Flexible Investment Casting Wax Patterns for 3D-Printing: Their Rheological and Mechanical Characterizations
Abstract
1. Introduction
2. Experimental
2.1. Chemicals
2.2. Sample Preparation
2.3. Instrumental Methods
3. Results and Discussion
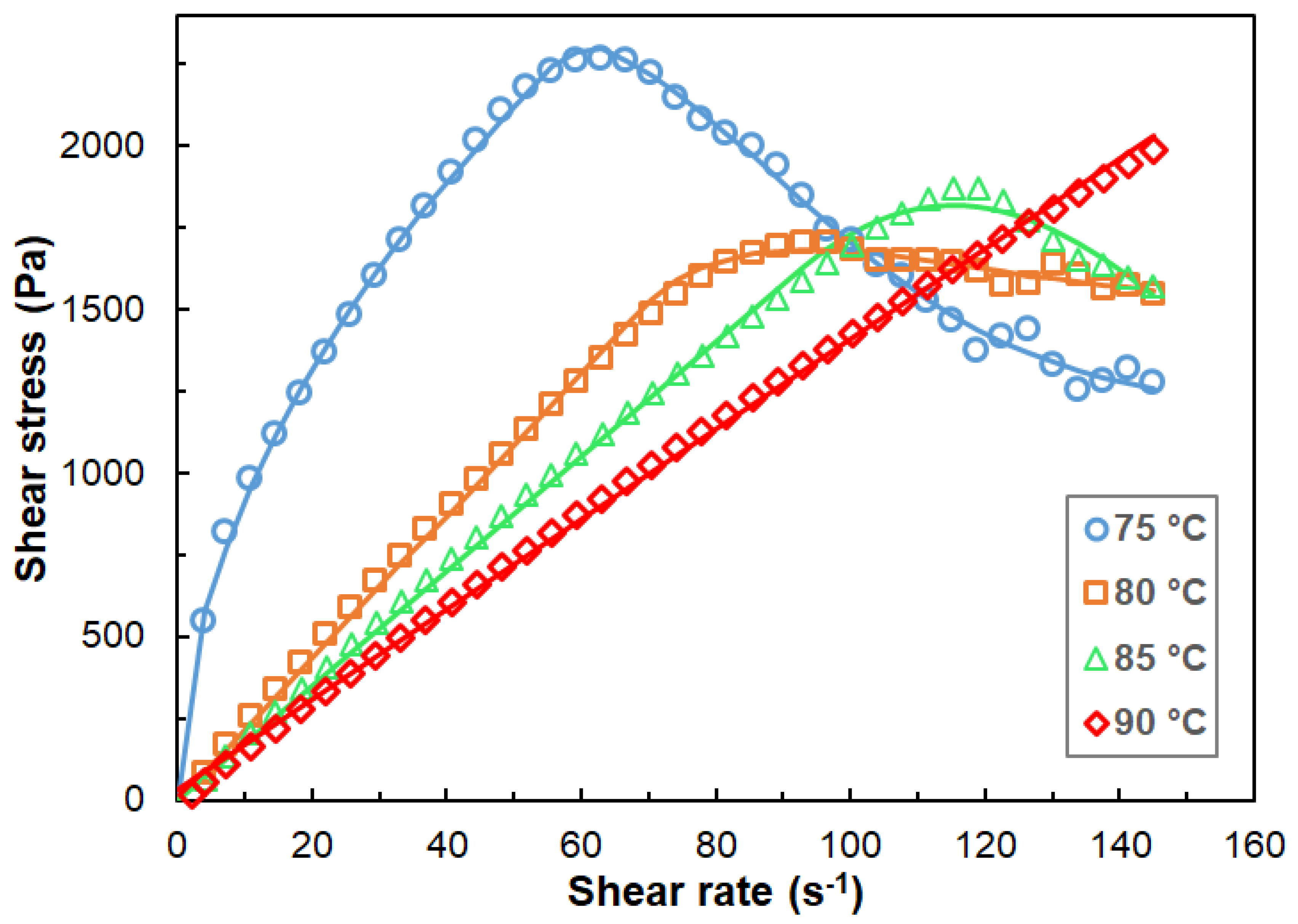
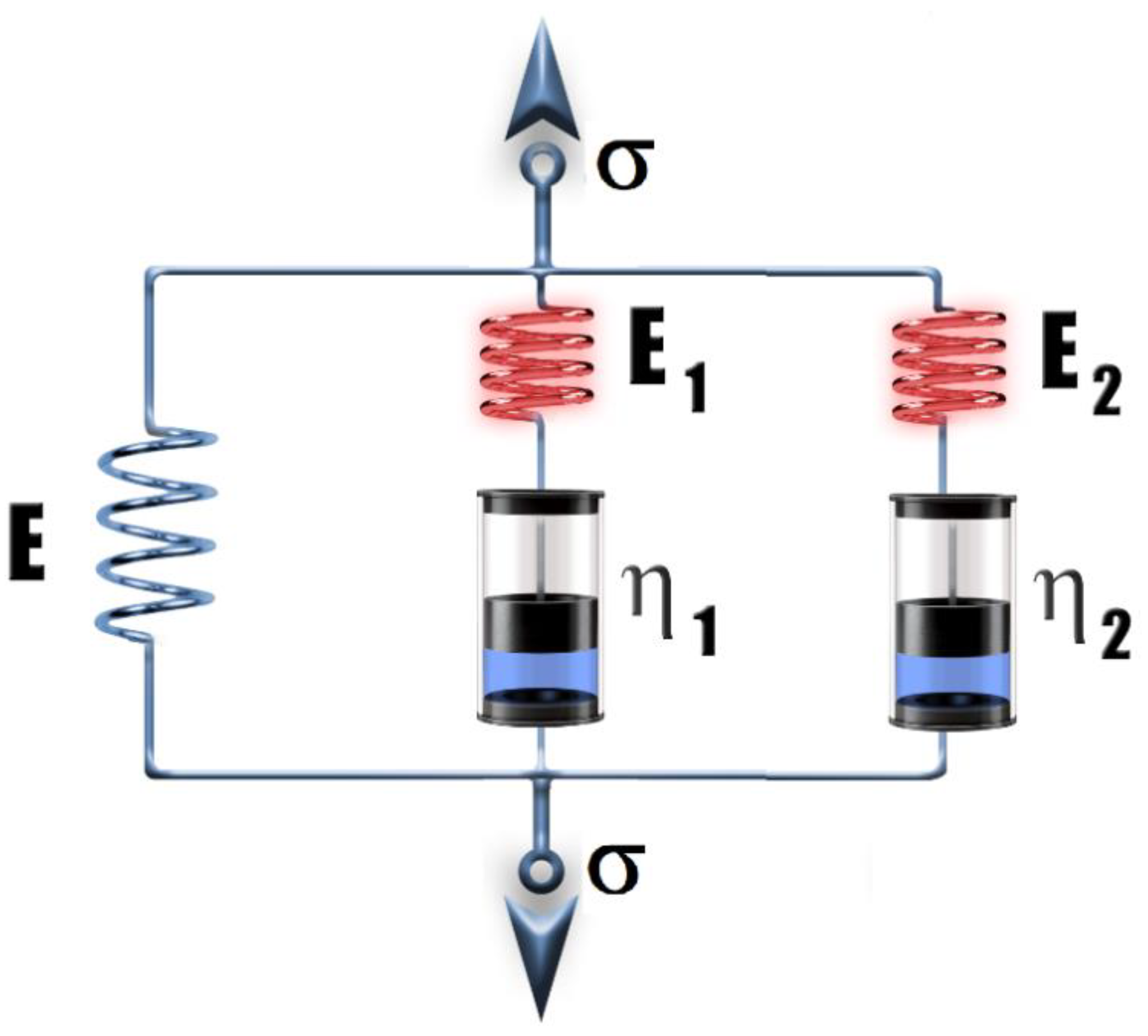
3.1. Rheological Properties
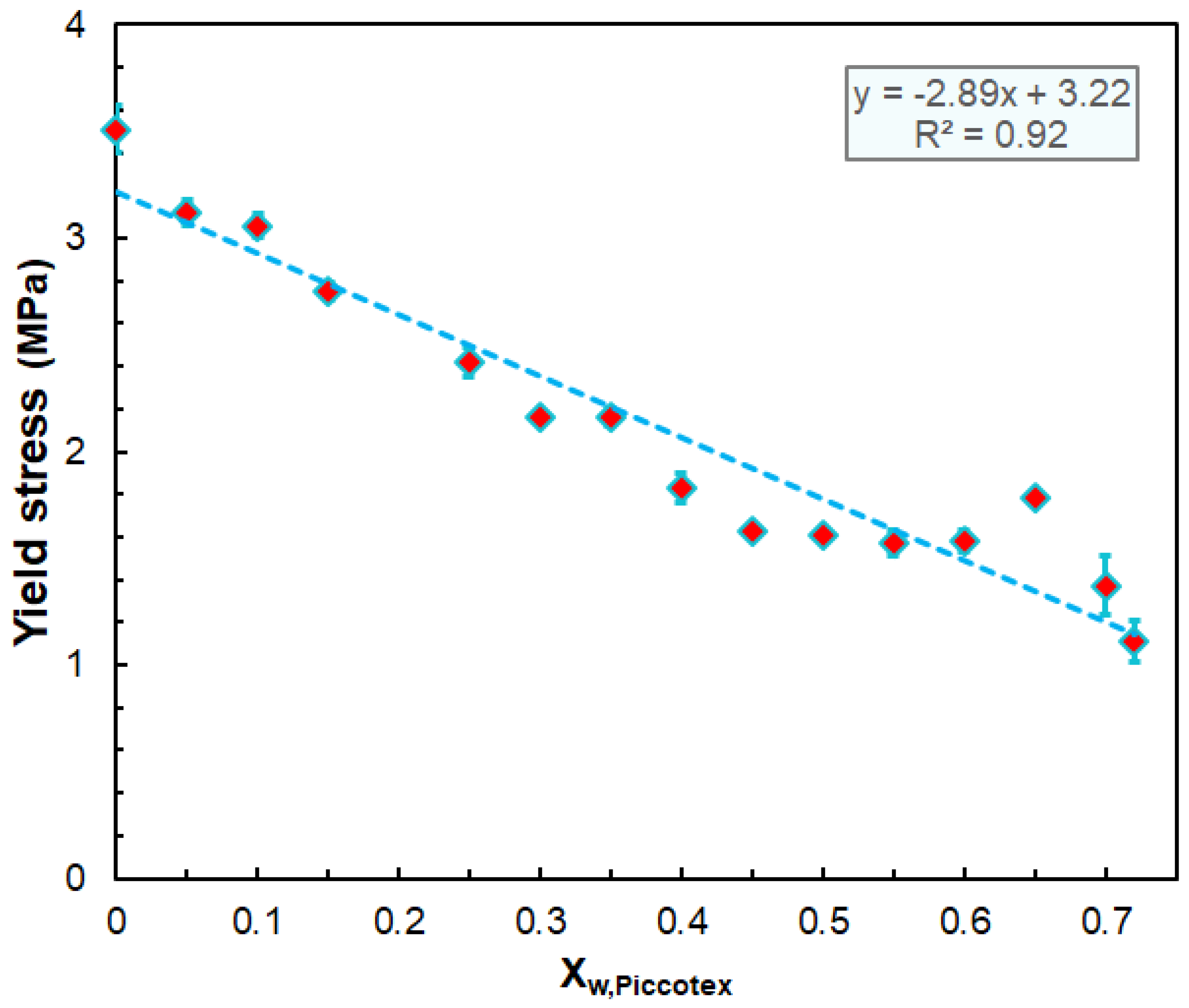
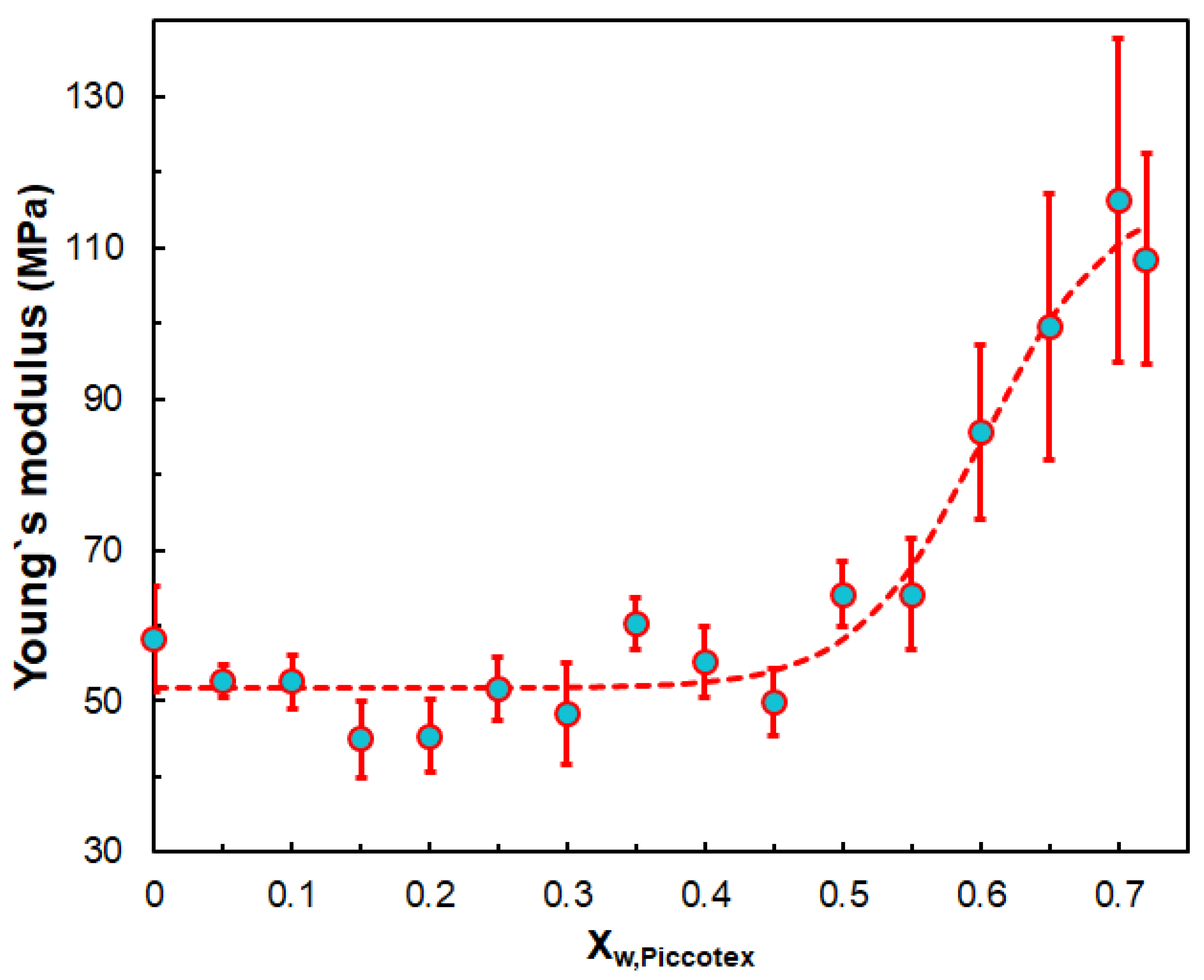
3.2. Mechanical Properties
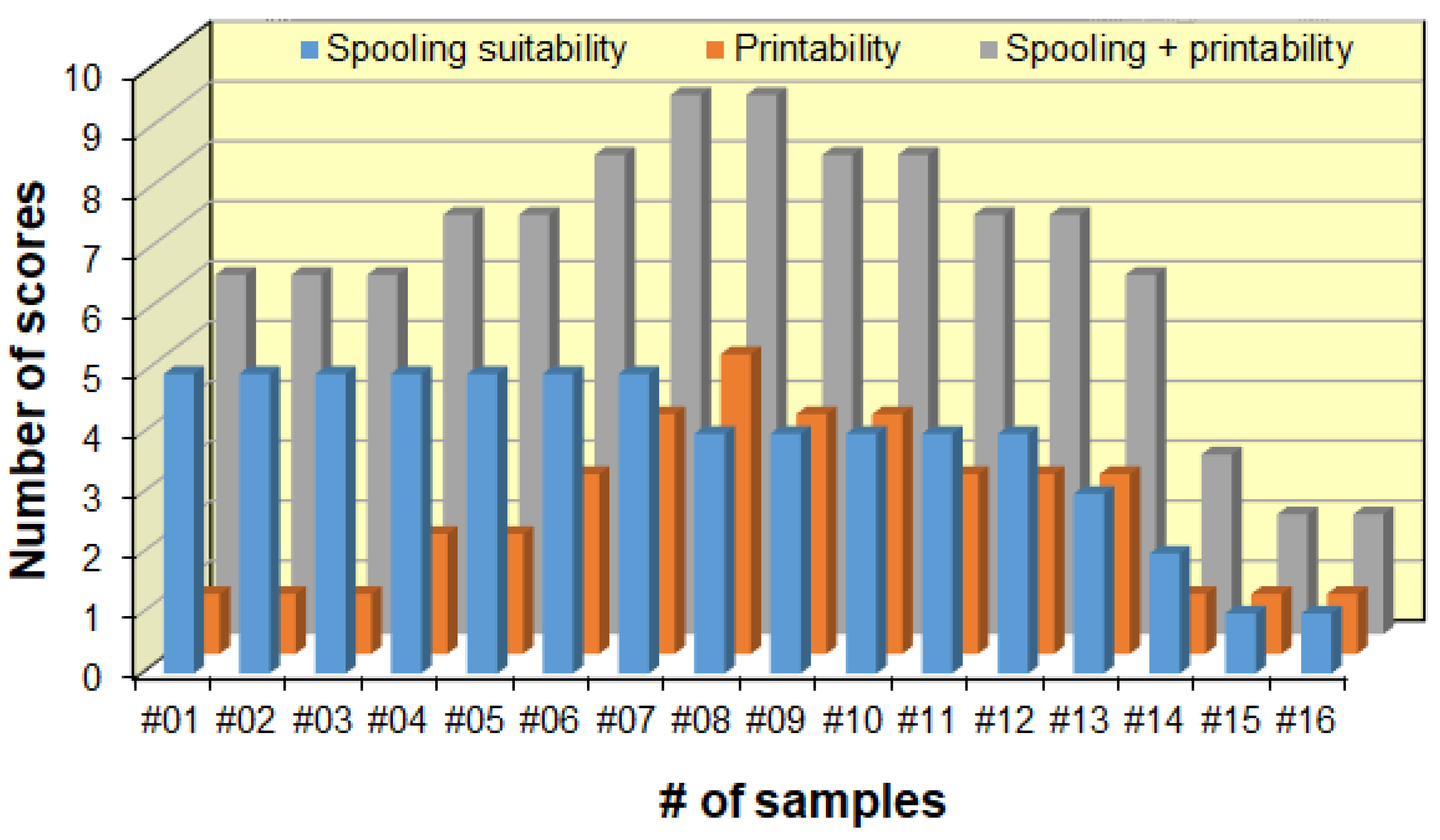
3.3. 3D Printing Application of the Blends
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S.; Singh, R. Precision investment casting: A state of art review and future trends. J. Eng. Manuf. 2017, 230, 2143–2164. [Google Scholar] [CrossRef]
- Pattnaik, S.; Karunakar, D.B.; Jha, P.K. Developments in investment casting process—A review. J. Mater. Process. Technol. 2012, 212, 2332–2348. [Google Scholar] [CrossRef]
- Tulloch, A.P. Beeswax—Composition and Analysis. Bee World 1980, 61, 47–62. [Google Scholar] [CrossRef]
- Jones, S.; Yuan, C. Advances in shell moulding for investment casting. J. Mater Process. Technol. 2003, 135, 258–265. [Google Scholar] [CrossRef]
- Cheah, C.M.; Chua, C.K.; Lee, C.W.; Feng, C.; Totong, K. Rapid prototyping and tooling techniques: A review of applications for rapid investment casting. Int. J. Adv. Manuf. Technol. 2005, 25, 308–320. [Google Scholar] [CrossRef]
