All-Solid State Potentiometric Sensors for Desvenlafaxine Detection Using Biomimetic Imprinted Polymers as Recognition Receptors
Abstract
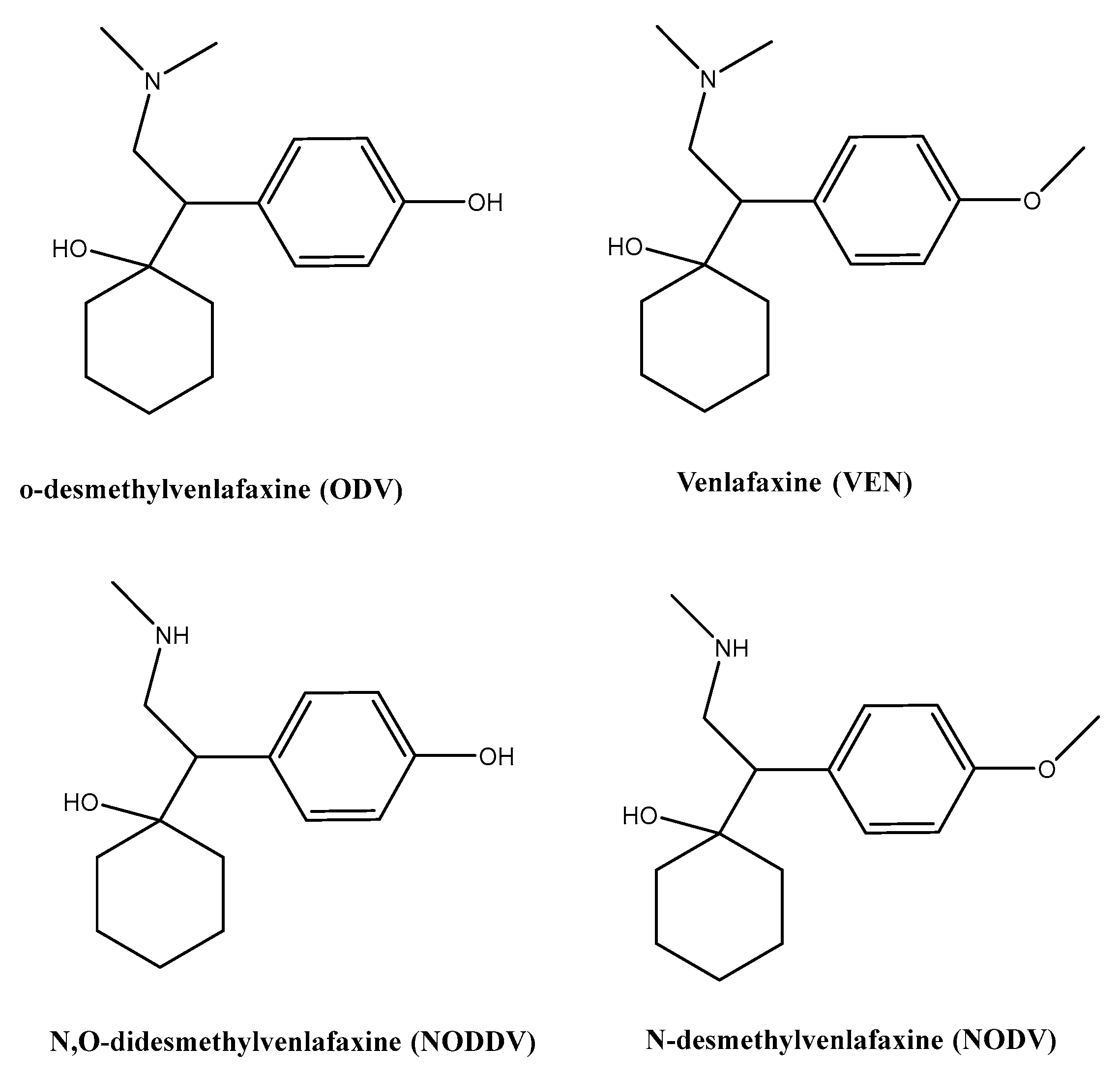
1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Reagents and Materials
2.3. MIPs and NIPs Synthesis
2.4. Sensor Fabrication and EMF Measurements
2.5. Electrochemical Impedance and Chronopotentiometric Measurements
2.6. Analytical Applications
3. Results and Discussion
3.1. Sensors’ Characteristics
3.2. Analytical Procedure Validation Study
3.2.1. Accuracy and Precision of the Method
3.2.2. Detection Limit and Quantification
3.3. pH Effect and Sensors’ Selectivity
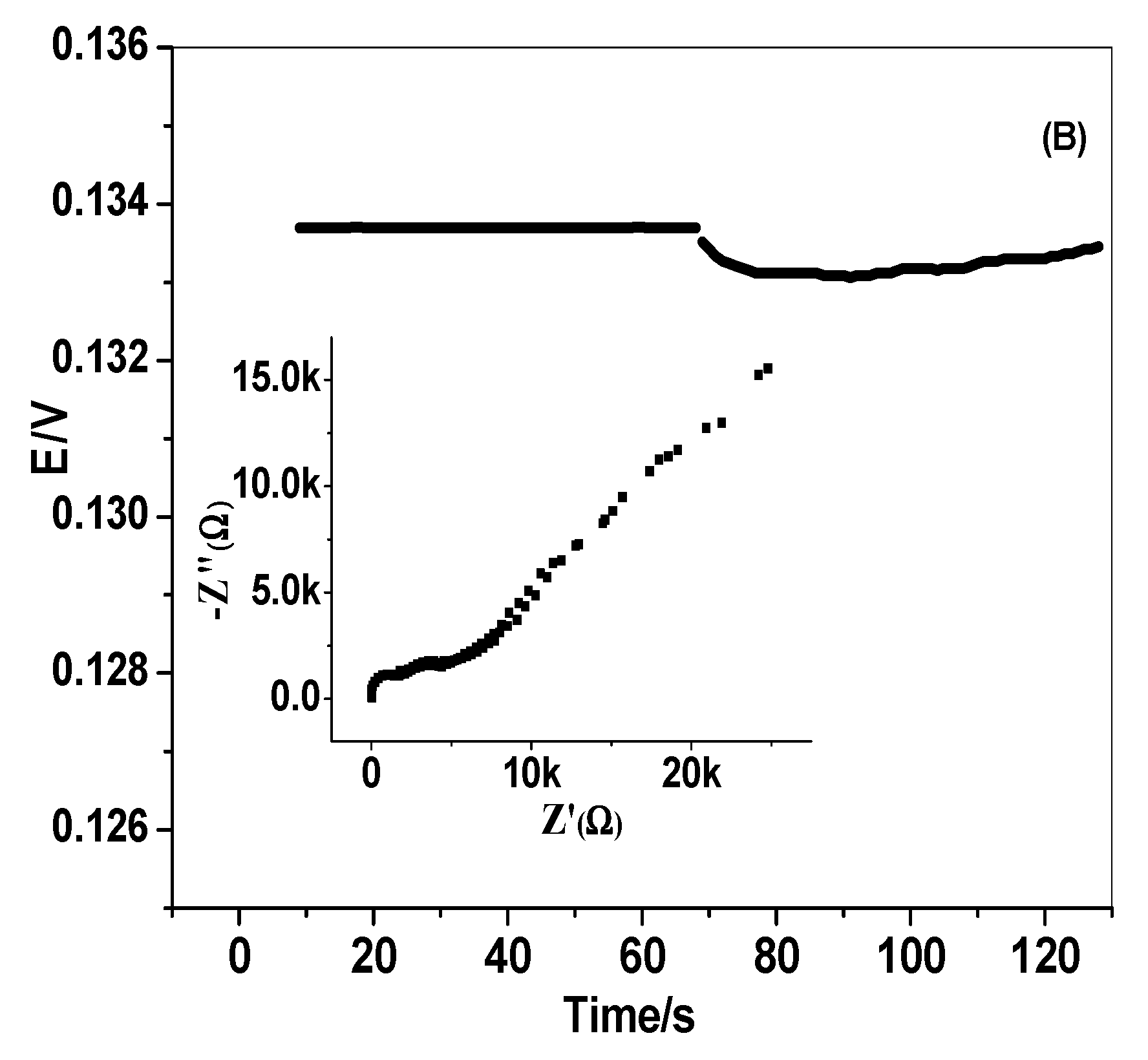
3.4. EIS and Chronopotentiometric Measurements
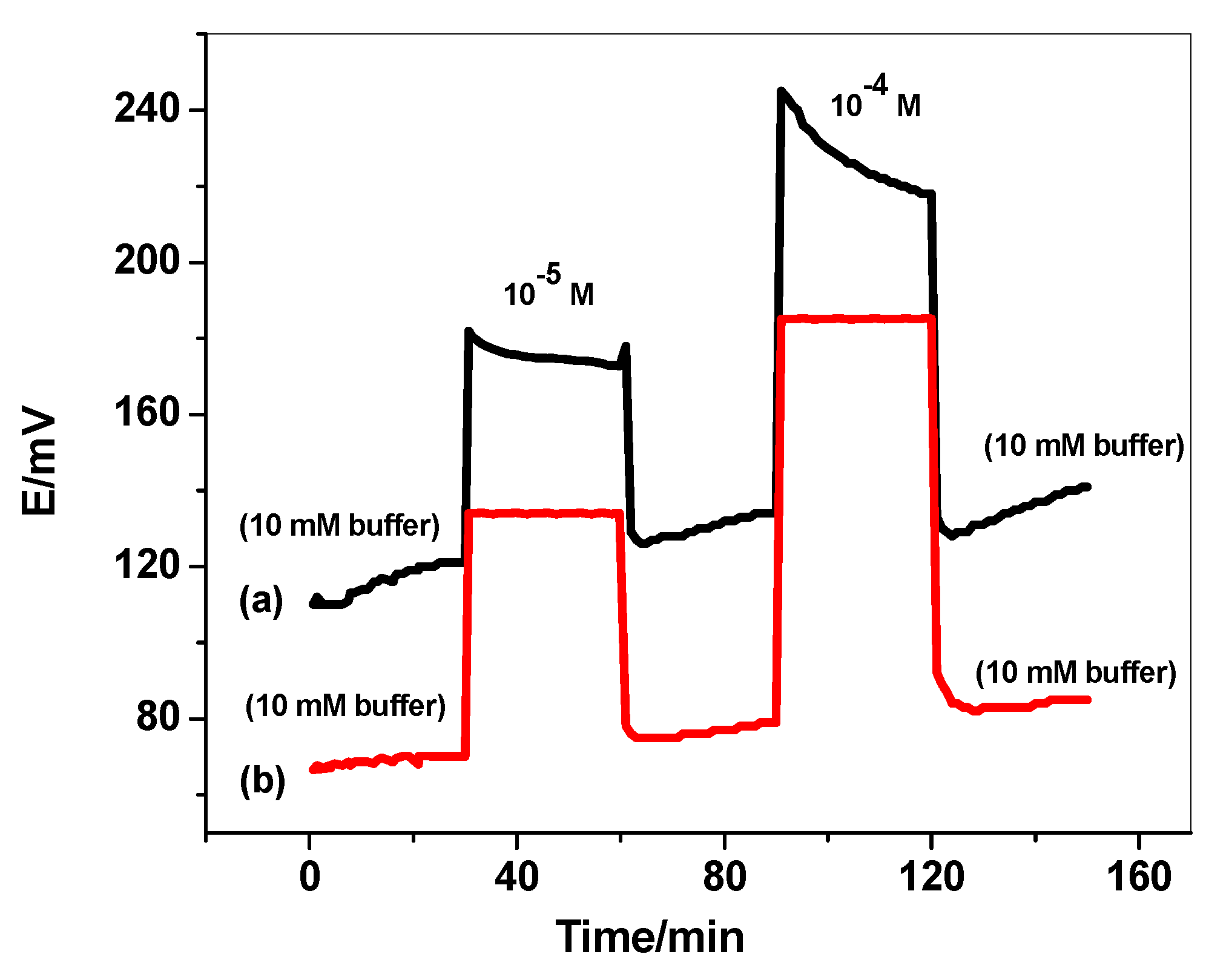
3.5. Sensor’s Durability
3.6. Assessment of ODV by Direct Potentiometric Measurements
3.7. ODV Recovery from Spiked Urine Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deecher, D.C.; Beyer, C.E.; Johnston, G. Desvenlafaxine Succinate: A New Serotonin and Norepinephrine Reuptake Inhibitor. J. Pharmacol. Exp Ther. 2006, 318, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Shams, M.E.; Arneth, B.; Hiemke, C. CYP2D6 polymorphism and clinical effect of the antidepressant venlafaxine. J. Clin. Pharm. Ther. 2006, 31, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.J.; Sohi, M.S.; Patkar, A.A.; Masand, P.S.; Pae, C.U. Desvenlafaxine Succinate: A Newer Antidepressant for the Treatment of Depression and Somatic Symptoms. Postgrad. Med. 2010, 122, 125–138. [Google Scholar] [CrossRef]
- Speroff, L.; Gass, M.; Constantine, G.; Olivier, S. Efficacy and Tolerability of Desvenlafaxine Succinate Treatment for Menopausal Vasomotor Symptoms. Obstet. Gynecol. 2008, 111, 77–87. [Google Scholar] [CrossRef]
- Pristiq (Desvenlafaxine Succinate), [Package Insert]; Wyeth Pharmaceuticals, Inc.: Philadelphia, PA, USA, April 2008.
