Novel Biocatalysts Based on Bromelain Immobilized on Functionalized Chitosans and Research on Their Structural Features
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Chitosan Derivatives
2.2.1. Synthesis of Carboxymethyl Chitosan (ChMC)
2.2.2. Synthesis of N-(2-Hydroxy) propyl-3-trimethylammonium Chitosan (HTCCh)
2.2.3. Synthesis of Chitosan Sulfate, Chitosan Acetate (ChS)
2.2.4. Synthesis of Chitosan Acetate (ChA)
2.3. Immobilization Procedure
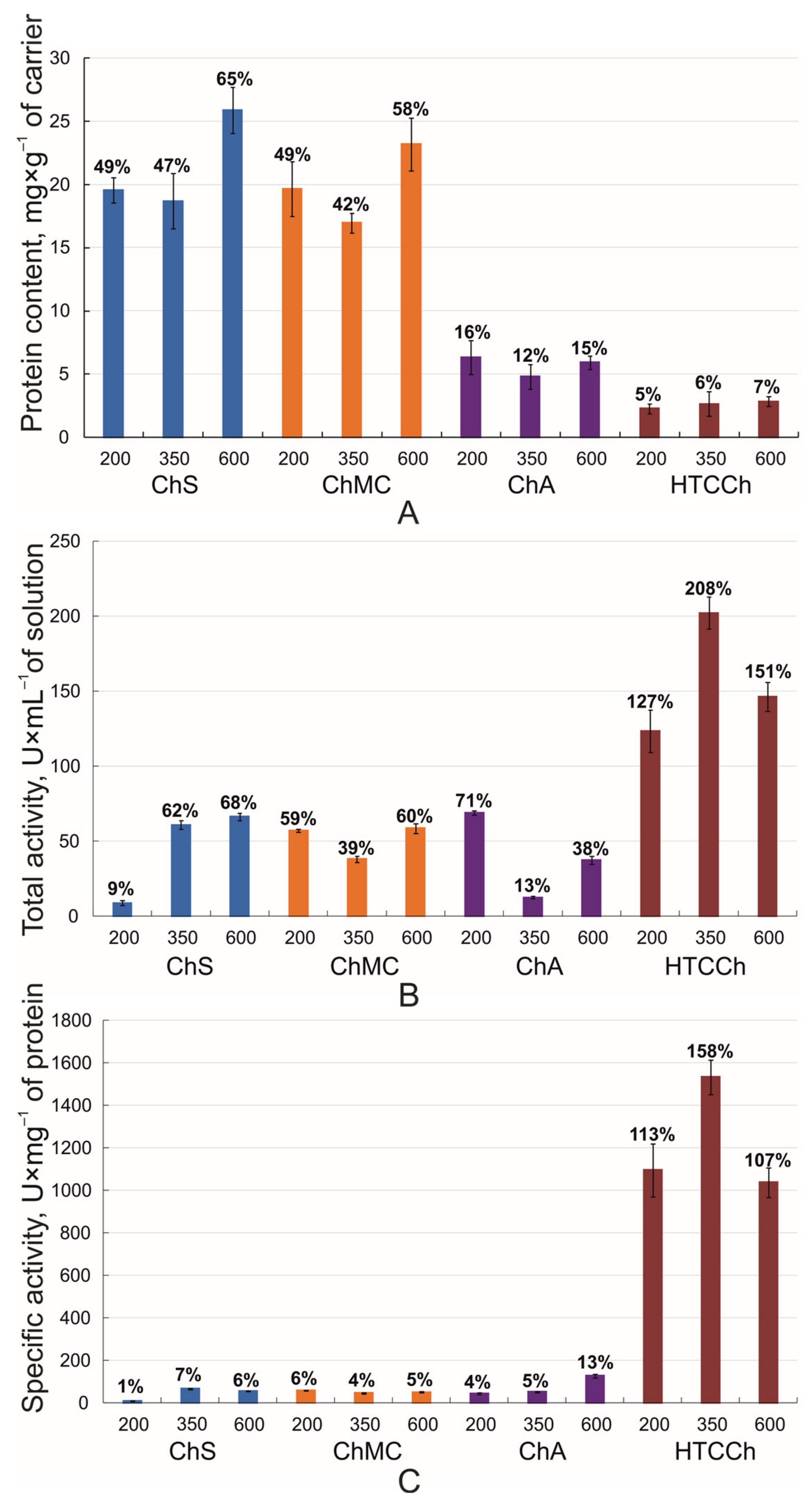
2.4. Protein Content Measurement
2.5. Evaluation of Proteolytic Activity of the Immobilized Enzymes
2.6. Molecular Docking
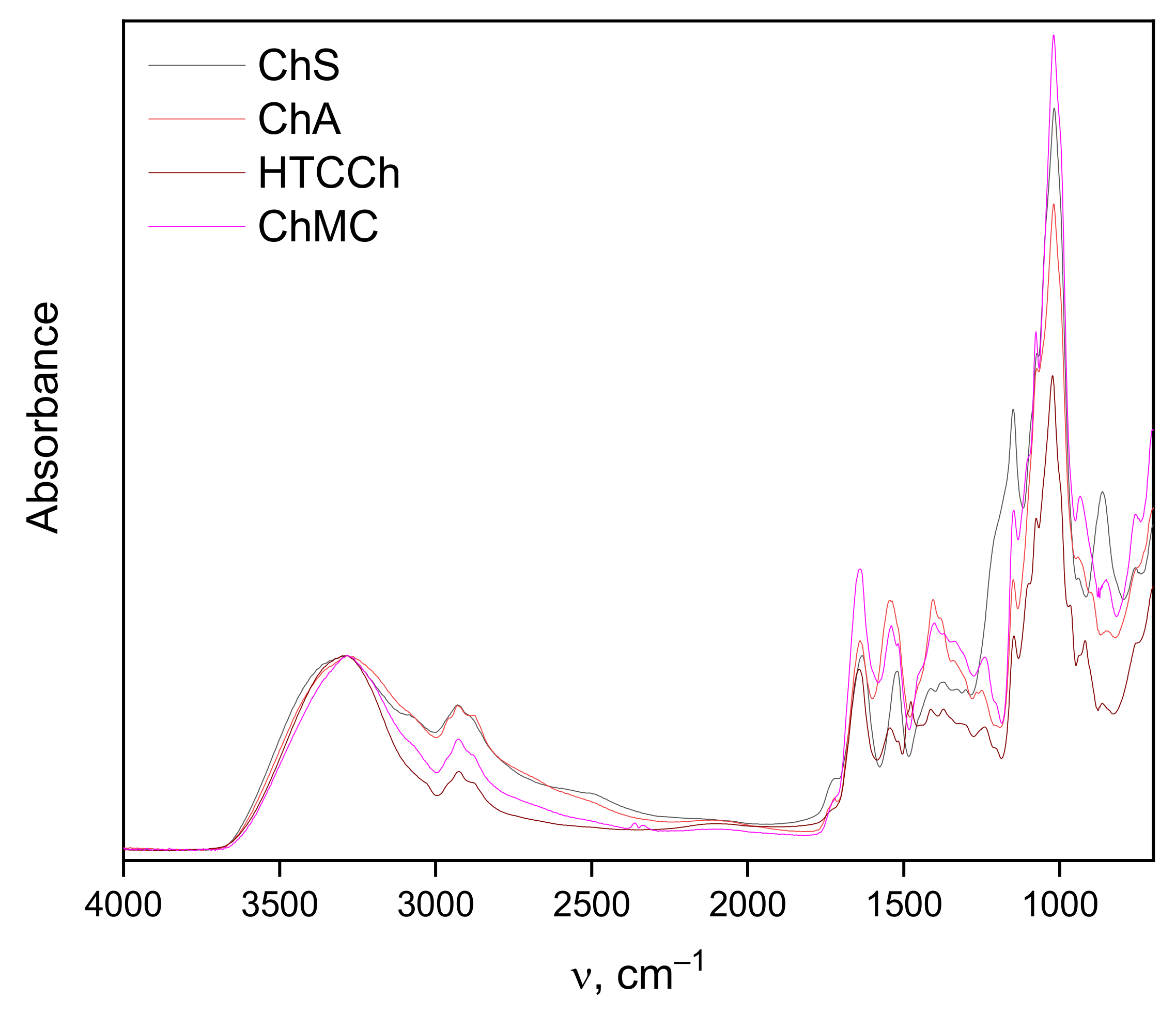
2.7. Infrared Spectroscopy
2.8. Bacterial Strains and Biofilm Assays
3. Results and Discussions
3.1. Bromelain’s Immobilization
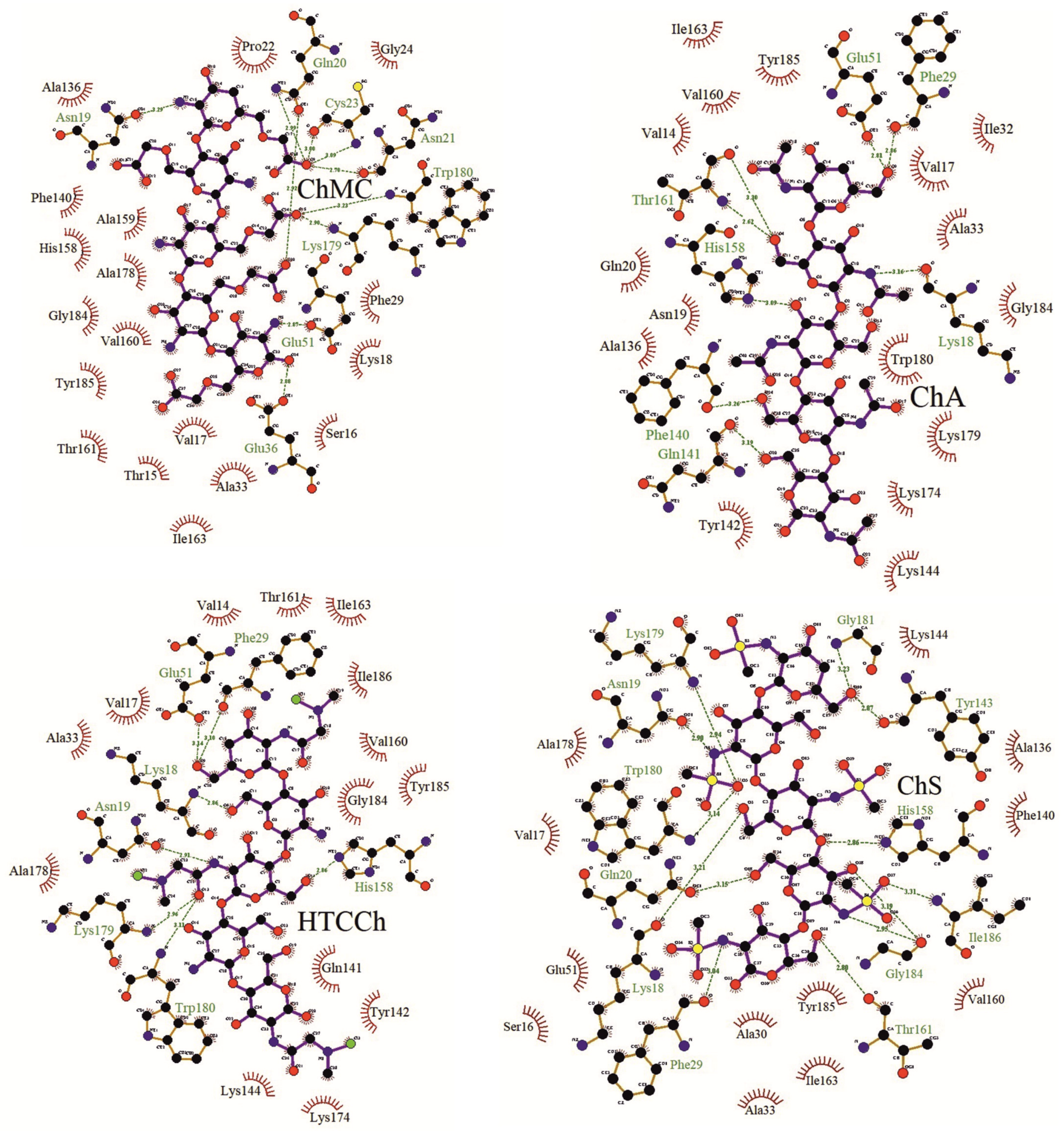
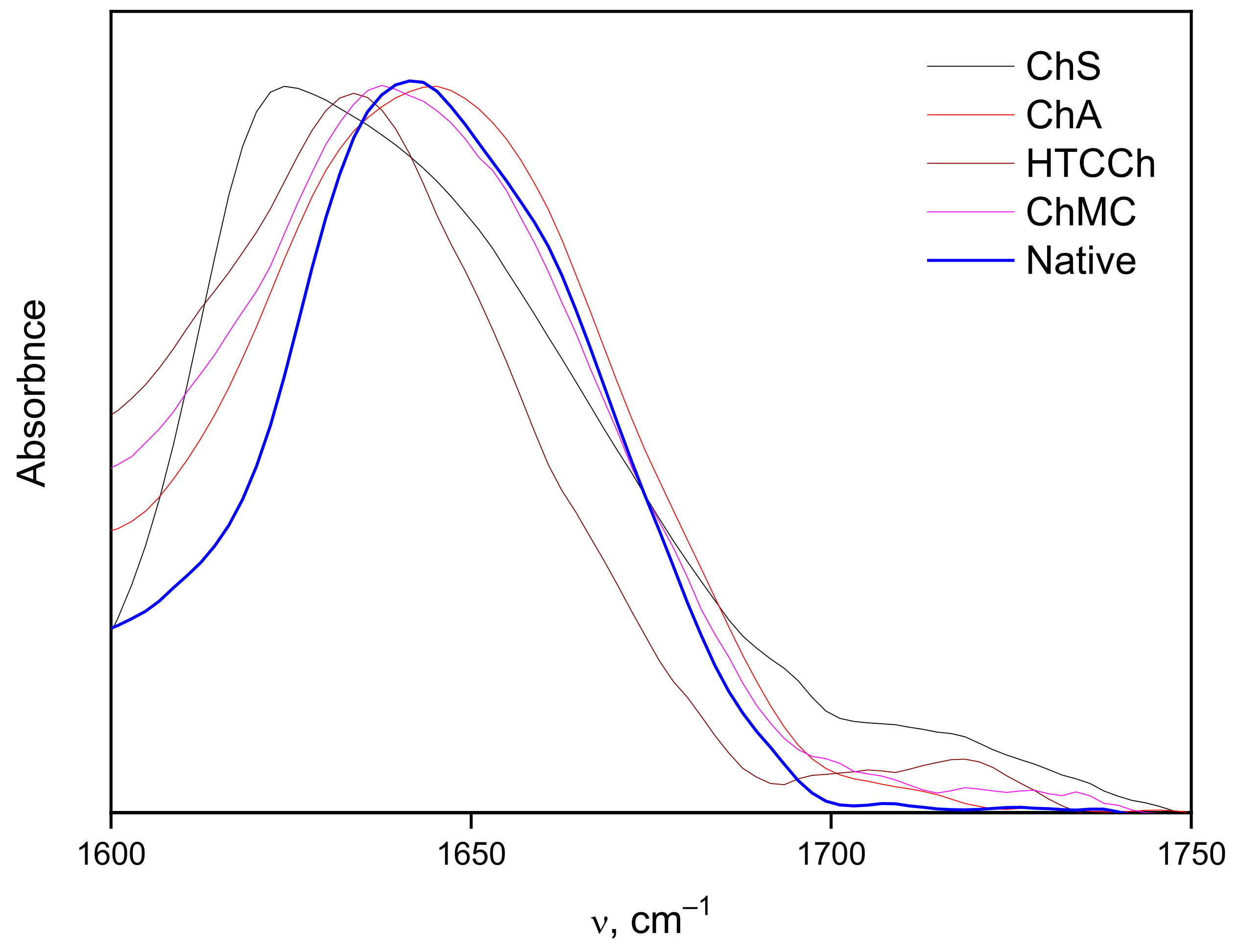
3.2. Interaction Mechanisms between Bromelain and Chitosan Derivatives
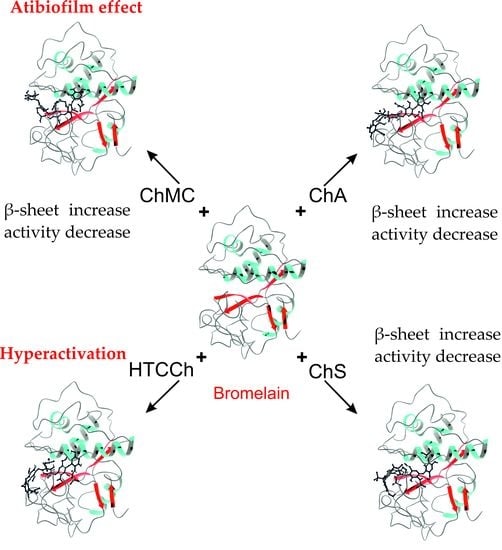
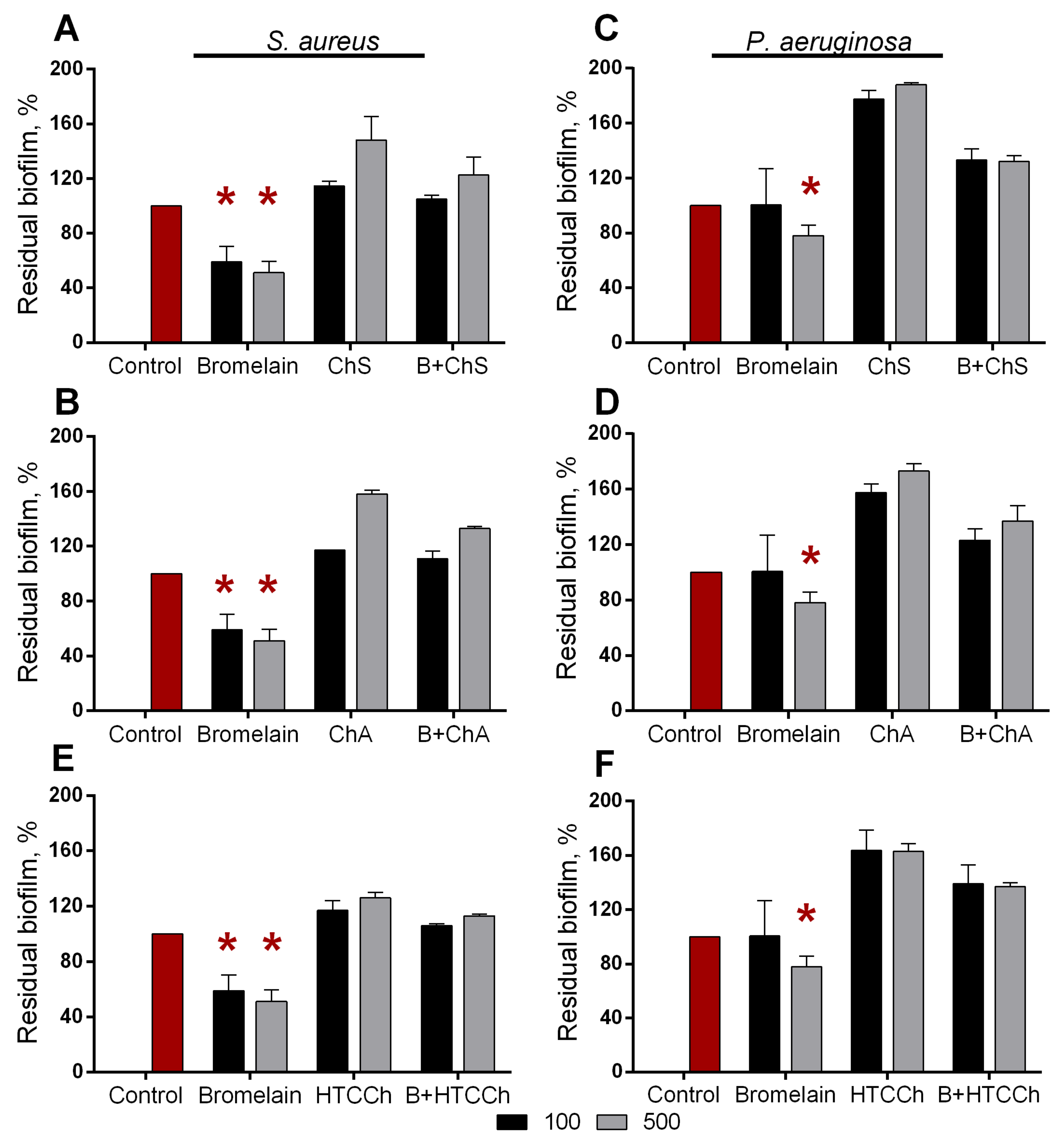
3.3. Antibiofilm Properties of Immobilized Bromelain
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Ige, O.O.; Umoru, L.E.; Aribo, S. Natural Products: A Minefield of Biomaterials. Int. Sch. Res. Not. Mater. Sci. 2012, 2012, 983062. [Google Scholar] [CrossRef]
- Joyce, K.; Fabra, G.T.; Bozkurt, Y.; Pandit, A. Bioactive potential of natural biomaterials: Identification, retention and assessment of biological properties. Signal Transduct. Target. Ther. 2021, 6, 122. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, L.; Zelenikhin, P.; Makarova, A.; Zueva, O.; Salnikov, V.; Zuev, Y.; Ilinskaya, O. Alginate-based hydrogel as delivery system for therapeutic bacterial RNase. Polymers 2022, 14, 2461. [Google Scholar] [CrossRef]
- Makarova, A.O.; Derkach, S.R.; Kadyirov, A.I.; Ziganshina, S.A.; Kazantseva, M.A.; Zueva, O.S.; Gubaidullin, A.T.; Zuev, Y.F. Supramolecular Structure and Mechanical Performance of k-Carrageenan–Gelatin Gel. Polymers 2022, 14, 4347. [Google Scholar] [CrossRef] [PubMed]
- Ramya, R.; Venkatesan, J.; Kim, S.K.; Sudha, P.N. Biomedical Applications of Chitosan: An Overview. J. Biomater. Tissue Eng. 2012, 2, 100–111. [Google Scholar] [CrossRef]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural modification, biological activity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef]
- Makshakova, O.N.; Zuev, Y.F. Interaction-Induced Structural Transformations in Polysaccharide and Protein-Polysaccharide Gels as Functional Basis for Novel Soft-Matter: A Case of Carrageenans. Gels 2022, 8, 287. [Google Scholar] [CrossRef]
