Bibliometrics of Functional Polymeric Biomaterials with Bioactive Properties Prepared by Radiation-Induced Graft Copolymerisation: A Review
Abstract
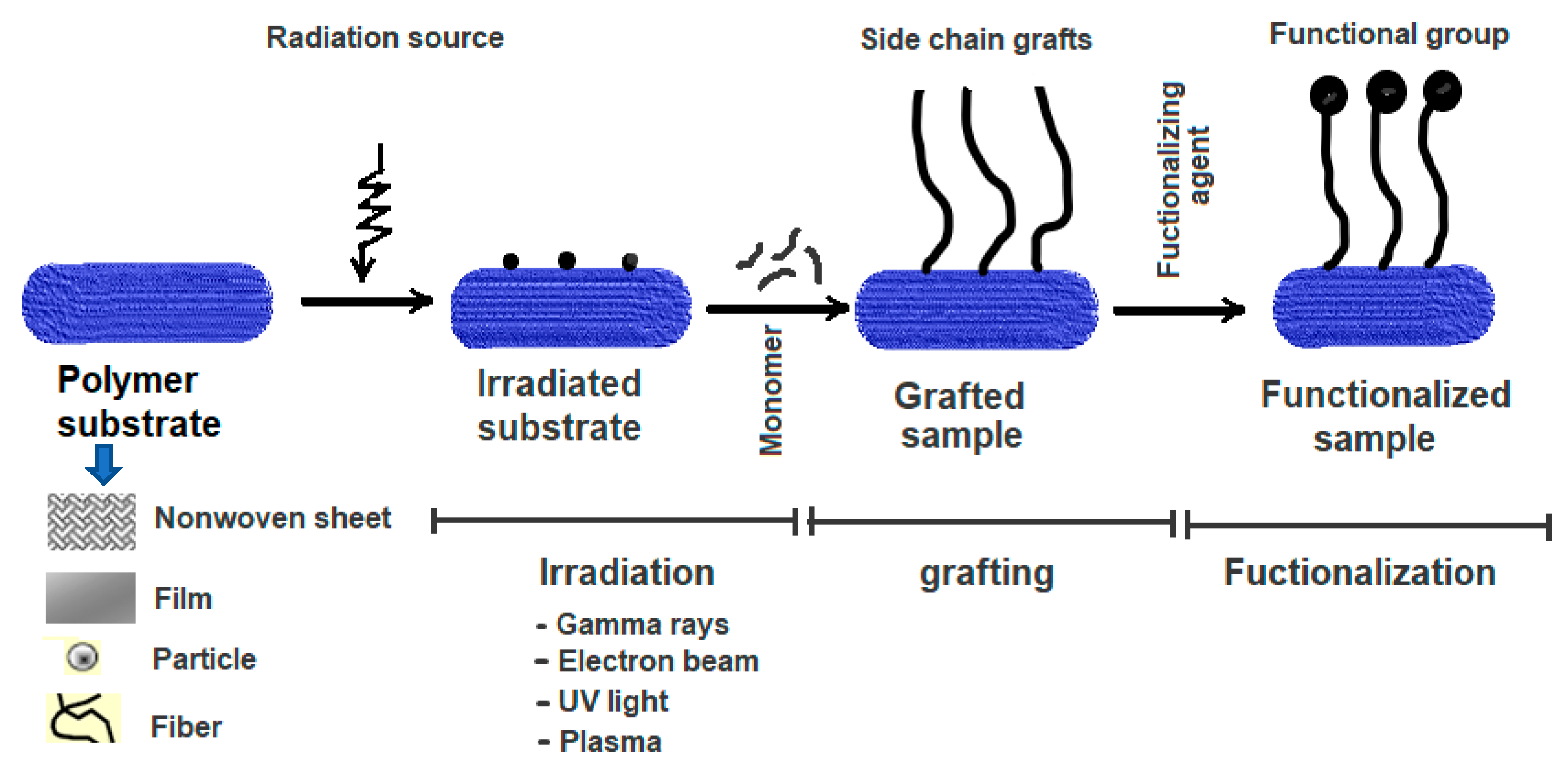
:1. Introduction
2. Data Collection and Methods
3. Results and Discussion
3.1. Classification and Applications of FPBMs Prepared by RIGC
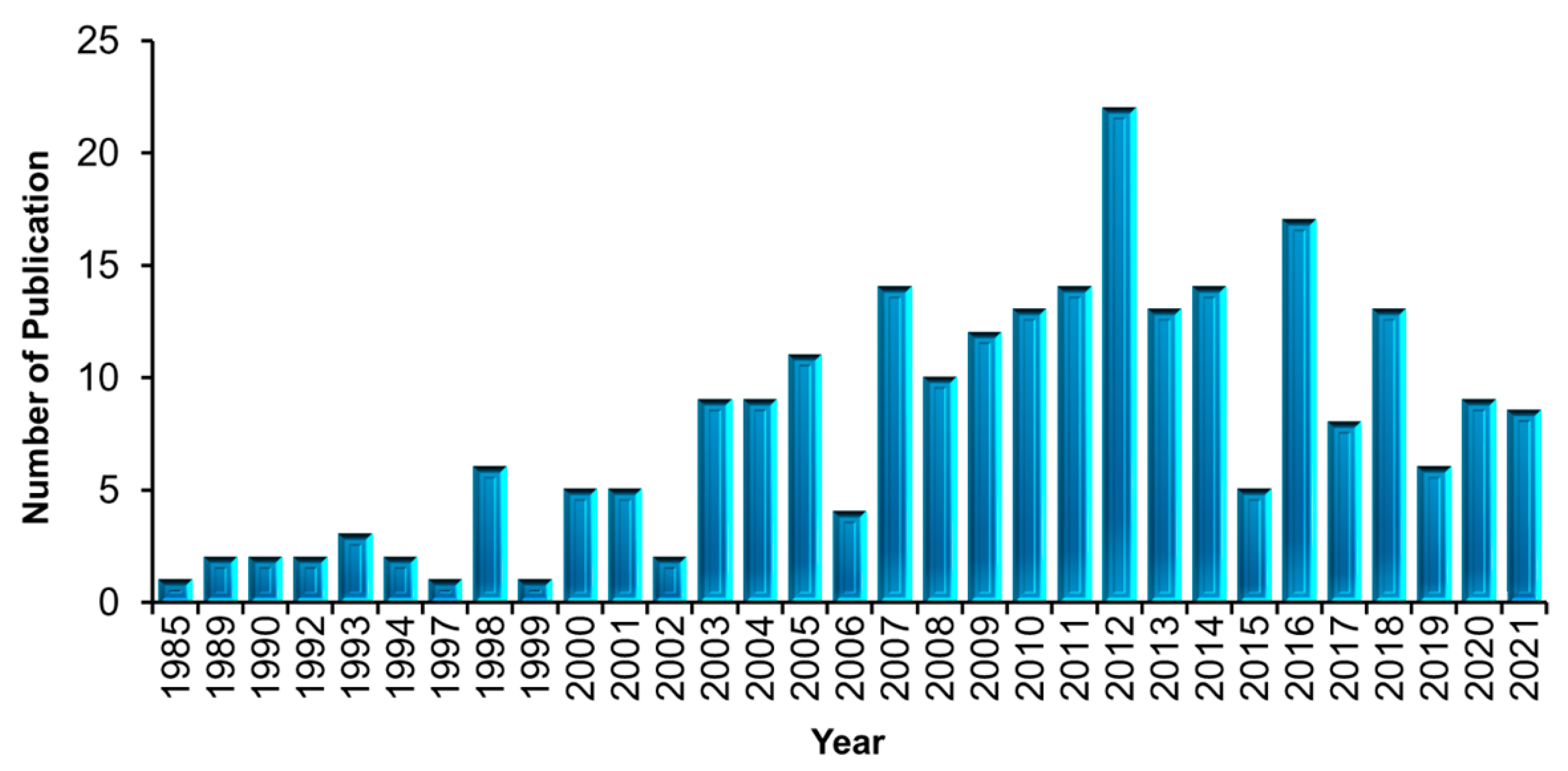
3.2. Distribution of Publications on FPBMs Prepared by RIGC (1985–2021)
3.3. Number Annual of Publications on Application of FPBMs Prepared by RIGC since 1985
3.4. Leading Journals for Publication Related to FPBMs Prepared by RIGC
3.5. Highly Cited Published Articles
Highly Cited Review Articles
3.6. Highly Cited Research Articles
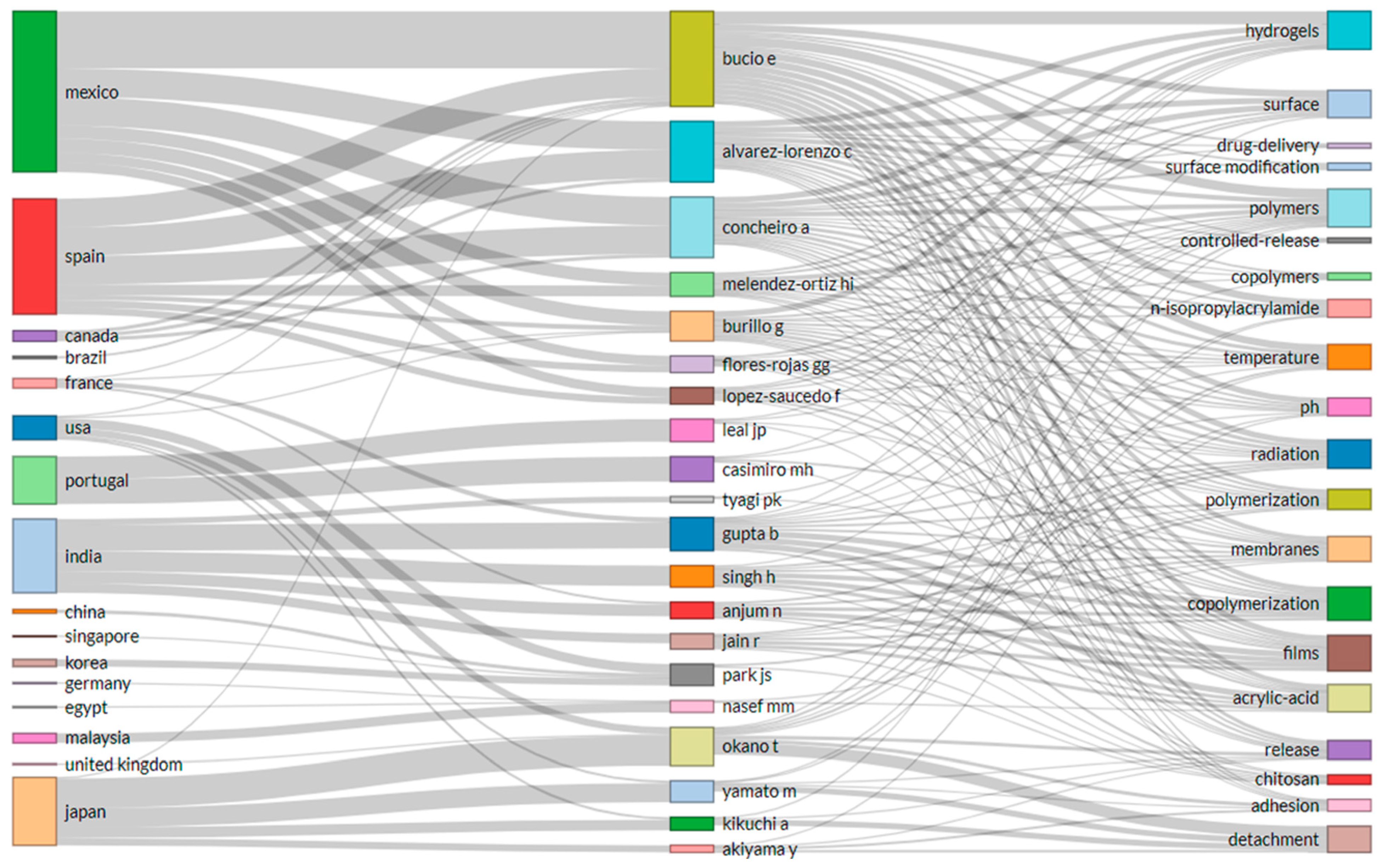
3.7. The Top Influential Authors on Development of FPBMs Prepared by RIGC
3.8. The Most Influential Countries to Provide Funding for FPBMs Prepared by RIGC
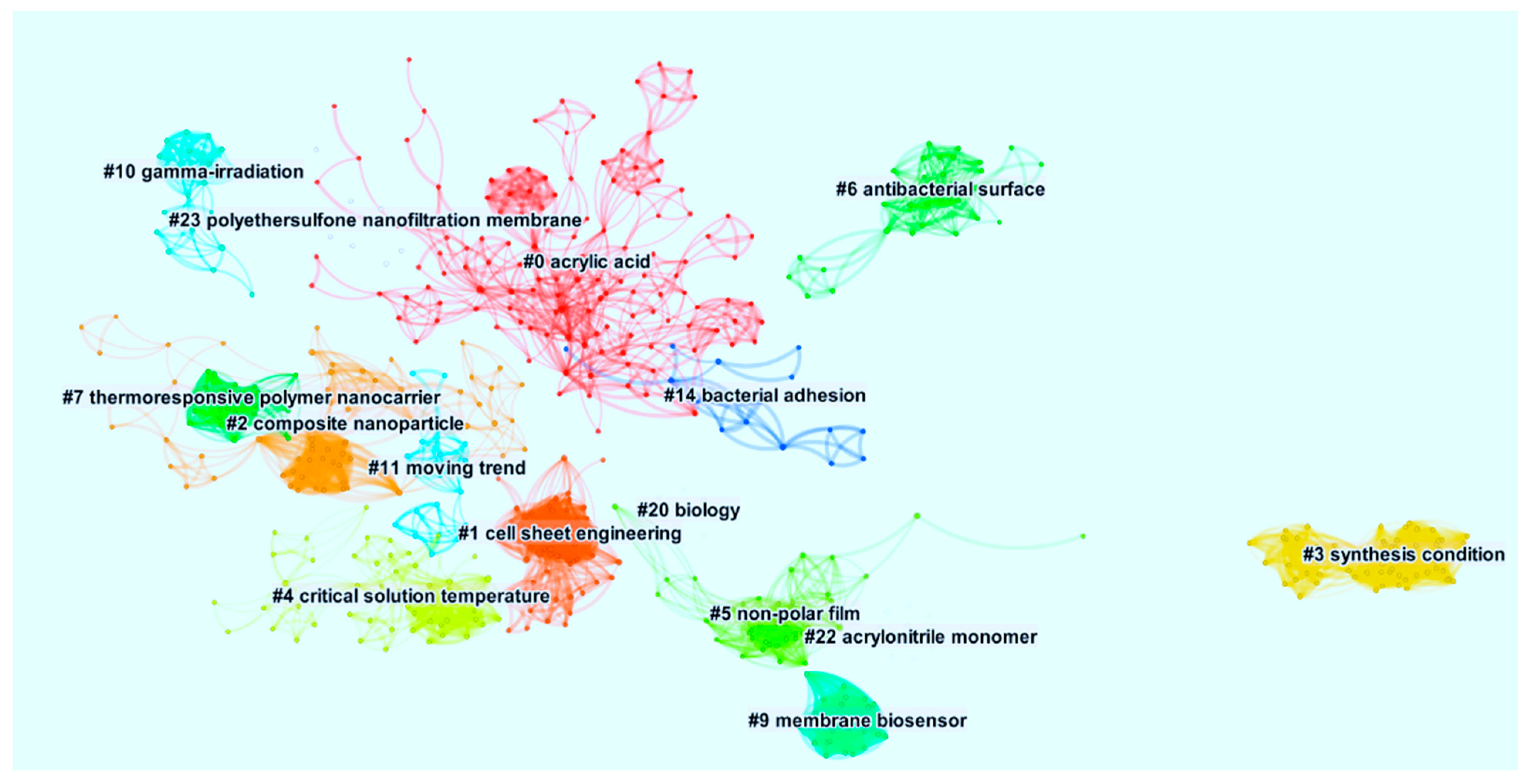
3.9. Keywords Analysis
3.10. Limitation of Study
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reddy, M.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, L.; Scott, R.A.; Kiick, K.L. 50th anniversary perspective: Polymeric biomaterials: Diverse functions enabled by advances in macromolecular chemistry. Macromolecules 2017, 50, 483–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osorio-Delgado, M.A.; Henao-Tamayo, L.J.; Velásquez-Cock, J.A.; Cañas-Gutierrez, A.I.; Restrepo-Múnera, L.M.; Gañán-Rojo, P.F.; Zuluaga-Gallego, R.O.; Ortiz-Trujillo, I.C.; Castro-Herazo, C.I. Biomedical applications of polymeric biomaterials. Dyna 2017, 84, 241–252. [Google Scholar] [CrossRef]
- Neděla, O.; Slepička, P.; Švorčík, V. Surface modification of polymer substrates for biomedical applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef] [PubMed]
- Nasef, M.M.; Hegazy, E.S.A. Preparation and applications of ion exchange membranes by radiation-induced graft copolymerization of polar monomers onto non-polar films. Prog. Polym. Sci. 2004, 29, 499–561. [Google Scholar] [CrossRef]
- Pino-Ramos, V.H.; Ramos-Ballesteros, A.; López-Saucedo, F.; López-Barriguete, J.E.; Varca, G.H.; Bucio, E. Radiation grafting for the functionalization and development of smart polymeric materials. Top. Curr. Chem. 2016, 374, 63. [Google Scholar] [CrossRef]
- Small, M.; Faglie, A.; Craig, A.J.; Pieper, M.; Fernand Narcisse, V.E.; Neuenschwander, P.F.; Chou, S.-F. Nanostructure-enabled and macromolecule-grafted surfaces for biomedical applications. Micromachines 2018, 9, 243. [Google Scholar] [CrossRef] [Green Version]
- Ashfaq, A.; Clochard, M.-C.; Coqueret, X.; Dispenza, C.; Driscoll, M.S.; Ulański, P.; Al-Sheikhly, M. Polymerization reactions and modifications of polymers by ionizing radiation. Polymers 2020, 12, 2877. [Google Scholar] [CrossRef]
- Nasef, M.M. Radiation-grafted membranes for polymer electrolyte fuel cells: Current trends and future directions. Chem. Rev. 2014, 114, 12278–12329. [Google Scholar] [CrossRef]
- Nasef, M.M.; Gürsel, S.A.; Karabelli, D.; Güven, O. Radiation-grafted materials for energy conversion and energy storage applications. Prog. Polym. Sci. 2016, 63, 1–41. [Google Scholar] [CrossRef]
- Nasef, M.M.; Gupta, B.; Shameli, K.; Verma, C.; Ali, R.R.; Ting, T.M. Engineered Bioactive Polymeric Surfaces by Radiation Induced Graft Copolymerization: Strategies and Applications. Polymers 2021, 13, 3102. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, I.; Sugo, T.; Senoo, K.; Okada, T.; Okamoto, J.; Machi, S. Graft polymerization of acrylic acid onto polyethylene film by preirradiation method. I. Effects of preirradiation dose, monomer concentration, reaction temperature, and film thickness. J. Appl. Polym. Sci. 1982, 27, 1033–1041. [Google Scholar] [CrossRef]
- Makuuchi, K.; Cheng, S. Radiation Processing of Polymer Materials and Its Industrial Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Yuan, J.; Yu, C.; Peng, J.; Wang, Y.; Ma, J.; Qiu, J.; Li, J.; Zhai, M. Facile synthesis of amphoteric ion exchange membrane by radiation grafting of sodium styrene sulfonate and N, N-dimethylaminoethyl methacrylate for vanadium redox flow battery. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 5194–5202. [Google Scholar] [CrossRef]
- Rajabalizadeh Mojarrad, N.; Sadeghi, S.; Yarar Kaplan, B.m.; Güler, E.; Alkan Gürsel, S. Metal-Salt Enhanced Grafting of Vinylpyridine and Vinylimidazole Monomer Combinations in Radiation Grafted Membranes for High-Temperature PEM Fuel Cells. ACS Appl. Energy Mater. 2019, 3, 532–540. [Google Scholar] [CrossRef] [Green Version]
- Lim, K.L.; Wong, C.Y.; Wong, W.Y.; Loh, K.S.; Selambakkannu, S.; Othman, N.A.F.; Yang, H. Radiation-Grafted Anion-Exchange Membrane for Fuel Cell and Electrolyzer Applications: A Mini Review. Membranes 2021, 11, 397. [Google Scholar] [CrossRef]
- Hoshina, H.; Kasai, N.; Amada, H.; Takahashi, M.; Tanaka, K.; Seko, N. Recovery of scandium from hot spring water with graft adsorbent containing phosphoric groups. J. Ion Exch. 2014, 25, 248–251. [Google Scholar] [CrossRef] [Green Version]
- Hanh, T.T.; Huy, H.T.; Hien, N.Q. Pre-irradiation grafting of acrylonitrile onto chitin for adsorption of arsenic in water. Radiat. Phys. Chem. 2015, 106, 235–241. [Google Scholar] [CrossRef]
- Gao, Q.; Hu, J.; Li, R.; Xing, Z.; Xu, L.; Wang, M.; Guo, X.; Wu, G. Radiation synthesis of a new amidoximated UHMWPE fibrous adsorbent with high adsorption selectivity for uranium over vanadium in simulated seawater. Radiat. Phys. Chem. 2016, 122, 1–8. [Google Scholar] [CrossRef]
- Sawada, S.-i.; Maekawa, Y. Radiation-Induced Asymmetric Grafting of Different Monomers into Base Films to Prepare Novel Bipolar Membranes. Molecules 2021, 26, 2028. [Google Scholar] [CrossRef]
- Ishihara, R.; Asai, S.; Saito, K. Recent Progress in Charged Polymer Chains Grafted by Radiation-Induced Graft Polymerization; Adsorption of Proteins and Immobilization of Inorganic Precipitates. Quantum Beam Sci. 2020, 4, 20. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Balaji, A.; Vellayappan, M.V.; Subramanian, A.P.; John, A.A.; Asokan, M.K.; Supriyanto, E. Radiation-induced surface modification of polymers for biomaterial application. J. Mater. Sci. 2015, 50, 2007–2018. [Google Scholar] [CrossRef]
- Chapiro, A. Radiation Chemistry of Polymer System; Interscience: New York, NY, USA, 1962. [Google Scholar]
- Charlsby, A. Atomic Radiation and Polymers; Pergamon Press: New York, NY, USA, 1960. [Google Scholar]
- Nasef, M.M.; Güven, O. Radiation-grafted copolymers for separation and purification purposes: Status, challenges and future directions. Prog. Polym. Sci. 2012, 37, 1597–1656. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, Y.; Wen, D.; Peng, J.; Zhao, L.; Zhai, M. Recent Progress in Environmental Applications of Functional Adsorbent Prepared by Radiation techniques: A review. J. Hazard. Mater. 2021, 424, 126887. [Google Scholar] [CrossRef] [PubMed]
- Gubler, L.; Scherer, G.G. Trends for fuel cell membrane development. Desalination 2010, 250, 1034–1037. [Google Scholar] [CrossRef]
- Hoffman, A.S.; Stayton, P.S.; Bulmus, V.; Chen, G.; Chen, J.; Cheung, C.; Chilkoti, A.; Ding, Z.; Dong, L.; Fong, R. Really smart bioconjugates of smart polymers and receptor proteins. J. Biomed. Mater. Res. 2000, 52, 577–586. [Google Scholar] [CrossRef]
- Gil, E.S.; Hudson, S.M. Stimuli-reponsive polymers and their bioconjugates. Prog. Polym. Sci. 2004, 29, 1173–1222. [Google Scholar] [CrossRef]