- Chakravorty, S. The Properties of Waxes Used in the Investment Casting Industry: Final Report; NPL Report; CMMT(A)245: Middlesex, UK, 1999; ISSN 1361-4061. [Google Scholar]
- Tewo, R.K.; Rutto, H.L.; Focke, W.; Seodigeng, T.; Koech, L.K. Formulations, development and characterization techniques of investment casting patterns. Rev. Chem. Eng. 2019, 35, 335–349. [Google Scholar] [CrossRef]
- Muschio, H.M. Filler and Wax Composition for Investment Casting. U.S. Patent US5518537A, 21 May 1995. [Google Scholar]
- Bemblage, O.; Karunakar, D.B. A study on the blended wax patterns in investment casting process. In Proceedings of the World Congress on Engineering, London, UK, 6–8 July 2011. [Google Scholar]
- Zhou, L.Y.; Fu, J.Z.; He, Y. A Review of 3D Printing Technologies for Soft Polymer Materials. Adv. Func. Mater. 2020, 30, 2000187. [Google Scholar] [CrossRef]
- Kiefer, L.A.; Lee, Y.J. Three–Dimensional Printable Wax Material for Casting Comprises Carnauba Wax and Polymer. U.S. Patent WO2021252828-A1, 16 December 2021. [Google Scholar]
- Mukhtarkhanov, M.; Shehab, E.; Ali, M.H. Process Parameter Optimization for 3D Printed Investment Casting Wax Pattern and Its Post–Processing Technique. Appl. Sci. 2022, 12, 6847. [Google Scholar] [CrossRef]
- Fan, Z.; Tang, C.; Fan, D. Laser Sintering 3D Printing Precision Casting Wax Powder Material Useful in 3D Printing, Comprises Petroleum Wax, Beeswax, Lignite, Polyethylene Wax, Resin, Toughening Agent, Stearic Acid, Flatting Agent, and Surfactant. Chinese Patent CN108165028-A, 15 June 2018. [Google Scholar]