- Ojeda-Hernández, D.D.; Canales-Aguirre, A.A.; Matias-Guiu, J.A.; Matias-Guiu, J.; Gómez-Pinedo, U.; Mateos-Díaz, J.C. Chitosan–Hydroxycinnamic Acids Conjugates: Emerging Biomaterials with Rising Applications in Biomedicine. Int. J. Mol. Sci. 2022, 23, 12473. [Google Scholar] [CrossRef]
- Amini, A.; Masoumi-Moghaddam, S.; Morris, D.L. Utility of Bromelain and N-Acetylcysteine in Treatment of Peritoneal Dissemination of Gastrointestinal Mucin-Producing Malignancies; Springer: Cham, Switzerland, 2016; pp. 63–80. [Google Scholar] [CrossRef]
- Hikisz, P.; Bernasinska-Slomczewska, J. Beneficial Properties of Bromelain. Nutrients 2021, 13, 4313. [Google Scholar] [CrossRef] [PubMed]
- Maurer, H.R. Bromelain: Biochemistry, pharmacology and medical use. Cell. Mol. Life Sci. 2001, 58, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, S.M.; Kelly, A.M. Natural treatment of perennial allergic rhinitis. Altern. Med. Rev. 2000, 5, 448–454. [Google Scholar] [PubMed]
- Hale, L.P.; Greer, P.K.; Trinh, C.T.; James, C.L. Proteinase activity and stability of natural bromelain preparations. Int. Immunopharmacol. 2005, 5, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Naveena, B.M.; Mendiratta, S.K.; Anjaneyulu, A.S.R. Tenderization of buffalo meat using plant proteases from Cucumis trigonus Roxb (Kachri) and Zingiber officinale roscoe (Ginger rhizome). Meat Sci. 2004, 68, 363–369. [Google Scholar] [CrossRef]
- Varilla, C.; Marcone, M.; Paiva, L.; Baptista, J. Bromelain, a Group of Pineapple Proteolytic Complex Enzymes (Ananas comosus) and Their Possible Therapeutic and Clinical Effects. A Summary. Foods 2021, 10, 2249. [Google Scholar] [CrossRef]
- Hatano, K.-I.; Takahashi, K.; Tanokura, M. Bromein, a Bromelain Inhibitor from Pineapple Stem: Structural and Functional Characteristics. Protein Pept. Lett. 2018, 25, 838–852. [Google Scholar] [CrossRef]
- Di Cosimo, R.; Mc Auliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial Use of Immobilized Enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef]
- Liese, A.; Hilterhaus, L. Evaluation of Immobilized Enzymes for Industrial Applications. Chem. Soc. Rev. 2013, 42, 6236–6249. [Google Scholar] [CrossRef]
- Almeida, F.L.C.; Prata, A.S.; Forte, M.B.S. Enzyme Immobilization: What Have We Learned in the Past Five Years? Biofuels Bioprod. Biorefining 2022, 16, 587–608. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Carballares, D.; Morellon-Sterling, R.; Fernandez-Lafuente, R. Stabilization of Enzymes via Immobilization: Multipoint Covalent Attachment and Other Stabilization Strategies. Biotechnol. Adv. 2021, 52, 107821. [Google Scholar] [CrossRef]
