Chemical and Mechanical Properties of Metakaolin-Based Geopolymers with Waste Corundum Powder Resulting from Erosion Testing
Abstract
1. Introduction
- Fe2O3, hematite (recommended to polish soft ores, glass, lapidary, and soft metals, MH = 5.5–6.5);
- Cr2O3, chromite (recommended for samples that cannot be exposed to aluminium oxide contamination, MH = 5.5);
- MgO, periclase, (recommended when fine polishing wrought aluminium alloys and magnesium-based metals and alloys, MH = 5.8);
- SiO2, natural pumice (ideal for processing glass, stone, MH = 6);
- SiO2, quartz sand (multipurpose abrasive, mainly used in sandblasting, MH = 7);
- Al2O3, natural corundum (ideal for all types of materials and for rough as well as fine grinding, MH = 9);
- SiC, synthetic silicon carbide (ideal for all types of materials for rough and fine grinding and polishing, MH = 9–10);
- C, diamond (multipurpose abrasive, MH = 10).
2. Materials and Methods
2.1. Materials
2.2. Preparation of Geopolymer Specimens
2.3. Geopolymers Characterisation
2.3.1. Reactivity Test in NaOH Solution
2.3.2. Microstructural Characterisation
2.3.3. Ionic Conductivity of the Geopolymer Leachate
2.3.4. Mineralogical Composition
2.3.5. Microstructural SEM Observation
2.3.6. Physical and Mechanical Properties
3. Results
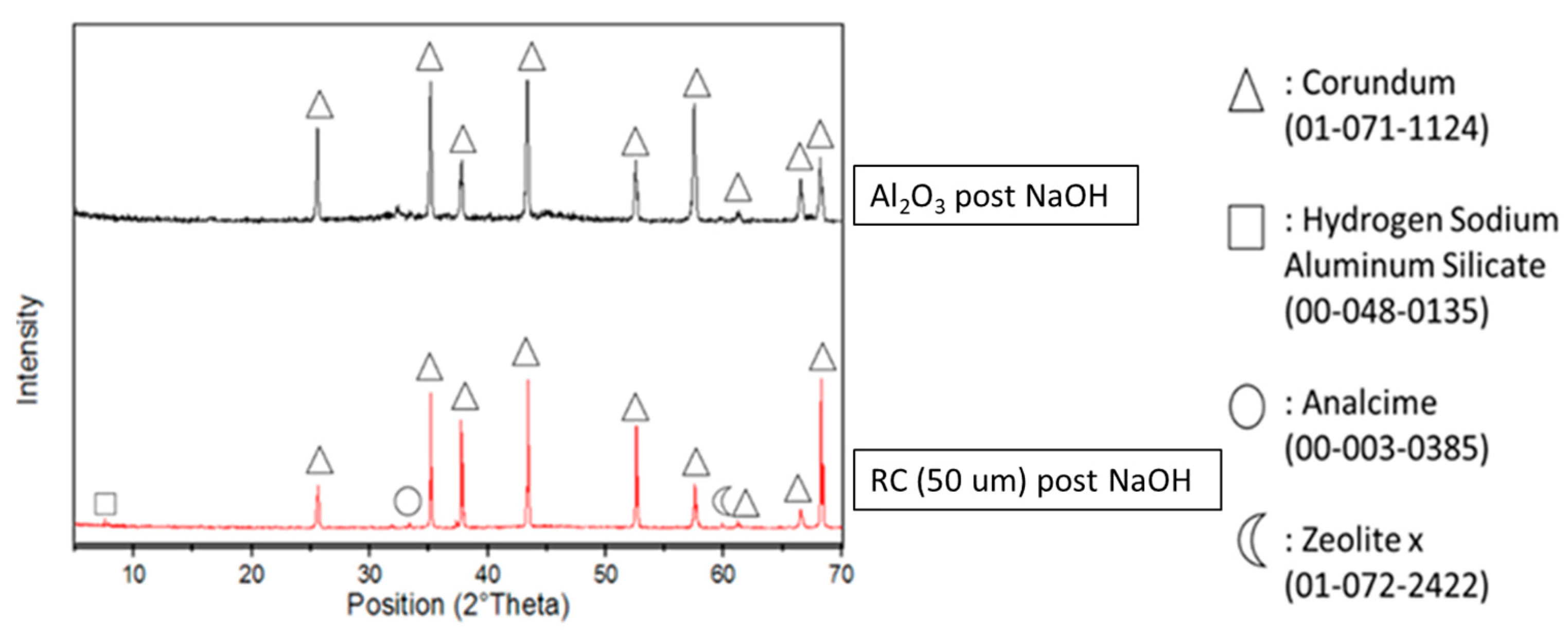
3.1. Reactivity of Waste Corundum Powders in NaOH
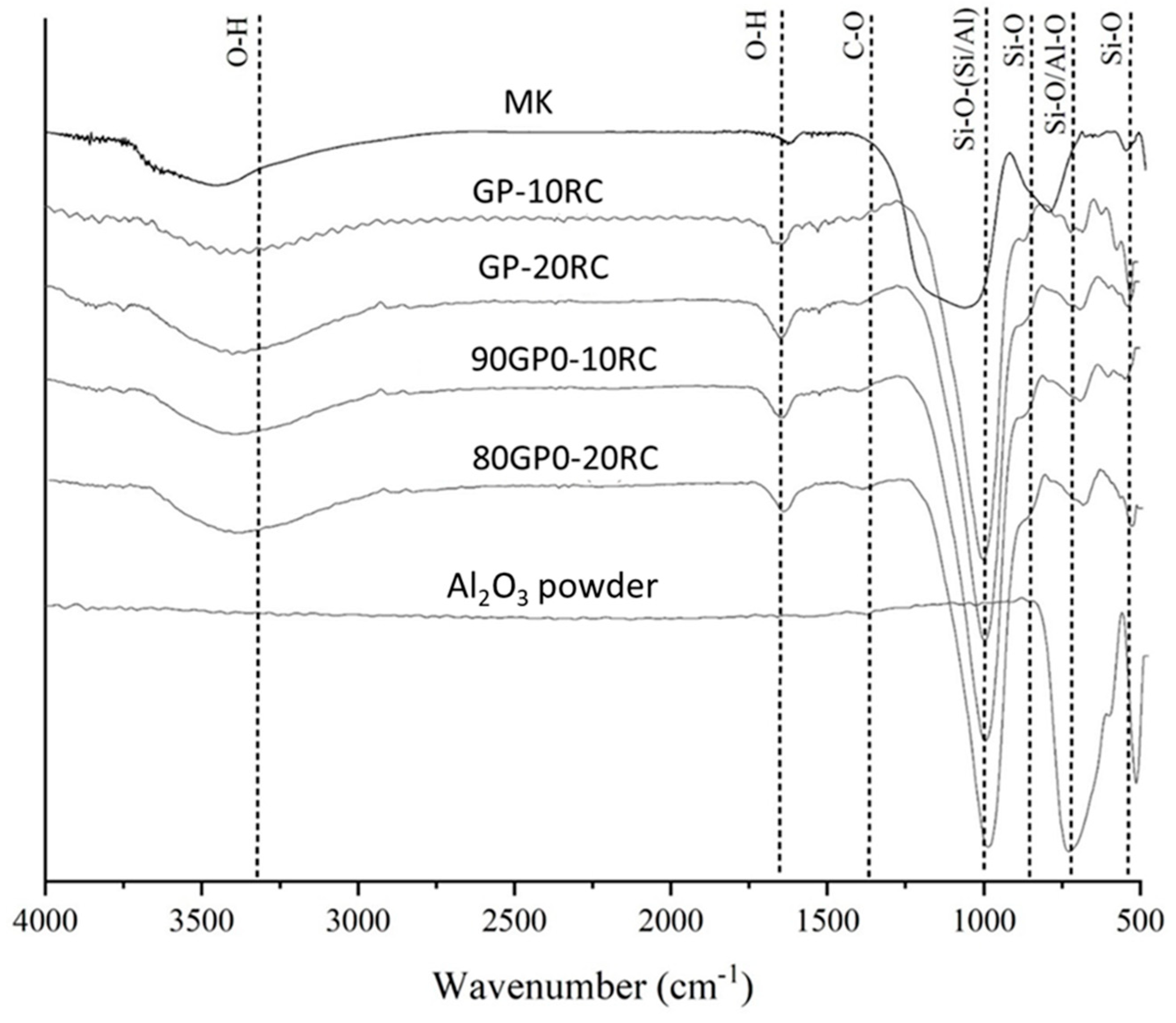
3.2. Microstructural Characterisation