- Ke, Y.; Ye, S.; Yu, C.; Zhao, W.; Pan, Y.; Guo, X. Industrial Casting Three–Dimensional Wax Printing Wire Material Comprises Industrial Wax, Polyolefin Plastomer Resin, Polyethylene Wax, and Stearic Acid. Chinese Patent CN106317913-A, 11 January 2017. [Google Scholar]
- Kuo, C.C.; Chen, W.H.; Li, J.F.; Zhu, Y.J. Development of a flexible modeling base for additive manufacturing. Int. J. Adv. Manuf. Technol. 2018, 94, 1533–1541. [Google Scholar] [CrossRef]
- Lan, Z.; Huang, J.; Li, D. Three–Dimensional Printing Wax Material Useful for Jewelry Comprises Petroleum Wax, Animal Wax, Vegetable Wax, Mineral Wax, Synthetic Wax, Viscosity Regulator, Toughening Agent, Hardness Regulator, Sodium Starch Octenyl Succinate and Toner. Chinese Patent CN113831744-A, 24 December 2021. [Google Scholar]
- Srinivasan, D.; Meignanamoorthy, M.; Ravichandran, M.; Mohanavel, V.; Alagarsamy, S.V.; Chanakyan, C.; Sakthivelu, S.; Karthick, A.; Prabhu, T.R.; Rajkumar, S. 3D Printing Manufacturing Techniques, Materials, and Applications: An Overview. Adv. Mater. Sci. Eng. 2022, 2021, 5756563. [Google Scholar] [CrossRef]
- Mezger, T.G. The Rheology Handbook; Vincentz Network: Hanover, Germany, 2011; p. 23. ISBN 9783866308909. [Google Scholar]
