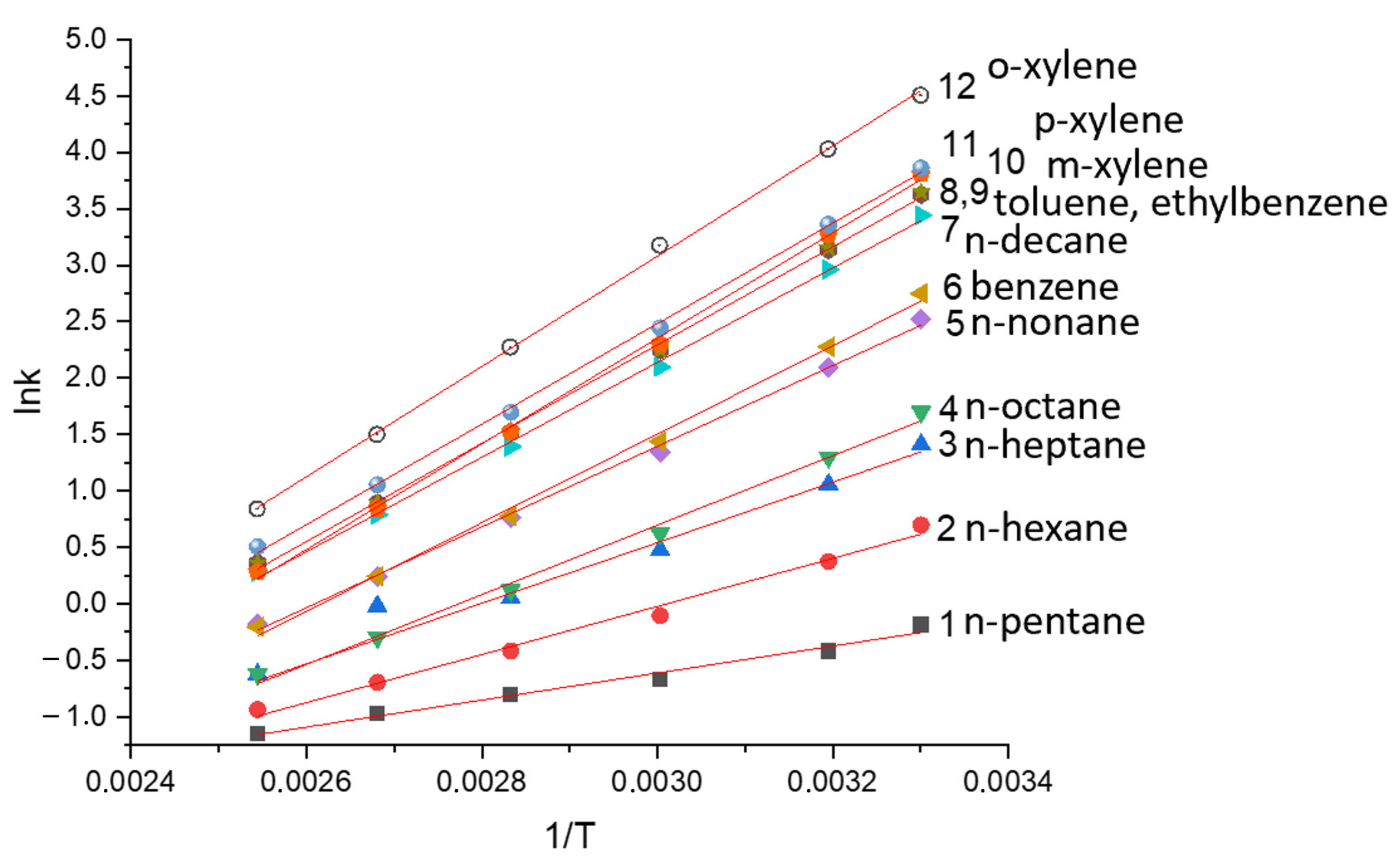
3.3. Thermodynamic Parameters of Sorption for Separations during AP and MP Stationary Phases
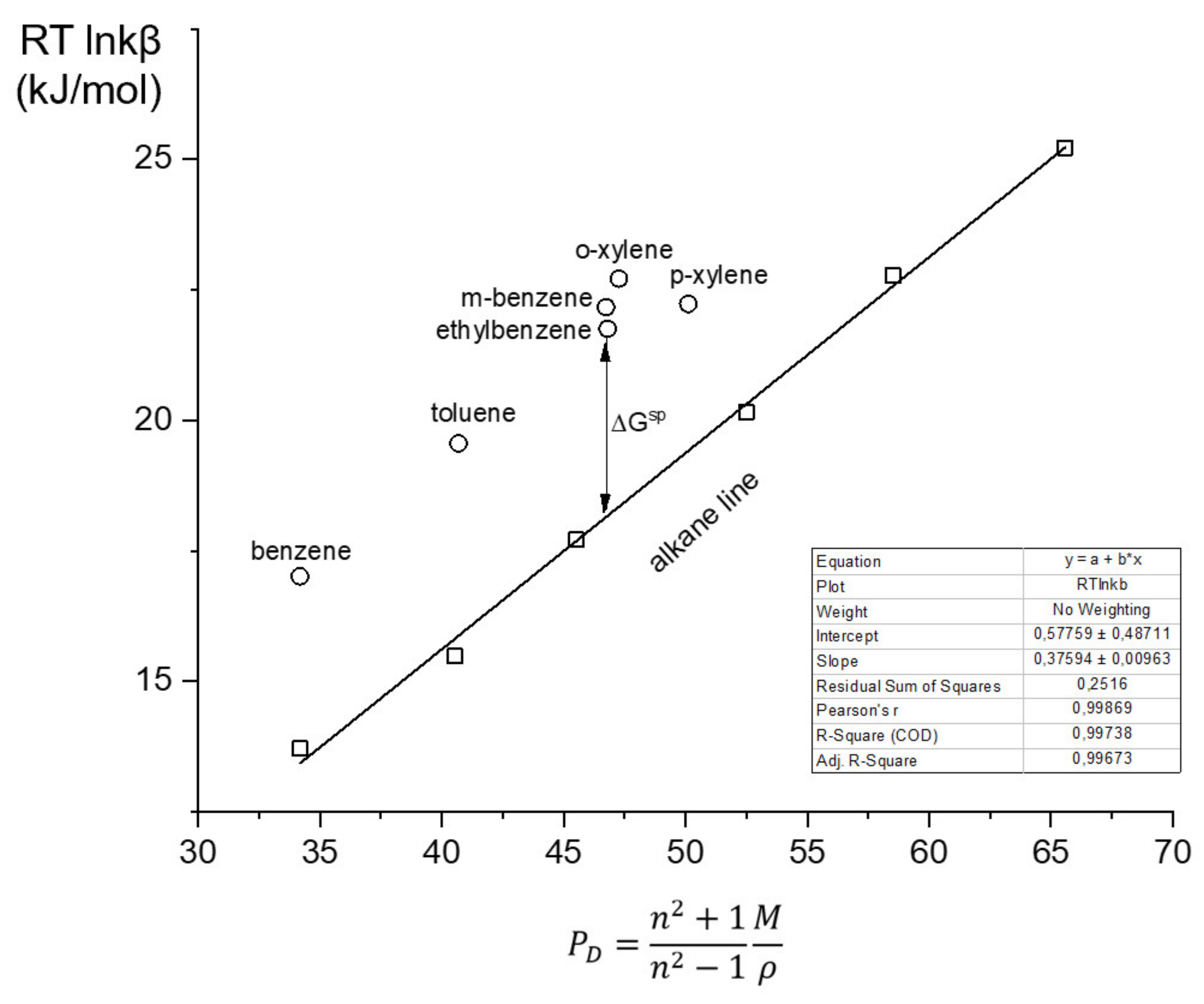
3.4. Evaluation of the Surface Free Energy for the AP and MP Stationary Phases
| Stationary Phase | Thermodynamic Parameters | Dispersive Surface Energy *) | Specific Surface Energy ∆GSP, kJ/mol (See Figure 7) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| −ΔH, kJ/mol | −ΔS, J/molK | Tcom | γsD, mJ/m2 | Benzene | Toluene | Ethylbenzene | m-Xylene | p-Xylene | o-Xylene | |
| AP | 7.2 | 16.2 | 415 | 56.1 | 3.6 | 3.7 | 3.6 | 4.0 | 2.8 | 4.4 |
| MP | 7.4 | 15.7 | 467 | 57.7 | 3.7 | 3.7 | 3.4 | 3.6 | 2.5 | 4.4 |
| PEG20M | 3.5 | 3.9 | 910 | 97.1 | 9.6 | 9.0 | 8.4 | 8.4 | 7.0 | 9.4 |
| PTMSP | 7.2 | 12.5 | 509 | 49.3 ** | 3.4 | 2.8 | 2.4 | 3.0 | 0.6 | 2.2 |
| SE-30 | 7.3 | 10.9 | 486 | 48.2 | 2.6 | 2.5 | 2.5 | 3.2 | 1.6 | 2.5 |
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dandeneau, R.D.; Zerenner, E.H. An investigation of glasses for capillary chromatography. J. High Resolut. Chromatogr. 1979, 2, 351–356. [Google Scholar] [CrossRef]
- Poole, C.F.; Poole, S.K. Separation characteristics of wall-coated open-tubular columns for gas chromatography. J. Chromatogr. A 2008, 1184, 254–280. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Hutt, S. A New Bonded Porous Polymer PLOT U Column with Increased Polarity. J. Chromatogr. Sci. 2000, 38, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.F. Gas Chromatography; (Handbooks in Separation Science); Elsevier: Amsterdam, The Netherlands, 2021; ISBN 13 978-0-12-820675-1; ISBN 10 0-12-820675-6. [Google Scholar]
