Self-Assembled Supramolecular Micelles Based on Multiple Hydrogen Bonding Motifs for the Encapsulation and Release of Fullerene
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Preparation of C60-Loaded Micelles
2.4. Determination of Fullerene Loading
2.5. Hydroxyl Radical (●OH) Scavenging Activity
3. Results and Discussion
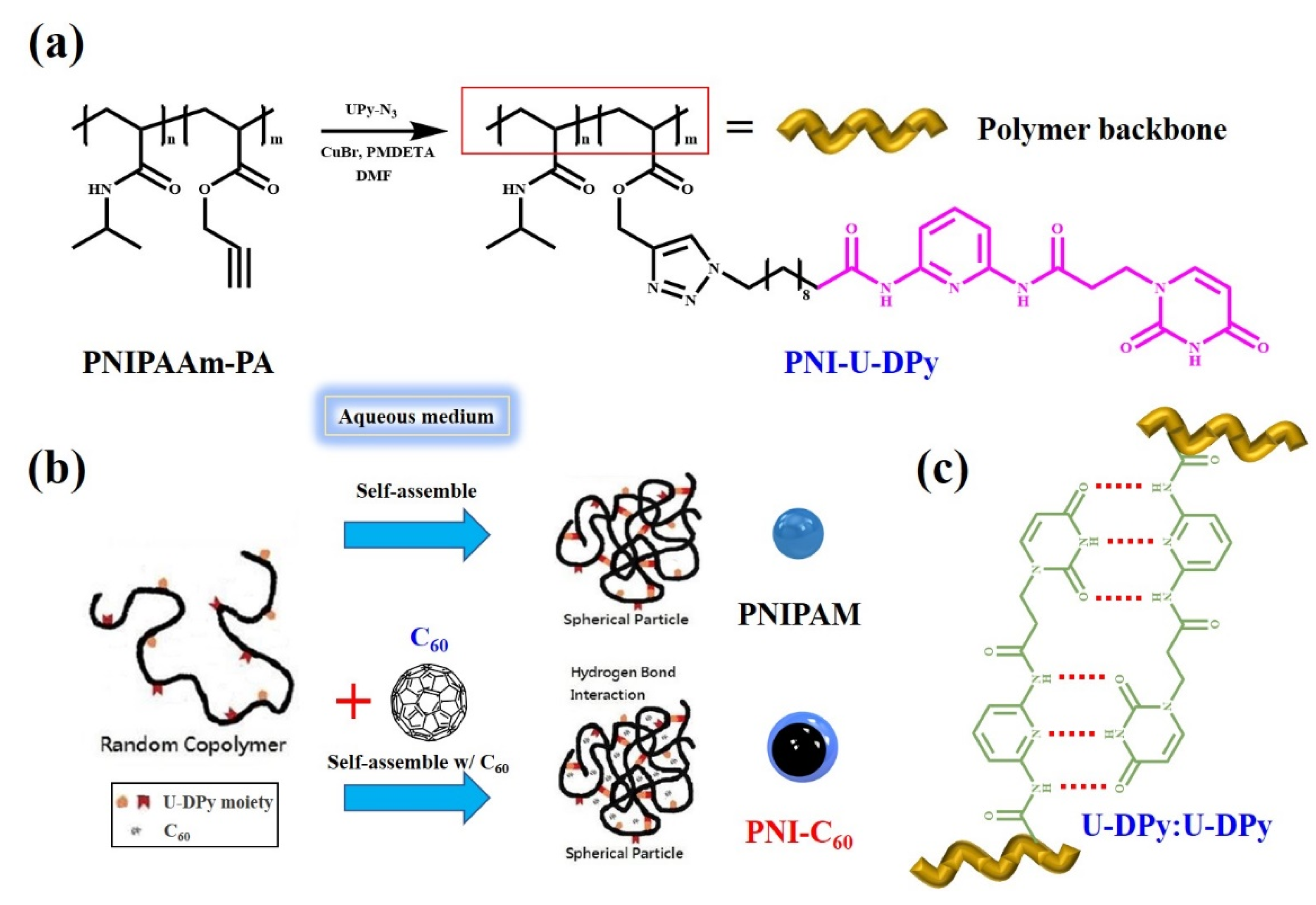
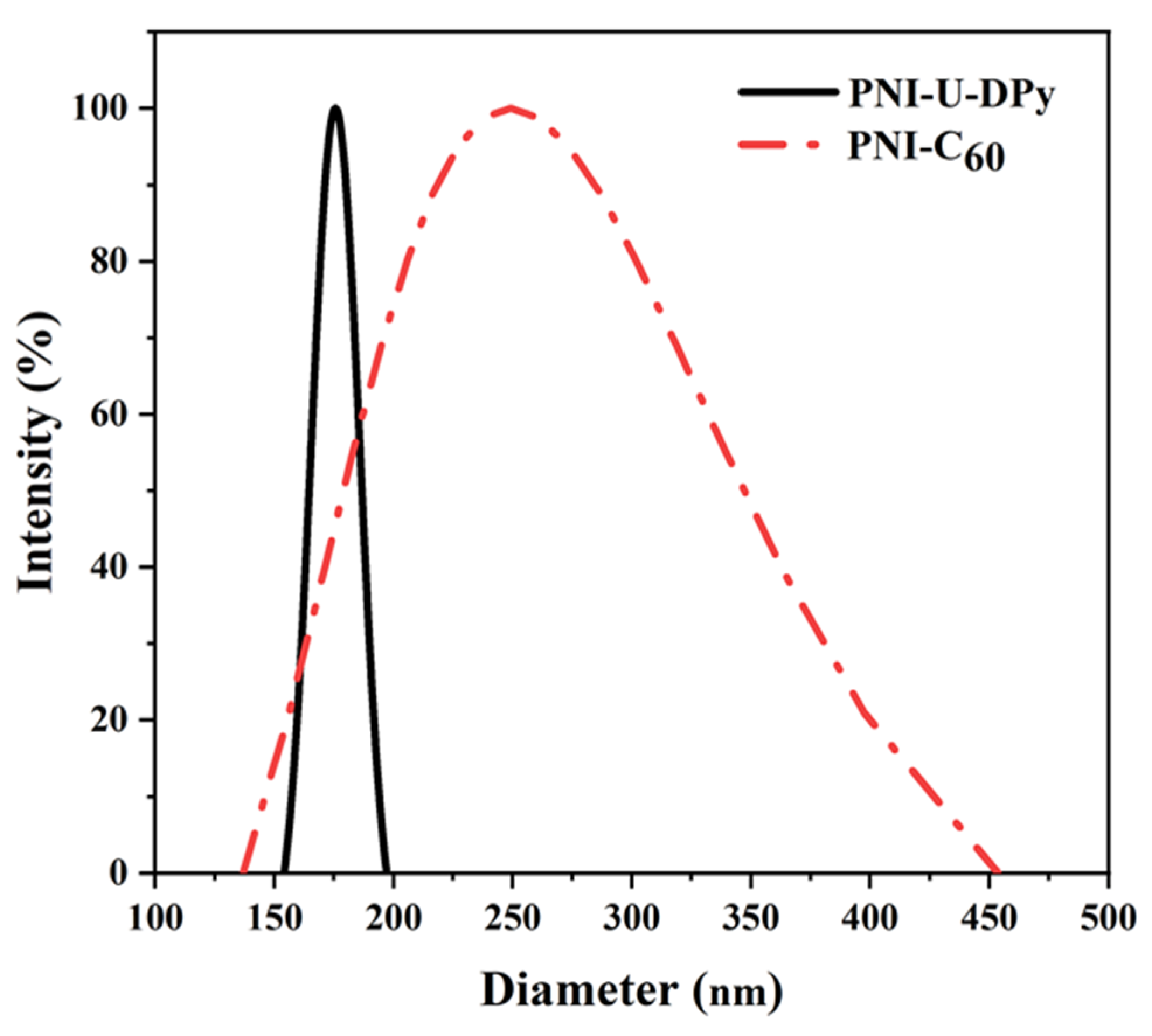
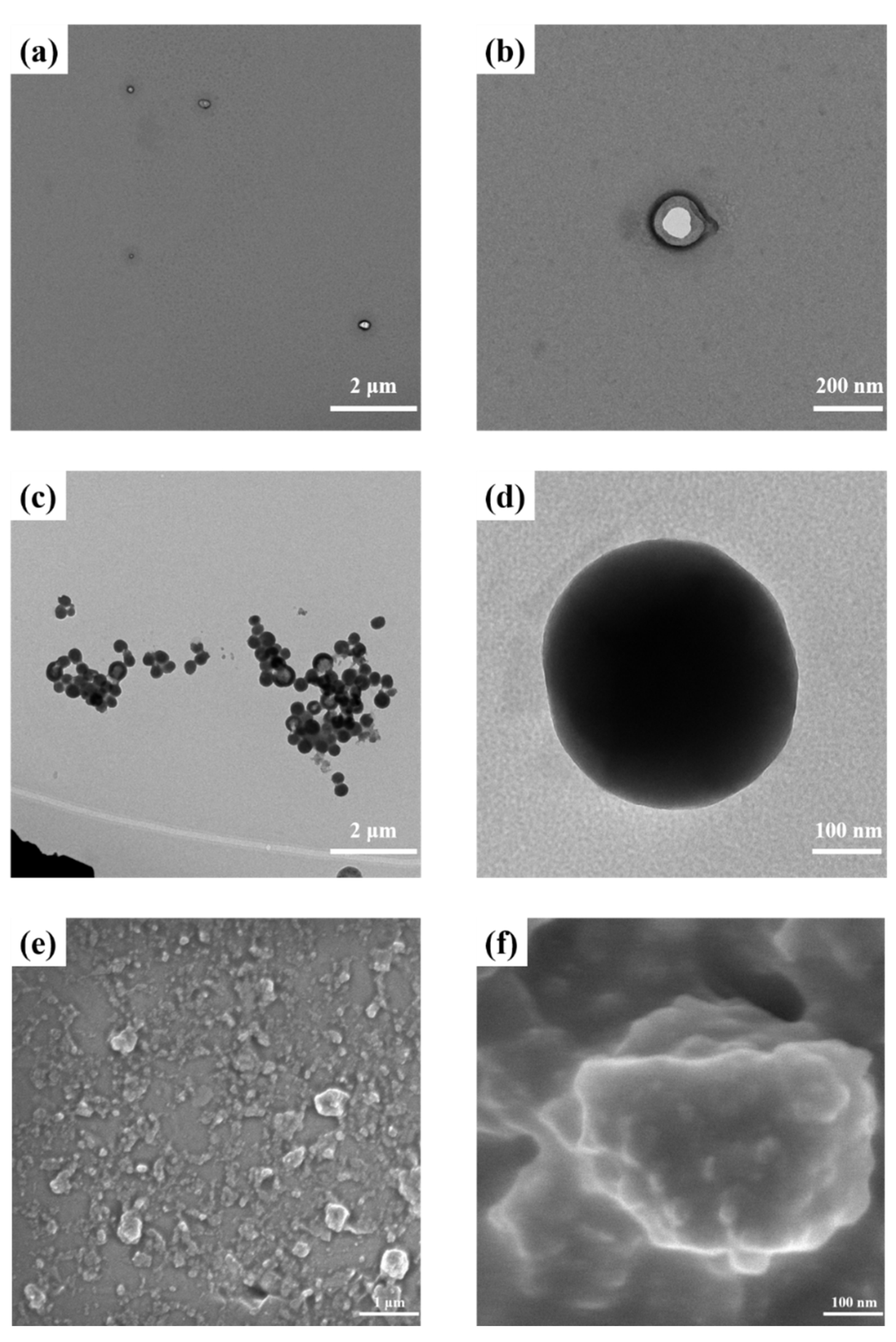
3.1. Preparation and Characterization of PNI-U-DPy and Supramolecular Micelles
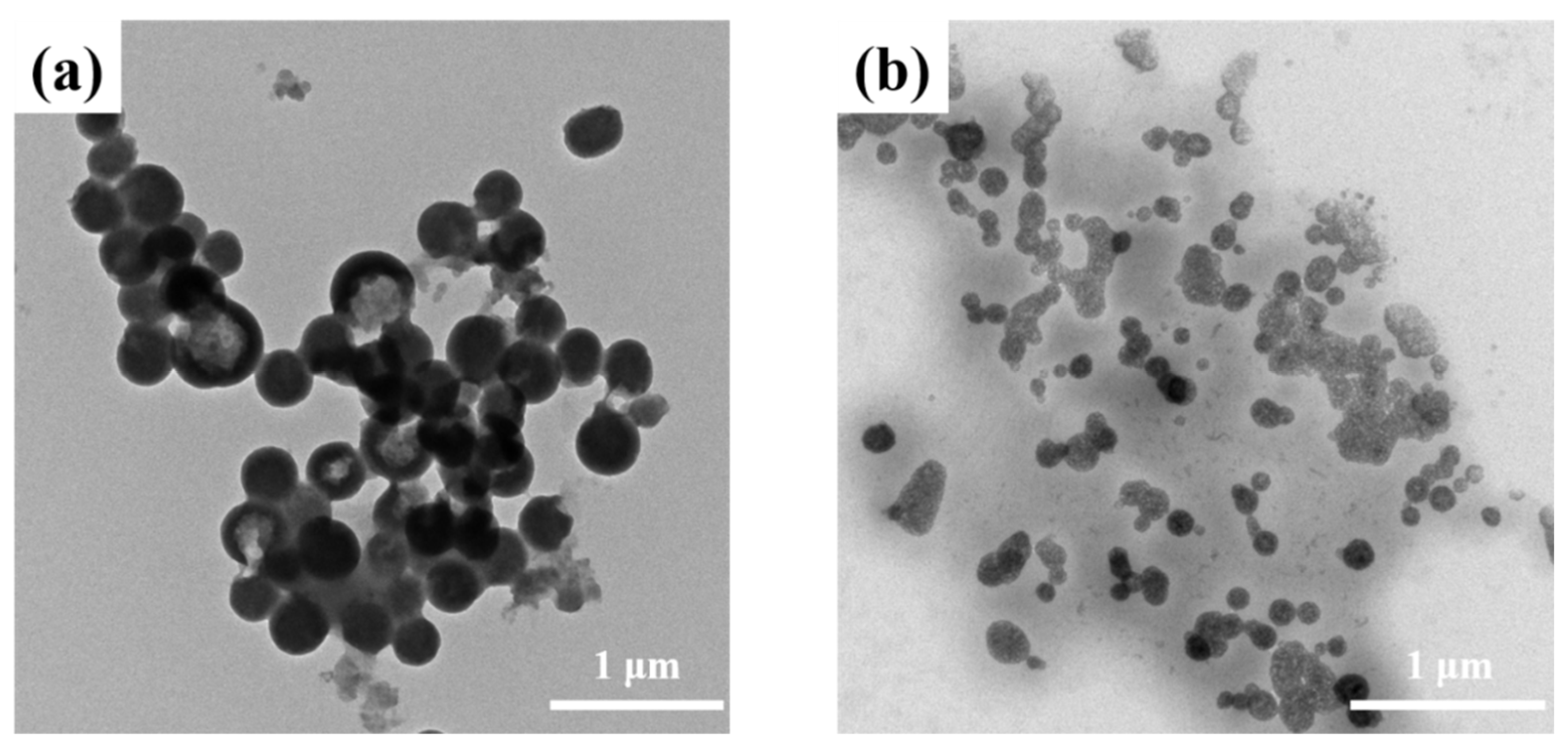
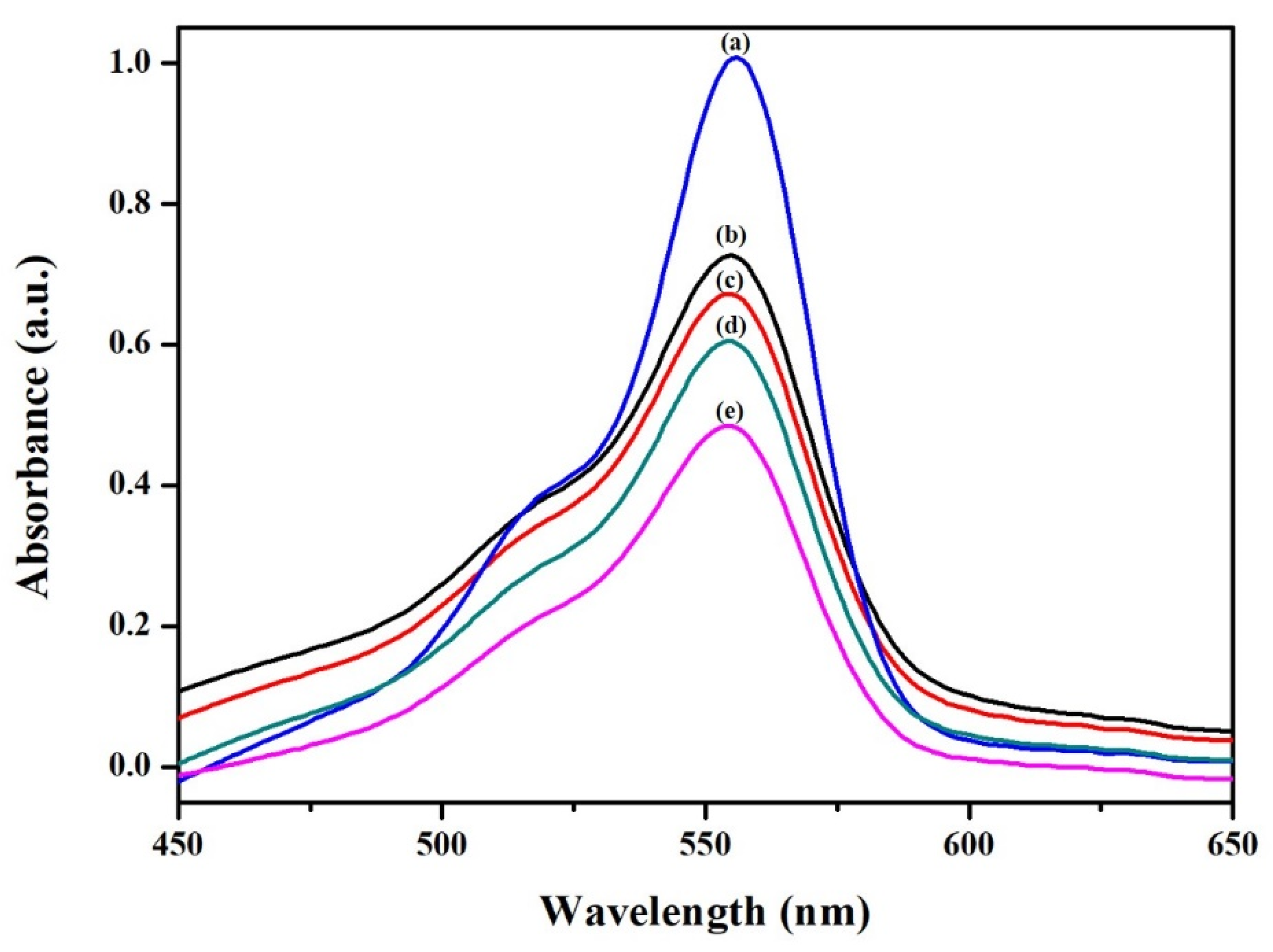
3.2. C60 Encapsulation
3.3. Drug Release Study of Micelles
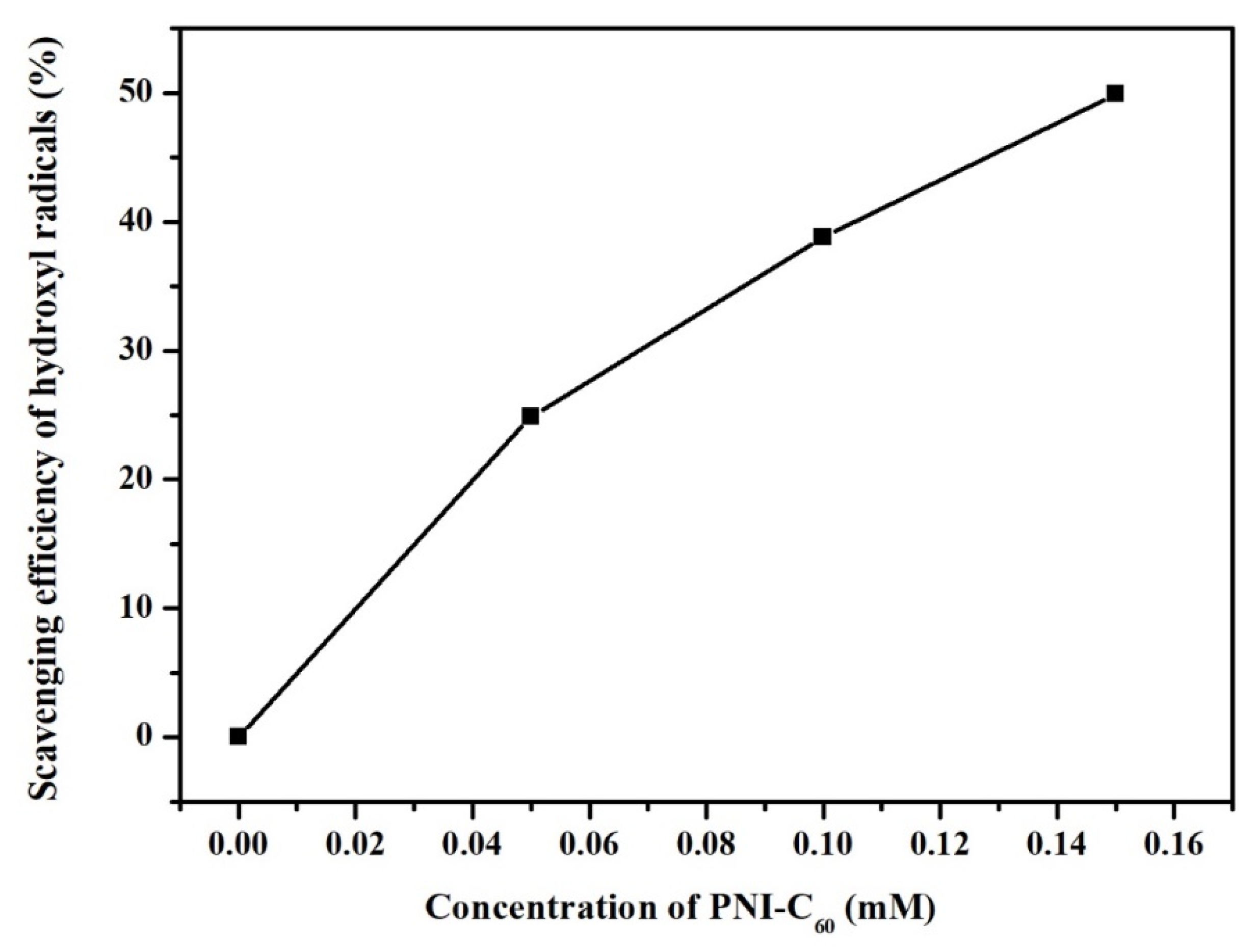
3.4. Hydroxyl Radical Scavenging Ability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishiyama, N.; Matsumura, Y.; Kataoka, K. Development of polymeric micelles for targeting intractable cancers. Cancer Sci. 2016, 107, 867–874. [Google Scholar] [CrossRef]
- Read, E.S.; Armes, S.P. Recent advances in shell cross-linked micelles. Chem. Commun. 2007, 29, 3021–3035. