Effect of Latex Purification and Accelerator Types on Rubber Allergens Prevalent in Sulphur Prevulcanized Natural Rubber Latex: Potential Application for Allergy-Free Natural Rubber Gloves
Abstract
1. Introduction
2. Materials and Methods
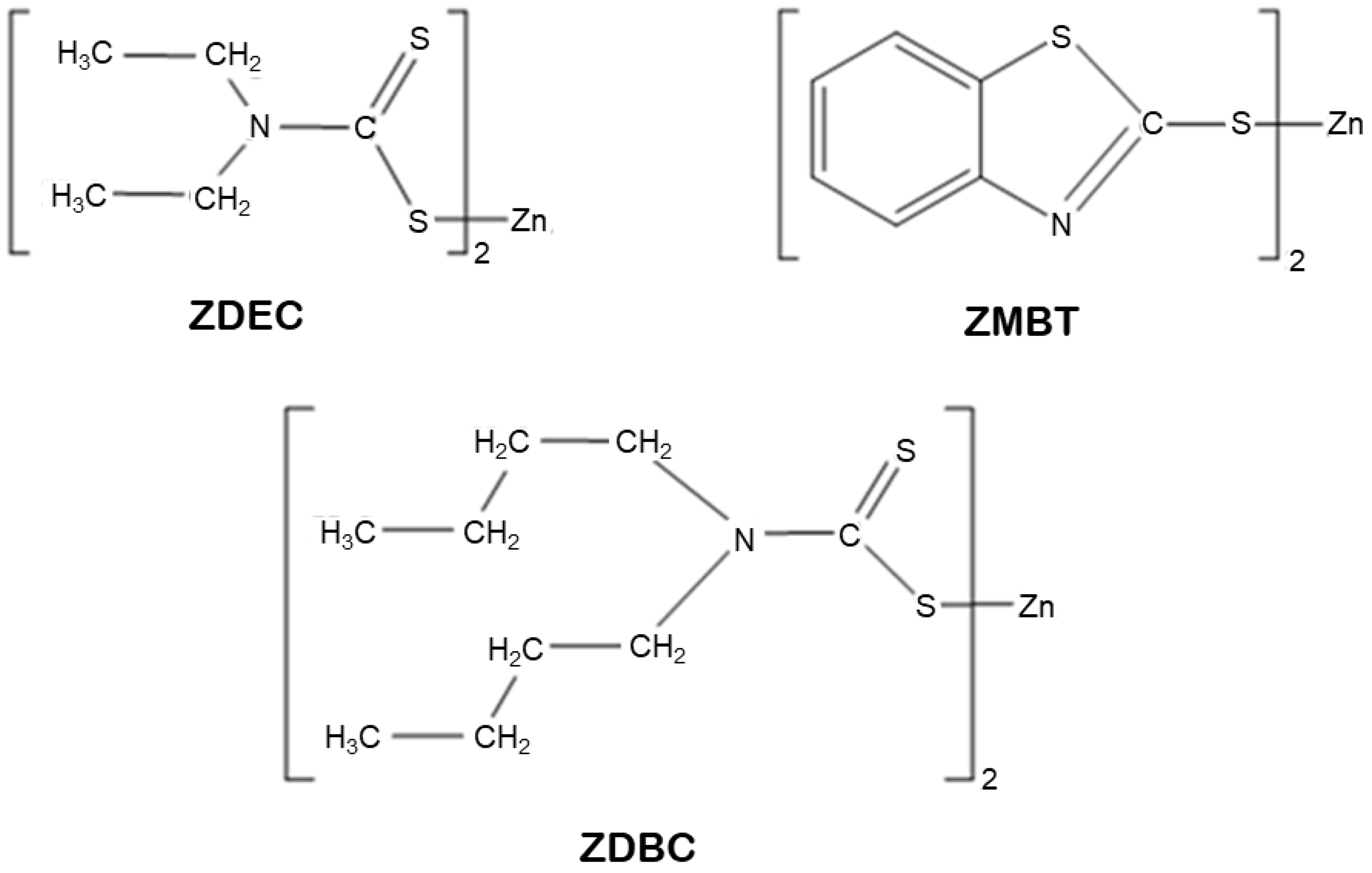
2.1. Materials
2.2. Methods
2.2.1. Preparation of Purified Latex
2.2.2. Preparation of NR Dipping Product