- Patrushev Yu, V.; Nikolaeva, O.A.; Sidelnikov, V.N. Multicapillary Columns with a Porous Layer Based on the Divinylbenzene Copolymer. Russian J. Phys. Chem. A. 2010, 84, 871–875. [Google Scholar] [CrossRef]
- De Zeeuw, J.; Duvekot, C.; Peene, J.; Dijkwel, P.; Heijnsdijk, P. A Review of the State-of-the-Art Column Technologies for the Determination of ppm to ppb Levels of Oxygenated, Sulfur, and Hydrocarbon Impurities in C1-C5 Hydrocarbon Streams. J. Chromatogr. Sci. 2003, 279, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Hollis, O.L. Porous Polymers Used in GC and LC. J. Chromatogr. Sci. 1973, 11, 335–342. [Google Scholar] [CrossRef]
- Wawrzyniak, R. Gas–Solid Chromatography (PLOT Columns). In Gas Chromatography; Handbooks in Separation Science; Poole, C., Ed.; Elsevier: New York, NY, USA, 2012; pp. 123–136. ISBN 13 978-0-12-385540-4 (paperback); ISBN 10 0-12-385540-3 (e-book). [Google Scholar] [CrossRef]
- Zeeuw, J. Column Technology: Porous Layer Open-Tubular Columns. In Gas Chromatography, (Handbooks in Separation Science), 2nd ed.; Colin, P., Ed.; Elsevier: New York, NY, USA, 2021; pp. 117–140. ISBN 13 978-0-12-820675-1 (paperback); ISBN 10 0-12-820675-6 (e-book). [Google Scholar] [CrossRef]