- Pawar, S.M.; Khatal, L.D.; Gabhe, S.Y.; Dhaneshwar, S.R. LC–UV and LC–MS evaluation of stress degradation behavior of desvenlafaxine. J. Pharm. Anal. 2012, 2, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Matoga, M.; Pehourcq, F.; Titier, K.; Dumora, F.; Jarry, C. Rapid high-performance liquid chromatographic measurement of venlafaxine and O-desmethylvenlafaxine in human plasma: Application to management of acute intoxications. J. Chromatogr. B Biomed. Sci. Appl. 2001, 760, 213–218. [Google Scholar] [CrossRef]
- Pawar, S.M.; Dhaneshwar, S.R. Application of stability indicating high performance thin layer chromatographic method for quantitation of desvenlafaxine in pharmaceutical dosage form. J. Liq. Chromatogr. Related Technol. 2012, 35, 499–510. [Google Scholar] [CrossRef]
- Hicks, D.R.; Wolaniuk, D.; Russell, A.; Cavanaugh, N.; Kraml, M. A high-performance liquid chromatographic method for the simultaneous determination of venlafaxine and o-desmethylvenlafaxine in biological fluids. Ther. Drug Monit. 1994, 16, 100–107. [Google Scholar] [CrossRef]
- Ardakani, K.H.; Foroumadi, A.; Rouini, M.R. Development and validation of a rapid HPLC- fluorescence method for simultaneous determination of venlafaxine and its major metabolites in human plasma. DARU 2010, 18, 97–102. [Google Scholar]
- Roberto, M.; Laura, M.; Roberta, C.; Salvatore, F.; Mario, A.; Maria, A.R. Analysis of the second generation antidepressant venlafaxine and its main active metabolite O-desmethylvenlafaxine in human plasma by HPLC with spectrofluorimetric detection. J. Chromatogr. B 2007, 856, 88–94. [Google Scholar]
- Waschgler, R.; Moll, W.; König, P.; Conca, A. Therapeutic drug monitoring of trazodone: Are there pharmacokinetic interactions involving citalopram and fluoxetine. Int. J. Clin. Pharmacol. Ther. 2004, 42, 120–124. [Google Scholar]
- Vu, R.L.; Helmeste, D.; Albers, L.; Reist, C. Rapid determination of venlafaxine and O-desmethylvenlafaxine in human plasma by high-performance liquid chromatography with fluorimetric detection. J. Chromatogr. B Biomed. Sci. Appl. 1997, 703, 195–201. [Google Scholar] [CrossRef]
- Fanali, S.; Catarcini, P.; Quaglia, M.G. Use of vancomycin silica stationary phase in packed capillary electrochromatography: III. Enantiomeric separation of basic compounds with the polar organic mobile phase. Electrophoresis 2002, 23, 477–485. [Google Scholar] [CrossRef]
- Labat, L.; Deveaux, M.; Dallet, P.; Dubost, J.P. Separation of new antidepressants and their metabolites by micellar electro kinetic capillary chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002, 773, 17–23. [Google Scholar] [CrossRef]
- Desiderio, C.; Aturki, Z.; Fanali, S. Use of vancomycin silica stationary phase in packed capillary electrochromatography I. Enantiomer separation of basic compounds. Electrophoresis 2001, 22, 535–543. [Google Scholar] [CrossRef]
- Fanali, S.; Rudaz, S.; Veuthey, J.L.; Desiderio, C. Use of vancomycin silica stationary phase in packed capillary electrochromatography: II. Enantiomer separation of venlafaxine and O-desmethylvenlafaxine in human plasma. J. Chromatogr. A 2001, 919, 195–203. [Google Scholar] [CrossRef]
- Rudaz, S.; Stella, C.; Balant-Gorgia, E.; Fanali, S.; Veuthey, J.L. Simultaneous stereoselective analysis of venlafaxine and O-desmethylvenlafaxine enantiomers in clinical samples by capillary electrophoresis using charged cyclodextrins. J. Pharm. Biomed. Anal. 2000, 23, 107–115. [Google Scholar] [CrossRef]
- Rudaz, S.; Veuthey, J.L.; Desiderio, C.; Fanali, S. Enantioseparation of venlafaxine andO-desmethylvenlafaxine by capillary electrophoresis with mixed cyclodextrins. Chromatographia 1999, 50, 369–372. [Google Scholar] [CrossRef]
- Sanghavi, B.J.; Srivastava, A.K. Adsorptive stripping differential pulse voltammetric determination of venlafaxine and desvenlafaxine employing nafion–carbon nanotube composite glassy carbon electrode. Electrochim. Acta 2011, 56, 4188–4196. [Google Scholar] [CrossRef]
- Liu, W.; Cai, H.L.; Li, H.D. High performance liquid chromatography-electrospry ionization mass spectrometry (HPLC-MS/ESI) method for the simultaneous determination of venlafaxine and its three metabolities in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 850, 405–411. [Google Scholar] [CrossRef]
- Moussa, B.A.; El-Bagary, R.I.; Al-Eryani, Y.I. Development and Validation of a Stability-Indicating HPLC Method for the Analysis of Desvenlafaxine Succinate in the Presence of its Acidic Induced Degradation Product in Bulk and Pharmaceutical Preparation. J. Chem. Pharm. Res. 2011, 3, 425–437. [Google Scholar]
- Shah, G.R.; Thaker, B.T.; Surati, K.R.; Parabia, M.H. Simultaneous determination of venlafaxine and its main active metabolite o-desmethyl venlafaxine in rat plasma by LC-MS/MS. Anal. Sci. 2009, 25, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Rajasekhar, D.; KumarJ, I.J.; Venkateswarlu, P. Rapid high-performance liquid chromatography–tandem mass spectrometry method for simultaneous measurement of venlafaxine and O-desmethylvenlafaxine in human plasma and its application in comparative bioavailability study. Biomed. Chromatogr. 2009, 23, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- de Castro, A.; Concheiro, M.; Quintela, O.; Cruz, A.; LÓpez-Rivadulla, M. LC–MS/MS method for the determination of nine antidepressants and some of their main metabolites in oral fluid and plasma: Study of correlation between venlafaxine concentrations in both matrices. J. Pharm. Biomed. Anal. 2008, 48, 183–193. [Google Scholar] [CrossRef]