2.2.3. Extraction of Proteins and Residual Accelerators
2.3. Characterizations
2.3.1. Analysis of Purified NR and Extractable Proteins
2.3.2. Analysis of Residual Accelerators
2.3.3. Physical Properties and Morphology of the NR Gloves
3. Results and Discussion
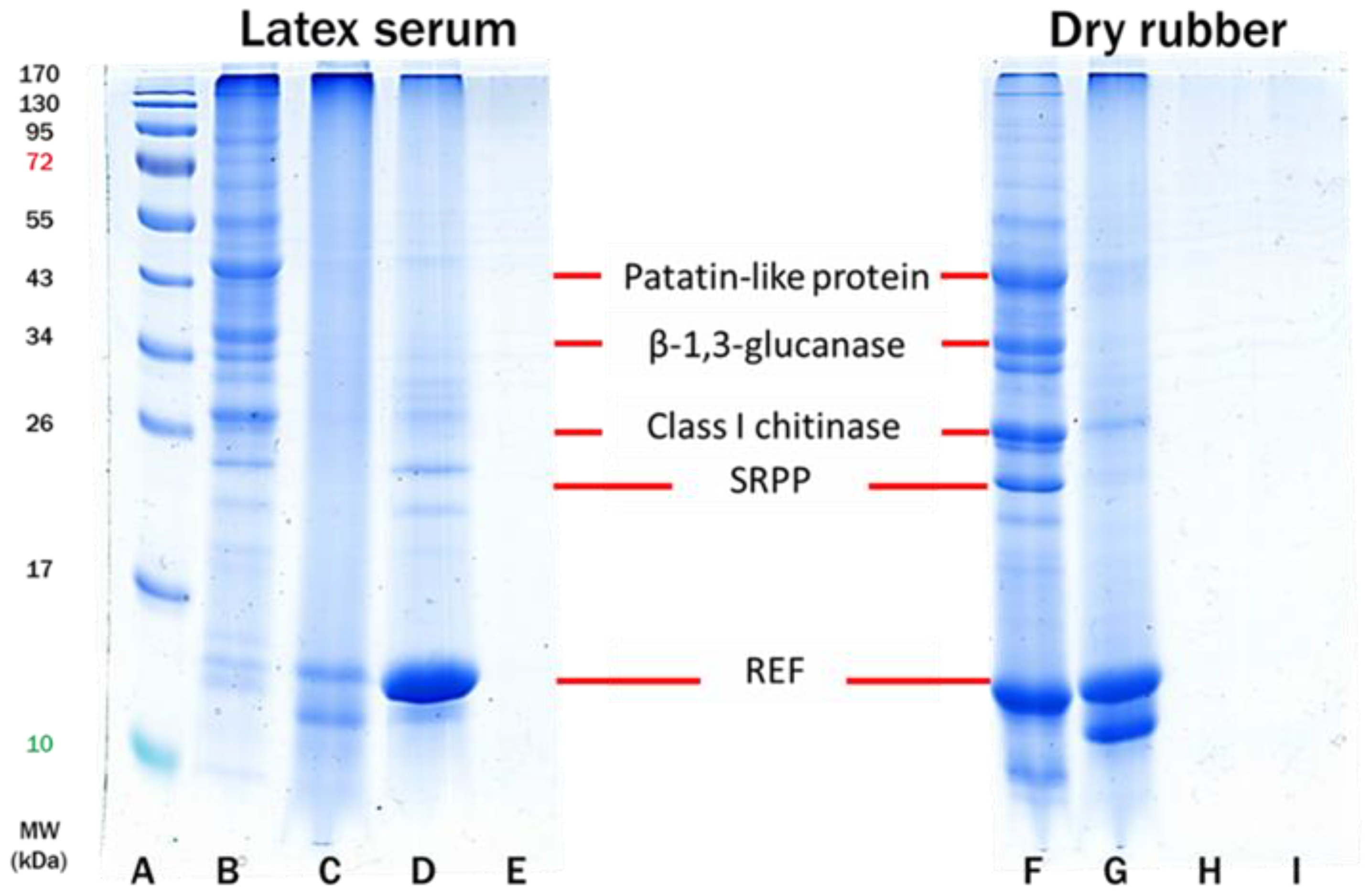
3.1. Characterization of Purified Rubber by FTIR and SDS-PAGE