- Hoffman, A.S. Conventional and environmentally-sensitive hydrogels for medical and industrial uses: A review paper. In Polymer Gels; Springer: Berlin/Heidelberg, Germany, 1991; pp. 289–297. [Google Scholar]
- Carenza, M. Recent achievements in the use of radiation polymerization and grafting for biomedical applications. Int. J. Radiat. Appl. Instrum. Part C Radiat. Phys. Chem. 1992, 39, 485–493. [Google Scholar] [CrossRef]
- Ulanski, P.; Janik, I.; Kadlubowski, S.; Kozicki, M.; Kujawa, P.; Pietrzak, M.; Stasica, P.; Rosiak, J.M. Polymeric biomaterials synthesized by radiation techniques–current studies at IARC, Poland. Polym. Adv. Technol. 2002, 13, 951–959. [Google Scholar] [CrossRef]
- Rosiak, J.; Janik, I.; Kadlubowski, S.; Kozicki, M.; Kujawa, P.; Stasica, P.; Ulanski, P. Nano-, micro-and macroscopic hydrogels synthesized by radiation technique. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2003, 208, 325–330. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Fu, W.; Yao, M.; Ding, Z.; Xuan, J.; Li, D.; Wang, S.; Xia, Y.; Cao, M. Poly (N-isopropylacrylamide)-based thermoresponsive composite hydrogels for biomedical applications. Polymers 2020, 12, 580. [Google Scholar] [CrossRef]
- Wach, R.A.; Rosiak, J.M.; Ulański, P. Polysaccharides hydrogel-radiation induced formation and medical applications. In Proceedings of the 26th Biomaterials in Medicine and Veterinary Medicine Conference, Rytro, Poland, 12–15 October 2017; Volume 20. [Google Scholar]
- Yang, J.; Yamato, M.; Kohno, C.; Nishimoto, A.; Sekine, H.; Fukai, F.; Okano, T. Cell sheet engineering: Recreating tissues without biodegradable scaffolds. Biomaterials 2005, 26, 6415–6422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamato, M.; Akiyama, Y.; Kobayashi, J.; Yang, J.; Kikuchi, A.; Okano, T. Temperature-responsive cell culture surfaces for regenerative medicine with cell sheet engineering. Prog. Polym. Sci. 2007, 32, 1123–1133. [Google Scholar] [CrossRef]
- Teles, M.N.O.; Santos, B.L.P.; Silva, D.P.; Teixeira, J.A.; Ruzene, D.S. A bibliometric description of lignin applicability for the removal of chemical pollutants in effluents. Water Air Soil Pollut. 2020, 231, 1–14. [Google Scholar] [CrossRef]
- Sweileh, W.M. Bibliometric analysis of peer-reviewed literature on antimicrobial stewardship from 1990 to 2019. Glob. Health 2021, 17, 1–14. [Google Scholar] [CrossRef]
- Almeida, F.L.C.; Castro, M.P.J.; Travália, B.M.; Forte, M.B.S. Trends in lipase immobilization: Bibliometric review and patent analysis. Process Biochem. 2021, 110, 37–51. [Google Scholar] [CrossRef]
- Salgado-Cruz, M.d.l.P.; Salgado-Cruz, J.; García-Hernández, A.B.; Calderón-Domínguez, G.; Gómez-Viquez, H.; Oliver-Espinoza, R.; Fernández-Martínez, M.C.; Yáñez-Fernández, J. Chitosan as a Coating for Biocontrol in Postharvest Products: A Bibliometric Review. Membranes 2021, 11, 421. [Google Scholar] [CrossRef]
- Ghule, B.; Laad, M. A Bibliometric Survey on Polymer Composites in Energy Storage Applications. Libr. Philos. Pract. 2020, 1–17. Available online: https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=8500&context=libphilprac (accessed on 6 November 2022).
- Khan, A.S.; Ur Rehman, S.; AlMaimouni, Y.K.; Ahmad, S.; Khan, M.; Ashiq, M. Bibliometric Analysis of Literature Published on Antibacterial Dental Adhesive from 1996–2020. Polymers 2020, 12, 2848. [Google Scholar] [CrossRef]
- Simmons, P.; McElroy, T.; Allen, A.R. A bibliometric review of artificial extracellular matrices based on tissue engineering technology literature: 1990 through 2019. Materials 2020, 13, 2891. [Google Scholar] [CrossRef]
- Abejón, R.; Pérez-Acebo, H.; Garea, A. A bibliometric analysis of research on supported ionic liquid membranes during the 1995–2015 period: Study of the main applications and trending topics. Membranes 2017, 7, 63. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, M.C.P.; Kieckbusch, T.G.; Perna, R.F.; Fujimoto, J.T.; Morales, S.A.V.; Romanelli, J.P. Trends on enzyme immobilization researches based on bibliometric analysis. Process Biochem. 2019, 76, 95–110. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Visualizing bibliometric networks. In Measuring Scholarly Impact; Springer: Berlin/Heidelberg, Germany, 2014; pp. 285–320. [Google Scholar]
- Aria, M.; Cuccurullo, C. bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Jeong, S.I.; Park, S.-C.; Park, S.-J.; Kim, E.-J.; Heo, H.; Park, J.-S.; Gwon, H.-J.; Lim, Y.-M.; Jang, M.-K. One-step synthesis of gene carrier via gamma irradiation and its application in tumor gene therapy. Int. J. Nanomed. 2018, 13, 525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turmanova, S.; Trifonov, A.; Kalaijiev, O.; Kostov, G. Radiation grafting of acrylic acid onto polytetrafluoroethylene films for glucose oxidase immobilization and its application in membrane biosensor. J. Membr. Sci. 1997, 127, 1–7. [Google Scholar] [CrossRef]
- Pino-Ramos, V.; Meléndez-Ortiz, H.; Ramos-Ballesteros, A.; Bucio, E. Radiation Grafting of Biopolymers and Synthetic Polymers. In Biopolymer Grafting: Applications; Elsevier: Oxford, UK, 2018. [Google Scholar]
- Alvarez-Lorenzo, C.; Bucio, E.; Burillo, G.; Concheiro, A. Medical devices modified at the surface by γ-ray grafting for drug loading and delivery. Expert Opin. Drug Deliv. 2010, 7, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Kumar, A. Radiation-induced graft copolymerization of N-vinyl imidazole onto moringa gum polysaccharide for making hydrogels for biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- García-Vargas, M.; González-Chomón, C.; Magariños, B.; Concheiro, A.; Alvarez-Lorenzo, C.; Bucio, E. Acrylic polymer-grafted polypropylene sutures for covalent immobilization or reversible adsorption of vancomycin. Int. J. Pharm. 2014, 461, 286–295. [Google Scholar] [CrossRef]
- Tang, Z.; Akiyama, Y.; Okano, T. Recent development of temperature-responsive cell culture surface using poly (N-isopropylacrylamide). J. Polym. Sci. Part B Polym. Phys. 2014, 52, 917–926. [Google Scholar] [CrossRef]
- Pasparakis, G.; Tsitsilianis, C. LCST polymers: Thermoresponsive nanostructured assemblies towards bioapplications. Polymer 2020, 211, 123146. [Google Scholar] [CrossRef]
- Jabbari, E.; Nozari, S. Swelling behavior of acrylic acid hydrogels prepared by γ-radiation crosslinking of polyacrylic acid in aqueous solution. Eur. Polym. J. 2000, 36, 2685–2692. [Google Scholar] [CrossRef]
- Costoya, A.; Becerra, L.E.V.; Meléndez-Ortiz, H.I.; Díaz-Gómez, L.; Mayer, C.; Otero, A.; Concheiro, A.; Bucio, E.; Alvarez-Lorenzo, C. Immobilization of antimicrobial and anti-quorum sensing enzymes onto GMA-grafted poly (vinyl chloride) catheters. Int. J. Pharm. 2019, 558, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Zuñiga-Zamorano, I.; Meléndez-Ortiz, H.I.; Costoya, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Poly (vinyl chloride) catheters modified with pH-responsive poly (methacrylic acid) with affinity for antimicrobial agents. Radiat. Phys. Chem. 2018, 142, 107–114. [Google Scholar] [CrossRef]
- Hidzir, N.M.; Radzali, N.A.M.; Rahman, I.A.; Shamsudin, S.A. Gamma irradiation-induced grafting of 2-hydroxyethyl methacrylate (HEMA) onto ePTFE for implant applications. Nucl. Eng. Technol. 2020, 52, 2320–2327. [Google Scholar] [CrossRef]
- Hiriart-Ramírez, E.; Contreras-García, A.; Garcia-Fernandez, M.J.; Concheiro, A.; Alvarez-Lorenzo, C.; Bucio, E. Radiation grafting of glycidyl methacrylate onto cotton gauzes for functionalization with cyclodextrins and elution of antimicrobial agents. Cellulose 2012, 19, 2165–2177. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Aoki, S.; Fujiwara, K.; Sugo, T.; Suzuki, K. Antimicrobial fabric adsorbed iodine produced by radiation-induced graft polymerization. Radiat. Phys. Chem. 2013, 84, 242–245. [Google Scholar] [CrossRef]