- Patel, B.N.; Sharma, N.; Sanyal, M.; Shrivastav, P.S. Liquid chromatography tandem mass spectrometry assay for the simultaneous determination of venlafaxine and O-desmethylvenlafaxine in human plasma and its application to a bioequivalence study. J. Pharm. Biomed. Anal. 2008, 47, 603–611. [Google Scholar] [CrossRef]
- Castaing, N.; Titier, K.; Receveur-Daurel, M.; Le-Deodic, M.; Le-Bars, D.; Moore, N.; Molimard, M. Quantification of eight new antidepressants and five of their active metabolites in whole blood by high-performance liquid chromatography-tandem mass spectrometry. J. Anal. Toxicol. 2007, 31, 334–341. [Google Scholar] [CrossRef]
- El-Didamony, M.; Ramadan, G.M.; Moustaf, M.A. Spectrofluorimetric Determination of Citalopram and Desvenlafaxine Antidepressant Drugs in Micellar Medium. Mor. J. Chem. 2022, 10, 235–247. [Google Scholar]
- Guerreiro, J.R.L.; Kamel, A.H.; Sales, M.G.F. Automatic potentiometric system based on periodate polymeric membrane sensors for the assessment of ascorbic acid in flavored waters. Food Chem. 2010, 120, 934. [Google Scholar] [CrossRef][Green Version]
- Ashmawy, N.H.; Almehizia, A.A.; Youssef, T.A.; Amr, A.E.; Al-Omar, M.A.; Kamel, A.H. Novel carbon/PEDOT/PSS-based screen-printed biosensors for acetylcholine neurotransmitter and acetyl cholinesterase detection in human serum. Molecules 2019, 24, 1539. [Google Scholar] [CrossRef]
- Abdalla, N.S.; Awwad, N.S.; Kamel, A.H. All solid-state poly(vinyl chloride) membrane potentiometric sensor integrated with nano-beads imprinted polymers for sensitive and rapid detection of bispyribac herbicide as organic pollutant. Molecules 2019, 24, 712. [Google Scholar] [CrossRef]
- Amr, S.A.E.; Al-Omar, M.A.; Kamel, A.H.; Elsayed, E.A. Single-Piece Solid Contact Cu2+-Selective Electrodes Based on a Synthesized Macrocyclic Calix[4]arene Derivative as a Neutral Carrier Ionophore. Molecules 2019, 24, 920. [Google Scholar] [CrossRef] [PubMed]
- Galal Eldin, A.; Amr, A.E.G.E.; Kamel, A.H.; Hassan, S.M. Screen-printed microsensors Using polyoctyl-thiophene (POT) conducting polymer as solid transducer for ultratrace determination of azides. Molecules 2019, 24, 1392. [Google Scholar] [CrossRef] [PubMed]
- Abd-Rabboh, H.S.M.; Kamel, A.H.; Amr, A.E. All-Solid-State Calcium Sensors Modified with Polypyrrol (PPY) and Graphene Oxide (GO) as Solid-Contact Ion-to-Electron Transducers. Chemosensors 2020, 8, 93. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Mahmoud, W.H.; Mohamed, A.H.K.; Kelany, A.E. Mercury(II) ion-selective polymeric membrane sensors for analysis of mercury in hazardous wastes. Anal. Sci. 2006, 22, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.M.; Marzouk, S.A.M.; Mohamed, A.H.K.; Badawy, N.M. Novel dicyanoargentate polymeric membrane sensors for selective determination of cyanide ions. Electroanalysis 2004, 16, 298–303. [Google Scholar] [CrossRef]
- Mohammed, S.A.; Ismail, N.S.; Zayed, M.A. Development of Carbon Paste Electrodes for the Selective Determination of Venlafaxine and its metabolite Desvenlafaxine in their Pure and Pharmaceutical Formulations. Egypt. J. Chem. 2020, 5, 27–28. [Google Scholar]
- Alturiqi, A.S. Quantitative analysis of desvenlafaxine HCl by ion selective electrode. Int. J. Pharm. Sci. Rev. Res. 2016, 40, 75–79. [Google Scholar]
- Amr, A.E.; Kamel, A.H.; Almehizia, A.A.; Sayed, A.Y.A.; Abd-Rabboh, H.S.M. Solid-Contact Potentiometric Sensors Based on Main-Tailored Bio-Mimics for Trace Detection of Harmine Hallucinogen in Urine Specimens. Molecules 2021, 26, 324. [Google Scholar] [CrossRef]
- Abd-Rabboh, H.S.M.; Amr, A.E.; Almehizia, A.A.; Kamel, A.H. All-Solid-State Potentiometric Ion-Sensors Based on Tailored Imprinted Polymers for Pholcodine Determination. Polymers 2021, 13, 1192. [Google Scholar] [CrossRef]
- Kamel, H.; Mohammad, S.G.; Awwad, N.S.; Mohammed, Y.Y. Survey on the Integration of Molecularly Imprinted Polymers as Artificial Receptors in Potentiometric Transducers for pharmaceutical Drugs. Int. J. Electrochem. Sci. 2019, 14, 2085–2124. [Google Scholar] [CrossRef]
- Suryanarayanan, V.; Wu, C.-T.; Ho, K.-C. Molecularly Imprinted Electrochemical Sensors. Electroanalysis 2020, 22, 1795–1811. [Google Scholar] [CrossRef]
- Sajini, T.; Mathew, B. A brief overview of molecularly imprinted polymers: Highlighting computational design, nano and photo-responsive imprinting. Talanta Open 2021, 4, 100072. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Kamel, A.H.; Fathy, M.A. A novel screen-printed potentiometric electrode with carbon nanotubes/polyaniline transducer and molecularly imprinted polymer for the determination of nalbuphine in pharmaceuticals and biological fluids. Anal. Chim. Acta 2022, 1227, 340239. [Google Scholar] [CrossRef] [PubMed]
- Abd-Rabboh, H.S.M.; Amr, A.E.; Almehizia, A.A.; Naglah, A.M.; Kamel, A.H. New Potentiometric Screen-Printed Platforms Modified with Reduced Graphene Oxide and Based on Man-Made Imprinted Receptors for Caffeine Assessment. Polymers 2022, 14, 1942. [Google Scholar] [CrossRef] [PubMed]
- Taleat, Z.; Khoshroo, A.; Mazloum-Ardakani, M. Screen-printed electrodes for biosensing: A review (2008–2013). Microchim. Acta 2014, 181, 865–891. [Google Scholar] [CrossRef]
- International Conference on Harmonization (ICH). In Validation of Analytical Procedures d PA/PH/OMCL (05) 47 DEF; OMCL Network/EDQM of the Council of Europe, 2005.