- Nikolaeva, O.A.; Patrushev, Y.V.; Sidelnikov, V.N. Chromatographic properties PLOT multicapillary columns. J. Chromatogr. A. 2017, 1488, 126–133. [Google Scholar] [CrossRef]
- Berezkin, V.G.; Korolev, A.A.; Khotimskii, V.S. Polytrimethylsilylpropyne as a chromatographic adsorbent in capillary gas chromatography. Dokl. Akad. Nauk 2000, 370, 1–4. [Google Scholar]
- Berezkin, V.G.; Popova, T.P.; Shiryaeva, V.E. Polytrimethylsilylpropyne as adsorbent for separation of gases. Russ. Chem. Bull. 2001, 50, 233–236. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Sadrzadeh, M.; Matsuura, T. Thermally stable polymers for advanced high-performance gas separation membranes. Prog. Energy Combust. Sci. 2018, 66, 1–41. [Google Scholar] [CrossRef]
- Belotserkovskaya, V.; Yakovleva, E. Chromatographic and adsorption properties of poly(1-trimethylsilyl-1-propyne) and their stabilization by adding poly(1-phenyl-1-propyne). J. Chromatogr. A 2013, 1298, 109–117. [Google Scholar] [CrossRef]
- Berezkin, V.G.; Popova, T.P.; Shiryaeva, V.E.; Korolev, A.A.; Malyukova, I.V. Equilibrium chromatographic characteristics of capillary column coated with polytrimethylpropyne for separating polar and non-polar organic compoundsby gas capillary chromatography. Russ. Chem. Bull. 2002, 51, 828–832. [Google Scholar] [CrossRef]
- Krylov, V.A.; Berezkin, V.G.; Chernova, O.Y.; Salganskii, Y.M.; Korolev, A.A. Gas-adsorption chromatography of reactive compounds on open-tubular columns with poly(trimethylsilylpropyne). J. Anal. Chem. 2003, 58, 372–374. [Google Scholar] [CrossRef]