- Riquet, A.-M.; Delattre, J.; Vitrac, O.; Guinault, A. Design of modified plastic surfaces for antimicrobial applications: Impact of ionizing radiation on the physical and mechanical properties of polypropylene. Radiat. Phys. Chem. 2013, 91, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Ping, X.; Wang, M.; Xuewu, G. Surface modification of poly (ethylene terephthalate)(PET) film by gamma-ray induced grafting of poly (acrylic acid) and its application in antibacterial hybrid film. Radiat. Phys. Chem. 2011, 80, 567–572. [Google Scholar] [CrossRef]
- Lim, S.J.; Shin, I.H. Graft copolymerization of GMA and EDMA on PVDF to hydrophilic surface modification by electron beam irradiation. Nucl. Eng. Technol. 2020, 52, 373–380. [Google Scholar] [CrossRef]
- Stasica, P.; Rosiak, J.; Ciach, M.; Radek, M. Approach to construct hydrogel intervertebral disc implants—Experimental and numerical investigations. Eng. Biomater. 2000, 3, 9–14. [Google Scholar]
- Yoshii, F.; Makuuchi, K.; Sudradjat, A.; Darwis, D.; Razzak, M. Heat stability of radiation crosslinked poly (vinyl alcohol) hydrogel. Ika Kikaigaku 1992, 62, 285–290. [Google Scholar] [CrossRef]
- Darwis, D.; Stasica, P.; Razzak, M.T.; Rosiak, J.M. Characterization of poly (vinyl alcohol) hydrogel for prosthetic intervertebral disc nucleus. Radiat. Phys. Chem. 2002, 63, 539–542. [Google Scholar] [CrossRef]
- Lee, S.-H.; An, S.-J.; Lim, Y.-M.; Huh, J.-B. The efficacy of electron beam irradiated bacterial cellulose membranes as compared with collagen membranes on guided bone regeneration in peri-implant bone defects. Materials 2017, 10, 1018. [Google Scholar] [CrossRef] [PubMed]
- Hidzir, N.M.; Hill, D.J.; Martin, D.; Grøndahl, L. Radiation-induced grafting of acrylic acid onto expanded poly (tetrafluoroethylene) membranes. Polymer 2012, 53, 6063–6071. [Google Scholar] [CrossRef]
- Magaña, H.; Becerra, C.D.; Serrano-Medina, A.; Palomino, K.; Palomino-Vizcaíno, G.; Olivas-Sarabia, A.; Bucio, E.; Cornejo-Bravo, J.M. Radiation Grafting of a Polymeric Prodrug onto Silicone Rubber for Potential Medical/Surgical Procedures. Polymers 2020, 12, 1297. [Google Scholar] [CrossRef] [PubMed]
- Kyomoto, M.; Moro, T.; Saiga, K.; Hashimoto, M.; Ito, H.; Kawaguchi, H.; Takatori, Y.; Ishihara, K. Biomimetic hydration lubrication with various polyelectrolyte layers on cross-linked polyethylene orthopedic bearing materials. Biomaterials 2012, 33, 4451–4459. [Google Scholar] [CrossRef]
- Moro, T.; Kawaguchi, H.; Ishihara, K.; Kyomoto, M.; Karita, T.; Ito, H.; Nakamura, K.; Takatori, Y. Wear resistance of artificial hip joints with poly (2-methacryloyloxyethyl phosphorylcholine) grafted polyethylene: Comparisons with the effect of polyethylene cross-linking and ceramic femoral heads. Biomaterials 2009, 30, 2995–3001. [Google Scholar] [CrossRef]
- Meléndez-Ortiz, H.I.; Alvarez-Lorenzo, C.; Concheiro, A.; Jimenez-Paez, V.M.; Bucio, E. Modification of medical grade PVC with N-vinylimidazole to obtain bactericidal surface. Radiat. Phys. Chem. 2016, 119, 37–43. [Google Scholar] [CrossRef]
- Meléndez-Ortiz, H.I.; Alvarez-Lorenzo, C.; Burillo, G.; Magariños, B.; Concheiro, A.; Bucio, E. Radiation-grafting of N-vinylimidazole onto silicone rubber for antimicrobial properties. Radiat. Phys. Chem. 2015, 110, 59–66. [Google Scholar] [CrossRef]
- Valencia-Mora, R.A.; Zavala-Lagunes, E.; Bucio, E. Grafting of thermo-sensitive N-vinylcaprolactam onto silicone rubber through the direct radiation method. Radiat. Phys. Chem. 2016, 124, 155–158. [Google Scholar] [CrossRef]
- Razzak, M.T.; Otsuhata, K.; Tabata, Y.; Ohashi, F.; Takeuchi, A. Modification of natural rubber tubes for biomaterials. II. Radiation-induced grafting of N, N-dimethylaminoethylacrylate (DMAEA) onto natural rubber (NR) tubes. J. Appl. Polym. Sci. 1989, 38, 829–839. [Google Scholar] [CrossRef]
- Razzak, M.T.; Otsuhata, K.; Tabata, Y.; Ohashi, F.; Takeuchi, A. Modification of natural rubber tubes for biomaterials I. Radiation-induced grafting of N, N-dimethyl acrylamide onto natural rubber tubes. J. Appl. Polym. Sci. 1988, 36, 645–653. [Google Scholar] [CrossRef]
- Nowatzki, P.J.; Koepsel, R.R.; Stoodley, P.; Min, K.; Harper, A.; Murata, H.; Donfack, J.; Hortelano, E.R.; Ehrlich, G.D.; Russell, A.J. Salicylic acid-releasing polyurethane acrylate polymers as anti-biofilm urological catheter coatings. Acta Biomater. 2012, 8, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Hosny, A.E.-D.M.; Farrag, H.A.; Helmy, O.M.; Hagras, S.A.; El-Hag Ali, A. In-vitro evaluation of antibacterial and antibiofilm efficiency of radiation-modified polyurethane–ZnO nanocomposite to be used as a self-disinfecting catheter. J. Radiat. Res. Appl. Sci. 2020, 13, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, A.; Gupta, B. Radiation-induced graft copolymerization of methacrylic acid onto polypropylene fibers. I. Effect of synthesis conditions. J. Appl. Polym. Sci. 1985, 30, 2643–2653. [Google Scholar] [CrossRef]
- Singh, H.; Tyagi, P. Radiation induced grafting of methacrylic acid onto silk for the immobilization of antimicrobial drug for sustained delivery. Die Angew. Makromol. Chem. Appl. Macromol. Chem. Phys. 1989, 172, 87–102. [Google Scholar] [CrossRef]
- Tyagi, P.; Gupta, B.; Singh, H. Radiation-induced grafting of 2-hydroxyethyl methacrylate onto polypropylene for biomedical applications. II. Evaluation as antimicrobial suture. J. Macromol. Sci. Part A Pure Appl. Chem. 1993, 30, 303–313. [Google Scholar] [CrossRef]
- Plessier, C.; Gupta, B.; Chapiro, A. Modification of polypropylene fiber by radiation-induced graft copolymerization of acrylonitrile monomer. J. Appl. Polym. Sci. 1998, 69, 1343–1348. [Google Scholar] [CrossRef]
- Gupta, B.; Anjum, N.; Gulrez, S.; Singh, H. Development of antimicrobial polypropylene sutures by graft copolymerization. II. Evaluation of physical properties, drug release, and antimicrobial activity. J. Appl. Polym. Sci. 2007, 103, 3534–3538. [Google Scholar] [CrossRef]
- Yuan, F.; Wei, J.; Tang, E.-Q.; Zhao, K.-Y.; Xue, Y. Synthesis and Modification of Polypropylene by Radiation-induced Grafting. Int. J. Chem. 2009, 1, 75. [Google Scholar] [CrossRef] [Green Version]
- López-Saucedo, F.; Flores-Rojas, G.G.; López-Saucedo, J.; Magariños, B.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Antimicrobial silver-loaded polypropylene sutures modified by radiation-grafting. Eur. Polym. J. 2018, 100, 290–297. [Google Scholar] [CrossRef]
- López-Saucedo, F.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Radiation-grafting of vinyl monomers separately onto polypropylene monofilament sutures. Radiat. Phys. Chem. 2017, 132, 1–7. [Google Scholar] [CrossRef]
- Marisol Arteaga-Luna, M.; Hugo Pino-Ramos, V.; Magaña, H.; Bucio, E. Polymeric pro-drug sutures for potential local release of salicylic acid. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 792–799. [Google Scholar] [CrossRef]
- López-Saucedo, F.; Flores-Rojas, G.; Bucio, E.; Alvarez-Lorenzo, C.; Concheiro, A.; González-Antonio, O. Achieving antimicrobial activity through poly (N-methylvinylimidazolium) iodide brushes on binary-grafted polypropylene suture threads. MRS Communications 2017, 7, 938–946. [Google Scholar] [CrossRef]
- Tummalapalli, M.; Anjum, S.; Kumari, S.; Gupta, B. Antimicrobial surgical sutures: Recent developments and strategies. Polym. Rev. 2016, 56, 607–630. [Google Scholar] [CrossRef]
