Preparation of Mannitol-Modified Loofah and Its High-Efficient Adsorption of Cu(II) Ions in Aqueous Solution
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Mannitol-Modified Loofah (MML)
2.3. Characterization Methods
2.4. Adsorption of Cu2+
2.5. Adsorption and Desorption
3. Results and Discussion
3.1. FTIR Analysis of MML
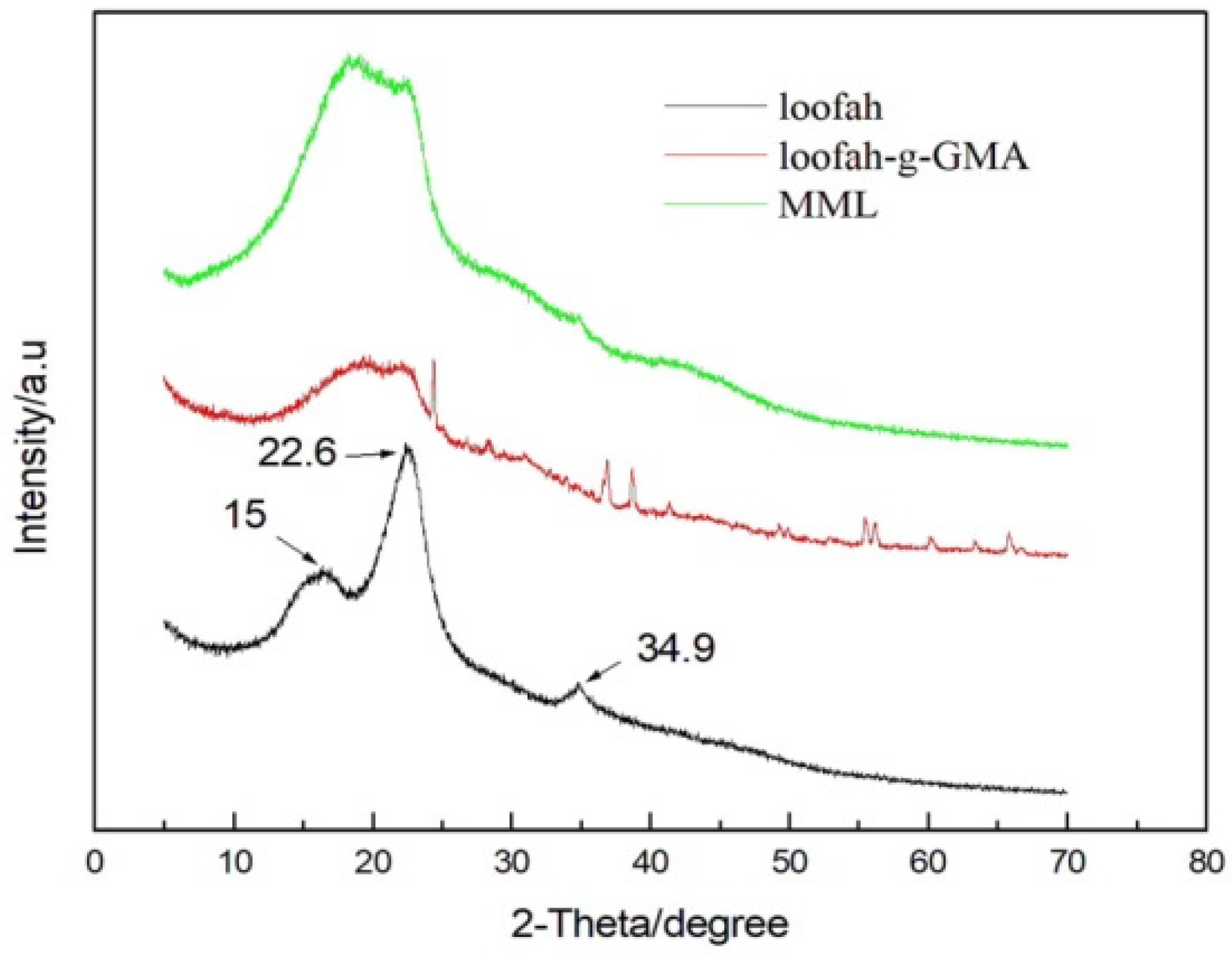
3.2. XRD Analysis of MML
3.3. SEM Image Analysis
3.4. Adsorption Behavior at Different pH
3.5. Adsorption Behavior at Different Adsorption Doses
3.6. Adsorption Behavior at Different Adsorption Times
3.7. Adsorption Behavior at Different Initial Concentrations of Cu(II) Ions
3.8. Isothermal Adsorption
3.9. Adsorption Kinetics
3.10. Adsorption Mechanism Analysis
3.11. Adsorbent Reuse
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dai, M.; Liu, Y.; Ju, B.; Tian, Y. Preparation of thermoresponsive alginate/starch ether composite hydrogel and its application to the removal of Cu(II) from aqueous solution. Bioresour. Technol. 2019, 294, 122192. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.W.; Ding, L.; Wang, Y.; Zhang, Y. High efficient adsorption for thorium in aqueous solution using a novel tentacle-type chitosan-based aerogel: Adsorption behavior and mechanism. Int. J. Biol. Macromol. 2022, 222, 1747–1757. [Google Scholar] [CrossRef] [PubMed]
- Rezania, S.; Ponraj, M.; Talaiekhozani, A.; Mohamad, S.E.; Din, M.F.M.; Taib, S.M. Perspectives of phytoremediation using water hyacinth for removal of heavy metals, organic and inorganic pollutants in wastewater. J. Environ. Manage. 2015, 163, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, T.; Afrin, R.; Mia, M.Y.; Hossen, M.Z. Phytoremediation of Chromium and some chemical parameters from Tannery effluent by using water Hyacinth (Eichhornia craassipes). Res. Agric. Livest. Fish. 2017, 4, 151–156. [Google Scholar] [CrossRef][Green Version]
- Liu, X.F.; Yin, H.; Liu, H.; Cai, Y.H.; Qi, X.; Dang, Z. Multicomponent adsorption of heavy metals onto biogenic hydroxyapatite: Surface functional groups and inorganic mineral facilitating stable adsorption of Pb(II). J. Hazard. Mater. 2022, 443, 130167. [Google Scholar] [CrossRef]