- Roschochowski, A.; Matuszak, A. Rapid tooling: The state of the art. J. Mater. Process. Technol. 2000, 106, 191–198. [Google Scholar] [CrossRef]
- Oliveira, L.M.S.L.; Nunes, R.C.P.; Pessoa, L.M.B.; Reis, L.G.; Spinelli, L.S.; Lucas, E.F. Influence of the chemical structure of additives poly(ethylene–co–vinyl acetate)–based on the pour point of crude oils. J. Appl. Polym. Sci. 2020, 137, 48969. [Google Scholar] [CrossRef]
- Kolczyk, J.; Jamrozowicz, Ł.; Kaźnica, N. Rheological Properties of Typical Ceramic Slurries Used in the Lost Wax Technology. Archi. Foundry Eng. 2017, 17, 197–201. [Google Scholar] [CrossRef][Green Version]
- Widemann, M.; Driest, P.J.; Orecchia, P.; Naline, F.; Golling, F.E.; Hecking, A.; Eggert, C.; Pires, R.; Danielmeier, K.; Richter, F.U. Structure–Property Relations in Oligomers of Linear Aliphatic Diisocyanates. ACS Sustain. Chem. Eng. 2018, 6, 9753–9759. [Google Scholar] [CrossRef]
- Spann, A.P.; Hancock, M.J.; Rostami, A.A.; Platt, S.P.; Rusyniak, M.J.; Sundar, R.S.; Lau, R.W.; Pithawalla, Y.B. Viscosity Model for Liquid Mixtures of Propylene Glycol, Glycerol, and Water. Ind. Eng. Chem. Res. 2021, 60, 670–677. [Google Scholar] [CrossRef]
- Mendes, R.; Vinay, G.; Ovarlez, G.; Coussot, P. Modeling the rheological behavior of waxy crude oils as a function of flow and temperature history. J. Rheol. 2015, 59, 703–732. [Google Scholar] [CrossRef]
- Shan, L.; Tan, Y.; Kim, Y.R. Applicability of the Cox–Merz relationship for asphalt binder. Constr. Build. Mater. 2012, 37, 716–722. [Google Scholar] [CrossRef]