- Buchenska, J.; Slomkowski, S.; Tazbir, J.; Sobolewska, E. Antibacterial poly (ethylene terephthalate) yarn containing cephalosporin type antibiotic. Fibres Text. East. Eur. 2003, 11, 41–47. [Google Scholar]
- Anjum, N.; Gulrez, S.; Singh, H.; Gupta, B. Development of antimicrobial polypropylene sutures by graft polymerization. I. Influence of grafting conditions and characterization. J. Appl. Polym. Sci. 2006, 101, 3895–3901. [Google Scholar] [CrossRef]
- Gupta, B.; Jain, R.; Anjum, N.; Singh, H. Preirradiation grafting of acrylonitrile onto polypropylene monofilament for biomedical applications: I. Influence of synthesis conditions. Radiat. Phys. Chem. 2006, 75, 161–167. [Google Scholar] [CrossRef]
- Gupta, B.; Jain, R.; Singh, H. Preparation of antimicrobial sutures by preirradiation grafting onto polypropylene monofilament. Polym. Adv. Technol. 2008, 19, 1698–1703. [Google Scholar] [CrossRef]
- Gupta, B.; Jain, R.; Anjum, N.; Singh, H. Preparation of antimicrobial sutures by preirradiation grafting of acrylonitrile onto polypropylene monofilament. III. Hydrolysis of the grafted suture. J. Appl. Polym. Sci. 2004, 94, 2509–2516. [Google Scholar] [CrossRef]
- Jain, R.; Gupta, B.; Anjum, N.; Revagade, N.; Singh, H. Preparation of antimicrobial sutures by preirradiation grafting of acrylonitrile onto polypropylene monofilament. II. mechanical, physical, and thermal characteristics. J. Appl. Polym. Sci. 2004, 93, 1224–1229. [Google Scholar] [CrossRef]
- Wu, M.; Bao, B.; Yoshii, F.; Makuuchi, K. Irradiation of crosslinked, poly (vinyl alcohol) blended hydrogel for wound dressing. J. Radioanal. Nucl. Chem. 2001, 250, 391–395. [Google Scholar] [CrossRef]
- Zhao, L.; Mitomo, H.; Zhai, M.; Yoshii, F.; Nagasawa, N.; Kume, T. Synthesis of antibacterial PVA/CM-chitosan blend hydrogels with electron beam irradiation. Carbohydr. Polym. 2003, 53, 439–446. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Q.; Chen, X.; Yu, F.; Zhu, Z. Investigation of PVA/ws-chitosan hydrogels prepared by combined γ-irradiation and freeze-thawing. Carbohydr. Polym. 2008, 73, 401–408. [Google Scholar] [CrossRef]
- Abou Taleb, M.F.; Ismail, S.A.; El-Kelesh, N.A. Radiation synthesis and characterization of polyvinyl alcohol/methacrylic acid–gelatin hydrogel for vitro drug delivery. J. Macromol. Sci. Part A 2008, 46, 170–178. [Google Scholar] [CrossRef]
- Kaur, I.; Bhati, P.; Sharma, S. Radiation induced synthesis of (gelatin-co-PVA)-g-poly (AAc) copolymer as wound dressing material. Adv. Mater. Res. 2014, 3, 183. [Google Scholar] [CrossRef]
- Razzak, M.T.; Darwis, D. Irradiation of polyvinyl alcohol and polyvinyl pyrrolidone blended hydrogel for wound dressing. Radiat. Phys. Chem. 2001, 62, 107–113. [Google Scholar] [CrossRef]
- Casimiro, M.; Gil, M.; Leal, J. Suitability of gamma irradiated chitosan based membranes as matrix in drug release system. Int. J. Pharm. 2010, 395, 142–146. [Google Scholar] [CrossRef]
- Cabane, E.; Zhang, X.; Langowska, K.; Palivan, C.G.; Meier, W. Stimuli-responsive polymers and their applications in nanomedicine. Biointerphases 2012, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Stuart, M.A.C.; Huck, W.T.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef]
- Meléndez-Ortiz, I.H.; Bucio, E. Stimuli-sensitive behaviour of binary graft Co-polymers (PP-g-DMAEMA)-g-NIPAAm and (PP-g-4VP)-g-NIPAAm in acidic and basic medium. Des. Monomers Polym. 2009, 12, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Luk, J.Z.; Cooper-White, J.; Rintoul, L.; Taran, E.; Grøndahl, L. Functionalised polycaprolactone films and 3D scaffolds via gamma irradiation-induced grafting. J. Mater. Chem. B 2013, 1, 4171–4181. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Sreearunothai, P.; Opaprakasit, P. Development and Characterization of Photoinduced Acrylamide-Grafted Polylactide Films for Biomedical Applications. Int. J. Polym. Sci. 2017, 2017, 5651398. [Google Scholar] [CrossRef]
- Casimiro, M.H.; Gomes, S.R.; Rodrigues, G.; Leal, J.P.; Ferreira, L.M. Chitosan/Poly (vinylpyrrolidone) matrices obtained by gamma-irradiation for skin scaffolds: Characterization and preliminary cell response studies. Materials 2018, 11, 2535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casimiro, M.H.; Lancastre, J.J.; Rodrigues, A.P.; Gomes, S.R.; Rodrigues, G.; Ferreira, L.M. Chitosan-Based matrices prepared by gamma irradiation for tissue regeneration: Structural properties vs. preparation method. In Applications of Radiation Chemistry in the Fields of Industry, Biotechnology and Environment; Springer: Berlin/Heidelberg, Germany, 2017; pp. 121–145. [Google Scholar]
- Tang, Z.; Akiyama, Y.; Okano, T. Temperature-responsive polymer modified surface for cell sheet engineering. Polymers 2012, 4, 1478–1498. [Google Scholar] [CrossRef] [Green Version]
- Elloumi-Hannachi, I.; Yamato, M.; Okano, T. Cell sheet engineering: A unique nanotechnology for scaffold-free tissue reconstruction with clinical applications in regenerative medicine. J. Intern. Med. 2010, 267, 54–70. [Google Scholar] [CrossRef]
- Takahashi, H.; Nakayama, M.; Yamato, M.; Okano, T. Controlled chain length and graft density of thermoresponsive polymer brushes for optimizing cell sheet harvest. Biomacromolecules 2010, 11, 1991–1999. [Google Scholar] [CrossRef]
- Haraguchi, Y.; Shimizu, T.; Yamato, M.; Okano, T. Scaffold-free tissue engineering using cell sheet technology. RSC Adv. 2012, 2, 2184–2190. [Google Scholar] [CrossRef]
- Riquet, A.; Rohman, G.; Guinault, A.; Demilly, M. Surface modification of polypropylene by radiation grafting of hydrophilic monomers: Physicochemical properties. Surf. Eng. 2011, 27, 234–241. [Google Scholar] [CrossRef]
- Yamada, N.; Okano, T.; Sakai, H.; Karikusa, F.; Sawasaki, Y.; Sakurai, Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Die Makromol. Chem. Rapid Commun. 1990, 11, 571–576. [Google Scholar] [CrossRef]
- Shimizu, T.; Yamato, M.; Kikuchi, A.; Okano, T. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials 2003, 24, 2309–2316. [Google Scholar] [CrossRef]
- Akiyama, Y.; Kikuchi, A.; Yamato, M.; Okano, T. Ultrathin poly (N-isopropylacrylamide) grafted layer on polystyrene surfaces for cell adhesion/detachment control. Langmuir 2004, 20, 5506–5511. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Maeda, N.; Watanabe, H.; Yamamoto, K.; Nagai, S.; Kikuchi, A.; Tano, Y. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation 2004, 77, 379–385. [Google Scholar] [CrossRef]
- Fukumori, K.; Akiyama, Y.; Yamato, M.; Kobayashi, J.; Sakai, K.; Okano, T. Temperature-responsive glass coverslips with an ultrathin poly (N-isopropylacrylamide) layer. Acta Biomater. 2009, 5, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Yamato, M.; Okano, T. Preparation of poly (N-isopropylacrylamide) grafted polydimethylsiloxane by using electron beam irradiation. J. Robot. Mechatron. 2013, 25, 631–636. [Google Scholar] [CrossRef]
- Kumar, P.A.; Sreenivasan, K.; Kumary, T. Alternate method for grafting thermoresponsive polymer for transferring in vitro cell sheet structures. J. Appl. Polym. Sci. 2007, 105, 2245–2251. [Google Scholar] [CrossRef]
- von Recum, H.; Okano, T.; Kim, S.W. Growth factor release from thermally reversible tissue culture substrates. J. Control. Release 1998, 55, 121–130. [Google Scholar] [CrossRef]
- Shimizu, T.; Yamato, M.; Kikuchi, A.; Okano, T. Two-dimensional manipulation of cardiac myocyte sheets utilizing temperature-responsive culture dishes augments the pulsatile amplitude. Tissue Eng. 2001, 7, 141–151. [Google Scholar] [CrossRef]
