Lung Extracellular Matrix Hydrogels-Derived Vesicles Contribute to Epithelial Lung Repair
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Lung ECM-Derived Hydrogels
2.2. Rat Bone Marrow Mesenchymal Stromal Cells Isolation
2.3. Extracellular Vesicles Isolation
2.4. F-Actin Staining
2.5. Scanning Electron Microscopy
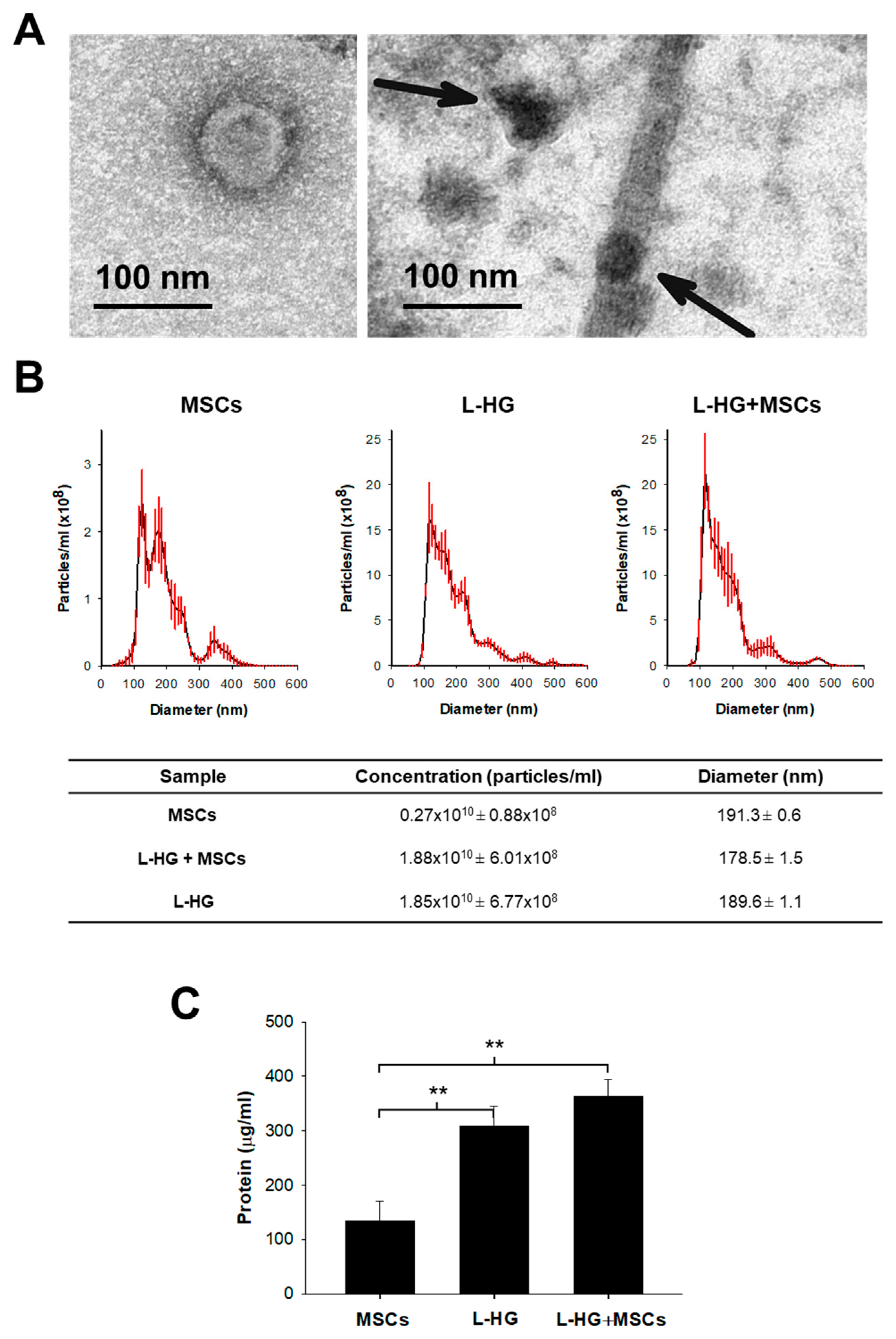
2.6. Transmission Electron Microscopy
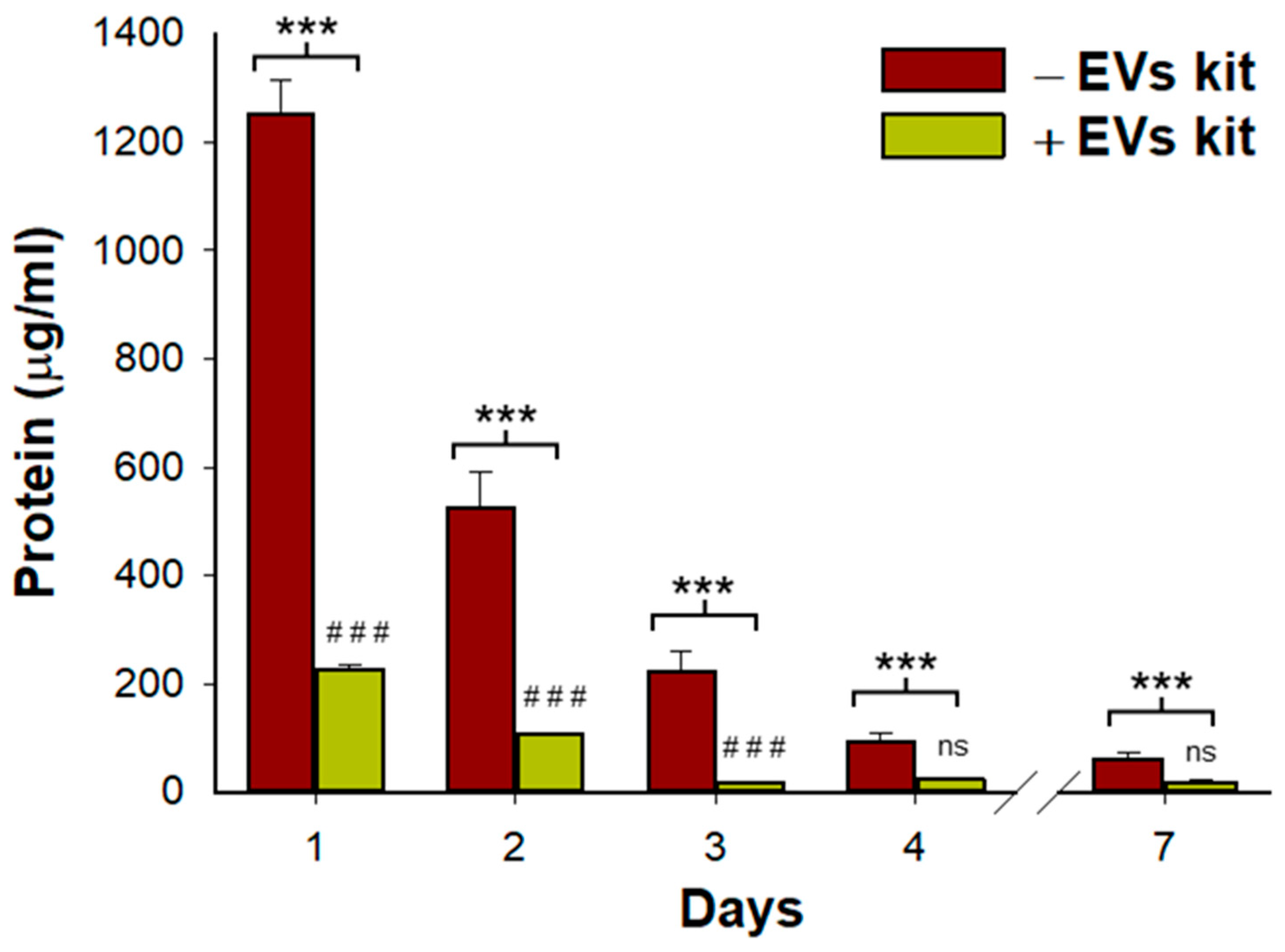
2.7. Protein Quantification
2.8. Wound Healing Assay
2.9. Statistical Analysis
3. Results
3.1. Acellular Lung Hydrogel Is an Important Source of Extracellular Vesicles
3.2. Woung Healing Is Enhanced by MSCs and L-HG-Derived EVs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jhala, D.; Vasita, R. A Review on Extracellular Matrix Mimicking Strategies for an Artificial Stem Cell Niche. Polym. Rev. 2015, 55, 561–595. [Google Scholar] [CrossRef]
- Marhuenda, E.; Villarino, A.; Narciso, M.; Elowsson, L.; Almendros, I.; Westergren-Thorsson, G.; Farré, R.; Gavara, N.; Otero, J. Development of a physiomimetic model of acute respiratory distress syndrome by using ECM hydrogels and organ-on-a-chip devices. Front. Pharmacol. 2022, 13, 945134. [Google Scholar] [CrossRef] [PubMed]
- Falcones, B.; Söderlund, Z.; Ibáñez-Fonseca, A.; Almendros, I.; Otero, J.; Farré, R.; Enes, S.R.; Rendin, L.E.; Westergren-Thorsson, G. hLMSC Secretome Affects Macrophage Activity Differentially Depending on Lung-Mimetic Environments. Cells 2022, 11, 1866. [Google Scholar] [CrossRef] [PubMed]
- Marhuenda, E.; Villarino, A.; Narciso, M.L.; Camprubí-Rimblas, M.; Farré, R.; Gavara, N.; Artigas, A.; Almendros, I.; Otero, J. Lung Extracellular Matrix Hydrogels Enhance Preservation of Type II Phenotype in Primary Alveolar Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 4888. [Google Scholar] [CrossRef] [PubMed]
- Falcones, B.; Sanz-Fraile, H.; Marhuenda, E.; Mendizábal, I.; Cabrera-Aguilera, I.; Malandain, N.; Uriarte, J.; Almendros, I.; Navajas, D.; Weiss, D.; et al. Bioprintable Lung Extracellular Matrix Hydrogel Scaffolds for 3D Culture of Mesenchymal Stromal Cells. Polymers 2021, 13, 2350. [Google Scholar] [CrossRef]