- Chen, Y.; Li, M.; Li, Y.; Liu, Y.; Chen, Y.; Li, H.; Li, L.; Xu, F.; Jiang, H.; Chen, L. Hydroxyapatite modified sludge-based biochar for the adsorption of Cu2+ and Cd2+: Adsorption behavior and mechanisms. Bioresour. Technol. 2021, 321, 124413. [Google Scholar] [CrossRef]
- Lee, S.; Choi, H. Persimmon leaf bio-waste for adsorptive removal of heavy metals from aqueous solution. J. Environ. Manag. 2018, 209, 382–392. [Google Scholar] [CrossRef]
- Park, J.; Cho, J.; Ok, Y.S.; Kim, S.H.; Heo, J.S.; Delaune, R.D.; Seo, D.C. Comparison of single and competitive metal adsorption by pepper stem biochar. Arch. Agron. Soil Sci. 2016, 62, 1899908230. [Google Scholar] [CrossRef]
- Nada, A.M.A.; El-Gendy, A.A.; Mohamed, S.H. Banana leaves as adsorbents for removal of metal ions from waste water. Carbohydr. Polym. 2010, 82, 1025–1030. [Google Scholar] [CrossRef]
- Shamsollahi, Z.; Partovinia, A. Recent advances on pollutants removal by rice husk as a bio -based adsorbent: A critical review. J. Environ. Manage. 2019, 246, 314–323. [Google Scholar] [CrossRef]
- Asere, T.G.; Stevens, C.V.; Du, G.; Laing, G. Use of (modified) natural adsorbents for arsenic remediation: A review. Sci. Total Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Chauhan, M.S. Adsorption of chromium (VI) from the synthetic aqueous solution using chemically modified dried water hyacinth roots. J. Environ. Chem. Eng. 2019, 7, 103218. [Google Scholar] [CrossRef]
- Gao, J.X.; Yuan, Y.H.; Yu, Q.H.; Yan, B.J.; Qian, Y.X.; Wen, J.; Ma, C.X.; Jiang, S.H.; Wang, X.L.; Wang, N. Bio-inspired antibacterial cellulose paper–poly(amidoxime) composite hydrogel for highly efficient uranium(VI) capture from seawater. Chem. Commun. 2020, 56, 3935–3938. [Google Scholar] [CrossRef]
- Chen, L.; Sun, Y.; Wang, J.; Ma, C.; Peng, S.; Cao, X.; Yang, L.; Ma, C.; Duan, G.; Liu, Z.; et al. A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption. e-Polymers 2022, 22, 468–477. [Google Scholar] [CrossRef]