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Wang, L.; Wu, F.; Gong, S.; Wei, J.; Lin, S. Self-assembly and stimuli-responsive behaviours of side-chain liquid crystalline copolymers: A dissipative particle dynamics simulation approach. Phys. Chem. Chem. Phys. 2019, 21, 7645–7653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.F.; Eisenberg, A. Multiple Morphologies of Crew-Cut Aggregates of Polystyrene-B-Poly(Acrylic Acid) Block-Copolymers. Science 1995, 268, 1728–1731. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.; Cheng, J. Anticancer Polymeric Nanomedicines. Polym. Rev. 2007, 47, 345–381. [Google Scholar] [CrossRef]
- Cagel, M.; Tesan, F.C.; Bernabeu, E.; Salgueiro, M.J.; Zubillaga, M.B.; Moretton, M.A.; Chiappetta, D.A. Polymeric mixed micelles as nanomedicines: Achievements and perspectives. Eur. J. Pharm. Biopharm. 2017, 113, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 2008, 126, 187–204. [Google Scholar] [CrossRef]
- Sui, M.; Liu, W.; Shen, Y. Nuclear drug delivery for cancer chemotherapy. J. Control. Release 2011, 155, 227–236. [Google Scholar] [CrossRef]
- Cheng, C.C.; Chang, F.C.; Kao, W.Y.; Hwang, S.M.; Liao, L.C.; Chang, Y.J.; Liang, M.C.; Chen, J.K.; Lee, D.J. Highly efficient drug delivery systems based on functional supramolecular polymers: In vitro evaluation. Acta Biomater. 2016, 33, 194–202. [Google Scholar] [CrossRef]