- Marusina, A.I.; Merleev, A.A.; Luna, J.I.; Olney, L.; Haigh, N.E.; Yoon, D.; Guo, C.; Ovadia, E.M.; Shimoda, M.; Luxardi, G.; et al. Tunable hydrogels for mesenchymal stem cell delivery: Integrin-induced transcriptome alterations and hydrogel optimization for human wound healing. Stem Cells 2020, 38, 231–245. [Google Scholar] [CrossRef]
- Han, S.; Mallampalli, R.K. The Acute Respiratory Distress Syndrome: From Mechanism to Translation. J. Immunol. 2015, 194, 855–860. [Google Scholar] [CrossRef]
- Wang, F.; Fang, B.; Qiang, X.; Shao, J.; Zhou, L. The efficacy of mesenchymal stromal cell-derived therapies for acute respiratory distress syndrome—A meta-analysis of preclinical trials. Respir. Res. 2020, 21, 307. [Google Scholar] [CrossRef]
- Matthay, M.A.; Calfee, C.S.; Zhuo, H.; Thompson, B.T.; Wilson, J.G.; E Levitt, J.; Rogers, A.J.; E Gotts, J.; Wiener-Kronish, J.P.; Bajwa, E.K.; et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): A randomised phase 2a safety trial. Lancet Respir. Med. 2019, 7, 154–162. [Google Scholar] [CrossRef]
- Nagpal, A.; Choy, F.C.; Howell, S.; Hillier, S.; Chan, F.; Hamilton-Bruce, M.A.; Koblar, S.A. Safety and effectiveness of stem cell therapies in early-phase clinical trials in stroke: A systematic review and meta-analysis. Stem Cell Res. Ther. 2017, 8, 191. [Google Scholar] [CrossRef]
- Nonaka, P.N.; Falcones, B.; Farre, R.; Artigas, A.; Almendros, I.; Navajas, D. Biophysically Preconditioning Mesenchymal Stem Cells Improves Treatment of Ventilator-Induced Lung Injury. Arch. Bronconeumol. 2020, 56, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Dun, Y.; He, D.; Shao, Y.; Liu, F.; Zhang, L.; Shen, J. RGD-Hydrogel Improves the Therapeutic Effect of Bone Marrow-Derived Mesenchymal Stem Cells on Phosgene-Induced Acute Lung Injury in Rats. Comput. Intell. Neurosci. 2022, 2022, 2743878. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, X.; Yang, L. Hydrogel Encapsulation: Taking the Therapy of Mesenchymal Stem Cells and Their Derived Secretome to the Next Level. Front. Bioeng. Biotechnol. 2022, 10, 859927. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.; Qin, S.; Vashi, M.; Predescu, D.N.; Jeganathan, N.; Bardita, C.; Ganesh, B.; DiBartolo, S.; Fogg, L.F.; Balk, R.A.; et al. Alk5/Runx1 signaling mediated by extracellular vesicles promotes vascular repair in acute respiratory distress syndrome. Clin. Transl. Med. 2018, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-G.; Feng, X.-M.; Abbott, J.; Fang, X.-H.; Hao, Q.; Monsel, A.; Qu, J.-M.; Matthay, M.A.; Lee, J.W. Human Mesenchymal Stem Cell Microvesicles for Treatment of Escherichia coli Endotoxin-Induced Acute Lung Injury in Mice. Stem Cells 2014, 32, 116–125. [Google Scholar] [CrossRef]