- Yang, W.S.; Wang, Y.F.; Wang, Q.M.; Wu, J.L.; Duan, G.G.; Xu, W.H.; Jian, S.J. Magnetically separable and recyclable Fe3O4@PDA covalent grafted by l-cysteine core-shell nanoparticles toward efficient removal of Pb2+. Vacuum 2021, 189, 110229. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, X.; Li, B. Adsorption of Hg2+ and Cd2+ by ethylenediamine modified peanut shells. Carbohydr. Polym. 2010, 81, 335–339. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, Y.; Wang, F. Fabrication of magnetic macroporous chitosan-g-poly (acrylic acid) hydrogel for removal of Cd2+ and Pb2+. Int. J. Biol. Macromol. 2016, 93, 483–492. [Google Scholar] [CrossRef]
- Gusmo, K.A.G.; Gurgel, L.V.A.; Melo, T.M.S. Adsorption studies of methylene blue and gentian violet on sugarcane bagasse modified with EDTA dianhydride( EDTAD) in aqueous solutions: Kinetic and equilibrium aspects. J. Environ. Manag. 2013, 118, 135–143. [Google Scholar] [CrossRef]
- Oboh, I.O.; Aluyor, E.O. Luffa cylindrical-an emerging cash crop. Afr. J. Agric. Res. 2009, 4, 684–688. [Google Scholar]
- Liu, G.T.; Gao, Y.D. Synthesis, characterization of aminothiourea modified walnut Shell and its adsorption for Pb(II) ions from aqueous solution. Polymer (Korea) 2016, 40, 194–200. [Google Scholar] [CrossRef]
- Cao, J.S.; Lin, J.X.; Fang, F.; Zhang, M.T.; Hu, Z.R. A new absorbent by modifying walnut shell for the removal of anionic dye: Kinetic and thermodynamic studies. Bioresour. Technol. 2014, 163, 199–205. [Google Scholar] [CrossRef]
- Liu, G.T.; Zhang, W.; Luo, R.S. Synthesis, characterization of amino-modified walnut shell and adsorption forPb(II) ions from aqueous solution. Polym. Bull. 2019, 76, 1099–1114. [Google Scholar] [CrossRef]
- Ding, Z.H.; Yu, R.; Hu, X.; Chen, Y.J.; Zhang, Y.F. Graft copolymerization of epichlorohydrin and ethylenediamine onto cellulose derived from agricultural by-products for adsorption of Pb(II) in aqueous solution. Cellulose 2014, 21, 1459–1469. [Google Scholar] [CrossRef]
- Naghizadeh, A.; Nasseri, S.; Nazmara, S. Removal of trichloroethylene from water by adsorption on tomultiwall carbon nanotubes. Iran. J. Environ. Health Sci. Eng. 2011, 8, 317–325. [Google Scholar]