- Liu, S.; Bilal, M.; Rizwan, K.; Gul, I.; Rasheed, T.; Iqbal, H.M.N. Smart Chemistry of Enzyme Immobilization Using Various Support Matrices—A Review. Int. J. Biol. Macromol. 2021, 190, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the One-Step Immobilization-Purification of Enzymes as Industrial Biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef]
- Nwagu, T.N.; Ugwuodo, C.J. Stabilizing bromelain for therapeutic applications by adsorption immobilization on spores of probiotic Bacillus. Int. J. Biol. Macromol. 2019, 127, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Zappino, M.; Cacciotti, I.; Benucci, I.; Nanni, F.; Liburdi, K.; Valentini, F.; Esti, M. Bromelain immobilization on microbial and animal source chitosan films, plasticized with glycerol, for application in wine-like medium: Microstructural, mechanical and catalytic characterisations. Food Hydrocoll. 2015, 45, 41–47. [Google Scholar] [CrossRef]
- Wei, B.; He, L.; Wang, X.; Yan, G.Q.; Wang, J.; Tang, R. Bromelain-decorated hybrid nano-particles based on lactobionic acid-conjugated chitosan for in vitro anti-tumor study. J. Biomater. Appl. 2017, 32, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Ataide, J.A.; Geraldes, D.C.; Gérios, E.F.; Bissaco, F.M.; Cefali, L.C.; Oliveira-Nascimento, L.; Mazzola, P.G. Freeze-dried chitosan nanoparticles to stabilize and deliver bromelain. J. Drug Deliv. Sci. Technol. 2021, 61, 102225. [Google Scholar] [CrossRef]
- Wang, X.; He, L.; Wei, B.; Yan, G.; Wang, J.; Tang, R. Bromelain-immobilized and lactobionic acid-modified chitosan nanoparticles for enhanced drug penetration in tumor tissues. Int. J. Biol. Macromol. 2018, 115, 129–142. [Google Scholar] [CrossRef]
- Tan, Y.-L.; Liu, C.-G.; Yu, L.-J.; Chen, X.-G. Effect of linoleic-acid modified carboxymethyl chitosan on bromelain immobilization onto self-assembled nanoparticles. Front. Mater. Sci. China 2008, 2, 209–213. [Google Scholar] [CrossRef]
- Ilaria, B.; Marco, E.; Katia, L.; Vittoria, G.A.M. Pineapple stem bromelain immobilized on different supports: Catalytic properties in model wine. Biotechnol. Prog. 2012, 28, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Itoyama, K.; Morimoto, K.; Takagishi, T.; Oka, M.; Hayashi, T. Spacer effects on enzymatic activity of bromelain immobilized onto porous chitosan beads. Eur. Polym. J. 1998, 34, 917–922. [Google Scholar] [CrossRef]
- Sorokin, A.; Lavlinskaya, M. Synthesis of the superabsorbents enriched in chitosan derivatives with excellent water absorption properties. Polym. Bull. 2022, 79, 407–427. [Google Scholar] [CrossRef]