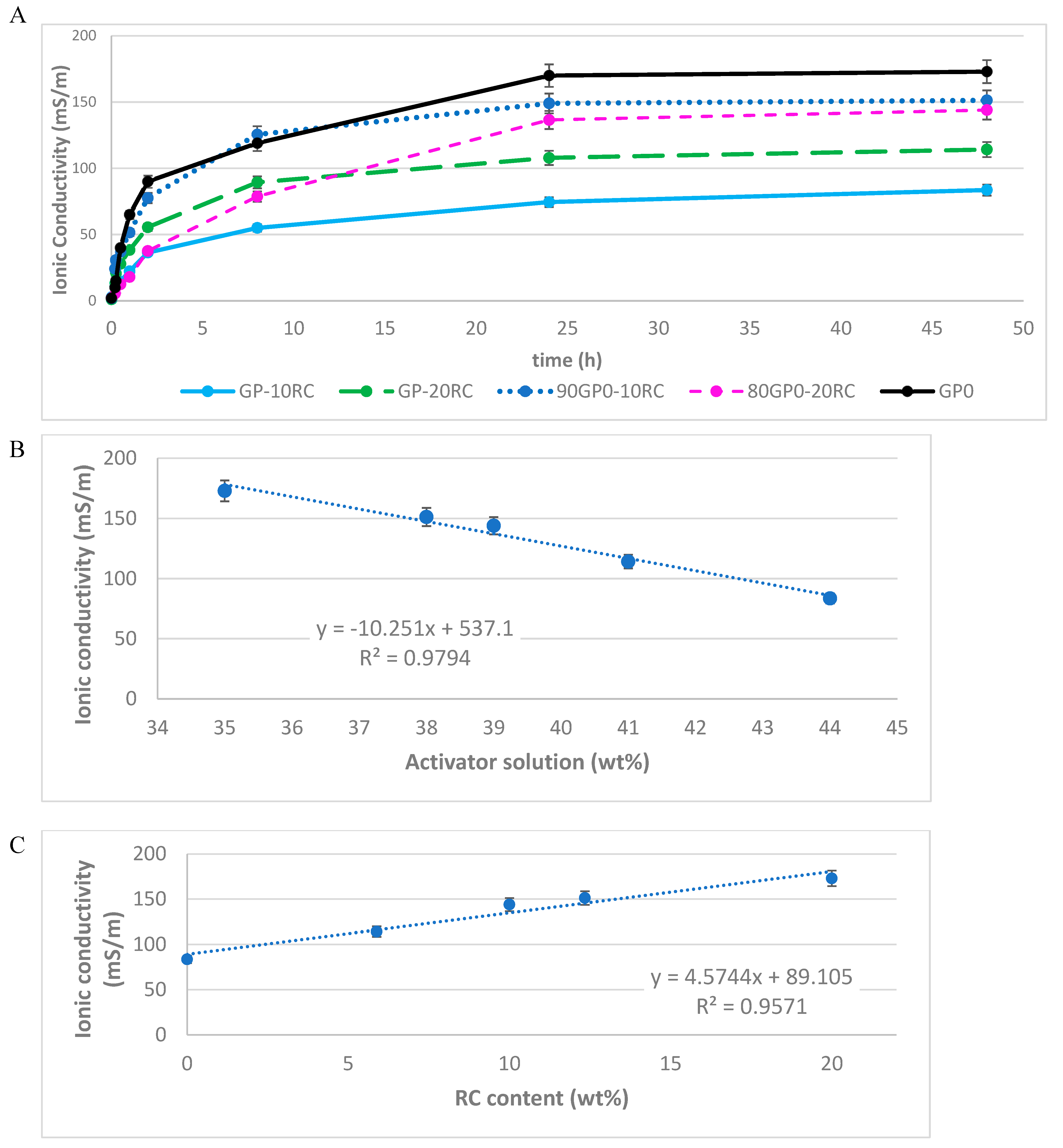
3.3. Ionic Conductivity of Leachate Solution
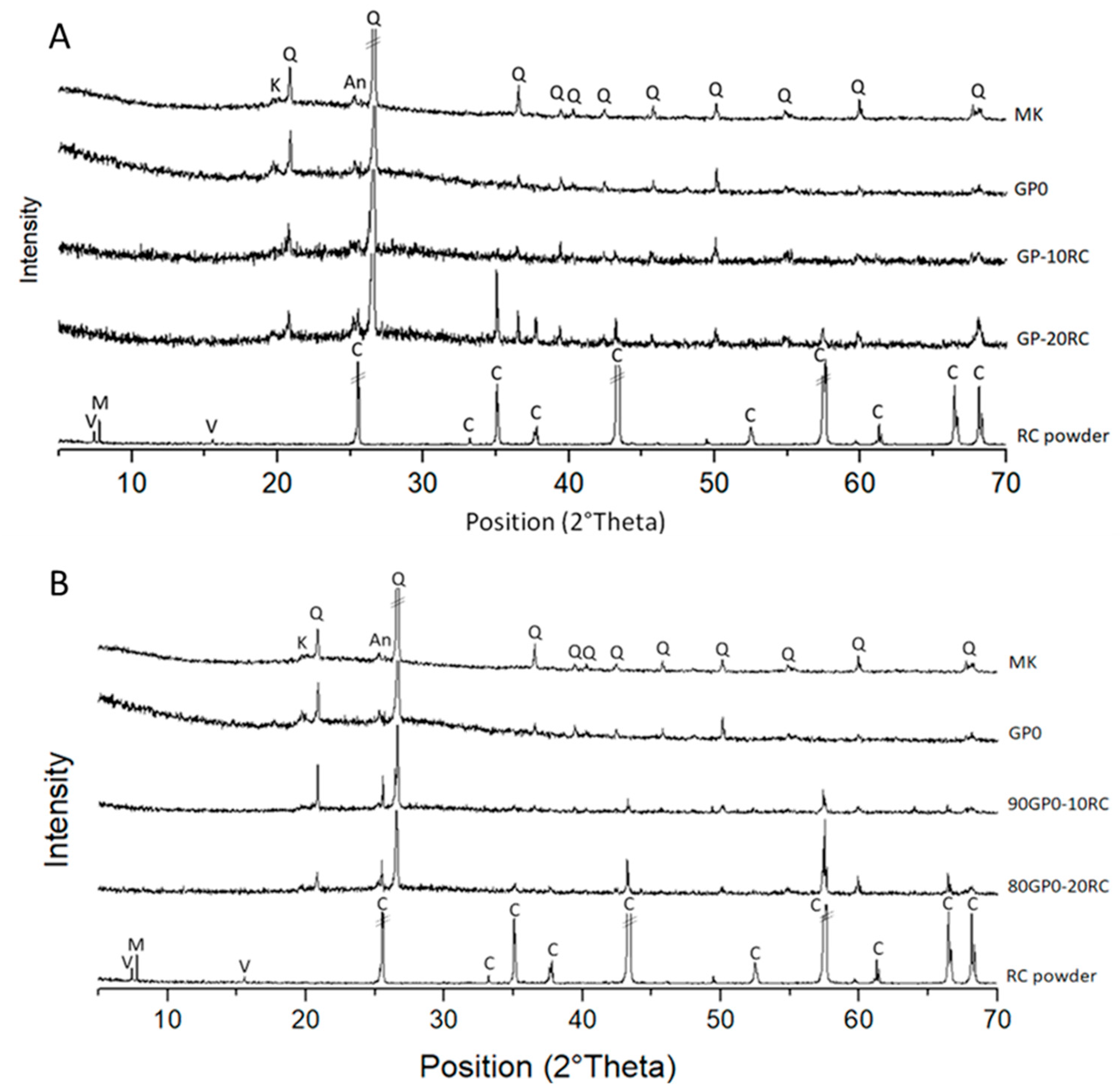
3.4. Mineralogical Composition
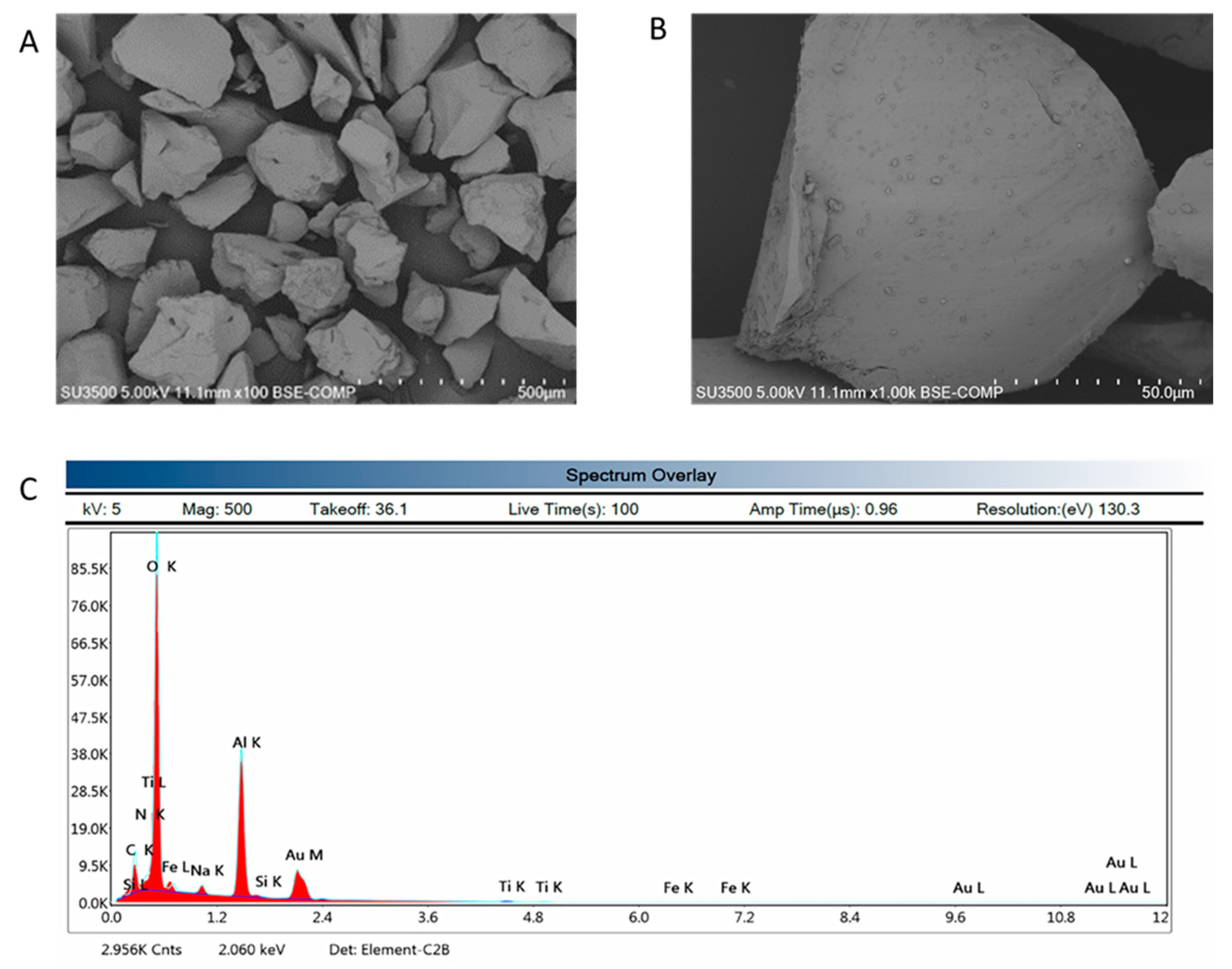
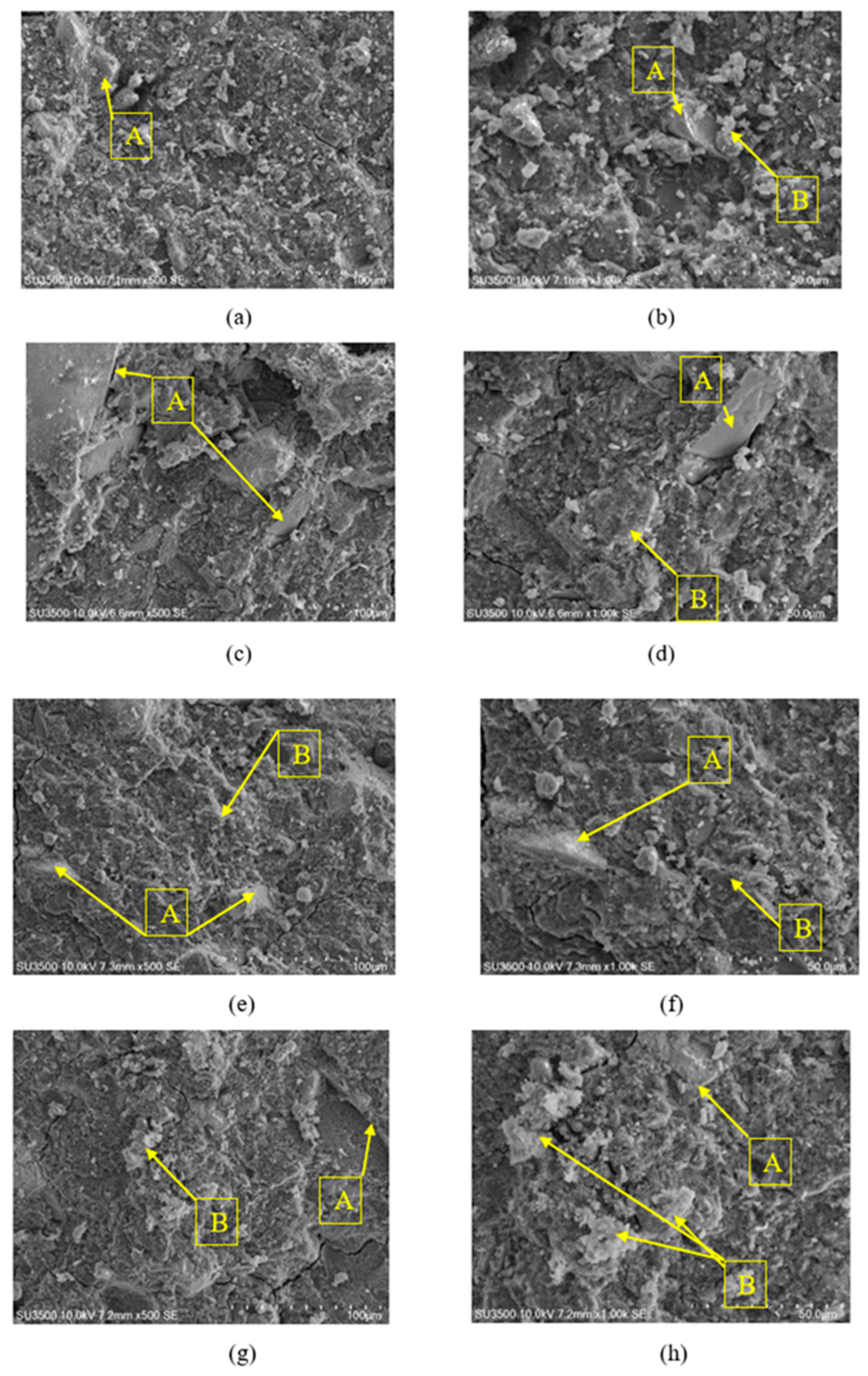
3.5. Microstructural SEM Observation
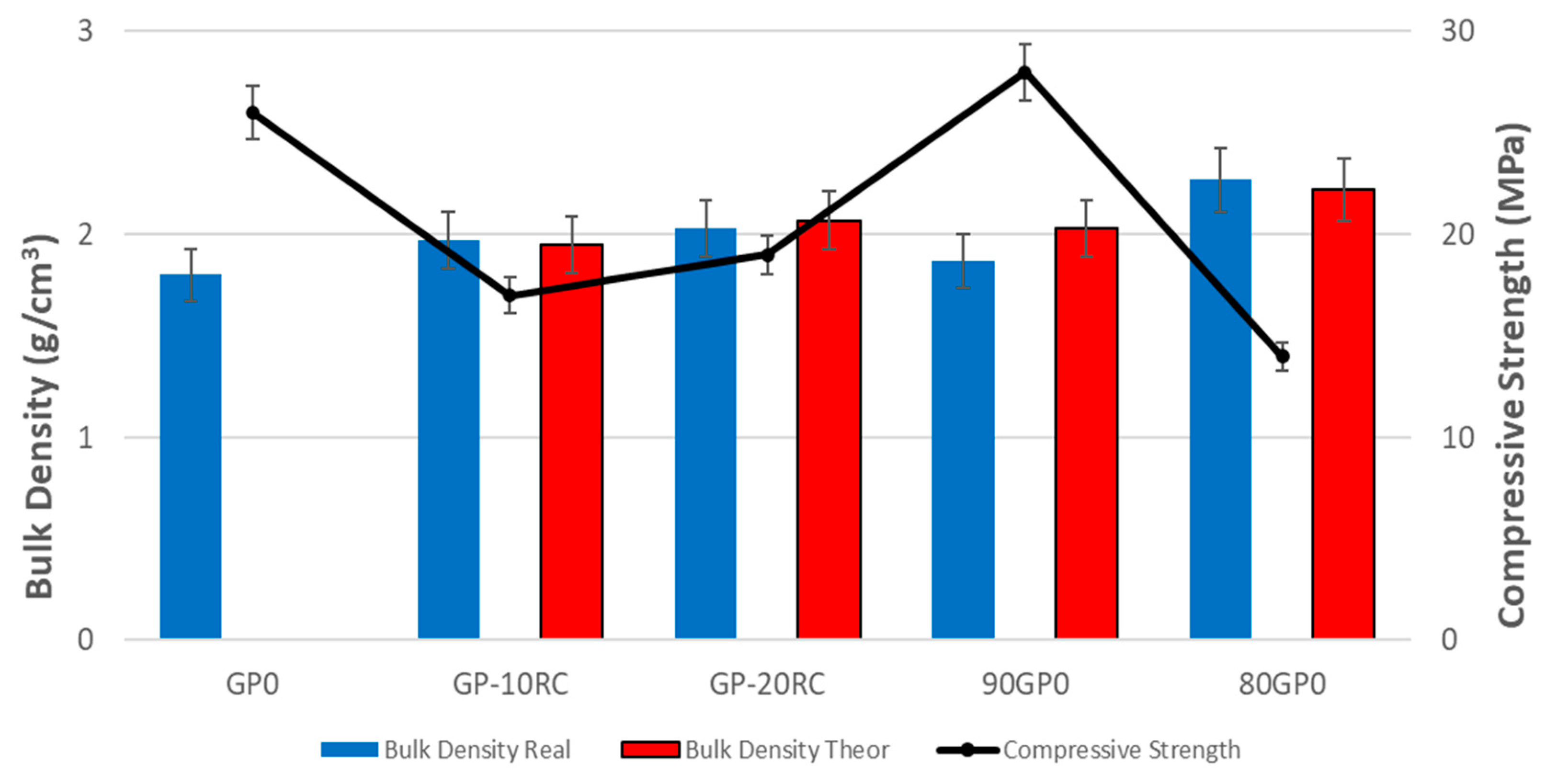
3.6. Physical and Mechanical Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davidovits, J. Geopolymers-inorganic polymeric new materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Torgal, F.; Labrincha, J.A.; Leonelli, C.; Palomo, A.; Chindaprasirt, P. Handbook of Alkali-Activated Cements, Mortars and Concretes, 1st ed.; Woodhead Publishing Series in Civil and Structural