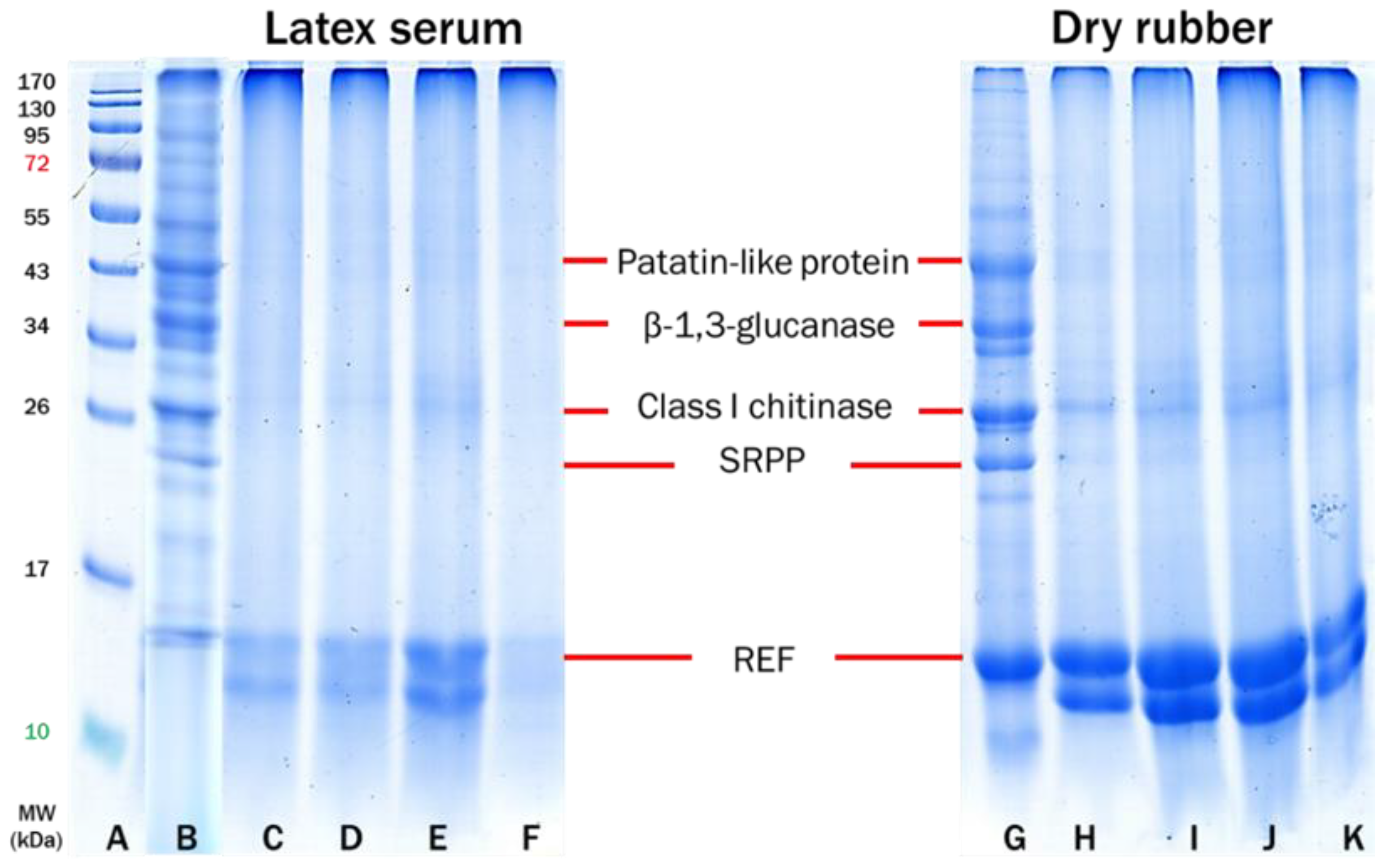
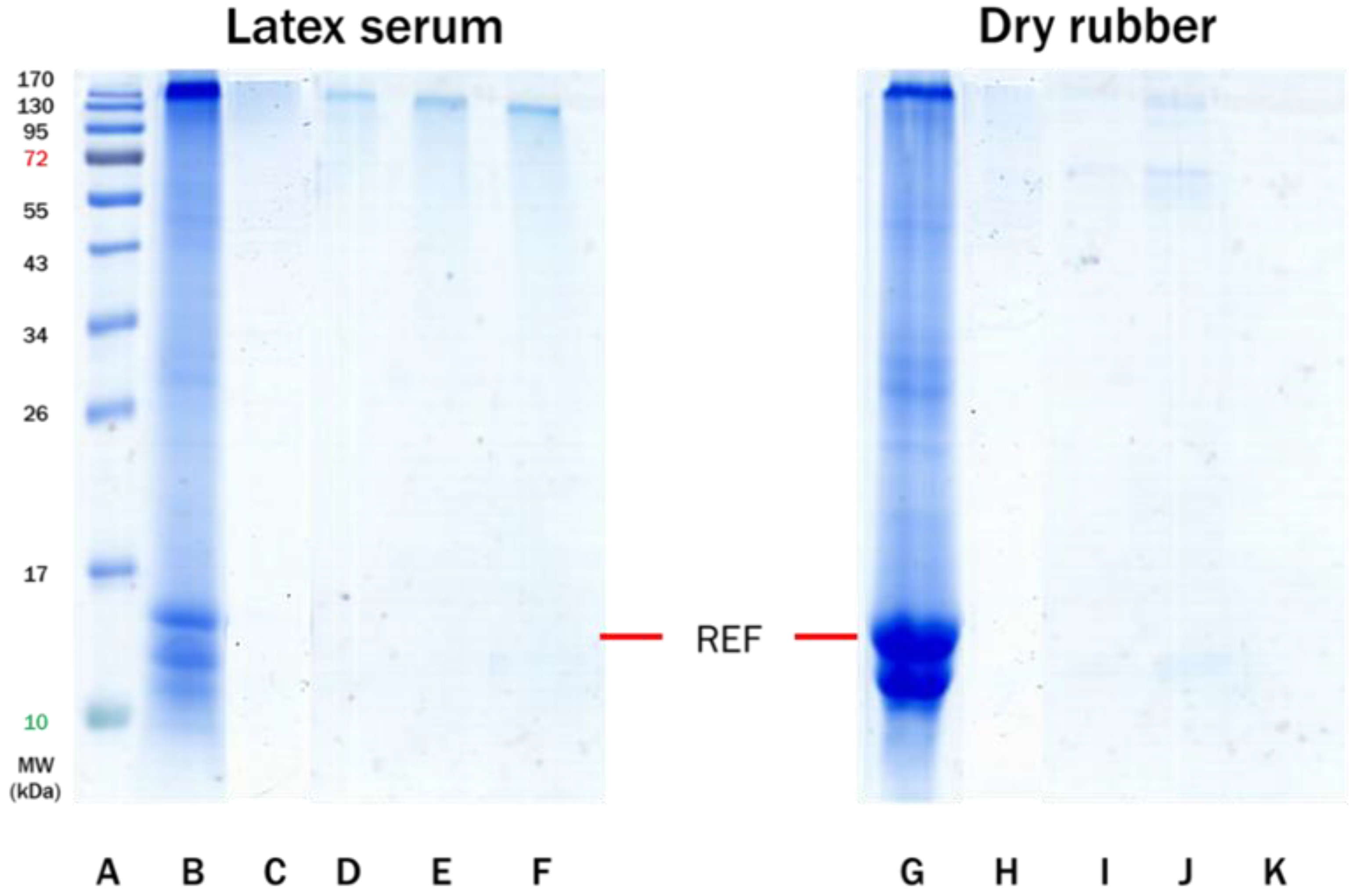
3.2. Effect of Accelerator Types on Allergenic Proteins of Purified NR
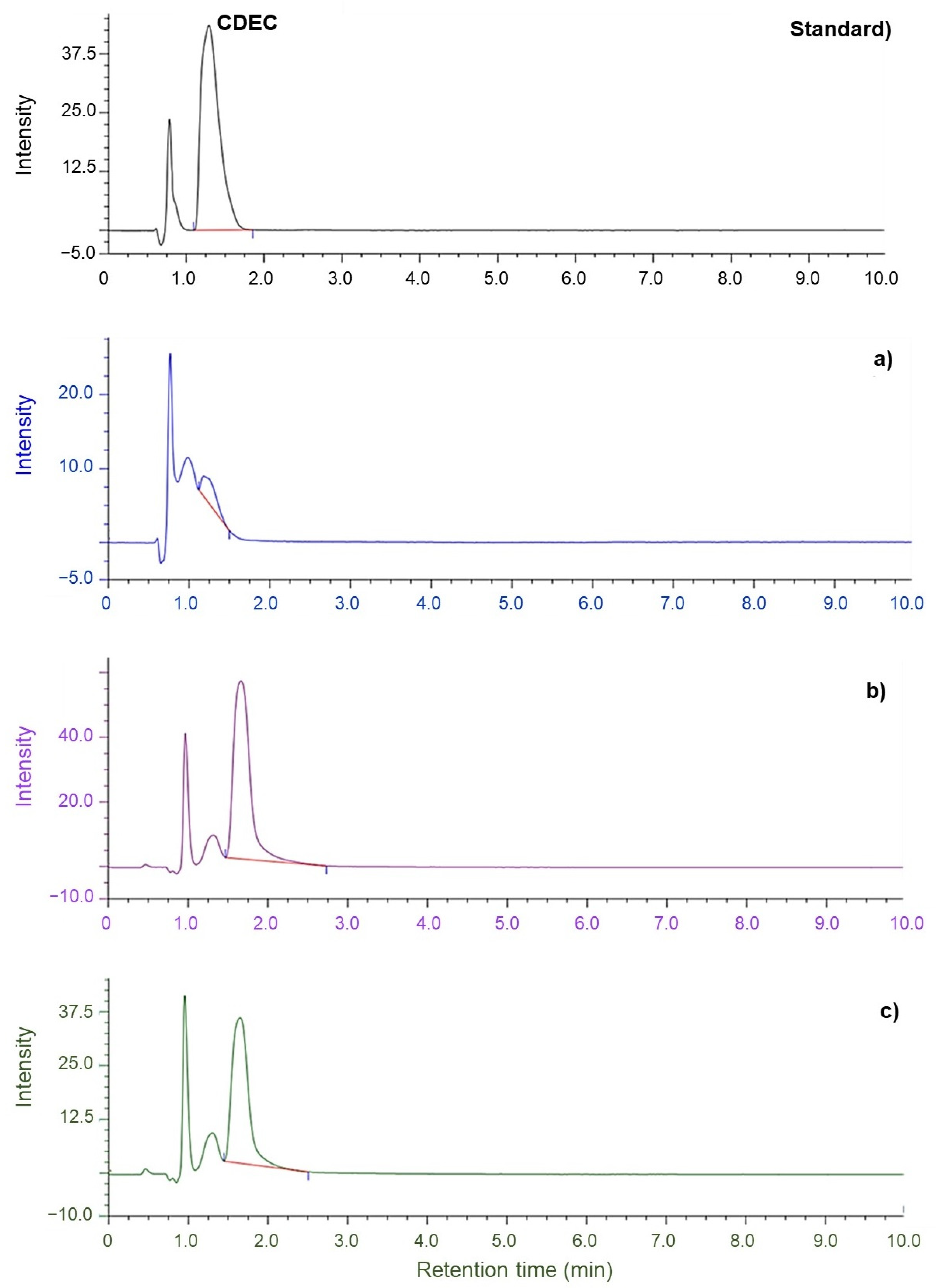