- Kobayashi, J.; Okano, T. Fabrication of a thermoresponsive cell culture dish: A key technology for cell sheet tissue engineering. Sci. Technol. Adv. Mater. 2010, 11, 014111. [Google Scholar] [CrossRef]
- Muñoz-Muñoz, F.; Ruiz, J.-C.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Novel interpenetrating smart polymer networks grafted onto polypropylene by gamma radiation for loading and delivery of vancomycin. Eur. Polym. J. 2009, 45, 1859–1867. [Google Scholar] [CrossRef]
- Sponchioni, M.; Palmiero, U.C.; Moscatelli, D. Thermo-responsive polymers: Applications of smart materials in drug delivery and tissue engineering. Mater. Sci. Eng. C 2019, 102, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.; Paul, A.; Sen, S.O.; Sen, K.K. Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications. Asian J. Pharm. Sci. 2015, 10, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Demirdirek, B.; Uhrich, K.E. Novel salicylic acid-based chemically crosslinked pH-sensitive hydrogels as potential drug delivery systems. Int. J. Pharm. 2017, 528, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-González, B.; Meléndez-Ortiz, H.I.; Díaz-Gómez, L.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Silicone rubber modified with methacrylic acid to host antiseptic drugs. Macromol. Mater. Eng. 2014, 299, 1240–1250. [Google Scholar] [CrossRef]
- Kayal, S. Thermoresponsive magnetic/polymer composite nanoparticles for biomedical applications. Mater. Today Proc. 2021, 41, 1116–1119. [Google Scholar] [CrossRef]
- Mutalik, S.; Suthar, N.A.; Managuli, R.S.; Shetty, P.K.; Avadhani, K.; Kalthur, G.; Kulkarni, R.V.; Thomas, R. Development and performance evaluation of novel nanoparticles of a grafted copolymer loaded with curcumin. Int. J. Biol. Macromol. 2016, 86, 709–720. [Google Scholar] [CrossRef] [Green Version]
- Magaña, H.; Palomino, K.; Cornejo-Bravo, J.M.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Radiation-grafting of acrylamide onto silicone rubber films for diclofenac delivery. Radiat. Phys. Chem. 2015, 107, 164–170. [Google Scholar] [CrossRef]
- Melendez-Ortiz, H.I.; Díaz-Rodríguez, P.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Binary graft modification of polypropylene for anti-inflammatory drug–device combo products. J. Pharm. Sci. 2014, 103, 1269–1277. [Google Scholar] [CrossRef]
- De Oliveira, M.; Parra, D.; Amato, V.; Lugão, A. Hydrogel membranes of PVAl/clay by gamma radiation. Radiat. Phys. Chem. 2013, 84, 111–114. [Google Scholar] [CrossRef]
- Gagliardi, M. In vitro haematic proteins adsorption and cytocompatibility study on acrylic copolymer to realise coatings for drug-eluting stents. Mater. Sci. Eng. C 2012, 32, 2445–2451. [Google Scholar] [CrossRef]
- Burillo, G.; Bucio, E.; Arenas, E.; Lopez, G.P. Temperature and pH-sensitive swelling behavior of binary DMAEMA/4VP grafts on poly (propylene) films. Macromol. Mater. Eng. 2007, 292, 214–219. [Google Scholar] [CrossRef]
- Bucio, E.; Burillo, G. Radiation grafting of pH and thermosensitive N-isopropylacrylamide and acrylic acid onto PTFE films by two-steps process. Radiat. Phys. Chem. 2007, 76, 1724–1727. [Google Scholar] [CrossRef]
- Stoica-Guzun, A.; Stroescu, M.; Tache, F.; Zaharescu, T.; Grosu, E. Effect of electron beam irradiation on bacterial cellulose membranes used as transdermal drug delivery systems. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2007, 265, 434–438. [Google Scholar] [CrossRef]
- Casimiro, M.; Gil, M.; Leal, J. Drug release assays from new chitosan/pHEMA membranes obtained by gamma irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2007, 265, 406–409. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Fuentes, Y.S.; Bucio, E.; Burillo, G. Radiation-induced grafting of N-isopropylacrylamide and acrylic acid onto polypropylene films by two step method. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2007, 265, 183–186. [Google Scholar] [CrossRef]
- Ikram, S.; Kumari, M.; Gupta, B. Thermosensitive membranes by radiation-induced graft polymerization of N-isopropyl acrylamide/acrylic acid on polypropylene nonwoven fabric. Radiat. Phys. Chem. 2011, 80, 50–56. [Google Scholar] [CrossRef]
- Contreras-García, A.; Alvarez-Lorenzo, C.; Taboada, C.; Concheiro, A.; Bucio, E. Stimuli–responsive networks grafted onto polypropylene for the sustained delivery of NSAIDs. Acta Biomater. 2011, 7, 996–1008. [Google Scholar] [CrossRef]
- Piao, M.-H.; Yang, D.-S.; Yoon, K.-R.; Lee, S.-H.; Choi, S.-H. Development of an electrogenerated chemiluminescence biosensor using carboxylic acid-functionalized MWCNT and Au nanoparticles. Sensors 2009, 9, 1662–1677. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.-H.; Lee, J.-C.; Choi, S.-H. Tyrosinase-immobilized biosensor based on the functionalized hydroxyl group-MWNT and detection of phenolic compounds in red wines. J. Sens. 2009, 2009, 916515. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.-S.; Jung, D.-J.; Choi, S.-H. One-step functionalization of multi-walled carbon nanotubes by radiation-induced graft polymerization and their application as enzyme-free biosensors. Radiat. Phys. Chem. 2010, 79, 434–440. [Google Scholar] [CrossRef]
- Kim, S.-K.; Kwen, H.-D.; Choi, S.-H. Fabrication of a microbial biosensor based on QD-MWNT supports by a one-step radiation reaction and detection of phenolic compounds in red wines. Sensors 2011, 11, 2001–2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.-I.; Kang, H.-Y.; Lee, J.-C.; Choi, S.-H. Fabrication of a multi-walled nanotube (MWNT) ionic liquid electrode and its application for sensing phenolics in red wines. Sensors 2009, 9, 6701–6714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.-J.; Chung, D.-J.; Oh, S.-H.; Choi, S.-H. Introduction of bifunctional group onto MWNT by radiation-induced graft polymerization and its use as biosensor-supporting materials. J. Nanomater. 2012, 2012, 127613. [Google Scholar] [CrossRef]
- Feil, H.; Bae, Y.H.; Feijen, J.; Kim, S.W. Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers. Macromolecules 1993, 26, 2496–2500. [Google Scholar] [CrossRef]
- Pino-Ramos, V.H.; Flores-Rojas, G.G.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Graft copolymerization by ionization radiation, characterization, and enzymatic activity of temperature-responsive SR-g-PNVCL loaded with lysozyme. React. Funct. Polym. 2018, 126, 74–82. [Google Scholar] [CrossRef]
- Abd Halin, N.I.; Al-Khatib, M.F.R.; Salleh, H.M.; Nasef, M.M. Preparation and Candida rugosa Lipase Immobilization on Nylon-6 Grafted and Aminated (Polyvinyl Benzyl Chloride) Microfibers. Bull. Chem. React. Eng. Catal. 2019, 14, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Alkhatib, M.; Bahrudin, N.A.; SALLEH, H.M.; Nasef, M.M.; Ting, T.M. Lipase immobilization on fibers grafted with polyglycidyl methachrylate. IIUM Eng. J. 2019, 20, 12–23. [Google Scholar] [CrossRef]
- Kamal, H.; Sabry, G.M.; Lotfy, S.; Abdallah, N.M.; Rosiak, J.; Hegazy, E.s.A. Immobilization of glucoamylase on polypropylene fibers modified by radiation induced graft copolymerization. J. Macromol. Sci. Part A Pure Appl. Chem. 2007, 45, 65–75. [Google Scholar] [CrossRef]
- Garnett, J.; Jankiewicz, S.; Levot, R.; Sangster, D. Insolubilisation of biologically active materials with novel radiation graft copolymers. Radiat. Phys. Chem. 1985, 25, 509–516. [Google Scholar] [CrossRef]
- Dong, L.C.; Hoffman, A.S. Thermally reversible hydrogels: III. Immobilization of enzymes for feedback reaction control. J. Control. Release 1986, 4, 223–227. [Google Scholar] [CrossRef]