- Hu, S.; Park, J.; Liu, A.; Lee, J.; Zhang, X.; Hao, Q.; Lee, J.-W. Mesenchymal Stem Cell Microvesicles Restore Protein Permeability Across Primary Cultures of Injured Human Lung Microvascular Endothelial Cells. Stem Cells Transl. Med. 2018, 7, 615–624. [Google Scholar] [CrossRef]
- Huleihel, L.; Hussey, G.S.; Naranjo, J.D.; Zhang, L.; Dziki, J.L.; Turner, N.J.; Stolz, D.B.; Badylak, S.F. Matrix-bound nanovesicles within ECM bioscaffolds. Sci. Adv. 2016, 2, e1600502. [Google Scholar] [CrossRef]
- Wu, J.; Ravikumar, P.; Nguyen, K.T.; Hsia, C.C.W.; Hong, Y. Lung protection by inhalation of exogenous solubilized extracellular matrix. PLoS ONE 2017, 12, e0171165. [Google Scholar] [CrossRef]
- Brennan, E.P.; Reing, J.; Chew, D.; Myers-Irvin, J.M.; Young, E.; Badylak, S.F. Antibacterial Activity within Degradation Products of Biological Scaffolds Composed of Extracellular Matrix. Tissue Eng. 2006, 12, 2949–2955. [Google Scholar] [CrossRef]
- Reing, J.E.; Zhang, L.; Myers-Irvin, J.; Cordero, K.E.; Freytes, D.O.; Heber-Katz, E.; Bedelbaeva, K.; McIntosh, D.; Dewilde, A.; Braunhut, S.J.; et al. Degradation Products of Extracellular Matrix Affect Cell Migration and Proliferation. Tissue Eng. Part A 2009, 15, 605–614. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Carreras, A.; Almendros, I.; Farré, R. Potential Role of Bone Marrow Mesenchymal Stem Cells in Obstructive Sleep Apnea. Int. J. Stem Cells 2011, 4, 43–49. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Campillo, N.; Falcones, B.; Montserrat, J.M.; Gozal, D.; Obeso, A.; Gallego-Martin, T.; Navajas, D.; Almendros, I.; Farré, R. Frequency and magnitude of intermittent hypoxia modulate endothelial wound healing in a cell culture model of sleep apnea. J. Appl. Physiol. 2017, 123, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Khayambashi, P.; Iyer, J.; Pillai, S.; Upadhyay, A.; Zhang, Y.; Tran, S.D. Hydrogel Encapsulation of Mesenchymal Stem Cells and Their Derived Exosomes for Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 684. [Google Scholar] [CrossRef]