- Ji, X.; Liang, Y.; Cao, W. Effect of Solid Volume Concentration on Rheological Properties of Chengdu Clay Slurry. Processes 2022, 10, 425. [Google Scholar] [CrossRef]
- Sestak, J.; Charles, M.E.; Cawkwell, M.G.; Houska, M. Start–up of gelled crude oil pipelines. J. Pipelines 1987, 6, 15–24. [Google Scholar]
- Cawkwell, M.G.; Charles, M.E. Characterization of Canadian artic thixotropic gelled crude oils utilizing an eight–parameter model. J. Pipelines 1989, 7, 251–264. [Google Scholar]
- Ashbaugh, H.S.; Guo, X.; Schwahn, D.; Prud’homme, R.K.; Richter, D.; Fetters, L.J. Interaction of Paraffin Wax Gels with Ethylene/Vinyl Acetate Co–polymers. Energy Fuels 2005, 19, 138–144. [Google Scholar] [CrossRef]
- Li, N.; Mao, G.; Shi, X.; Tian, S.; Liu, Y. Advances in the research of polymeric pour point depressant for waxy crude oil. J. Dispers. Sci. Technol. 2018, 39, 1165–1171. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, N.; Ran, X.; Han, C.; Han, L.; Zhuang, Y.; Dong, L. Toughening of Polylactide by Melt Blending with Methyl Methacrylate–Butadiene–Styrene Copolymer. J. Appl. Polym. Sci. 2012, 125, 36952. [Google Scholar] [CrossRef]
- Ward, I.M.; Sweeney, J. An Introduction to the Mechanical Properties of Solid Polymers, 2nd ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2004; pp. 59–70. ISBN 9780471496267. [Google Scholar]