- Wade, R.C.; Sobolev, V.; Ortiz, A.R.; Peters, G. Computational approaches to modeling receptor flexibility upon ligand binding: Application to interfacially activated enzymes. Struct.-Based Drug Des. 1998, 352, 223–232. [Google Scholar] [CrossRef][Green Version]
- Rathinam, S.; Solodova, S.; Kristjánsdóttir, I.; Hjálmarsdóttir, M.A.; Másson, M. The antibacterial structure-activity relationship for common chitosan derivatives. Int. J. Biol. Macromol. 2020, 165, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.; Qin, C.; Zeng, L.; Li, W.; Wang, W. Microbiocidal activity of chitosan-N-2-hydroxypropyl trimethyl ammonium chloride. J. Appl. Polym. Sci. 2006, 103, 3851–3856. [Google Scholar] [CrossRef]
- Pires, N.R.; Cunha, P.L.R.; Maciel, J.S.; Angelim, A.L.; Melo, V.M.M.; Paula, R.C.M.; Feitosa, J.P.A. Sulfated chitosan as tear substitute with no antimicrobial activity. Carbohydr. Polym. 2013, 91, 92–99. [Google Scholar] [CrossRef]
- Dai, T.; Tegos, G.P.; Burkatovskaya, M.; Castano, A.P.; Hamblin, M.R. Chitosan Acetate Bandage as a Topical Antimicrobial Dressing for Infected Burns. Antimicrobal Agents Chemother. 2009, 3, 393–400. [Google Scholar] [CrossRef]
- Aguanell, A.; Pozo, M.L.; Pérez-Martín, C.; Pontes, G.; Bastida, A.; Fernández-Mayoralas, A.; García-Junceda, E.; Revuelta, J. Chitosan sulfate-lysozyme hybrid hydrogels as platforms with fine-tuned degradability and sustained inherent antibiotic and antioxidant activities. Carbohydr. Polym. 2022, 291, 119611. [Google Scholar] [CrossRef]
- Chen, S.C.; Wu, Y.C.; Mi, F.L.; Lin, Y.H.; Yu, L.C.; Sung, H.W. A novel pH-sensitive hydrogel composed of N,O-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery. J. Control. Release 2004, 96, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Gorshkova, M.Y.; Volkova, I.F.; Alekseeva, S.G.; Molotkova, N.N.; Skorikova, E.E.; Izumrudov, V.A. Water-soluble modified chitosan and its interaction with a polystyrenesulfonate anion. Polym. Sci. Ser. A 2011, 53, 57–66. [Google Scholar] [CrossRef]
- Olshannikova, S.S.; Malykhina, N.V.; Lavlinskaya, M.S.; Sorokin, A.V.; Yudin, N.E.; Vyshkvorkina, Y.M.; Lukin, A.N.; Holyavka, M.G.; Artyukhov, V.G. Novel Immobilized Biocatalysts Based on Cysteine Proteases Bound to 2-(4-Acetamido-2-sulfanilamide) Chitosan and Research on Their Structural Features. Polymers 2022, 14, 3223. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Faar, A.L.; Randall, R.J. Protein measurement with Folin-phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Artyukhov, V.G.; Kovaleva, T.A.; Kholyavka, M.G.; Bityutskaya, L.A.; Grechkina, M.V. Thermal inactivation of free and immobilized inulinase. Appl. Biochem. Microbiol. 2010, 46, 385–389. [Google Scholar] [CrossRef]