- Abebe Alemayehu, Y.; Tewabe Gebeyehu, B.; Cheng, C.-C. Photosensitive Supramolecular Micelles with Complementary Hydrogen Bonding Motifs To Improve the Efficacy of Cancer Chemotherapy. Biomacromolecules 2019, 20, 4535–4545. [Google Scholar] [CrossRef]
- Cheng, C.-C.; Sun, Y.-T.; Lee, A.-W.; Huang, S.-Y.; Fan, W.-L.; Chiao, Y.-H.; Chiu, C.-W.; Lai, J.-Y. Hydrogen-bonded supramolecular micelle-mediated drug delivery enhances the efficacy and safety of cancer chemotherapy. Polym. Chem. 2020, 11, 2791–2798. [Google Scholar] [CrossRef]
- Hu, J.; Yang, L.; Yang, P.; Jiang, S.; Liu, X.; Li, Y. Polydopamine free radical scavengers. Biomater. Sci. 2020, 8, 4940–4950. [Google Scholar] [CrossRef] [PubMed]
- Eom, T.; Barat, V.; Khan, A.; Stuparu, M.C. Aggregation-free and high stability core-shell polymer nanoparticles with high fullerene loading capacity, variable fullerene type, and compatibility towards biological conditions. Chem. Sci. 2021, 12, 4949–4957. [Google Scholar] [CrossRef]
- Sukhodub, L.; Sukhodub, L.; Kumeda, M.; Prylutska, S.; Deineka, V.; Prylutskyy, Y.I.; Ritter, U. C60 fullerene loaded hydroxyapatite-chitosan beads as a promising system for prolonged drug release. Carbohydr. Polym. 2019, 223, 115067. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.