Engineering; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Burciaga-Díaz, O.; Magallanes-Rivera, R.; Escalante-García, J. Alkali-activated slag-metakaolin pastes: Strength, structural, and microstructural characterization. J. Sustain. Cem. Mater. 2013, 2, 111–127. [Google Scholar] [CrossRef]
- Aboshia, A.M.A.; Rahmat, R.A.; Zain, M.F.M.; Ismail, A. Early age shrinkage cracking of restrained metakaolin-slag-palm oil fuel ash binder geopolymer mortars. J. Sustain. Cem. Mater. 2018, 7, 271–295. [Google Scholar] [CrossRef]
- Wongkeo, W.; Torkittikul, P.; Nochaiya, T.; Pakawanit, P. 3D pore structure, thermal and physical properties of metakaolin-black rice husk ash-based alkali-activated cement. J. Sustain. Cem. Mater. 2021, 11, 223–238. [Google Scholar] [CrossRef]
- Brabrand, D.J.; Loehr, R.C. Solidification/stabilization of spent abrasives and use as nonstructural concrete. Waste Manag. 1993, 13, 333–339. [Google Scholar] [CrossRef]
- Mathews, S.; Wilson, K. Reuse of waste cutting sand at lawrence livermore national laboratory. In Proceedings of the Air and Waste Mangement Association Annual Meetings and Exposition, San Diego, CA, USA, 14–18 June 1998. [Google Scholar]
- US Department of Labor. Abrasive Blasting Hazards in Shipyard Employment. Occupational Safety and Health Administration (OSHA) Guidance Document, December 2006. Available online: https://www.osha.gov/maritime/guidance/shipyard-guidance (accessed on 7 September 2022).