- Mutunga, M.F.; Wycliffe, C.W.; Gilbert, O. Adsorption of anionic dye (Reactive black 5) using macadamia seed Husks: Kinetics and equilibrium studies. Sci. Afr. 2020, 7, e00283. [Google Scholar]
- Zhang, Y.; Li, H.J.; Li, M.C.; Xin, M.H. Adsorption of aniline on aminated chitosan/graphene oxide composite material. J. Mol. Struct. 2020, 1209, 127973. [Google Scholar]
- Hyeong, Y.C.; Jong, H.B.; Yohei, H.; Sol, A.; Kim, I.S.; Lee, H.; Kim, M. Thiol-functionalized cellulose nanofiber membranes for the effective adsorption of heavy metal ions in water. Carbohydr. Polym. 2020, 234, 115881. [Google Scholar]
- Sutirman, Z.A.; AmiraRahim, E.; MarsinSanagi, M.; Karim, K.J.A.; Ibrahim, W.A. New efficient chitosan derivative for Cu(II) ions removal: Characterization and adsorption performance. Int. J. Biol. Macromol. 2020, 153, 513–522. [Google Scholar] [CrossRef]
- Wang, J.J.; Cao, M.S.; Jiang, C.Y.; Zheng, Y.X.; Zhang, C.S.; Wei, J. Adsorption and coadsorption mechanisms of Hg2+ and methyl orange by branched polyethyleneimine modified magnetic straw. Mater. Lett. 2018, 229, 160–163. [Google Scholar] [CrossRef]
- Ranasinghe, S.H.; Navaratne, A.N.; Priyantha, N. Enhancement of adsorption characteristics of Cr(III) and Ni(II) by surface modification of jackfruit peel biosorbent. J. Environ. Chem. Eng. 2018, 6, 5670–5682. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Siafaka, P.I.; Lambropoulou, D.A.; Lazaridis, N.K.; Bikiaris, D.N. Poly(itaconic acid) -grafted chitosan adsorbents with different cross -linking for Pb(II) and Cd(II) uptake. Langmuir. 2013, 30, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Y.; Li, L.P.; Kong, L.C.; Cai, G.Y.; Wang, P.; Zhang, J.; Zuo, W.; Tian, Y. Compressible amino-modified carboxymethyl chitosan aerogel for efficient Cu(II) adsorption from wastewater. Sep. Purif. Technol. 2022, 293, 121146. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, F.; Zhao, Y.C.; Zhong, L.L.; Gao, R.H.; Zhang, X.M.; Wang, T.; Xue, J.Q. Preparation of thiosemicarbazide-modified polyvinyl alcohol and its selective adsorption of Cu(II). Colloid Interface Sci. Commun. 2021, 43, 100377. [Google Scholar] [CrossRef]