-i.; Banya, S.; Hosoi, I.; Koyama, N.; Suzuki, K.; Jeyadevan, B.; Oku, T.; Fujita, K.; Yamada, S.; Akiyama, T. Preparation of silver-nanoparticle-loaded C60-ethylenediamine adduct microparticles and their application to photoelectric conversion. Appl. Phys. Express 2021, 14, 067003. [Google Scholar] [CrossRef]
- Saura—Sanmartin, A.; Martinez-Cuezva, A.; Marin-Luna, M.; Bautista, D.; Berna, J. Effective Encapsulation of C60 by Metal—Organic Frameworks with Polyamide Macrocyclic Linkers. Angew. Chem. 2021, 133, 10909–10914. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Wang, X.; Xu, J.; Luo, Q.; Liu, J. Self-assembled nanostructures from C60-containing supramolecular complex: Its stimuli-responsive reversible transition and biological antioxidative capacity. New J. Chem. 2011, 35. [Google Scholar] [CrossRef]
- Santra, S.; Kolay, S.; Sk, S.; Ghosh, D.; Mishra, A.; Roy, L.; Sarkar, K.; Molla, M.R. Supramolecularly cross-linked nanoassemblies of self-immolative polyurethane from recycled plastic waste: High encapsulation stability and the triggered release of guest molecules. Polym. Chem. 2022, 13, 3294–3303. [Google Scholar] [CrossRef]
- Cheng, C.-C.; Yen, Y.-C.; Chang, F.-C. Hierarchical structures formed from self-complementary sextuple hydrogen-bonding arrays. RSC Adv. 2011, 1, 1190–1194. [Google Scholar] [CrossRef]