- Pankova, S.M.; Sakibaev, F.A.; Holyavka, M.G.; Vyshkvorkina, Y.M.; Lukin, A.N.; Artyukhov, V.G. Studies of the Processes of the Trypsin Interactions with Ion Exchange Fibers and Chitosan. Russ. J. Bioorganic Chem. 2021, 47, 765–776. [Google Scholar] [CrossRef]
- Sabirova, A.R.; Rudakova, N.L.; Balaban, N.P.; Ilyinskaya, O.N.; Demidyuk, I.V.; Kostrov, S.V.; Rudenskaya, G.N.; Sharipova, M.R. A novel secreted metzincin metalloproteinase from Bacillus intermedius. FEBS Lett. 2010, 584, 4419–4425. [Google Scholar] [CrossRef] [PubMed]
- Holyavka, M.; Faizullin, D.; Koroleva, V.; Olshannikova, S.; Zakhartchenko, N.; Zuev, Y.; Kondtatyev, M.; Zakharova, E.; Artyukhov, V. Novel biotechnological formulations of cysteine proteases, immobilized on chitosan. Structure, stability and activity. Int. J. Biol. Macromol. 2021, 180, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Holyavka, M.G.; Kondratyev, M.S.; Samchenko, A.A.; Kabanov, A.V.; Komarov, V.M.; Artyukhov, V.G. In silico design of high-affinity ligands for the immobilization of inulinase. Comput. Biol. Med. 2016, 71, 198–204. [Google Scholar] [CrossRef]
- Byler, D.M.; Susi, H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers 1986, 25, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Kayumov, A.R.; Khakimullina, E.N.; Sharafutdinov, I.S.; Trizna, E.Y.; Latypova, L.Z.; Lien, H.T.; Margulis, A.B.; Bogachev, M.I.; Kurbangalieva, A.R. Inhibition of biofilm formation in Bacillus subtilis by new halogenated furanones. J. Antibiot. 2015, 68, 297–301. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A.; Kolter, R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: A genetic analysis. Mol. Microbiol. 1998, 28, 449–461. [Google Scholar] [CrossRef]
- Cui, J.; Ren, S.; Lin, T.; Feng, Y.; Jia, S. Shielding effects of Fe3+-tannic acid nanocoatings for immobilized enzyme on magnetic 611 Fe3O4@silica core shell nanosphere. Chem. Eng. J. 2018, 343, 629–637. [Google Scholar] [CrossRef]
- Ramezani, R.; Aminlari, M.; Fallahi, H. Effect of chemically modified soy proteins and ficin-tenderized meat on the quality attributes of sausage. J. Food Sci. 2006, 68, 85–88. [Google Scholar] [CrossRef]
- Marques, A.C.; Marostica, M.R.; Pastore, G.M. Some nutritional, technological and environmental advances in the use of enzymes in meat products. Enzym. Res. 2010, 2010, 480923. [Google Scholar] [CrossRef] [PubMed]