- Heyden, Y.V.; Massart, D. Review of the use of robustness and ruggedness in analytical chemistry. Data Handl. Sci. Technol. 1996, 19, 79–147. [Google Scholar]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Desvenlafaxine (accessed on 6 October 2022).
- Bakker, E. Determination of improved selectivity coefficients of polymer membrane ion-selective electrodes by conditioning with a discriminated ion. J. Electrochem. Soc. 1996, 143, L83. [Google Scholar] [CrossRef]
- Lyu, Y.; Gan, S.; Bao, Y.; Zhong, L.; Xu, J.; Wang, W.; Liu, Z.; Ma, Y.; Yang, G.; Niu, L. Solid-contact ion-selective electrodes: Response mechanisms, transducer materials and wearable sensors. Membranes 2020, 10, 128. [Google Scholar] [CrossRef]
- Bobacka, J. Potential stability of all-solid-state ion-selective electrodes using conducting polymers as ion-to-electron transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef]
- Klamerus, K.J.; Moloney, K.; Rudolph, R.L.; Sisenwine, S.F.; Jusko, W.J.; Chiang, S.T. Introduction of a composite parameter to the pharmacokinetics of venlafaxine and its active O-desmethyl metabolite. J. Clin. Pharmacol. 1992, 32, 716–724. [Google Scholar] [CrossRef]
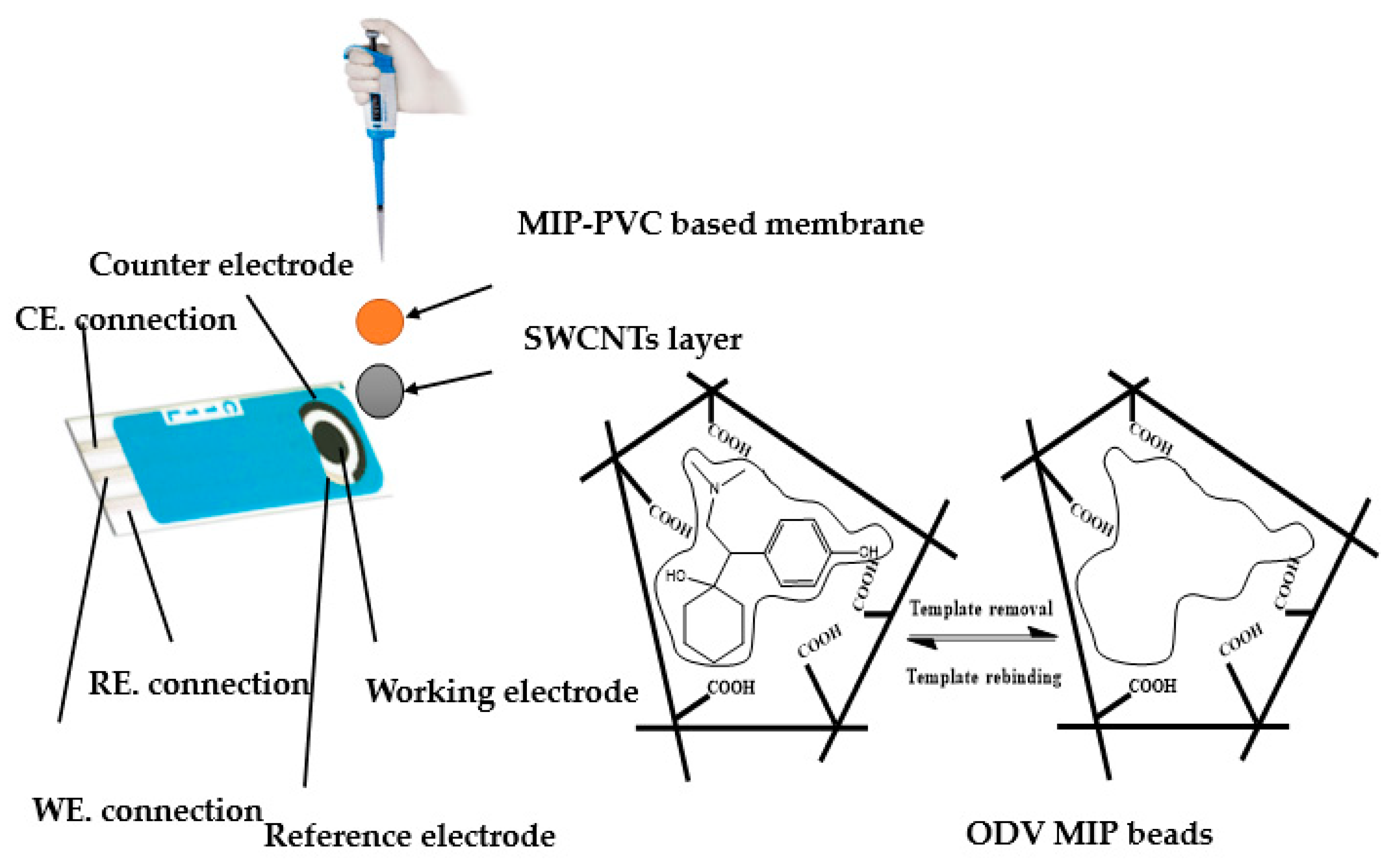
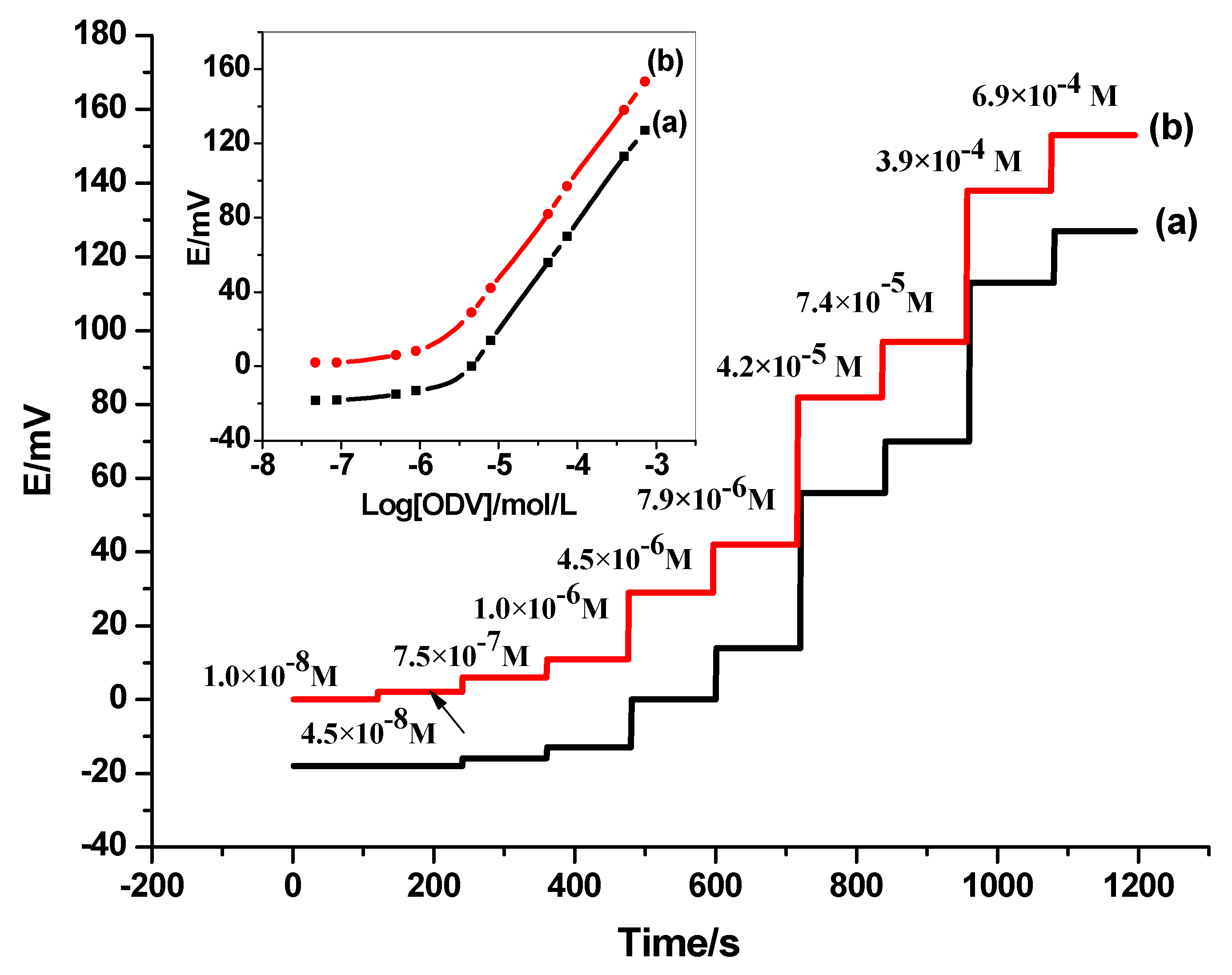