- Dong, L.C.; Hoffman, A.S. A new method for immobilization of biomolecules using preirradiation grafting at low temperature. Int. J. Radiat. Appl. Instrum. Part C. Radiat. Phys. Chem. 1986, 28, 177–182. [Google Scholar] [CrossRef]
- Hongfei, H.; Guanghui, W.; Jilan, W. Immobilization of peroxidase on SPEU film via radiation grafting. Int. J. Radiat. Appl. Instrum. Part C. Radiat. Phys. Chem. 1988, 31, 761–767. [Google Scholar] [CrossRef]
- Schmidt, M.; Prager, A.; Schönherr, N.; Gläser, R.; Schulze, A. Reagent-free immobilization of industrial lipases to develop lipolytic membranes with self-cleaning surfaces. Membranes 2022, 12, 599. [Google Scholar] [CrossRef]
- Xu, C.; Huang, W.; Zhou, Y.; Yan, D.; Chen, S.; Huang, H. Graft copolymerization of N-vinyl-2-pyrrolidone onto pre-irradiated poly (vinylidene fluoride) powder. Radiat. Phys. Chem. 2012, 81, 426–431. [Google Scholar] [CrossRef]
- Qin, Q.; Hou, Z.; Lu, X.; Bian, X.; Chen, L.; Shen, L.; Wang, S. Microfiltration membranes prepared from poly (N-vinyl-2-pyrrolidone) grafted poly (vinylidene fluoride) synthesized by simultaneous irradiation. J. Membr. Sci. 2013, 427, 303–310. [Google Scholar] [CrossRef]
- Shen, L.; Feng, S.; Li, J.; Chen, J.; Li, F.; Lin, H.; Yu, G. Surface modification of polyvinylidene fluoride (PVDF) membrane via radiation grafting: Novel mechanisms underlying the interesting enhanced membrane performance. Sci. Rep. 2017, 7, 2721. [Google Scholar] [CrossRef] [Green Version]
- Xi, Z.-Y.; Xu, Y.-Y.; Zhu, L.-P.; Zhu, B.-K. Modification of polytetrafluoroethylene porous membranes by electron beam initiated surface grafting of binary monomers. J. Membr. Sci. 2009, 339, 33–38. [Google Scholar] [CrossRef]
- Deng, B.; Yang, X.; Xie, L.; Li, J.; Hou, Z.; Yao, S.; Liang, G.; Sheng, K.; Huang, Q. Microfiltration membranes with pH dependent property prepared from poly (methacrylic acid) grafted polyethersulfone powder. J. Membr. Sci. 2009, 330, 363–368. [Google Scholar] [CrossRef]
- Deng, B.; Li, J.; Hou, Z.; Yao, S.; Shi, L.; Liang, G.; Sheng, K. Microfiltration membranes prepared from polyethersulfone powder grafted with acrylic acid by simultaneous irradiation and their pH dependence. Radiat. Phys. Chem. 2008, 77, 898–906. [Google Scholar] [CrossRef]
- Mok, S.; Worsfold, D.; Fouda, A.; Matsuura, T. Surface modification of polyethersulfone hollow-fiber membranes by γ-ray irradiation. J. Appl. Polym. Sci. 1994, 51, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Van der Bruggen, B. Chemical modification of polyethersulfone nanofiltration membranes: A review. J. Appl. Polym. Sci. 2009, 114, 630–642. [Google Scholar] [CrossRef]
- Schmidt, M.; Zahn, S.; Gehlhaar, F.; Prager, A.; Griebel, J.; Kahnt, A.; Knolle, W.; Konieczny, R.; Gläser, R.; Schulze, A. Radiation-Induced Graft Immobilization (RIGI): Covalent Binding of Non-Vinyl Compounds on Polymer Membranes. Polymers 2021, 13, 1849. [Google Scholar] [CrossRef] [PubMed]
- Adem, E.; Avalos-Borja, M.; Bucio, E.; Burillo, G.; Castillon, F.; Cota, L. Surface characterization of binary grafting of AAc/NIPAAm onto poly (tetrafluoroethylene)(PTFE). Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2005, 234, 471–476. [Google Scholar] [CrossRef]
- Casimiro, M.; Leal, J.; Gil, M. Characterisation of gamma irradiated chitosan/pHEMA membranes for biomedical purposes. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2005, 236, 482–487. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Gu, Z. Studies on acrylic acid–grafted polyester fabrics by electron beam preirradiation method. I. Effects of process parameters on graft ratio and characterization of grafting products. J. Appl. Polym. Sci. 2003, 89, 3931–3938. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, J.H.; Nho, Y.C.; Kwon, O.H. Antibacterial activities of acrylic acid-grafted polypropylene fabric and its metallic salt. J. Appl. Polym. Sci. 1998, 69, 2213–2220. [Google Scholar] [CrossRef]
- Hassan, M.S.; Ibrahim, H.M. Characterization and antimicrobial properties of metal complexes of polypropylene fibers grafted with acrylic acid using gamma irradiation. Polym. Adv. Technol. 2016, 27, 532–541. [Google Scholar] [CrossRef]
- Montoya-Villegas, K.A.; Ramírez-Jiménez, A.; Licea-Claverie, Á.; Pérez-Sicairos, S.; Bucio, E.; Bernáldez-Sarabia, J.; Licea-Navarro, A.F. Surface Modification of Polyester-Fabric with Hydrogels and Silver Nanoparticles: Photochemical Versus Gamma Irradiation Methods. Materials 2019, 12, 3284. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Bhardwaj, Y.; Rawat, K.; Sabharwal, S. Radiation-induced grafting of vinylbenzyltrimethylammonium chloride (VBT) onto cotton fabric and study of its anti-bacterial activities. Radiat. Phys. Chem. 2005, 73, 175–182. [Google Scholar] [CrossRef]
- Flores-Rojas, G.; López-Saucedo, F.; Vázquez, E.; Hernández-Mecinas, E.; Huerta, L.; Cedillo, G.; Concheiro, A.; Alvarez-Lorenzo, C.; Bucio, E. Synthesis of polyamide-6@ cellulose microfilms grafted with N-vinylcaprolactam using gamma-rays and loading of antimicrobial drugs. Cellulose 2020, 27, 2785–2801. [Google Scholar] [CrossRef]
- Huang, K.-S.; Yang, C.-H.; Huang, S.-L.; Chen, C.-Y.; Lu, Y.-Y.; Lin, Y.-S. Recent advances in antimicrobial polymers: A mini-review. Int. J. Mol. Sci. 2016, 17, 1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morais, D.S.; Guedes, R.M.; Lopes, M.A. Antimicrobial approaches for textiles: From research to market. Materials 2016, 9, 498. [Google Scholar] [CrossRef] [PubMed]
- Seino, S.; Imoto, Y.; Kitagawa, D.; Kubo, Y.; Kosaka, T.; Kojima, T.; Nitani, H.; Nakagawa, T.; Yamamoto, T.A. Radiochemical synthesis of silver nanoparticles onto textile fabrics and their antibacterial activity. J. Nucl. Sci. Technol. 2016, 53, 1021–1027. [Google Scholar] [CrossRef]
- Ferraz, C.C.; Varca, G.H.; Ruiz, J.-C.; Lopes, P.S.; Mathor, M.B.; Lugão, A.B.; Bucio, E. Radiation-grafting of thermo-and pH-responsive poly (N-vinylcaprolactam-co-acrylic acid) onto silicone rubber and polypropylene films for biomedical purposes. Radiat. Phys. Chem. 2014, 97, 298–303. [Google Scholar] [CrossRef]
- Li, X.; Li, P.; Saravanan, R.; Basu, A.; Mishra, B.; Lim, S.H.; Su, X.; Tambyah, P.A.; Leong, S.S.J. Antimicrobial functionalization of silicone surfaces with engineered short peptides having broad spectrum antimicrobial and salt-resistant properties. Acta Biomater. 2014, 10, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Goel, N.; Kumar, V.; Rao, M.; Bhardwaj, Y.; Sabharwal, S. Functionalization of cotton fabrics by radiation induced grafting of quaternary salt to impart antibacterial property. Radiat. Phys. Chem. 2011, 80, 1233–1241. [Google Scholar] [CrossRef]
- Yang, J.M.; Lin, H.T.; Wu, T.H.; Chen, C.C. Wettability and antibacterial assessment of chitosan containing radiation-induced graft nonwoven fabric of polypropylene-g-acrylic acid. J. Appl. Polym. Sci. 2003, 90, 1331–1336. [Google Scholar] [CrossRef]
- Terada, A.; Yuasa, A.; Tsuneda, S.; Hirata, A.; Katakai, A.; Tamada, M. Elucidation of dominant effect on initial bacterial adhesion onto polymer surfaces prepared by radiation-induced graft polymerization. Colloids Surf. B. Biointerfaces 2005, 43, 99–107. [Google Scholar] [CrossRef]
- Murata, H.; Koepsel, R.R.; Matyjaszewski, K.; Russell, A.J. Permanent, non-leaching antibacterial surfaces—2: How high density cationic surfaces kill bacterial cells. Biomaterials 2007, 28, 4870–4879. [Google Scholar] [CrossRef]