- Yavari, M.; Le, T.; Lin, H. Physical aging of glassy perfluoro polymers in thin film composite membranes. Part I. Gas transport properties. J. Membr. Sci. 2017, 525, 387–398. [Google Scholar] [CrossRef]
- Struik, L.C.E. Physical Aging in Amorphous Polymers and Other Materials; Elsevier: New York, NY, USA, 1978; p. 1019. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.; Paul, D.R. Physical aging of thin glassy polymer films: Free volume interpretation. J. Membr. Sci. 2006, 277, 219–229. [Google Scholar] [CrossRef]
- Alentiev, D.A.; Chapala, P.P.; Filatova, M.P.; Finkelshtein, E.S.; Bermeshev, M.V. Synthesis of novel tricyclononenes containing alkoxysilyl groups. Mendeleev Commun. 2016, 26, 530–531. [Google Scholar] [CrossRef]
- Alentiev, D.A.; Egorova, E.S.; Bermeshev, M.V.; Starannikova, L.E.; Topchiy, M.A.; Asachenko, A.F.; Gribanov, P.S.; Nechaev, M.S.; Yampolskii, Y.P.; Finkelshtein, E.S. Janus tricyclononene polymers bearing tri(n-alkoxy)silyl side groups for membrane gas separation. J. Mater. Chem. A 2018, 6, 19393. [Google Scholar] [CrossRef]
- Kurganov, A.; Kanateva, A.; Shiryaeva, V.; Popova, T.; Korolev, A. Investigation of poly[oligo(ethylene glycol)diacrylates] as potential stationary phases in GC. J Sep Sci. 2017, 40, 3675–3681. [Google Scholar] [CrossRef]
- Yampolskii, Y.; Belov, N. Investigation of Polymers by Inverse Gas Chromatography. Macromolecules 2015, 48, 6751–6767. [Google Scholar] [CrossRef]