- Farhadihosseinabadi, B.; Zarebkohan, A.; Eftekhary, M.; Heiat, M.; Moghaddam, M.M.; Gholipourmalekabadi, M. Crosstalk between chitosan and cell signaling pathways. Cell. Mol. Life Sci. 2019, 76, 2697–2718. [Google Scholar] [CrossRef] [PubMed]
- Nunes, Y.L.; de Menezes, F.L.; de Sousa, I.G.; Cavalcante, A.L.G.; Cavalcante, F.T.T.; da Silva Moreira, K.; de Oliveira, A.L.B.; Mota, G.F.; da Silva Souza, J.E.; de Aguiar Falcão, I.R.; et al. Chemical and physical Chitosan modification for designing enzymatic industrial biocatalysts: How to choose the best strategy? Int. J. Biol. Macromol. 2021, 181, 1124–1170. [Google Scholar] [CrossRef] [PubMed]
- Tavano, O.L.; Berenguer-Murcia, A.; Secundo, F.; Fernandez-Lafuente, R. Biotechnological applications of proteases in food technology. Compr. Rev. Food Sci. Food Saf. 2018, 17, 412–436. [Google Scholar] [CrossRef] [PubMed]
- Hoarau, M.; Badieyan, S.; Marsh, E.N.G. Immobilized enzymes: Understanding enzyme–surface interactions at the molecular level. Org. Biomol. Chem. 2017, 15, 9539–9551. [Google Scholar] [CrossRef]
- Drenth, J.; Jansonius, J.N.; Koekoek, R.; Wolthers, B.G. The structure of papain. Adv. Protein Chem. 1971, 25, 79–115. [Google Scholar] [CrossRef]
- Kamphuis, I.G.; Kalk, K.H.; Swarte, M.B.A.; Drenth, J. Structure of papain refined at 1.65 Å resolution. J. Mol. Biol. 1984, 179, 233–256. [Google Scholar] [CrossRef]
- Novinec, M.; Lenarcic, B. Papain-like peptidases: Structure, function, and evolution. Biomol. Concepts 2013, 4, 287–308. [Google Scholar] [CrossRef]
- Holyavka, M.; Pankova, S.; Koroleva, V.; Vyshkvorkina, Y.; Lukin, A.; Kondratyev, M.; Artyukhov, V. Influence of UV radiation on molecular structure and catalytic activity of free and immobilized bromelain, ficin and papain. J. Photochem. Photobiol. B Biol. 2019, 201, 111681. [Google Scholar] [CrossRef]
- Makshakova, O.N.; Faizullin, D.A.; Zuev, Y.F. Interplay between secondary structure and ion binding upon thermoreversible gelation of κ-carrageenan. Carbohydr. Polym. 2020, 227, 115342. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Olshannikova, S.S.; Lavlinskaya, M.S.; Holyavka, M.G.; Faizullin, D.A.; Zuev, Y.F.; Artukhov, V.G. Chitosan Graft Copolymers with N-Vinylimidazole as Promising Matrices for Immobilization of Bromelain, Ficin, and Papain. Polymers 2022, 14, 2279. [Google Scholar] [CrossRef] [PubMed]
- Nahar, S.; Mizan, M.F.R.; Ha, A.J.W.; Ha, S.D. Advances and Future Prospects of Enzyme-Based Biofilm Prevention Approaches in the Food Industry. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1484–1502. [Google Scholar] [CrossRef] [PubMed]