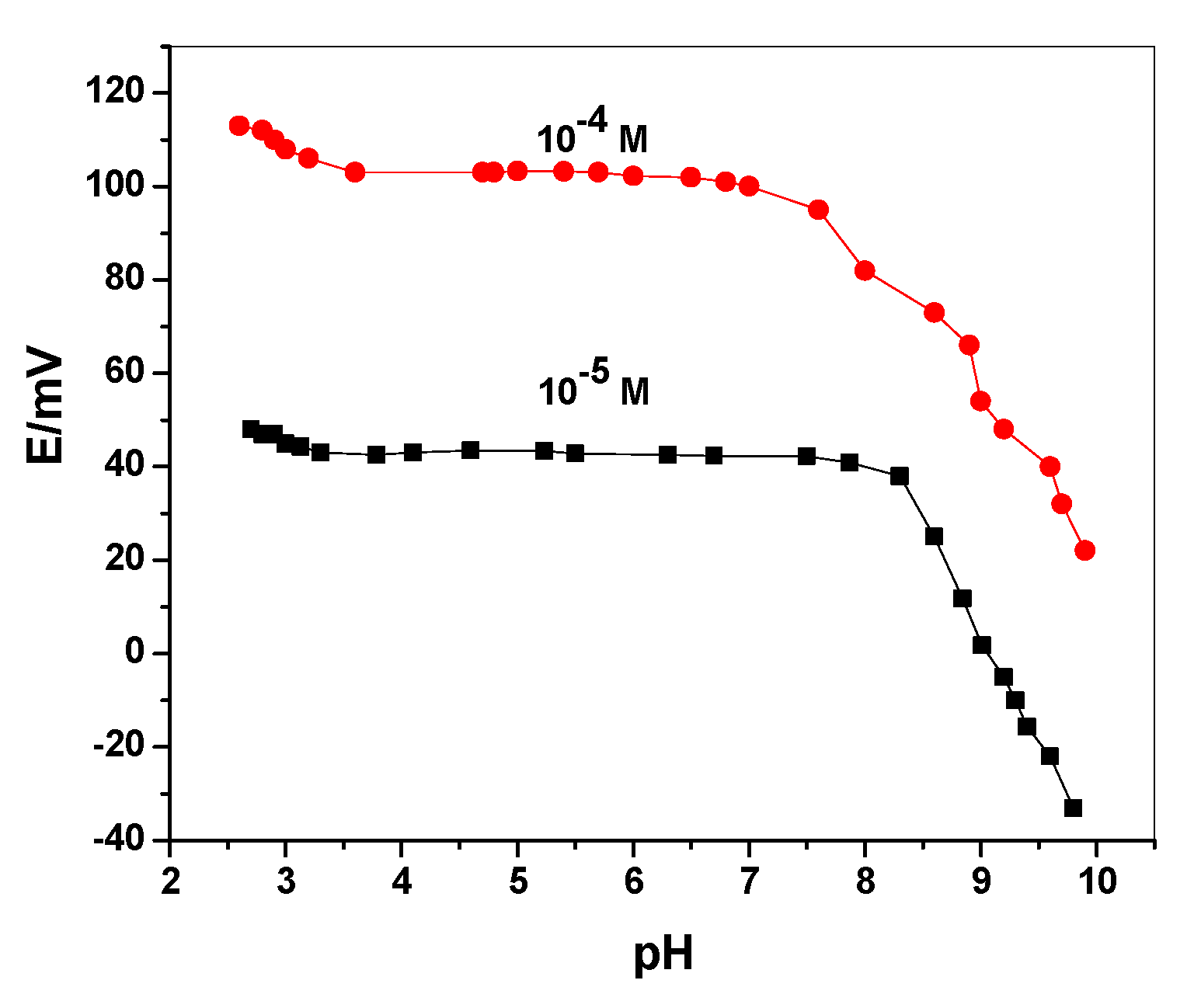
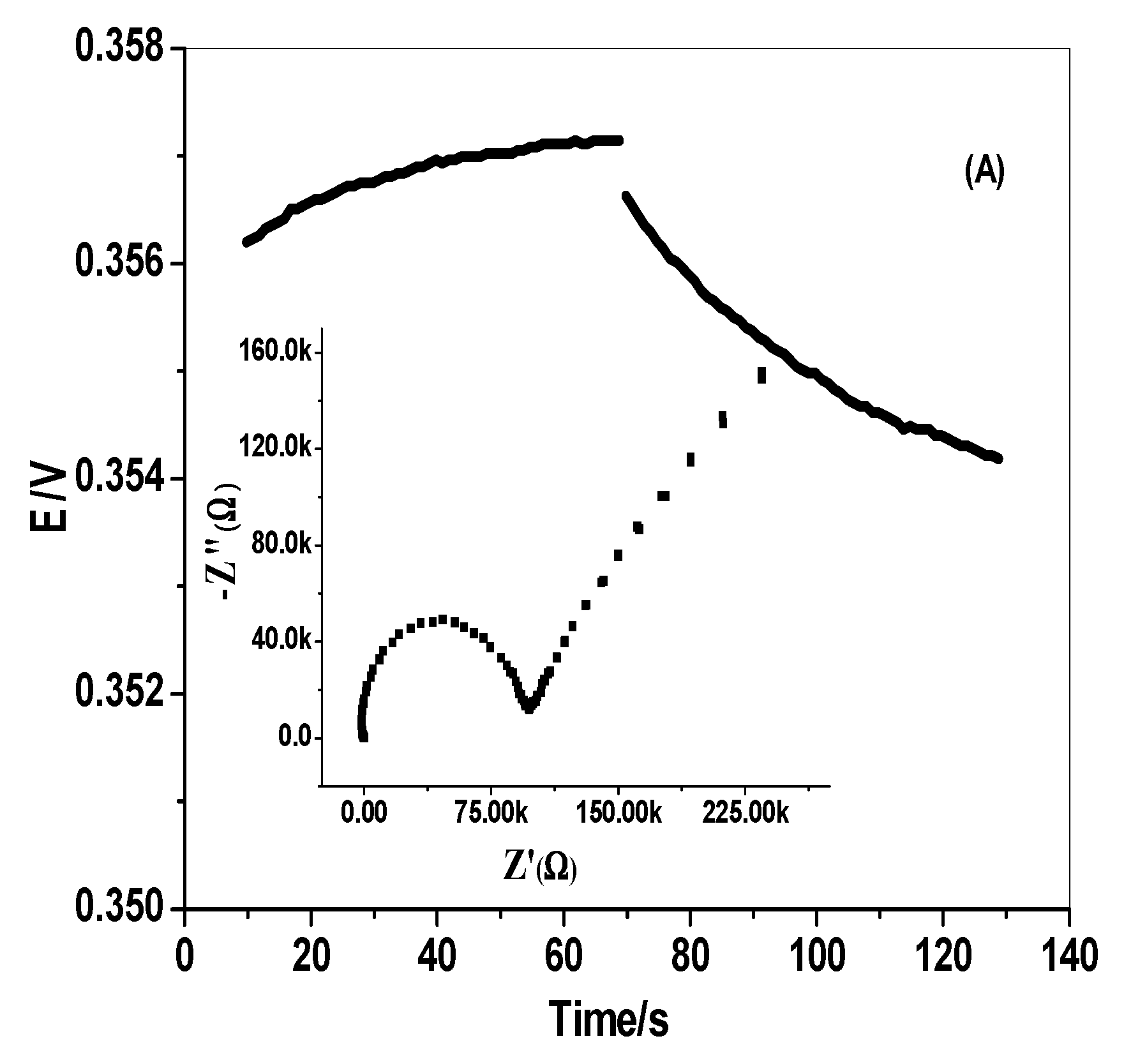
| Sensing Material | Electrode Type | Slope, (mV/Decade) | Detection Limit (mol/L) | Working or Linear Range (mol/L) | pH Range | Response Time, s | Ref. |
|---|---|---|---|---|---|---|---|
| Desvenlafaxine- tetraphenyl borate | carbon paste electrodes | 57.9 | 1.5 × 10−4 | 5.6 × 10−4–1.0 × 10−2 | 2.0–6.0 | 8–14 | [37] |
| desvenlafaxine- phosphotungstate | 59.2 | 6.3 × 10−5 | 5.6 × 10−4–1.0 × 10−2 | 2.0–6.0 | 5–12 | ||
| Desvenlafaxine-silicomolybdate | glass tube-ISE | 58.0 | 3.4 × 10−6 | 1.0 × 10−5–1.0 × 10−2 | 4–8.5 | 20 | [38] |
| C/MIP-ODV | screen-printed | 57.5 ± 1.2 | 2.1 × 10−6 | 4.5 × 10−6–1.0 × 10−2 | 3.5–8.0 | <5 | This work |
| C/SWCNTs/MIP-ODV | 58.1 ± 1.3 | 1.5 × 10−6 | 4.2 × 10−6–1.0 ×10−2 | 3.5–8.0 | <5 |
| Parameter | C/MIP-ODV | C/SWCNTs/MIP-ODV | C/SWCNTs/NIP-ODV |
|---|---|---|---|
| Slope(mV/decade) | 56.4 ± 1.1 | 57.2 ± 0.8 | 38.3 ± 2.3 |
| Detection limit, (M) | 3.0 × 10−6 | 2.0 × 10−6 | 5.0 × 10−5 |
| Correlation coefficient (R2) | 0.999 | 0.999 | 0.999 |
| Lower limit of linear range, (M) | 6.0 × 10−6 | 5.0 × 10−6 | 7.0 × 10−5 |
| Response time, (s) | <5 | <5 | <5 |
| pH range | 3.5–8.0 | 3.5–8.0 | 3.5–8.0 |
| Life span (days) | 12 | 15 | 10 |
| Precision, (%) | 1.1 | 0.4 | 1.4 |