- Tang, L.; Gou, S.H.; He, Y.; Liu, L.; Fang, S.W.; Duan, W.M.; Liu, T. An efficient chitosan-based adsorption material containing phosphoric acid and amidoxime groups for the enrichment of Cu(II) and Ni(II) from water. J. Mol. Liq. 2021, 331, 115815. [Google Scholar] [CrossRef]







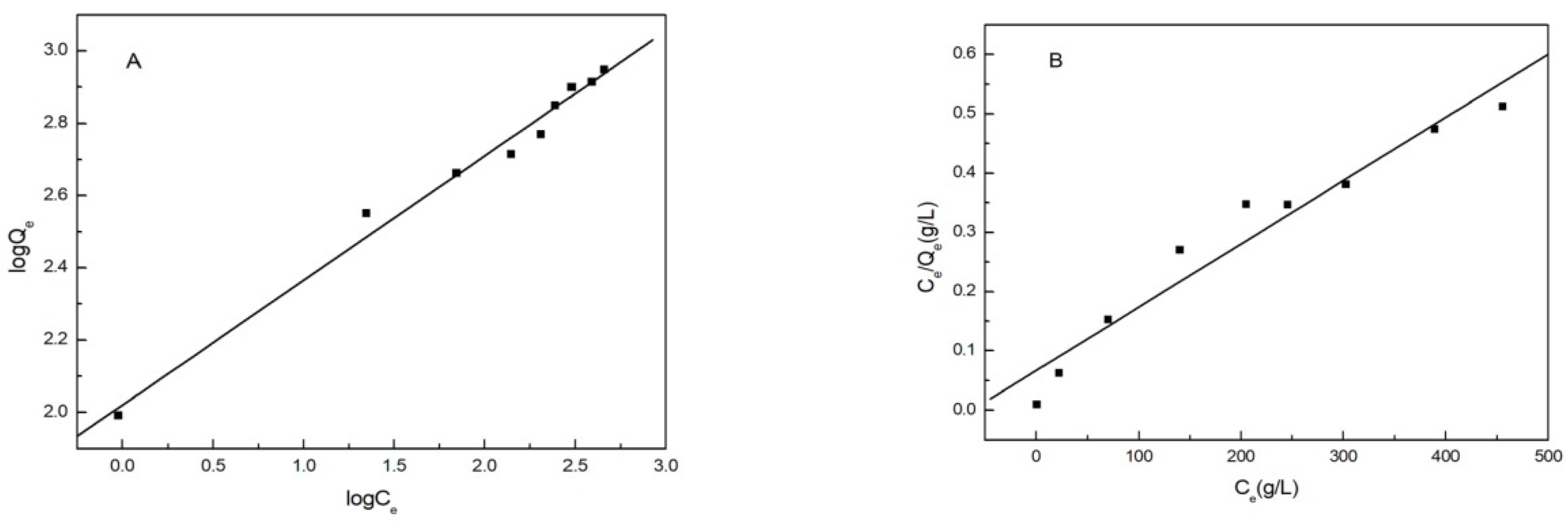


| Qe*/mg·g−1 | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|
| Qm**/mg·g−1 | KL/L·mg−1 | R2 | SD | KF | n | R2 | SD | |
| 888.89 | 934.58 | 0.0161 | 0.9470 | 0.043 | 104.80 | 2.900 | 0.9858 | 0.037 |
| Qe*/mg·g−1 | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||||
|---|---|---|---|---|---|---|---|---|
| k1/min−1 | Qe**/mg·g−1 | R2 | SD | k2/g·mg−1·min−1 | Qe**/mg·g−1 | R2 | SD | |
| 489.01 | 0.030 | 495.52 | 0.9031 | 0.263 | 0.0001 | 540.54 | 0.9827 | 0.018 |
| Adsorbents | Adsorption Capacity (mg/g) |
|---|---|
| Amino-modified carboxymethyl chitosan [32] | 175.56 |
| Thiosemicarbazide-modified polyvinyl alcohol [33] | 82.36 |
| CS-g-AOPAM [34] | 215.5 |
| MML(in this paper) | 888.89 |
| Desorption Times | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Removal efficiency/% | 97.48 | 96.21 | 95.38 | 94.91 | 93.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Liang, J.; Zhang, J. Preparation of Mannitol-Modified Loofah and Its High-Efficient Adsorption of Cu(II) Ions in Aqueous Solution. Polymers 2022, 14, 4883. https://doi.org/10.3390/polym14224883
Liu G, Liang J, Zhang J. Preparation of Mannitol-Modified Loofah and Its High-Efficient Adsorption of Cu(II) Ions in Aqueous Solution. Polymers. 2022; 14(22):4883. https://doi.org/10.3390/polym14224883
Chicago/Turabian StyleLiu, Guangtian, Jianjian Liang, and Jie Zhang. 2022. "Preparation of Mannitol-Modified Loofah and Its High-Efficient Adsorption of Cu(II) Ions in Aqueous Solution" Polymers 14, no. 22: 4883. https://doi.org/10.3390/polym14224883
APA StyleLiu, G., Liang, J., & Zhang, J. (2022). Preparation of Mannitol-Modified Loofah and Its High-Efficient Adsorption of Cu(II) Ions in Aqueous Solution. Polymers, 14(22), 4883. https://doi.org/10.3390/polym14224883