- Cheng, C.-C.; Lin, I.H.; Yen, Y.-C.; Chu, C.-W.; Ko, F.-H.; Wang, X.; Chang, F.-C. New self-assembled supramolecular polymers formed by self-complementary sextuple hydrogen bond motifs. RSC Adv. 2012, 2, 9952–9957. [Google Scholar] [CrossRef]
- Wang, J.-H.; Cheng, C.-C.; Yen, Y.-C.; Miao, C.-C.; Chang, F.-C. Block-copolymer-like supramolecules confined in nanolamellae. Soft Matter 2012, 8, 3747–3750. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Sandeep, S. Synthesis and characterization of a novel pH-controllable composite hydrogel for anticancer drug delivery. New J. Chem. 2011, 35, 2869–2876. [Google Scholar] [CrossRef]
- Morgan, B.; Lahav, O. The effect of pH on the kinetics of spontaneous Fe(II) oxidation by O2 in aqueous solution--basic principles and a simple heuristic description. Chemosphere 2007, 68, 2080–2084. [Google Scholar] [CrossRef] [PubMed]
- Atere, T.G.; Akinloye, O.A.; Ugbaja, R.N.; Ojo, D.A.; Dealtry, G. In vitro antioxidant capacity and free radical scavenging evaluation of standardized extract of Costus afer leaf. Food Sci. Hum. Wellness 2018, 7, 266–272. [Google Scholar] [CrossRef]