- Madany, I.M.; Al-Sayed, M.H.; Raveendran, E. Utilization of copper blasting grit waste as a construction material. Waste Manag. 1991, 11, 35–40. [Google Scholar] [CrossRef]
- Zain, M.; Islam, M.; Radin, S.; Yap, S. Cement-based solidification for the safe disposal of blasted copper slag. Cem. Concr. Compos. 2004, 26, 845–851. [Google Scholar] [CrossRef]
- dos Anjos, M.; Sales, A.; Andrade, N. Blasted copper slag as fine aggregate in Portland cement concrete. J. Environ. Manag. 2017, 196, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Borucka-Lipska, J.; Techman, M.; Skibicki, S. Use of Contaminated Sand Blasting Grit for Production of Cement Mortars. IOP Conf. Ser. Mater. Sci. Eng. 2019, 471, 032055. [Google Scholar] [CrossRef]
- Sua-Iam, G.; Makul, N. Use of recycled alumina as fine aggregate replacement in self-compacting concrete. Constr. Build. Mater. 2013, 47, 701–710. [Google Scholar] [CrossRef]
- Muttashar, H.L.; Ariffin, M.A.M.; Hussin, M.W.; Bin Ishaq, S. Realisation of enhanced self-compacting geopolymer concrete using spent garnet as sand replacement. Mag. Concr. Res. 2018, 70, 558–569. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Budiea, A.; Mirza, J. Utilizing spend garnets as sand replacement in alkali-activated mortars containing fly ash and GBFS. Constr. Build. Mater. 2019, 225, 132–145. [Google Scholar] [CrossRef]
- Dal Poggetto, G.; D’Angelo, A.; Catauro, M.; Barbieri, L.; Leonelli, C. Recycling of Waste Corundum Abrasive Powder in Mk-Based Geopolymers. Polymers 2022, 14, 2173. [Google Scholar] [CrossRef] [PubMed]
- Coppola, B.; Tardivat, C.; Richaud, S.; Tulliani, J.-M.; Montanaro, L.; Palmero, P. 3D printing of dense and porous alkali-activated refractory wastes via Direct Ink Writing (DIW). J. Eur. Ceram. Soc. 2021, 41, 3798–3808. [Google Scholar] [CrossRef]
- Tiffo, E.; Belibi, P.D.B.; Mbah, J.B.B.; Thamer, A.; Pougnong, T.E.; Baenla, J.; Elimbi, A. Effect of various amounts of aluminium oxy-hydroxide coupled with thermal treatment on the performance of alkali-activated metakaolin and volcanic scoria. Sci. Afr. 2021, 14, e01015. [Google Scholar] [CrossRef]
- Kumar, R.; Alex, T.C. Elucidation of the Nature of Structural Heterogeneity During Alkali Leaching of Non-activated and Mechanically Activated Boehmite (γ-AlOOH). Metall. Mater. Trans. B 2015, 46, 1684–1701. [Google Scholar] [CrossRef]
- Qi, C.; Weinell, C.E.; Dam-Johansen, K.; Wu, H. A review of blasting waste generation and management in the ship repair industry. J. Environ. Manag. 2021, 300, 113714. [Google Scholar] [CrossRef]
- Commission Notice on Technical Guidance on the Classification of Waste, Report ID: C/2018/1447. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.C_.2018.124.01.0001.01.ENG&toc=OJ:C:2018:124:TOC (accessed on 7 September 2022).