- Ettre, L.S.; Hinshaw, J.V. Basic Relationships of Gas Chromatography; Advanstar: Cleveland, OH, USA, 1993; pp. 122–123. ISBN 3-7785-2418-6. [Google Scholar]
- Shiryaeva, V.E.; Korolev, A.A.; Popova, T.P.; Kanateva AYu Kurganov, A.A. The stability study of thermodynamic parameters of sorption of light hydrocarbons on poly[trimethylsilyl(propyn-1)] at different temperatures. J. Chromatogr. Sci. 2019, 57, 778–783. [Google Scholar] [CrossRef]
- Alentiev, D. Synthesis and Polymerization of Monosubstituted Silicon-Containing bi- and Tricyclic Hydrocarbons. PhD Thesis, A.V. Topchiev Institute of Petrochemical Synthesis, Moscow, Russia, 2019. [Google Scholar]
- Barry, E.F.; Grob, R.L. Columns for Gas Chromatography. Performance and Selection; J. Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; p. 178. ISBN 978-0-471-74043-8. [Google Scholar]
- Mohammadi-Jam, S.; Waters, K.E. Inverse gas chromatography applications: A review. Adv. Colloid. Interf. Sci. 2014, 212, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Conder, J.R.; Young, C.L. Physicochemical Measurements by Gas Chromatography; Wiley: Chichester, UK, 1979. [Google Scholar] [CrossRef]
- Frauenhofer, E.; Cimmerer, C.; Yu, J.; Al-Saigh, Z.Y.; Kim, J. Investigation of sorption and diffusion of hydrocarbons into polydimethylsiloxane in the headspace-solid phase microextraction sampling process via inverse gas chromatography. J. Chromatogr. A 2021, 1639, 461894. [Google Scholar] [CrossRef] [PubMed]