- Perkins, S.J. Protein volumes and hydration effects. The calculations of partial specific volumes, neutron scattering matchpoints and 280-nm absorption coefficients for proteins and glycoproteins from amino acid sequences. Eur. J. Biochem. 1986, 157, 169–180. [Google Scholar] [CrossRef]
- Lu, H.-C.; Lin, M.-Y.; Peng, Y.-C.; Chou, S.-L.; Lo, J.-I.; Cheng, B.-M. Absorption, emission and photolysis of C60 with far-UV excitation. Mon. Not. R. Astron. Soc. 2015, 452, 2788–2793. [Google Scholar] [CrossRef]
- Cataldo, F.; Iglesias-Groth, S. On The Molar Extinction Coefficients Of The Electronic Absorption Spectra Of C 60 And C 70 Fullerenes Radical Cation Introduction. Eur. Chem. Bull 2013, 2, 1013–1018. [Google Scholar]
- Wang, H.; Ullah, A. Synthesis and Evaluation of Thermoresponsive Renewable Lipid-Based Block Copolymers for Drug Delivery. Polymers 2022, 14, 3436. [Google Scholar] [CrossRef]
- Ibrahim, M.; Abuwatfa, W.H.; Awad, N.S.; Sabouni, R.; Husseini, G.A. Encapsulation, Release, and Cytotoxicity of Doxorubicin Loaded in Liposomes, Micelles, and Metal-Organic Frameworks: A Review. Pharmaceutics 2022, 14, 254. [Google Scholar] [CrossRef]
- Wong, H.C.; Cabral, J.T. Mechanism and Kinetics of Fullerene Association in Polystyrene Thin Film Mixtures. Macromolecules 2011, 44, 4530–4537. [Google Scholar] [CrossRef]