| Accuracy, (%) | 99.2 | 99.5 | 98.3 |
| Standard deviation, (mV) | ±0.8 | ±0.4 | ±1.4 |
| Interfering Ion | log K potODV,J ± SD * | |
|---|---|---|
| C/MIP-ODV | C/SWCNTs/MIP-ODV | |
| K+ | −5.1 ± 0.3 | −5.15 ± 0.2 |
| Na+ | −5.2 ± 0.7 | −5.3 ± 0.3 |
| Mg2+ | −5.5 ± 0.2 | −5.4 ± 0.4 |
| Ca2+ | −4.8 ± 0.4 | −4.7 ± 0.5 |
| Arginine | −5.8 ± 0.2 | −5.8 ± 0.3 |
| Alanine | −4.3 ± 0.2 | −4.4 ± 0.4 |
| Glycine | −4.4 ± 0.1 | −4.5 ± 0.3 |
| Caffeine | −4.2 ± 0.1 | −4.3 ± 0.2 |
| Glucose | −5.5 ± 0.2 | −5.6 ± 0.1 |
| Lactose | −5.4 ± 0.3 | −5.5 ± 0.2 |
| Tramadol | −3.1 ± 0.3 | −3.1 ± 0.2 |
| Aspirin | −2.8 ± 0.1 | −2.9 ± 0.3 |
| Venlafaxine | −2.6 ± 0.3 | −2.6 ± 0.1 |
| Parameter | C/MIP-ODV | C/SWCNTs/MIP-ODV |
|---|---|---|
| Rb* (MΩ) | 0.1 ± 0.04 | 0.005 ± 0.0002 |
| Cdl (µF) | 11.6 ± 0.6 | 91.7 ± 3.4 |
| Cg (nF) | 0.03 ± 0.001 | 0.88 ± 0.06 |
| Rb**(MΩ) | 0.45 ± 0.04 | 0.15 ± 0.03 |
| ∆E/∆t (µV/s) | 57.8 ± 1.1 | 10.6 ± 2.1 |
| CL (µF) | 13.2 ± 1.4 | 76.2 ± 1.6 |
| Pharmaceutical Product | Nominal Content Taken, mg/Capsule | Found, mg/Capsule a | bF-Test | t-Student Test | |||
|---|---|---|---|---|---|---|---|
| Proposed Method | Recovery% ± SD | Reference Method [6] | Recovery% ± SD | ||||
| Pristiq® (Pfizer) | 50 | 48.7 | 97.4 ± 1.1 | 49.7 | 99.4 ± 0.4 | 3.4 | 2.4 |