- Anjum, N.; Bellon-Fontaine, M.N.; Herry, J.M.; Riquet, A.M. A novel process to develop modified polymeric surfaces for the analysis of bacterial adhesion: Surface properties and adhesion test. J. Appl. Polym. Sci. 2008, 109, 1746–1756. [Google Scholar] [CrossRef]
- Huang, C.; Wang, H.; Xu, Y.H. Functional finishing on silk fabric with acrylamide monomer and chitosan. Adv. Mater. Res. 2011, 175–176, 696–702. [Google Scholar]
- Ye, F.; Huang, C.; Jiang, X.; He, W.; Gao, X.; Ma, L.; Ao, J.; Xu, L.; Wang, Z.; Li, Q. Reusable fibrous adsorbent prepared via Co-radiation induced graft polymerization for iodine adsorption. Ecotoxicol. Environ. Saf. 2020, 203, 111021. [Google Scholar] [CrossRef] [PubMed]
- Goel, N.; Rao, M.; Kumar, V.; Bhardwaj, Y.; Chaudhari, C.; Dubey, K.; Sabharwal, S. Synthesis of antibacterial cotton fabric by radiation-induced grafting of [2-(Methacryloyloxy) ethyl] trimethylammonium chloride (MAETC) onto cotton. Radiat. Phys. Chem. 2009, 78, 399–406. [Google Scholar] [CrossRef]
- Salmieri, S.; Khan, R.A.; Safrany, A.; Lacroix, M. Gamma rays-induced 2-hydroxyethyl methacrylate graft copolymerization on methylcellulose-based films: Structure analysis and physicochemical properties. Ind. Crops Prod. 2015, 70, 64–71. [Google Scholar] [CrossRef]
- Hoffman, A. Radiation technology for immobilization of bioactive materials. In IAEA Publication TECDOC-486; IAEA: Vienna, Austria, 1988. [Google Scholar]
- IAEA. International Atomic Energy Agency, Radiation Processing Technology Applications in Bioengineering; IAEA: Vienna, Austria, 1994. [Google Scholar]
- IAEA. Radiation Synthesis and Modification of Polymers for Biomedical Applications; International Atomic Energy Agency: Vienna, Austria, 2002; Volume 34. [Google Scholar]
- IAEA. Nanoscale Radiation Engineering of Advanced Materials for Potential Biomedical Applications; International Atomic Energy Agency: Vienna, Austria, 2015. [Google Scholar]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Guimard, N.K.; Gomez, N.; Schmidt, C.E. Conducting polymers in biomedical engineering. Prog. Polym. Sci. 2007, 32, 876–921. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive polymers for biomedical applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef] [Green Version]
- Ramanavičius, A.; Ramanavičienė, A.; Malinauskas, A. Electrochemical sensors based on conducting polymer—Polypyrrole. Electrochim. Acta 2006, 51, 6025–6037. [Google Scholar] [CrossRef]
- Hasan, J.; Crawford, R.J.; Ivanova, E.P. Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Webb, R.C.; Bonifas, A.P.; Behnaz, A.; Zhang, Y.; Yu, K.J.; Cheng, H.; Shi, M.; Bian, Z.; Liu, Z.; Kim, Y.-S. Ultrathin conformal devices for precise and continuous thermal characterization of human skin. Nat. Mater. 2013, 12, 938–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Peppas, N.A. Synthesis and characterization of pH-and temperature-sensitive poly (methacrylic acid)/poly (N-isopropylacrylamide) interpenetrating polymeric networks. Macromolecules 2000, 33, 102–107. [Google Scholar] [CrossRef]
- Kingshott, P.; Wei, J.; Bagge-Ravn, D.; Gadegaard, N.; Gram, L. Covalent attachment of poly (ethylene glycol) to surfaces, critical for reducing bacterial adhesion. Langmuir 2003, 19, 6912–6921. [Google Scholar] [CrossRef]
- Kobayashi, M.; Chang, Y.-S.; Oka, M. A two year in vivo study of polyvinyl alcohol-hydrogel (PVA-H) artificial meniscus. Biomaterials 2005, 26, 3243–3248. [Google Scholar] [CrossRef]
- Chen, C. Science mapping: A systematic review of the literature. J. Data Inf. Sci. 2017, 2, 1–40. [Google Scholar] [CrossRef]
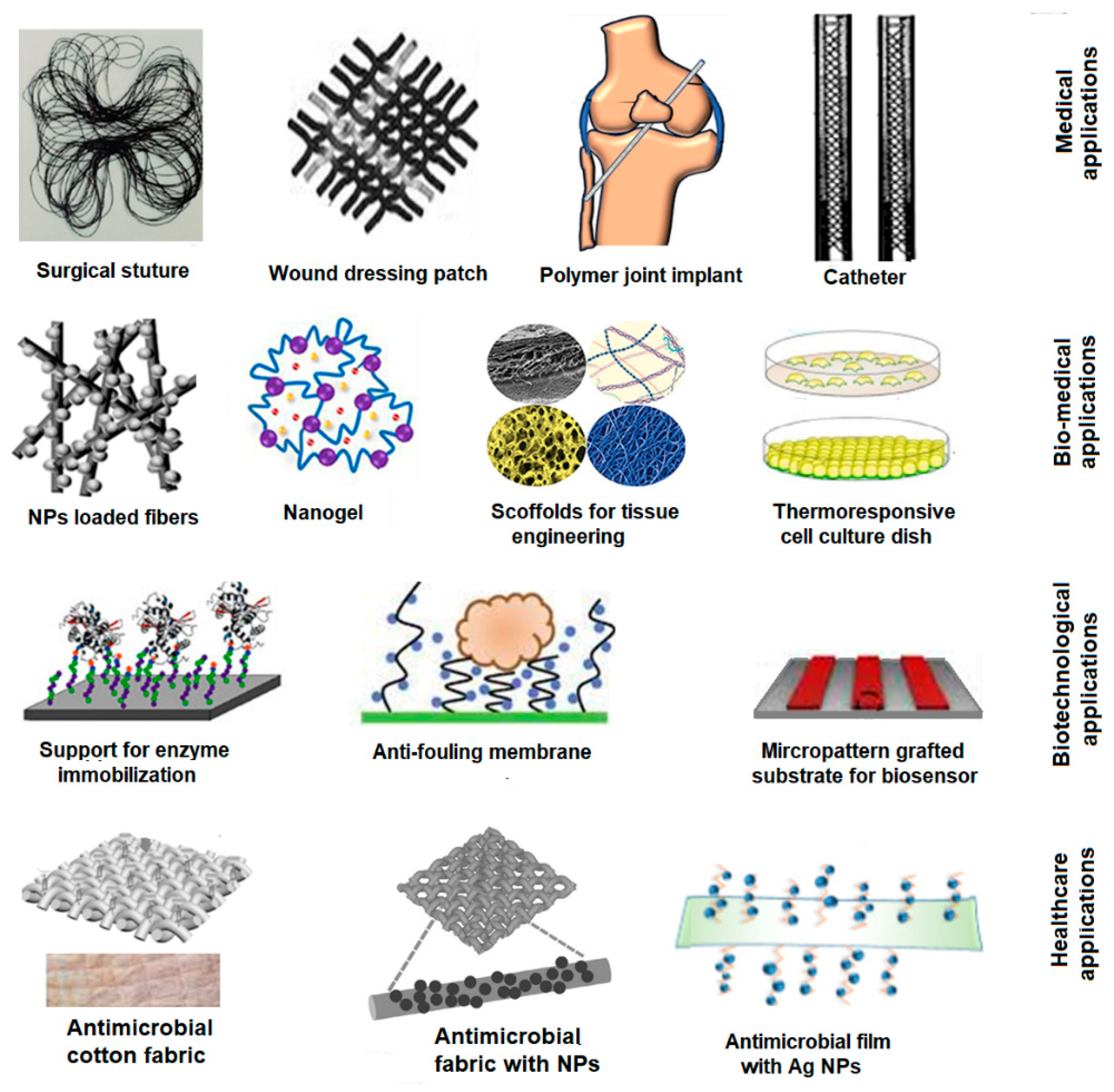

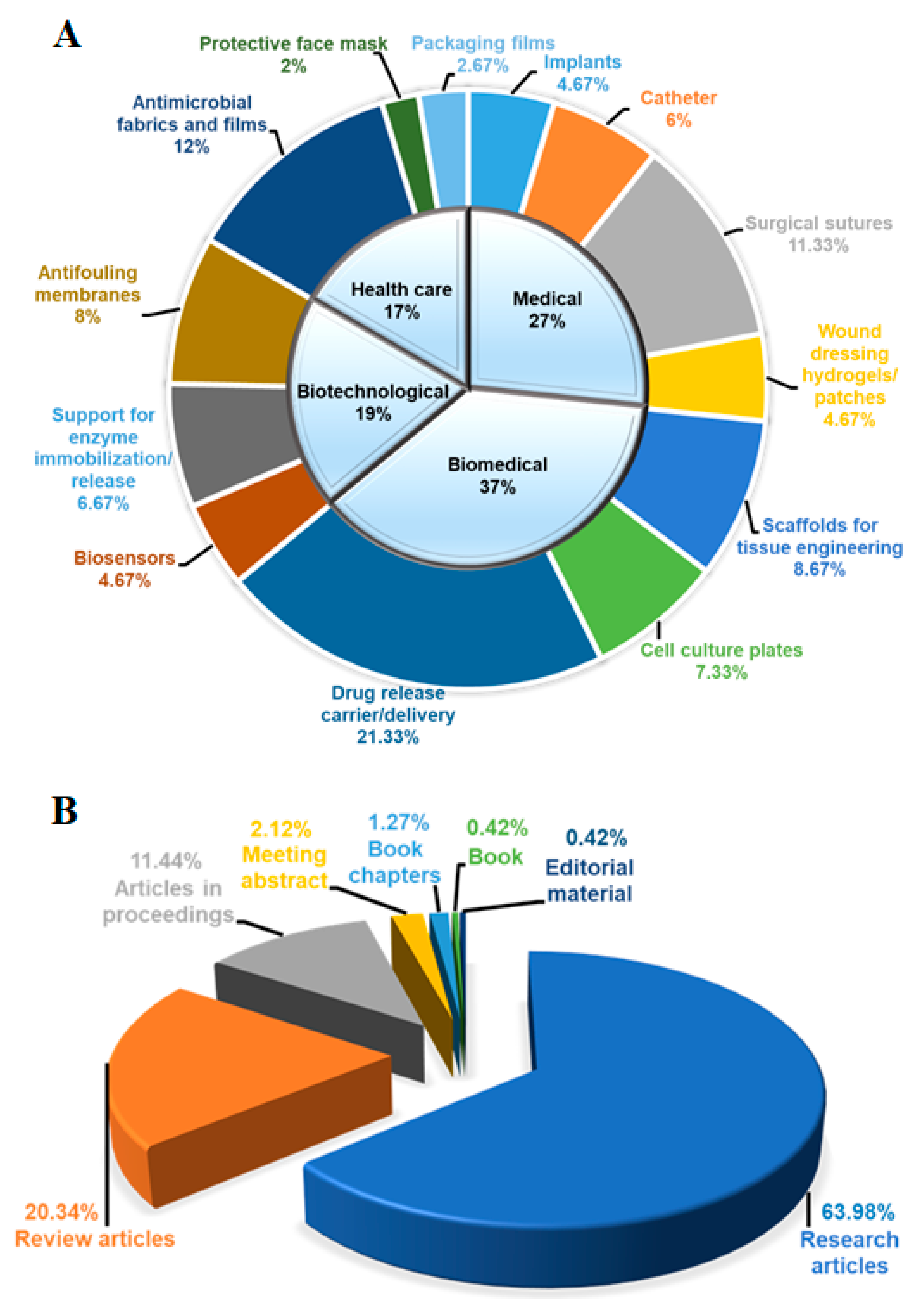



| Field | Applications | References |
|---|---|---|
| Medical | Implants | [60,67,68,69,70,71,72,73,74] |
| Catheter | [58,59,75,76,77,78,79,80,81] | |
| Surgical sutures | [54,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98] | |
| Wound dressing hydrogels/patches | [61,99,100,101,102,103,104,105] | |
| Biomedical | Scaffolds for tissue engineering | [56,106,107,108,109,110,111,112,113,114,115,116,117] |
| Cell culture plates | [55,118,119,120,121,122,123,124,125,126,127] | |
| Drug release carrier/delivery | [49,50,52,53,54,83,102,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152] | |
| Biotechnological | Biosensors | [50,146,147,148,149,150,151] |
| Support for enzyme immobilization/release | [58,153,154,155,156,157,158,159,160,161] | |
| Antifouling membranes | [66,162,163,164,165,166,167,168,169,170,171,172] | |
| Health care | Antimicrobial fabrics and films | [63,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189] |
| Protective face mask | [62,63,190] | |
| Packaging films | [64,65,191,192] |
| Source | TP (%), 1985–2021 | TC | Publisher | Country | IF 2021 | Quartile (Q) |
|---|---|---|---|---|---|---|
| Radiation Physics and Chemistry | 28 (11.96) | 904 | Elsevier | UK | 2.858 | 1 |
| Journal of Applied Polymer Science | 16 (6.83) | 856 | Wiley | USA | 3.057 | 2 |
| ACTA Biomaterialia | 9 (3.84) | 640 | Elsevier | Netherlands | 10.633 | 1 |
| Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms | 8 (3.40) | 395 | Elsevier | Netherlands | 1.377 | 2 |
| Progress in Polymer Science | 8 (3.40) | 5506 | Elsevier | UK | 31.281 | 1 |
| Biomaterials | 9 (3.01) | 2241 | Elsevier | UK | 12.479 | 1 |
| Polymer | 6 (2.56) | 754 | Elsevier | Netherlands | 4.967 | 1 |
| Macromolecules | 5 (2.13) | 378 | American Chemical Society | USA | 6.057 | 1 |
| Materials | 5 (2.13) | 270 | MDPI | Switzerland | 3.748 | 2 |
| Langmuir | 4 (1.71) | 1010 | American Chemical Society | USA | 4.331 | 1 |
| Rank | Title, Ref. | DOI | First Author—Corresponding Author * | Countries’ Contribution | Source, (Q 2021), IF 2021 | TC (1985–2021) | C/Y * | Open Access Designation |
|---|---|---|---|---|---|---|---|---|
| 1 | Emerging applications of stimuli-responsive polymer materials, [107] | 10.1038/NMAT2614 | Stuart, Martien A. Cohen—Igor Luzinov *, Sergiy Minko * | Netherlands, UK, USA | Nature materials, (1), 43.841 | 4011 | 364.63 | - |
| 2 | Hydrogels in biology and medicine: from molecular principles to bionanotechnology, [197] | 10.1002/adma.200501612 | Peppas, Nicholas A. *—Langer, Robert * | USA | Advanced materials, (1), 32.086 | 2689 | 179.26 | - |
| 3 | Foreign body reaction to biomaterials, [198] | 10.1016/j.smim.2007.11.004 | Anderson, James M. -Analiz Rodriguez * | USA | Seminars in immunology, (1), 8.856 | 2648 | 203.69 | Green accepted |
| 4 | Stimuli-responsive polymers and their bioconjugates, [29] | 10.1016/j.progpolymsci.2004.08.003 | Eun Seok Gil—Samuel M.Hudson * | USA | Progress in polymer science (1), 31.281 | 1976 | 116.23 | - |
| 5 | Conducting polymers in biomedical engineering, [199] | 10.1016/j.progpolymsci.2007.05.012 | Nathalie K.Guimard—Christine E.Schmidt * | USA | Progress in polymer science (1), 31.281 | 1059 | 75.64 | - |
| 6 | Conductive polymers: towards a smart biomaterial for tissue engineering, [200] | 10.1016/j.actbio.2014.02.015 | Richard Balint—Sarah H.Cartmell * | UK | Acta Biomaterialia, (1), 10.121 | 860 | 122.85 | Green published, hybrid |
| 7 | Polymeric materials with antimicrobial activity, [62] | - | Alexandra Muñoz-Bonilla *—MartaFernández-García * | Spain | Progress in polymer science (1), 31.281 | 816 | 90.66 | - |
| 8 | Thermoresponsive Polymers for Biomedical Applications, [201] | 10.3390/polym3031215 | Mark A. Ward * | UK | Polymers (1), 4.967 | 678 | 67.8 | Green submitted, gold |
| 9 | Electrochemical sensors based on conducting polymer- polypyrrole, [202] | 10.1016/j.electacta.2005.11.052 | Arunas Ramanaviciu * | Lithuania | Electrochimica Acta (1), 6.901 | 640 | 40 | Open access |