- Korolev, A.A.; Shiryaeva, V.E.; Popova, T.P.; Kurganov, A.A. Enthalpy–entropy compensation effect on adsorption of light hydrocarbons on monolithic stationary phases. J. Sep. Sci. 2011, 34, 2362–2369. [Google Scholar] [CrossRef] [PubMed]
- Hamieh, T. Study of the temperature effect on the surface area of model organic molecules, the dispersive surface energy and the surface properties of solids by inverse gas chromatography. J. Chromatogr. A 2020, 1627, 461372. [Google Scholar] [CrossRef]
- Charmas, B.; Leboda, R. Effect of surface heterogeneity on adsorption on solid surfaces: Application of inverse gas chromatography in the studies of energetic heterogeneity of adsorbents. J. Chromatogr. A 2000, 886, 133–152. [Google Scholar] [CrossRef]
- Dorris, G.M.; Gray, D.G. Adsorption of n-alkanes at zero surface coverage on cellulose paper and wood fibers. J. Colloid Interface Sci. 1980, 77, 353–362. [Google Scholar] [CrossRef]
- Dong, S.; Brendle, M.; Donnet, J. Study of solid surface polarity by inverse gas chromatography at infinite dilution. Chromatographia 1989, 28, 469–472. [Google Scholar] [CrossRef]

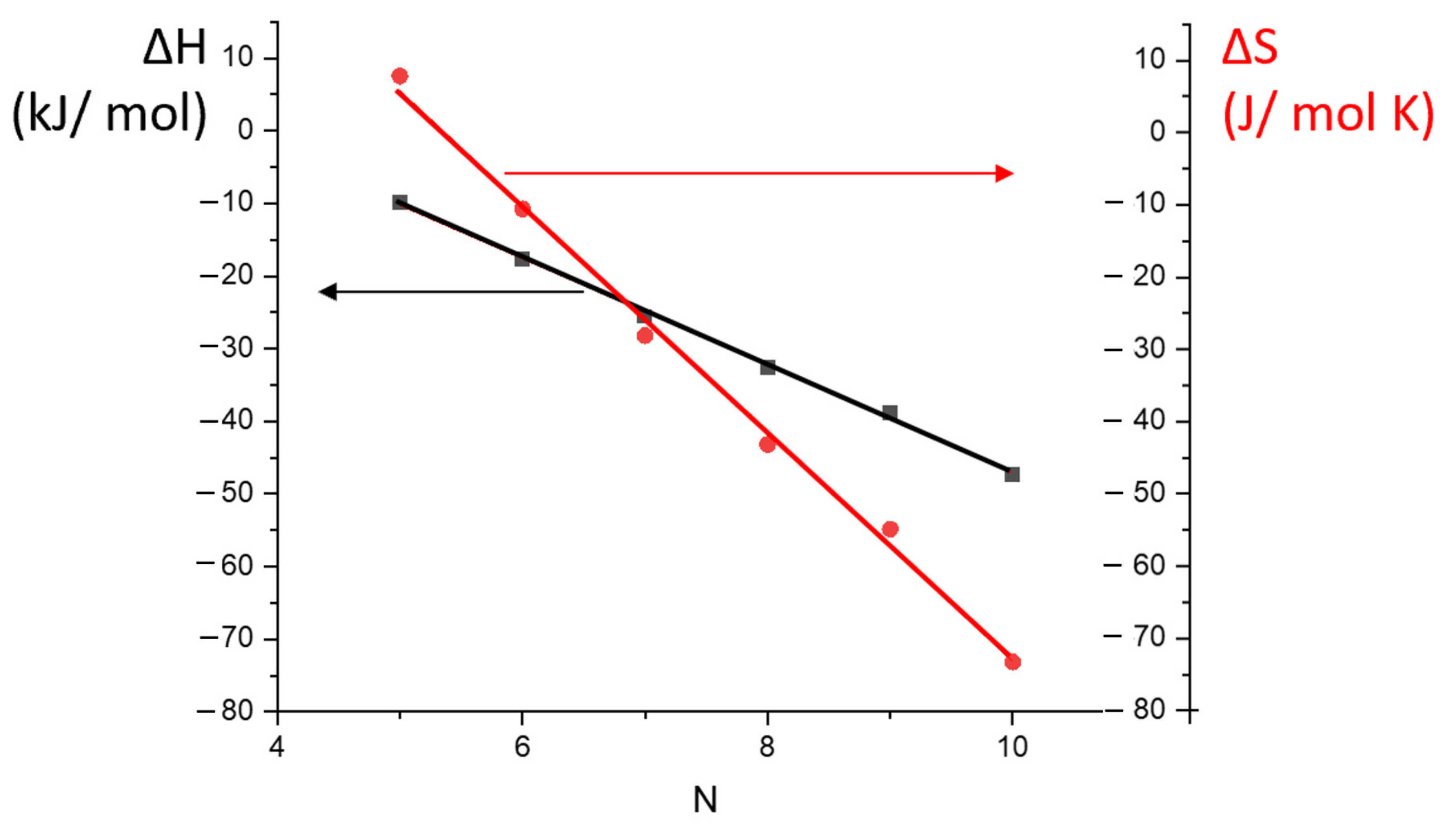
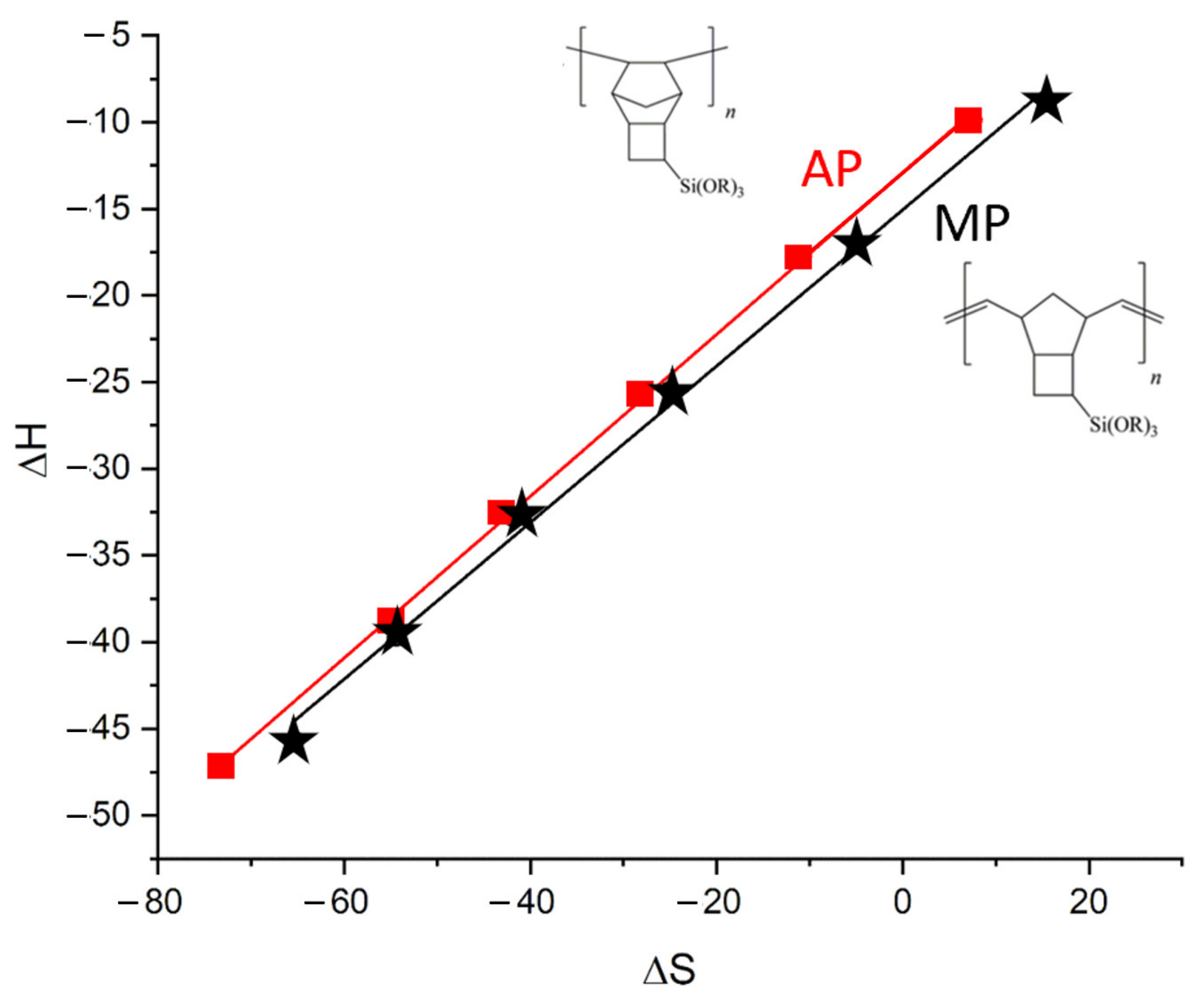
| Column | Stationary Phase | Molar Mass/Polydispersity, kDa | Glass Transition Temperature Tg, °C | Decomposition Temperature Tc, °C | Column Length L, m | Column Inner Diameter dc, mm | Stationary Phase Thickness df, μm | Phase Ratio β |