- Karger–Kocsis, J.; Kéki, S. Review of Progress in Shape Memory Epoxies and Their Composites. Polymers 2018, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Nagy, L.; Nagy, M.; Vadkerti, B.; Daróczi, L.; Deák, G.; Zsuga, M.; Kéki, S. Designed Polyurethanes for Potential Biomedical and Pharmaceutical Applications: Novel Synthetic Strategy for Preparing Sucrose Containing Biocompatible and Biodegradable Polyurethane Networks. Polymers 2019, 11, 825. [Google Scholar] [CrossRef] [PubMed]
- Kordován, M.A.; Hegedűs, C.; Czifrák, K.; Lakatos, C.; Kálmán-Szabó, I.; Daróczi, L.; Zsuga, M.; Kéki, S. Novel Polyurethane Scaffolds Containing Sucrose Crosslinker for Dental Application. Int. J. Mol. Sci. 2022, 23, 7904. [Google Scholar] [CrossRef] [PubMed]
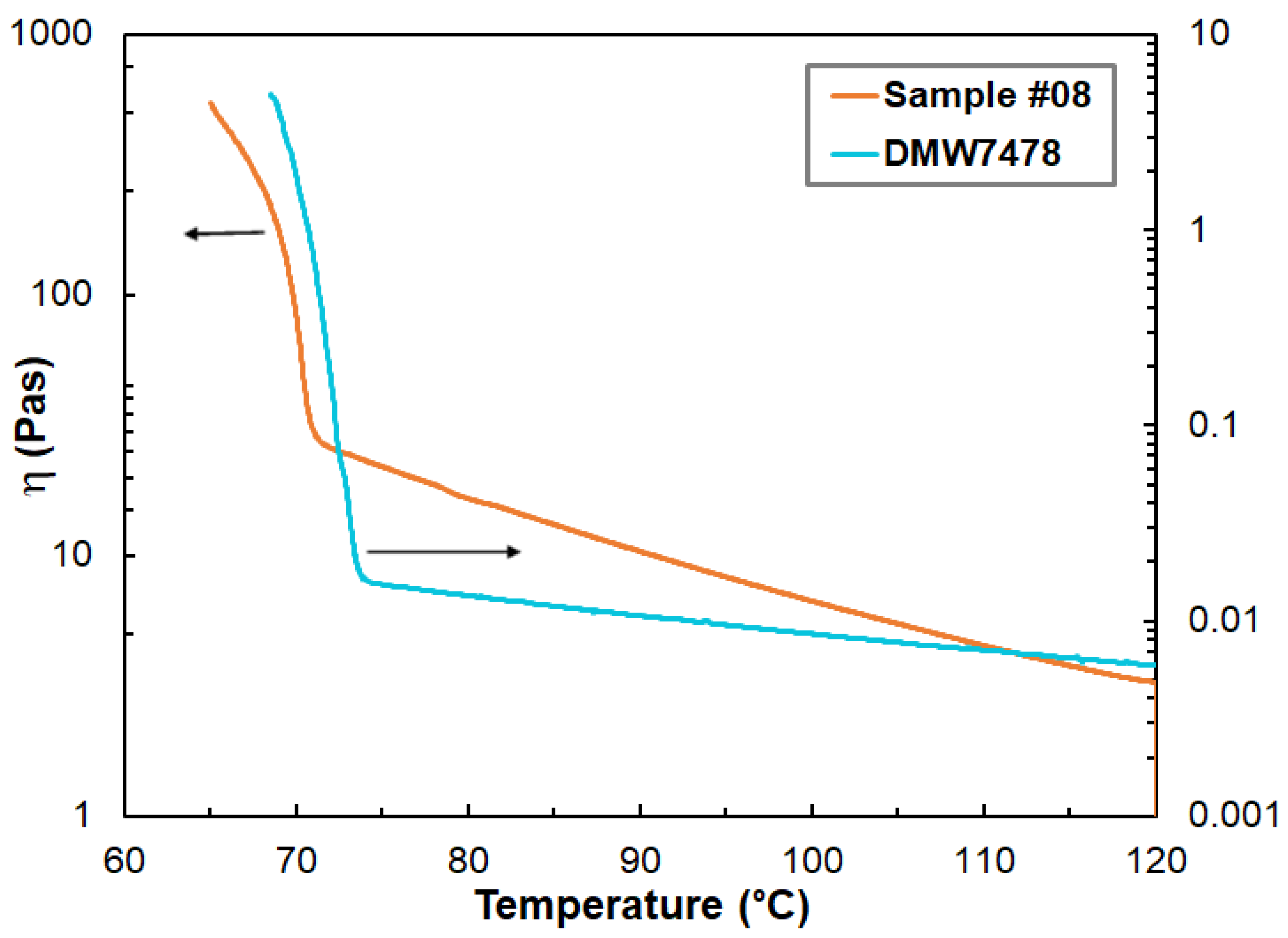
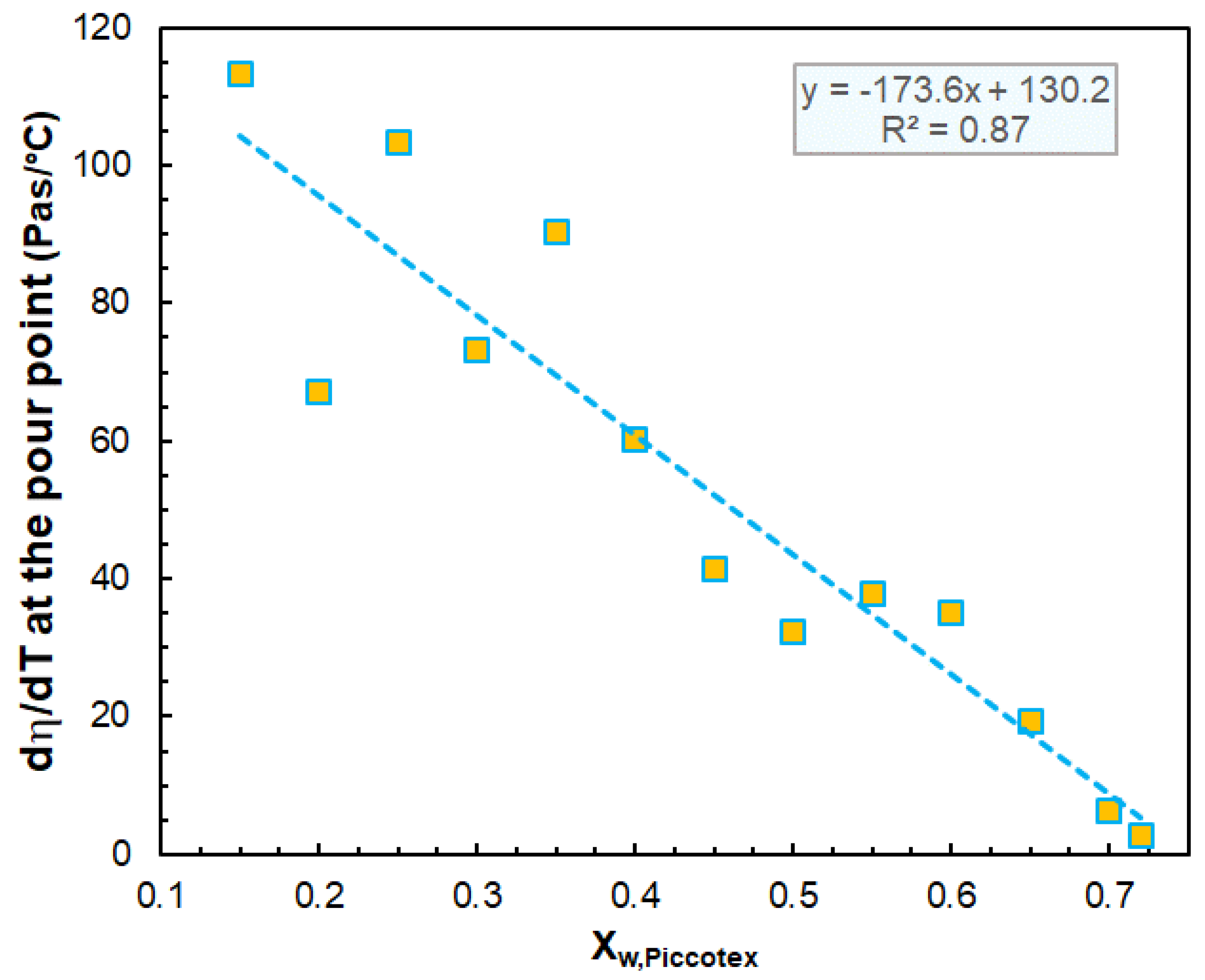
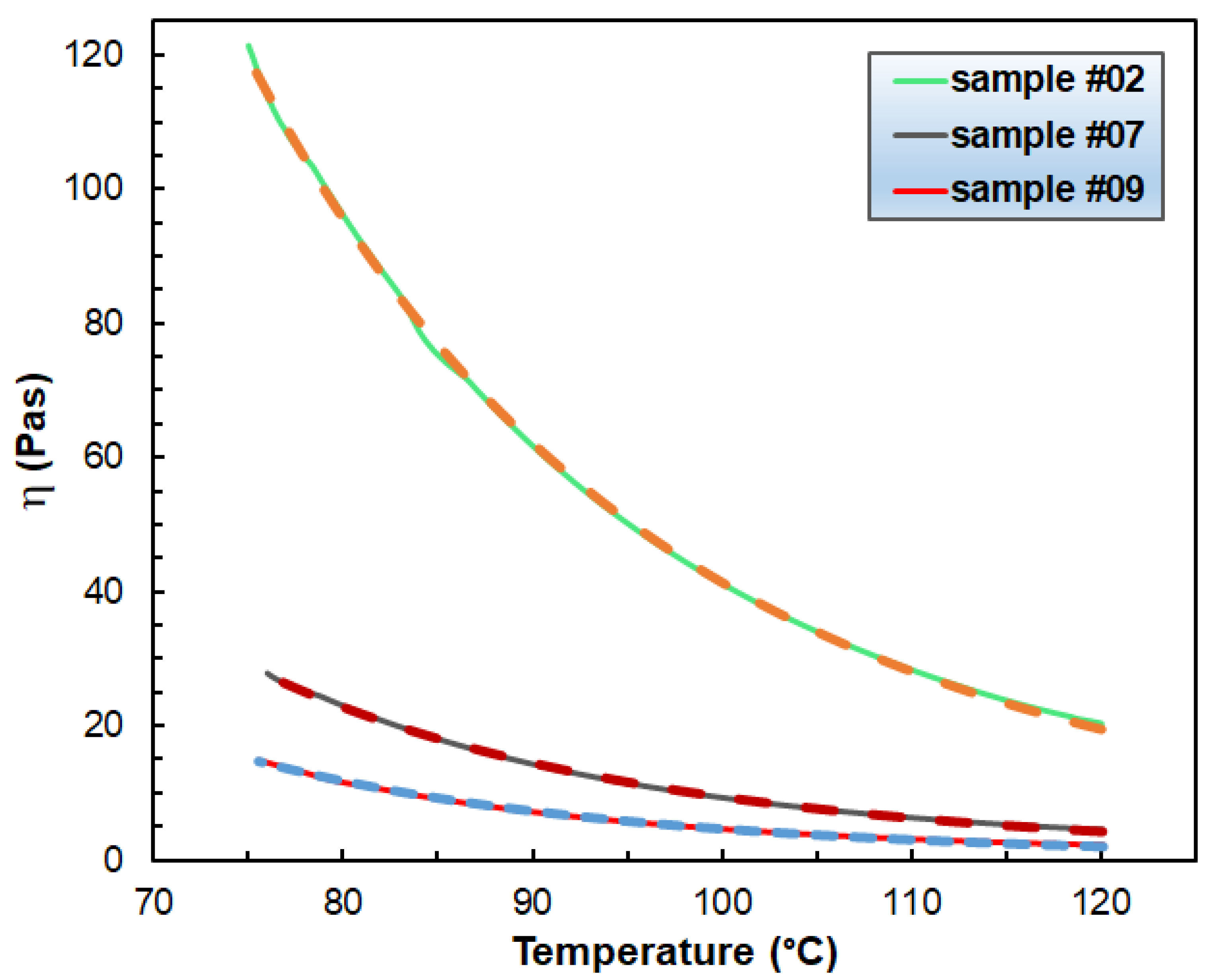
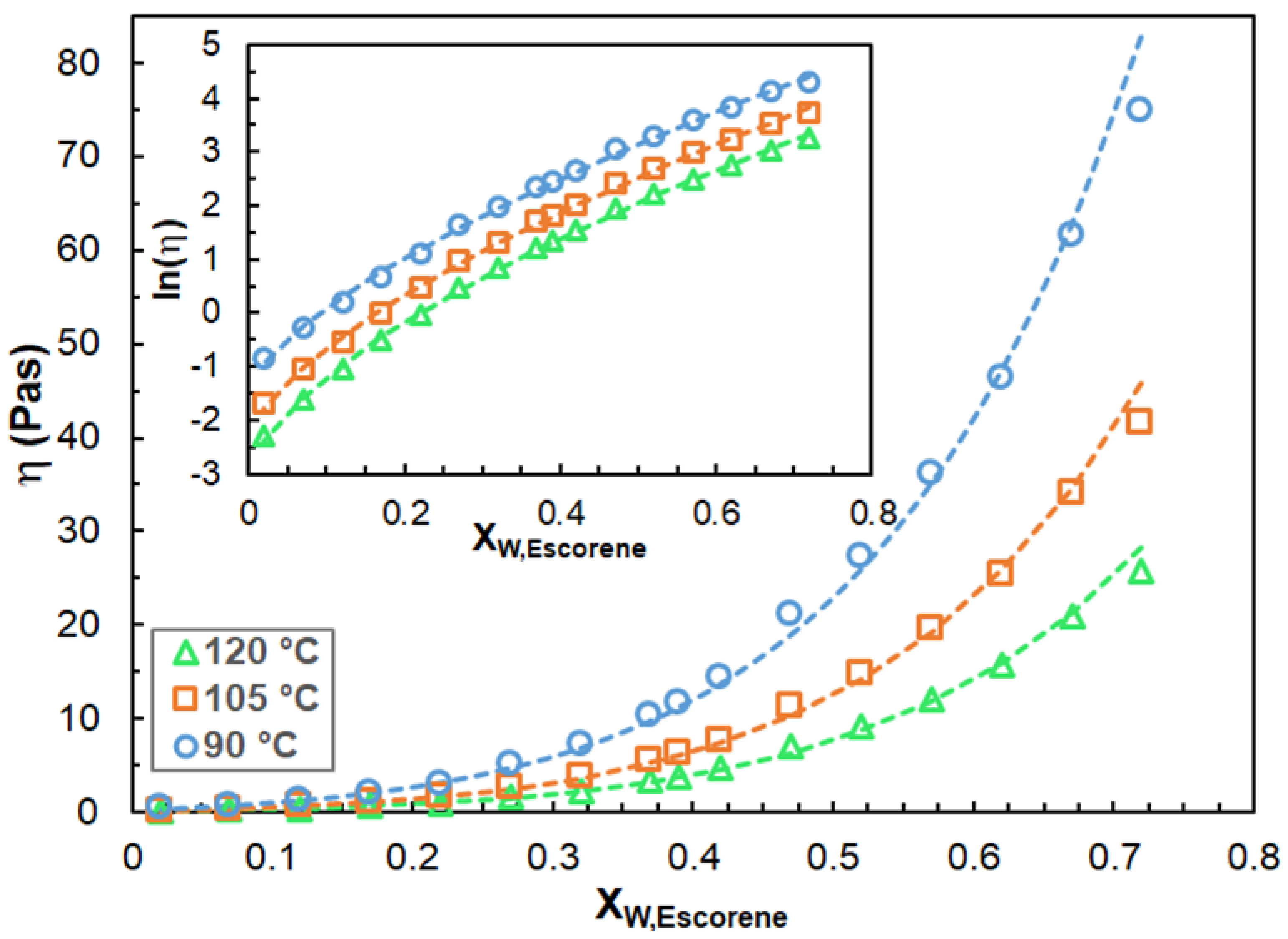



| Sample | DMW7478 % (m/m) | Piccotex 75 % (m/m) | Escorene % (m/m) |
|---|---|---|---|
| #01 | 28 | 0 | 72 |
| #02 | 28 | 5 | 67 |
| #03 | 28 | 10 | 62 |
| #04 | 28 | 15 | 57 |
| #05 | 28 | 20 | 52 |
| #06 | 28 | 25 | 47 |
| #07 | 28 | 30 | 42 |
| #08 | 28 | 35 | 37 |
| #09 | 28 | 40 | 32 |
| #10 | 28 | 45 | 27 |
| #11 | 28 | 50 | 22 |
| #12 | 28 | 55 | 17 |
| #13 | 28 | 60 | 12 |
| #14 | 28 | 65 | 7 |
| #15 | 28 | 70 | 2 |
| #16 | 28 | 72 | 0 |
| Sample | #01 | #02 | #03 | #04 | #05 | #06 | #07 | #08 |