- Thallinger, B.; Prasetyo, E.N.; Nyanhongo, G.S.; Guebitz, G.M. Antimicrobial enzymes: An emerging strategy to fight microbes and microbial biofilms. Biotechnol. J. 2013, 8, 97. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Mohamed, M.S.; Khalil, M.S.; Mohamed, W.S.; Mabrouk, M.I. Antibiofilm activity of papain enzyme against pathogenic Klebsiella pneumoniae. J. Appl. Pharm. Sci. 2018, 6, 163–168. [Google Scholar] [CrossRef]
- Atacan, K.; Ozacar, M. Investigation of antibacterial properties of novel papain immobilized on tannic acid modified Ag/CuFe2O4 magnetic nanoparticles. Int. J. Biol. Macromol. 2018, 109, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Trizna, E.; Baydamshina, D.; Kholyavka, M.; Sharafutdinov, I.; Hairutdinova, A.; Khafizova, F.; Zakirova, E.; Hafizov, R.; Kayumov, A. Soluble and immobilized papain and trypsin as destroyers of bacterial biofilms. Genes Cells 2016, 10, 106–112. [Google Scholar]
- Baidamshina, D.R.; Koroleva, V.A.; Trizna, E.Y.; Pankova, S.M.; Agafonova, M.N.; Chirkova, M.N.; Vasileva, O.S.; Akhmetov, N.; Shubina, V.V.; Porfiryev, A.G.; et al. Anti-biofilm and wound-healing activity of chitosan-immobilized Ficin. Int. J. Biol. Macromol. 2020, 164, 4205–4217. [Google Scholar] [CrossRef]
- Baidamshina, D.R.; Koroleva, V.A.; Olshannikova, S.S.; Trizna, E.Y.; Bogachev, M.I.; Artyukhov, V.G.; Holyavka, M.G.; Kayumov, A.R. Biochemical Properties and Anti-Biofilm Activity of Chitosan-Immobilized Papain. Mar. Drugs 2021, 19, 197. [Google Scholar] [CrossRef]
- Baidamshina, D.R.; Trizna, E.Y.; Holyavka, M.G.; Bogachev, M.I.; Artyukhov, V.G.; Akhatova, F.S.; Rozhina, E.V.; Fakhrullin, R.F.; Kayumov, A.R. Targeting microbial biofilms using Ficin, a nonspecific plant protease. Sci. Rep. 2017, 7, srep46068. [Google Scholar] [CrossRef]




| Affinity, kcal/mol | Amino Acids Forming | |
|---|---|---|
| Hydrogen Bonds and Their Lengths, Å | Any Other Interactions | |
| Amino acids of bromelain that form bonds and interactions with ChMC | ||
| −7.8 | Asn19, 3.29 Å; Gln20, 2.92 and 2.99 Å; Asn21, 2.70 Å; Cys23, 3.00 and 3.09 Å; Glu36 (αLI), 2.88 Å; Glu51 (αLII), 2.87 Å; Lys179, 2.90 Å; Trp180, 3.23 Å | Thr15, Ser16, Val17, Lys18, Pro22, Gly24, Phe29 (αLI), Ala33 (αLI), Ala136, Phe140, His158 (βR), Ala159 (βR), Val160 (βR), Thr161 (βR), Ile163 (βR), Ala178, Gly184, Tyr185 |
| Amino acids of bromelain that form bonds and interactions with HTCCh | ||
| −8 | Lys18, 2.86 Å; Asn19, 2.91 Å; Phe29 (αLI), 3.10 Å; Glu51 (αLII), 3.14 Å; His158 (βR), 2.86 Å; Lys179, 2.96 Å; Trp180, 3.11 Å | Val14, Val17, Ala33 (αLI), Gln141, Tyr142, Lys144, Val160 (βR), Thr161 (βR), Ile163 (βR), Lys174, Ala178, Gly184, Tyr185, Ile186 |