| 10 | Antibacterial surfaces: the quest for a new generation of biomaterials, [203] | 10.1016/j.tibtech.2013.01.017 | JafarHasan, Elena P. Ivanova * | Australia | Trends in biotechnology (1), 19.53 | 548 | 60.88 | Open Access |
| Rank | Title/Reference | Application | DOI | First Author—Research Leader * | Countries’ Contribution | Source, (Q 2021), (IF 2021) | TC (1985–2021) | C/Y |
|---|---|---|---|---|---|---|---|---|
| 1 | Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers, [152] | Controlled drug delivery | 10.1021/ma00062a016 | H. Feil * | Netherlands | Macromolecules, (1), (5.985) | 919 | 32.82 |
| 2 | Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells, [118] | Cell culture | 10.1002/marc.1990.030111109 | N Yamada/T. Okano * | Japan | Die makromolekulare chemie, rapid communication, (-), (4.839) | 750 | 35.71 |
| 3 | Ultrathin conformal devices for precise and continuous thermal characterization of human skin, [204] | Biosensor | doi.org/10.1038/nmat3755 | R. Chad Webb/J. A. Rogers * | USA, China, Singapore | Nature materials, (1), (47.656) | 689 | 86.12 |
| 4 | Permanent, non-leaching antibacterial surfaces—2: How high density cationic surfaces kill bacterial cells, [187] | Antimicrobial surface | 10.1016/j.biomaterials.2007.06.012 | H. Murata/A. J. Russell * | USA | Biomaterials, (1), (12.479) | 492 | 35.14 |
| 5 | Synthesis and characterization of pH- and temperature-sensitive poly(methacrylic acid)/poly(N-isopropylacrylamide) interpenetrating polymeric networks, [205] | Controlled drug delivery | 10.1021/ma00128a007 | C. S. Brazel * | USA | Macromolecules (1), (5.985) | 436 | 16.76 |
| 6 | Ultrathin poly(N-isopropylacrylamide) grafted layer on polystyrene surfaces for cell adhesion/detachment control, [120] | Cell culture plates | 10.1021/la036139f | Y. Akiyama/ T. Okano * | Japan | Langmuir, (1), (4.331) | 432 | 25.41 |
| 7 | Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface, [121] | Cell culture plates | 10.1097/01.TP.0000110320.45678.30 | K. Nishida/ T. Okano * | Japan | Transplantation, (2), (4.74) | 424 | 24.94 |
| 8 | Covalent attachment of poly(ethylene glycol) to surfaces, critical for reducing bacterial adhesion, [206] | Antifouling surface | 10.1021/LA034032M | P. Kingshott * | Denmark | Langmuir, (1), (4.331) | 266 | 14.77 |
| 9 | A two year in vivo study of polyvinyl alcohol-hydrogel (PVA-H) artificial meniscus, [207] | Knee implant | 10.1016/j.biomaterials.2004.08.028 | M. Kobayashi * | Japan | Biomaterials, (1), (12.47) | 259 | 16.18 |
| 10 | Two-Dimensional Manipulation of Cardiac Myocyte Sheets Utilizing Temperature-Responsive Culture Dishes Augments the Pulsatile Amplitude, [126] | Cell culture plates (Cardiac myocyte tissue) | 10.1089/107632701300062732 | T. Shimizu/ T. Okano * | Japan | Tissue Engineering: Part A (2), (3.845) | 186 | 18.60 |
| Rank | Author | TC (1985–2021) | No of Publication | Affiliation | Country |
|---|---|---|---|---|---|
| 1 | T. Okano | 4854 | 20 | Tokyo Women Med Univ, Inst Adv Biomed Engn & Sci, TWIns, Shinjuku Ku, Tokyo 1628666, Japan | Japan |
| 2 | M. Yamato | 2855 | 11 | Tokyo Women Med Univ, Ctr Excellence Century 21, Inst Adv Biomed Engn & Sci, Shinjuku Ku, Tokyo 1628666, Japan | Japan |
| 3 | A. Kikuchi | 2075 | 7 | Tokyo Women Med Univ, Ctr Excellence Century 21, Inst Adv Biomed Engn & Sci, Shinjuku Ku, Tokyo 1628666, Japan | Japan |
| 4 | B. Emilio | 592 | 30 | Univ Nacl Autonoma Mexico, Inst Ciencias Nucl, Dept Quim Radiac & Radioquim, Ciudad Univ, Mexico City 04510, DF, Mexico | Mexico |
| 5 | B. Gupta | 365 | 12 | Indian Inst Technol, Dept Text Technol, New Delhi 110016, India | India |
| 6 | A. Concheiro | 300 | 16 | Univ Santiago de Compostela, Dept Farm & Tecnol Farmaceut, Santiago De Compostela 15782, Spain | Spain |
| 7 | C. Alvarez-Lorenzo | 300 | 16 | Univ Santiago de Compostela, Dept Farm & Tecnol Farmaceut, Santiago De Compostela 15782, Spain | Spain |
| 8 | G. Burillo | 289 | 8 | Univ Nacl Autonoma Mexico, Inst Ciencias Nucl, Dept Quim Radiac & Radioquim, Mexico City 04510, DF, Mexico | Mexico |
| 9 | H. Singh | 264 | 10 | Indian Inst Technol, Dept Text Technol, New Delhi 110016, India | India |
| 10 | H. I. Melendez-Ortiz | 82 | 7 | Univ Nacl Autonoma Mexico, Inst Ciencias Nucl, Dept Quim Radiac & Radioquim, Ciudad Univ, Mexico City 04510, DF, Mexico | Mexico |
| Rank | Country | h-Index | All Citations | All Articles | % of Total Fund |
|---|---|---|---|---|---|
| 1 | USA | 28 | 17,558 | 37 | 15.7 |
| 2 | Japan | 25 | 5135 | 35 | 14.9 |
| 3 | India | 21 | 2041 | 28 | 11.9 |
| 4 | China | 19 | 2179 | 26 | 11.1 |
| 5 | Mexico | 16 | 592 | 30 | 12.8 |
| 6 | Spain | 12 | 1217 | 19 | 8.1 |
| 7 | Portugal | 9 | 1458 | 10 | 4.3 |
| 8 | Canada | 8 | 4514 | 8 | 3.4 |
| 9 | Germany | 7 | 4673 | 9 | 3.8 |
| 10 | UK | 7 | 5863 | 8 | 3.4 |
| Cluster ID | Size | Silhouette | Mean (Year) | Start | End | Duration | Activeness | Theme |
|---|---|---|---|---|---|---|---|---|
| 0 | 133 | 0.955 | 2004 | 1962 | 2020 | 58 | Active | Acrylic Acid |
| 1 | 68 | 0.942 | 1999 | 1969 | 2014 | 45 | Active | Cell Sheet Engineering |
| 2 | 65 | 0.941 | 2008 | 1992 | 2018 | 26 | Active | Composite Nanoparticle |
| 3 | 59 | 1.000 | 1975 | 1959 | 1989 | 30 | Inactive | Synthesis Condition |
| 4 | 51 | 0.959 | 1990 | 1945 | 2006 | 61 | Inactive | Critical Solution Temperature |
| 5 | 47 | 0.922 | 1993 | 1974 | 2003 | 29 | Inactive | Non-Polar Film |
| 6 | 35 | 0.995 | 2005 | 1997 | 2011 | 14 | Active | Antibacterial Surfaces |
| 7 | 28 | 0.994 | 1972 | 1899 | 2017 | 18 | Active | Thermo-responsive Polymer Nanocarrier |
| 9 | 26 | 0.984 | 1987 | 1956 | 1995 | 39 | Inactive | Membrane Biosensor |
| 10 | 20 | 0.990 | 2010 | 1969 | 2020 | 51 | Active | Gamma-Irradiation |
| 11 | 19 | 0.997 | 2001 | 1991 | 2006 | 15 | Inactive | Moving Trend |
| 14 | 16 | 0.991 | 2000 | 1989 | 2008 | 19 | Inactive | Bacterial Adhesion |
| 20 | 11 | 0.994 | 1999 | 1989 | 2005 | 16 | Inactive | Biology |
| 22 | 11 | 0.996 | 1985 | 1962 | 1998 | 36 | Inactive | Acrylonitrile Monomer |
| 23 | 9 | 0.999 | 2003 | 1986 | 2007 | 21 | Inactive | Polyethersulfone Nanofiltration Membrane |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusefi, M.; Nasef, M.M.; Tareq, M.A.; Gupta, B.; Shameli, K.; Ali, R.R.; Ting, T.M.; El Enshasy, H.A. Bibliometrics of Functional Polymeric Biomaterials with Bioactive Properties Prepared by Radiation-Induced Graft Copolymerisation: A Review. Polymers 2022, 14, 4831. https://doi.org/10.3390/polym14224831
Yusefi M, Nasef MM, Tareq MA, Gupta B, Shameli K, Ali RR, Ting TM, El Enshasy HA. Bibliometrics of Functional Polymeric Biomaterials with Bioactive Properties Prepared by Radiation-Induced Graft Copolymerisation: A Review. Polymers. 2022; 14(22):4831. https://doi.org/10.3390/polym14224831
Chicago/Turabian StyleYusefi, Mostafa, Mohamed Mahmoud Nasef, Mohammad Ali Tareq, Bhuvanesh Gupta, Kamyar Shameli, Roshafima Rasit Ali, Teo Ming Ting, and Hesham Ali El Enshasy. 2022. "Bibliometrics of Functional Polymeric Biomaterials with Bioactive Properties Prepared by Radiation-Induced Graft Copolymerisation: A Review" Polymers 14, no. 22: 4831. https://doi.org/10.3390/polym14224831
APA StyleYusefi, M., Nasef, M. M., Tareq, M. A., Gupta, B., Shameli, K., Ali, R. R., Ting, T. M., & El Enshasy, H. A. (2022). Bibliometrics of Functional Polymeric Biomaterials with Bioactive Properties Prepared by Radiation-Induced Graft Copolymerisation: A Review. Polymers, 14(22), 4831. https://doi.org/10.3390/polym14224831