| Pour point (°C) | 72.8 | – | – | 72.2 | 71.9 | 72.0 | 71.5 | 70.6 |
| Sample | #09 | #10 | #11 | #12 | #13 | #14 | #15 | #16 |
| Pour point (°C) | 71.3 | 71.2 | 70.8 | 70.6 | 69.5 | 70.7 | 71.1 | 72.0 |
| Sample | Young’s Modulus (MPa) | Yield Stress (MPa) | Yield Strain % | Stress at Break (MPa) | Strain at Break (%) | Flexural Modulus (MPa) |
|---|---|---|---|---|---|---|
| #01 | 58.24 ± 6.99 | 3.51 ± 0.11 | 27.5 ± 2.1 | 3.08 ± 0.16 | 29.9 ± 2.4 | 62.50 ± 1.46 |
| #02 | 52.63 ± 2.18 | 3.12 ± 0.06 | 29.2 ± 1.8 | 2.80 ± 0.08 | 32.7 ± 2.0 | 57.10 ± 1.51 |
| #03 | 52.57 ± 3.54 | 3.06 ± 0.06 | 28.9 ± 0.8 | 2.76 ± 0.07 | 31.7 ± 1.4 | 50.00 ± 1.93 |
| #04 | 45.01 ± 5.10 | 2.75 ± 0.04 | 29.4 ± 1.6 | 2.43 ± 0.12 | 34.6 ± 0.9 | 54.10 ± 2.31 |
| #05 | 45.40 ± 4.91 | 1.35 ± 0.42 | 6.3 ± 3.2 | 0.95 ± 0.51 | 9.6 ± 3.2 | 51.52 ± 1.76 |
| #06 | 51.60 ± 4.22 | 2.42 ± 0.07 | 24.3 ± 1.3 | 1.75 ± 0.38 | 26.3 ± 1.6 | 45.81 ± 3.39 |
| #07 | 48.41 ± 6.74 | 2.16 ± 0.02 | 28.0 ± 0.8 | 1.68 ± 0.22 | 37.6 ± 1.4 | 50.63 ± 2.98 |
| #08 | 60.30 ± 3.44 | 2.16 ± 0.04 | 22.5 ± 0.5 | 1.17 ± 0.05 | 27.6 ± 2.1 | 51.53 ± 0.59 |
| #09 | 55.09 ± 4.67 | 1.83 ± 0.07 | 21.1 ± 1.8 | 1.09 ± 0.07 | 30.7 ± 3.6 | 55.06 ± 3.73 |
| #10 | 49.90 ± 4.47 | 1.63 ± 0.01 | 18.8 ± 1.3 | 1.10 ± 0.05 | 37.8 ± 1.0 | 58.35 ± 2.52 |
| #11 | 64.16 ± 4.36 | 1.61 ± 0.01 | 18.0 ± 0.8 | 1.06 ± 0.03 | 36.9 ± 1.8 | 55.91 ± 3.69 |