|---|---|---|---|---|---|---|---|---|
| 1 | AP | 600/3.2 | −63 [21] | 400 ** | 10.0 | 0.165 | 0.25 | 165 |
| 2 | MP | 1000/2.5 | −71 [21] | 360 ** | 10.0 | 0.165 | 0.25 | 165 |
| 3 | SE-30 | 1500/2.5 | −120 [22] | >350 | 15.0 | 0.23 | 0.22 | 229 |
| 4 | PEG 20M | 20/1.05 | −52/+50 * [22] | 290 ** [22] | 16.0 | 0.165 | 0.25 | 165 |
| 5 | PTMSP | 200/1.2 | >300 [23] | >350 [23] | 15.0 | 0.165 | 0.25 | 165 |
| Polymer | AP | MP | SE-30 | PEG 20M | PTMSP | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Solute | ΔH, kJ/mol | ΔS, J/molK | ΔH, kJ/mol | ΔS, J/molK | ΔH, kJ/mol | ΔS, J/molK | ΔH, kJ/mol | ΔS, J/molK | ΔH, kJ/mol | ΔS, J/molK |
| C5 | −8.9 | 15.5 | −9.9 | 7.53 | − | − | −17.6 | −42.16 | −45.6 | −78.5 |
| C6 | −17.1 | −5.0 | −17.7 | −10.76 | −27.1 | −48.1 | −21.6 | −45.23 | −48.2 | −74.3 |
| Bzl | −21.4 | −13.9 | −22.3 | −19.65 | − | − | −30.4 | −50.0 | −51.7 | −86.0 |
| C7 | −25.5 | −25.0 | −25.6 | −28.22 | −30.3 | −50.6 | −25.4 | −50.2 | −55.4 | −88.5 |
| Tol | −29.7 | −32.3 | −29.8 | −34.87 | − | − | −33.4 | −53.1 | −56.4 | −89.3 |
| C8 | −32.8 | −41.9 | −32.6 | −43.18 | −33.8 | −54.3 | −29.4 | −53.8 | −60.4 | −93.5 |
| EtBzl | −35.3 | −43.3 | −34.9 | −48.59 | − | − | −36.7 | −58.4 | −60.8 | −94.3 |
| m-Xyl | −36.6 | −46.1 | −36.0 | −46.51 | − | − | −36.2 | −56.2 | −59.7 | −95.1 |
| p-Xyl | −36.9 | −46.8 | −36.2 | −47.34 | − | − | −37.1 | −58.6 | −60.8 | −93.5 |