- Wang, X.-S.; Metanawin, T.; Zheng, X.-Y.; Wang, P.-Y.; Ali, M.; Vernon, D. Structure-Defined C60/Polymer Colloids Supramolecular Nanocomposites in Water. Langmuir 2008, 24, 9230–9232. [Google Scholar] [CrossRef] [PubMed]
- Sathish, M.; Miyazawa, K.I. Synthesis and Characterization of Fullerene Nanowhiskers by Liquid-Liquid Interfacial Precipitation: Influence of C-60 Solubility. Molecules 2012, 17, 3858–3865. [Google Scholar] [CrossRef] [PubMed]
- Sathish, M.; Miyazawa, K.; Hill, J.P.; Ariga, K. Solvent engineering for shape-shifter pure fullerene (C60). J. Am. Chem. Soc. 2009, 131, 6372–6373. [Google Scholar] [CrossRef]
- Brands, S.; Schein, P.; Castro-Ochoa, K.F.; Galinski, E.A. Hydroxyl radical scavenging of the compatible solute ectoine generates two N-acetimides. Arch. Biochem. Biophys 2019, 674, 108097. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Xu, D.; Lei, R.; Li, N.; Li, K.A. Free-Radical Scavenging Capacity Using the Fenton Reaction with Rhodamine B as the Spectrophotometric Indicator. J. Agric. Food. Chem. 2008, 56, 730–735. [Google Scholar] [CrossRef]





| Temperature | 25 °C | 35 °C | 45 °C | 55 °C | 65 °C |
|---|---|---|---|---|---|
| Dh (nm) | 345 ± 3 | 268 ± 2 | 247 ± 4 | 235 ± 2 | 234 ± 2 |