- Azhdari, M.H.; Goodarzi, N.; Doroudian, M.; MacLoughlin, R. Molecular Insight into the Therapeutic Effects of Stem Cell-Derived Exosomes in Respiratory Diseases and the Potential for Pulmonary Delivery. Int. J. Mol. Sci. 2022, 23, 6273. [Google Scholar] [CrossRef]
- Ji, H.-L.; Liu, C.; Zhao, R.-Z. Stem cell therapy for COVID-19 and other respiratory diseases: Global trends of clinical trials. World J. Stem Cells 2020, 12, 471–480. [Google Scholar] [CrossRef]
- Horie, S.; Masterson, C.; Devaney, J.; Laffey, J. Stem cell therapy for acute respiratory distress syndrome. Curr. Opin. Crit. Care 2016, 22, 14–20. [Google Scholar] [CrossRef]
- Gorman, E.; Shankar-Hari, M.; Hopkins, P.; Tunnicliffe, W.S.; Perkins, G.D.; Silversides, J.; McGuigan, P.; Krasnodembskaya, A.; Jackson, C.; Boyle, R.; et al. Repair of acute respiratory distress syndrome by stromal cell administration (REALIST) trial: A phase 1 trial. eClinicalMedicine 2021, 41, 101167. [Google Scholar] [CrossRef]
- Fernández-Francos, S.; Eiro, N.; González-Galiano, N.; Vizoso, F. Mesenchymal Stem Cell-Based Therapy as an Alternative to the Treatment of Acute Respiratory Distress Syndrome: Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 7850. [Google Scholar] [CrossRef]
- Al Halawani, A.; Mithieux, S.M.; Yeo, G.C.; Hosseini-Beheshti, E.; Weiss, A.S. Extracellular Vesicles: Interplay with the Extracellular Matrix and Modulated Cell Responses. Int. J. Mol. Sci. 2022, 23, 3389. [Google Scholar] [CrossRef]
- Kusuma, G.D.; Carthew, J.; Lim, R.; Frith, J.E. Effect of the Microenvironment on Mesenchymal Stem Cell Paracrine Signaling: Opportunities to Engineer the Therapeutic Effect. Stem Cells Dev. 2017, 26, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Hussey, G.S.; Molina, C.P.; Cramer, M.C.; Tyurina, Y.Y.; Tyurin, V.A.; Lee, Y.C.; El-Mossier, S.O.; Murdock, M.H.; Timashev, P.S.; Kagan, V.E.; et al. Lipidomics and RNA sequencing reveal a novel subpopulation of nanovesicle within extracellular matrix biomaterials. Sci. Adv. 2020, 6, eaay4361. [Google Scholar] [CrossRef] [PubMed]