| o-Xyl | −37.7 | −46.9 | −38.9 | −54.82 | − | − | −37.4 | −57.2 | −60.3 | −91.8 |
| C9 | −39.7 | −54.3 | −37.1 | −48.17 | −37.6 | −59.0 | −32.0 | −57.7 | −70.8 | −110.9 |
| C10 | −45.7 | −65.3 | −40.7 | −53.99 | −41.5 | −63.9 | −35.3 | −61.0 | −75.8 | −116.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanatieva, A.Y.; Alentiev, D.A.; Shiryaeva, V.E.; Korolev, A.A.; Kurganov, A.A. Impact of the Polymer Backbone Structure on the Separation Properties of New Stationary Phases Based on Tricyclononenes. Polymers 2022, 14, 5120. https://doi.org/10.3390/polym14235120
Kanatieva AY, Alentiev DA, Shiryaeva VE, Korolev AA, Kurganov AA. Impact of the Polymer Backbone Structure on the Separation Properties of New Stationary Phases Based on Tricyclononenes. Polymers. 2022; 14(23):5120. https://doi.org/10.3390/polym14235120
Chicago/Turabian StyleKanatieva, Anastasiia Yu., Dmitry A. Alentiev, Valeria E. Shiryaeva, Alexander A. Korolev, and Alexander A. Kurganov. 2022. "Impact of the Polymer Backbone Structure on the Separation Properties of New Stationary Phases Based on Tricyclononenes" Polymers 14, no. 23: 5120. https://doi.org/10.3390/polym14235120
APA StyleKanatieva, A. Y., Alentiev, D. A., Shiryaeva, V. E., Korolev, A. A., & Kurganov, A. A. (2022). Impact of the Polymer Backbone Structure on the Separation Properties of New Stationary Phases Based on Tricyclononenes. Polymers, 14(23), 5120. https://doi.org/10.3390/polym14235120