- Carroll-Webb, S.A.; Walther, J.V. A surface complex reaction model for the pH-dependence of corundum and kaolinite dissolution rate. Geochim. Cosmochim. Acta 1988, 52, 2609–2623. [Google Scholar] [CrossRef]
- Wu, Y.S.; Li, H.L.; Shi, F.L.; Su, G.Q.; Qu, Y.P. Corundum dissolution in concentrated sodium hydroxide solution. China Foundry Res. Dev. 2016, 13, 422–426. [Google Scholar] [CrossRef]
- Sato, T.; Sato, S.; Okuwaki, A.; Tanaka, S.-I. Corrosion Behavior of Alumina Ceramics in Caustic Alkaline Solutions at High Temperatures. J. Am. Ceram. Soc. 1991, 74, 3081–3084. [Google Scholar] [CrossRef]
- Pantongsuk, T.; Kittisayarm, P.; Muenglue, N.; Benjawan, S.; Thavorniti, P.; Tippayasam, C.; Nilpairach, S.; Heness, G.; Chaysuwan, D. Effect of hydrogen peroxide and bagasse ash additions on thermal conductivity and thermal resistance of geopolymer foams. Mater. Today Commun. 2021, 26, 102149. [Google Scholar] [CrossRef]
- Tippayasam, C.; Balyore, P.; Thavorniti, P.; Kamseu, E.; Leonelli, C.; Chindaprasirt, P.; Chaysuwan, D. Potassium alkali concentration and heat treatment affected metakaolin-based geopolymer. Constr. Build. Mater. 2016, 104, 293–297. [Google Scholar] [CrossRef]
- Reed, J.S. Principles of Ceramics Processing; Wiley & Sons Inc.: New York, NY, USA, 1995. [Google Scholar]
- Saleem, N.; Rashid, K.; Fatima, N.; Hanif, S.; Naeem, G.; Aslam, A.; Fatima, M.; Aslam, K. Appraisal of geopolymer lightweight aggregates sintered by microwave radiations. J. Asian Concr. Fed. 2020, 6, 37–49. [Google Scholar] [CrossRef]
- Mak, G.Y.; Lam, E.Y.; Choi, H.W. Liquid-immersion laser micromachining of GaN grown on sapphire. Appl. Phys. A 2010, 102, 441–447. [Google Scholar] [CrossRef]
- Dal Poggetto, G.; Catauro, M.; Crescente, G.; Leonelli, C. Efficient Addition of Waste Glass in MK-Based Geopolymers: Microstructure, Antibacterial and Cytotoxicity Investigation. Polymers 2021, 13, 1493. [Google Scholar] [CrossRef] [PubMed]
- Dal Poggetto, G.; D’Angelo, A.; Blanco, I.; Piccolella, S.; Leonelli, C.; Catauro, M. FT-IR Study, Thermal Analysis, and Evaluation of the Antibacterial Activity of a MK-Geopolymer Mortar Using Glass Waste as Fine Aggregate. Polymers 2021, 13, 2970. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Dal Poggetto, G.; Sgarlata, C.; Ciprioti, S.V.; Pacifico, S.; Leonelli, C. Thermal and microbiological performance of metakaolin-based geopolymers cement with waste glass. Appl. Clay Sci. 2020, 197, 105763. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, L.; Ramey, D.; Waterman, B.T.; Ormsby, S.T. Utilization of aluminum sludge (AS) to enhance mine tailings-based geopolymer. J. Mater. Sci. 2014, 50, 1370–1381. [Google Scholar] [CrossRef]
- Ghanbari, M.; Hadian, A.; Nourbakhsh, A.; MacKenzie, K. Modeling and optimization of compressive strength and bulk density of metakaolin-based geopolymer using central composite design: A numerical and experimental study. Ceram. Int. 2017, 43, 324–335. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; Van Deventer, J.S.J. Physical evolution of Na-geopolymer derived from metakaolin up to 1000 °C. J. Mater. Sci. 2007, 42, 3044–3054. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, S.; Zuo, Y.; Chen, W.; Ye, G. Chemical deformation of metakaolin based geopolymer. Cem. Concr. Res. 2019, 120, 108–118. [Google Scholar] [CrossRef]
- Xin, Y.L.; Yin, H.F.; Tang, Y.; Wan, Q.F.; Gao, K.; Yuan, H.D.; Wang, Z.W. Formation mechanism and characterization of gradient density in corundum–spinel refractory. Ceram. Int. 2018, 45, 8023–8026. [Google Scholar] [CrossRef]
- Yin, H.; Xin, Y.; Dang, J.; Gao, K.; Tang, Y.; Yuan, H. Preparation and properties of lightweight corundum-spinel refractory with density gradient. Ceram. Int. 2018, 44, 20478–20483. [Google Scholar] [CrossRef]
- Perec, A. Disintegration and recycling possibility of selected abrasives for water jet cutting. DYNA 2017, 84, 249–256. [Google Scholar] [CrossRef]
- Valasek, P.; Müller, M.; Hloch, S. Recycling of corundum particles—Two-body abrasive wear of polymeric composites based on waste. Teh. Vjesn.-Tech. Gaz. 2015, 22, 567–572. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, W.; Sorge, L.P.; Seo, D.-K. Exploratory Synthesis of Low-Silica Nanozeolites through Geopolymer Chemistry. Cryst. Growth Des. 2019, 19, 1167–1171. [Google Scholar] [CrossRef]
- Ruiz-Santaquiteria, C.; Fernández-Jiménez, A.; Palomo, A. Quantitative determination of reactive SiO2 and Al2O3 in aluminosilicate materials. In Proceedings of the XIII International Congress on the Chemistry of Cement, Madrid, Spain, 3–8 July 2011; Available online: https://materconstrucc.revistas.csic.es/index.php/materconstrucc/article/view/636 (accessed on 7 September 2022).