- Man, K.; Brunet, M.Y.; Federici, A.S.; Hoey, D.A.; Cox, S.C. An ECM-Mimetic Hydrogel to Promote the Therapeutic Efficacy of Osteoblast-Derived Extracellular Vesicles for Bone Regeneration. Front. Bioeng. Biotechnol. 2022, 10, 829969. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Swindell, H.S.; Ramasubramanian, L.; Liu, R.; Lam, K.S.; Farmer, D.; Wang, A. Extracellular Matrix Mimicking Nanofibrous Scaffolds Modified With Mesenchymal Stem Cell-Derived Extracellular Vesicles for Improved Vascularization. Front. Bioeng. Biotechnol. 2020, 8, 633. [Google Scholar] [CrossRef]
- Huleihel, L.; Bartolacci, J.G.; Dziki, J.L.; Vorobyov, T.; Arnold, B.; Scarritt, M.E.; Pineda Molina, C.; Lopresti, S.T.; Brown, B.N.; Naranjo, J.D.; et al. Matrix-Bound Nanovesicles Recapitulate Extracellular Matrix Effects on Macrophage Phenotype. Tissue Eng. Part A 2017, 23, 1283–1294. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, P.; Sun, H.; Zhou, H.; Zhang, Y.; Xiao, Z. Lung tissue extracellular matrix-derived hydrogels protect against radiation-induced lung injury by suppressing epithelial–mesenchymal transition. J. Cell. Physiol. 2020, 235, 2377–2388. [Google Scholar] [CrossRef]
- Pouliot, R.A.; Link, P.A.; Mikhaiel, N.S.; Schneck, M.B.; Valentine, M.S.; Gninzeko, F.J.K.; Herbert, J.A.; Sakagami, M.; Heise, R.L. Development and characterization of a naturally derived lung extracellular matrix hydrogel. J. Biomed. Mater. Res. Part A 2016, 104, 1922–1935. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulldemolins, A.; Jurado, A.; Herranz-Diez, C.; Gavara, N.; Otero, J.; Farré, R.; Almendros, I. Lung Extracellular Matrix Hydrogels-Derived Vesicles Contribute to Epithelial Lung Repair. Polymers 2022, 14, 4907. https://doi.org/10.3390/polym14224907
Ulldemolins A, Jurado A, Herranz-Diez C, Gavara N, Otero J, Farré R, Almendros I. Lung Extracellular Matrix Hydrogels-Derived Vesicles Contribute to Epithelial Lung Repair. Polymers. 2022; 14(22):4907. https://doi.org/10.3390/polym14224907
Chicago/Turabian StyleUlldemolins, Anna, Alicia Jurado, Carolina Herranz-Diez, Núria Gavara, Jorge Otero, Ramon Farré, and Isaac Almendros. 2022. "Lung Extracellular Matrix Hydrogels-Derived Vesicles Contribute to Epithelial Lung Repair" Polymers 14, no. 22: 4907. https://doi.org/10.3390/polym14224907
APA StyleUlldemolins, A., Jurado, A., Herranz-Diez, C., Gavara, N., Otero, J., Farré, R., & Almendros, I. (2022). Lung Extracellular Matrix Hydrogels-Derived Vesicles Contribute to Epithelial Lung Repair. Polymers, 14(22), 4907. https://doi.org/10.3390/polym14224907