| polydispersity | 0.277 ± 0.01 | 0.247 ± 0.01 | 0.227 ± 0.01 | 0.198 ± 0.01 | 0.194 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-W.; Chang, Y.-Y.; Cheng, C.-C.; Hung, M.-T.; Mohamed, M.G. Self-Assembled Supramolecular Micelles Based on Multiple Hydrogen Bonding Motifs for the Encapsulation and Release of Fullerene. Polymers 2022, 14, 4923. https://doi.org/10.3390/polym14224923
Huang C-W, Chang Y-Y, Cheng C-C, Hung M-T, Mohamed MG. Self-Assembled Supramolecular Micelles Based on Multiple Hydrogen Bonding Motifs for the Encapsulation and Release of Fullerene. Polymers. 2022; 14(22):4923. https://doi.org/10.3390/polym14224923
Chicago/Turabian StyleHuang, Cheng-Wei, Ya-Ying Chang, Chih-Chia Cheng, Meng-Ting Hung, and Mohamed Gamal Mohamed. 2022. "Self-Assembled Supramolecular Micelles Based on Multiple Hydrogen Bonding Motifs for the Encapsulation and Release of Fullerene" Polymers 14, no. 22: 4923. https://doi.org/10.3390/polym14224923
APA StyleHuang, C.-W., Chang, Y.-Y., Cheng, C.-C., Hung, M.-T., & Mohamed, M. G. (2022). Self-Assembled Supramolecular Micelles Based on Multiple Hydrogen Bonding Motifs for the Encapsulation and Release of Fullerene. Polymers, 14(22), 4923. https://doi.org/10.3390/polym14224923