| 100 | 103.5 | 103.5 ± 0.9 | 100.4 | 100.4 ± 0.2 | 2.3 | 3.1 | |
| Sample, No. | * ODV Drug, µM | Recovery, % | |
|---|---|---|---|
| Spiked | Found | ||
| 1 | 20.0 | 18.4 ± 3.4 | 92.0 ± 2.4 |
| 2 | 50.0 | 52.6 ± 0.4 | 105.2 ± 4.2 |
| 3 | 75.0 | 73.2 ± 0.4 | 97.6 ± 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajaber, M.A.; Kamel, A.H. All-Solid State Potentiometric Sensors for Desvenlafaxine Detection Using Biomimetic Imprinted Polymers as Recognition Receptors. Polymers 2022, 14, 4814. https://doi.org/10.3390/polym14224814
Bajaber MA, Kamel AH. All-Solid State Potentiometric Sensors for Desvenlafaxine Detection Using Biomimetic Imprinted Polymers as Recognition Receptors. Polymers. 2022; 14(22):4814. https://doi.org/10.3390/polym14224814
Chicago/Turabian StyleBajaber, Majed A., and Ayman H. Kamel. 2022. "All-Solid State Potentiometric Sensors for Desvenlafaxine Detection Using Biomimetic Imprinted Polymers as Recognition Receptors" Polymers 14, no. 22: 4814. https://doi.org/10.3390/polym14224814
APA StyleBajaber, M. A., & Kamel, A. H. (2022). All-Solid State Potentiometric Sensors for Desvenlafaxine Detection Using Biomimetic Imprinted Polymers as Recognition Receptors. Polymers, 14(22), 4814. https://doi.org/10.3390/polym14224814