- Ribeiro, R.A.S.; Ribeiro, M.G.S.; Kutyla, G.P.; Kriven, W.M. Amazonian Metakaolin Reactivity for Geopolymer Synthesis. Adv. Mater. Sci. Eng. 2019, 2019, 8950764. [Google Scholar] [CrossRef]
- Bouchikhi, A.; Mamindy-Pajany, Y.; Maherzi, W.; Albert-Mercier, C.; El-Moueden, H.; Benzerzour, M.; Peys, A.; Abriak, N.-E. Use of residual waste glass in an alkali-activated binder–Structural characterization, environmental leaching behavior and comparison of reac-tivity. J. Build. Eng. 2021, 34, 101903. [Google Scholar] [CrossRef]
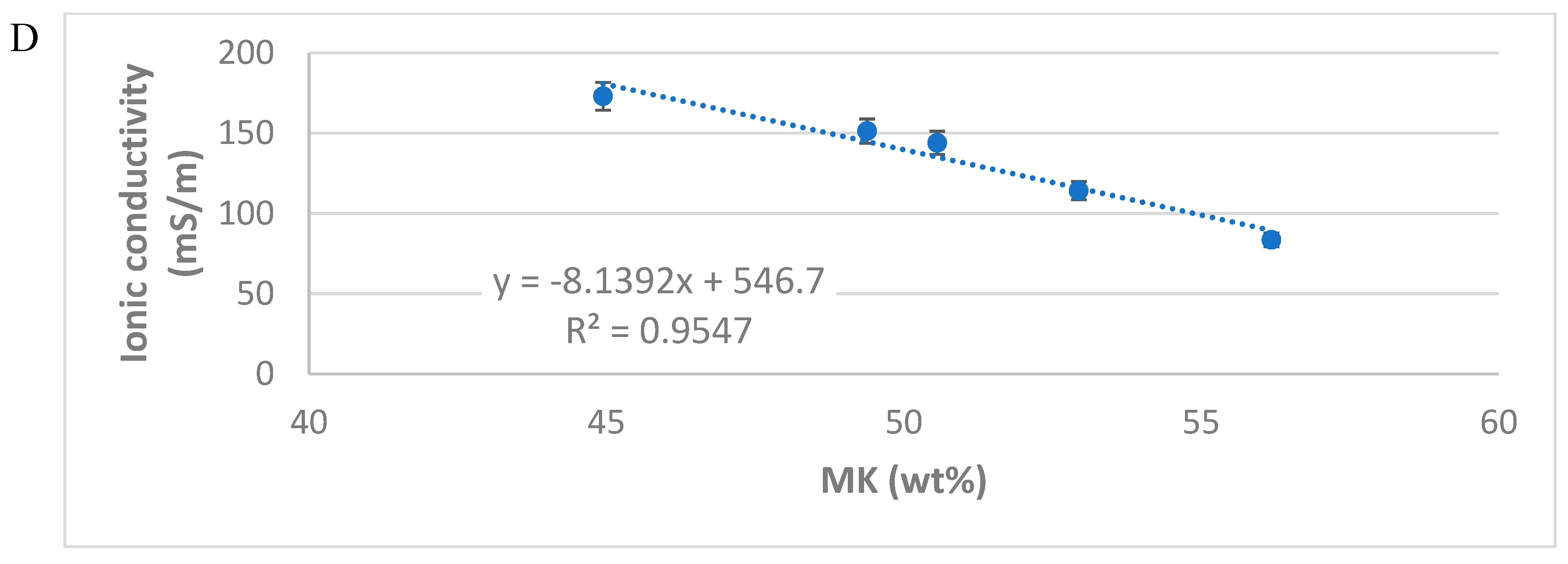
| Mix ID | MK (g) | RC Powder (wt%) | NaOH (g) | NaSilicate (g) | L/MK (wt/wt) | L/S (wt/wt) | SiO2 (wt%) | Na2O (wt%) | Al2O3 (wt%) | H2O (wt%) | Other Oxides (wt%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GP0 | 100 | 0 | 38 | 40 | 0.78 | 0.78 | 37 | 6 | 22 | 32 | 3 |
| GP-10RC | 90 | 5.88 | 34 | 36 | 0.778 | 0.7 | 35 | 6 | 27 | 30 | 2 |
| GP-20RC | 80 | 12.34 | 30 | 32 | 0.775 | 0.62 | 33 | 5 | 31 | 28 | 3 |
| 90GP0-10RC | 51 | 10 | 19 | 20 | 0.765 | 0.64 | 34 | 5 | 30 | 28 | 3 |
| 80GP0-20RC | 45 | 20 | 17 | 18 | 0.778 | 0.54 | 30 | 5 | 36 | 25 | 4 |
| Wavenumber (cm−1) | Interpretation | Reference |
|---|---|---|
| 3695−3660 cm−1 | Hydroxyl groups in the kaolinite | [25,30,31] |
| 3450 and 1650 cm−1 | Hydration water | [30,31] |
| 1454 cm−1 | Na2CO3 | [31] |
| 1080−1050 cm−1 | Asymmetrical Si−O−Si Stretching vibration | [25,30] |
| 1010−980 cm−1 | Si−O−Al stretching vibration | [16,30,31] |
| 912 cm−1 | Al (VI)−OH stretching | [30,31] |
| 800−500 cm−1 | Al−O−Si bending vibrations | [30,31] |
| 450−470 cm−1 | Si−O−Si bending vibration | [16,25,30,31] |
| 1089 cm−1 | Al−O vibration | [16,31] |
| 1089, 800 and 780 cm−1 | Disorders in Corundum network | [16] |
| 459 cm−1 | Al−O bending | [16,30,31] |
| 474 cm−1 | Al−O vibration Al2O3 | [16] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dal Poggetto, G.; Kittisayarm, P.; Pintasiri, S.; Chiyasak, P.; Leonelli, C.; Chaysuwan, D. Chemical and Mechanical Properties of Metakaolin-Based Geopolymers with Waste Corundum Powder Resulting from Erosion Testing. Polymers 2022, 14, 5091. https://doi.org/10.3390/polym14235091
Dal Poggetto G, Kittisayarm P, Pintasiri S, Chiyasak P, Leonelli C, Chaysuwan D. Chemical and Mechanical Properties of Metakaolin-Based Geopolymers with Waste Corundum Powder Resulting from Erosion Testing. Polymers. 2022; 14(23):5091. https://doi.org/10.3390/polym14235091
Chicago/Turabian StyleDal Poggetto, Giovanni, Pakamon Kittisayarm, Suphahud Pintasiri, Pongpak Chiyasak, Cristina Leonelli, and Duangrudee Chaysuwan. 2022. "Chemical and Mechanical Properties of Metakaolin-Based Geopolymers with Waste Corundum Powder Resulting from Erosion Testing" Polymers 14, no. 23: 5091. https://doi.org/10.3390/polym14235091
APA StyleDal Poggetto, G., Kittisayarm, P., Pintasiri, S., Chiyasak, P., Leonelli, C., & Chaysuwan, D. (2022). Chemical and Mechanical Properties of Metakaolin-Based Geopolymers with Waste Corundum Powder Resulting from Erosion Testing. Polymers, 14(23), 5091. https://doi.org/10.3390/polym14235091