| Amino acids of bromelain that form bonds and interactions with ChS | ||
| −9.0 | Lys18, 3.21 Å; Asn19, 2.90 Å; Gln20, 3.15 Å; Phe29 (αLI), 3.04 Å; Tyr143, 2.87 Å; His158 (βR), 2.86 Å; Trp161 (βR), 2.80 Å; Lys179, 2.94 Å; Trp180, 3.14 Å; Gly181, 3.23 Å; Gly184, 2.95 and 3.19 Å; Ile186, 3.31 Å | Ser16, Val17, Ala30 (αLI), Ala33 (αLI), Glu51 (αLII), Ala136, Phe140, Lys144, Val160 (βR), Ile163 (βR), Ala178, Tyr185 |
| Amino acids of bromelain that form bonds and interactions with ChA | ||
| −9.4 | Lys18, 3.16 Å; Phe29 (αLI), 2.86 Å; Glu51 (αLII), 2.81 Å; Phe140, 3.26 Å; Gln141, 3.19 Å; His158 (βR), 3.09 Å; Thr161(βR), 2.62 and 3.30 Å | Val14, Val17, Asn19, Gln20, Ile32 (αLI), Ala33 (αLI), Ala136, Tyr142, Lys144, Val160 (βR), Ile163 (βR), Lys174, Lys179, Trp180, Gly184, Tyr185 |
| Structure | Sample | |||
|---|---|---|---|---|
| Bromelain in Solution | Bromelain with ChMC | Bromelain with ChS | Bromelain with ChA | |
| α-helix | 15 | 13 | 13 | 19 |
| β-sheet | 29 | 43 | 52 | 40 |
| other | 56 | 44 | 35 | 41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holyavka, M.G.; Goncharova, S.S.; Sorokin, A.V.; Lavlinskaya, M.S.; Redko, Y.A.; Faizullin, D.A.; Baidamshina, D.R.; Zuev, Y.F.; Kondratyev, M.S.; Kayumov, A.R.; et al. Novel Biocatalysts Based on Bromelain Immobilized on Functionalized Chitosans and Research on Their Structural Features. Polymers 2022, 14, 5110. https://doi.org/10.3390/polym14235110
Holyavka MG, Goncharova SS, Sorokin AV, Lavlinskaya MS, Redko YA, Faizullin DA, Baidamshina DR, Zuev YF, Kondratyev MS, Kayumov AR, et al. Novel Biocatalysts Based on Bromelain Immobilized on Functionalized Chitosans and Research on Their Structural Features. Polymers. 2022; 14(23):5110. https://doi.org/10.3390/polym14235110
Chicago/Turabian StyleHolyavka, Marina G., Svetlana S. Goncharova, Andrey V. Sorokin, Maria S. Lavlinskaya, Yulia A. Redko, Dzhigangir A. Faizullin, Diana R. Baidamshina, Yuriy F. Zuev, Maxim S. Kondratyev, Airat R. Kayumov, and et al. 2022. "Novel Biocatalysts Based on Bromelain Immobilized on Functionalized Chitosans and Research on Their Structural Features" Polymers 14, no. 23: 5110. https://doi.org/10.3390/polym14235110
APA StyleHolyavka, M. G., Goncharova, S. S., Sorokin, A. V., Lavlinskaya, M. S., Redko, Y. A., Faizullin, D. A., Baidamshina, D. R., Zuev, Y. F., Kondratyev, M. S., Kayumov, A. R., & Artyukhov, V. G. (2022). Novel Biocatalysts Based on Bromelain Immobilized on Functionalized Chitosans and Research on Their Structural Features. Polymers, 14(23), 5110. https://doi.org/10.3390/polym14235110