| #12 | 64.10 ± 7.32 | 1.57 ± 0.06 | 12.8 ± 1.7 | 1.00 ± 0.10 | 21.3 ± 5.2 | 70.95 ± 6.57 |
| #13 | 85.67 ± 11.50 | 1.58 ± 0.05 | 10.9 ± 0.25 | 0.98 ± 0.07 | 25.6 ± 6.5 | 70.34 ± 3.12 |
| #14 | 99.50 ± 17.68 | 1.78 ± 0.03 | 11.9 ± 0.6 | 1.05 ± 0.05 | 40.6 ± 7.3 | 74.62 ± 4.65 |
| #15 | 116.22 ± 21.46 | 1.37 ± 0.14 | 5.9 ± 0.6 | 1.18 ± 0.27 | 11.5 ± 3.6 | 128.29 ± 7.94 |
| #16 | 108.50 ± 12.10 | 1.11 ± 0.05 | 6.3 ± 0.3 | 0.37 ± 0.06 | 31.4 ± 8.5 | 200.48 ± 27.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, L.; Deák, G.; Nyul, D.; Kéki, S. Flexible Investment Casting Wax Patterns for 3D-Printing: Their Rheological and Mechanical Characterizations. Polymers 2022, 14, 4744. https://doi.org/10.3390/polym14214744
Szabó L, Deák G, Nyul D, Kéki S. Flexible Investment Casting Wax Patterns for 3D-Printing: Their Rheological and Mechanical Characterizations. Polymers. 2022; 14(21):4744. https://doi.org/10.3390/polym14214744
Chicago/Turabian StyleSzabó, László, György Deák, Dávid Nyul, and Sándor Kéki. 2022. "Flexible Investment Casting Wax Patterns for 3D-Printing: Their Rheological and Mechanical Characterizations" Polymers 14, no. 21: 4744. https://doi.org/10.3390/polym14214744
APA StyleSzabó, L., Deák, G., Nyul, D., & Kéki, S. (2022). Flexible Investment Casting Wax Patterns for 3D-Printing: Their Rheological and Mechanical Characterizations. Polymers, 14(21), 4744. https://doi.org/10.3390/polym14214744