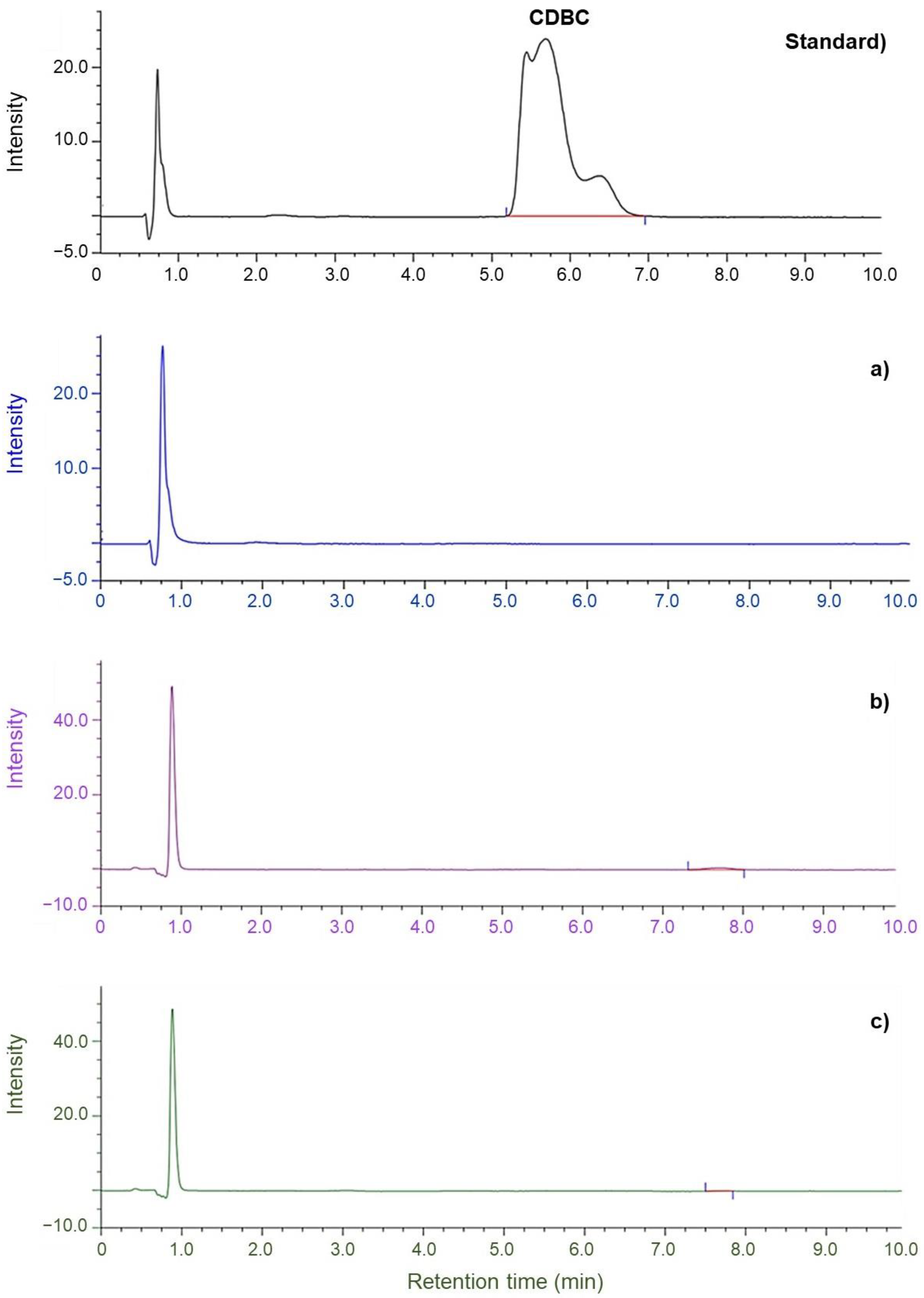
3.3. Effect of Purified NR and Accelerator Types on the Migration of Residual Accelerators from Glove to Artificial Sweat
3.4. Properties of Products
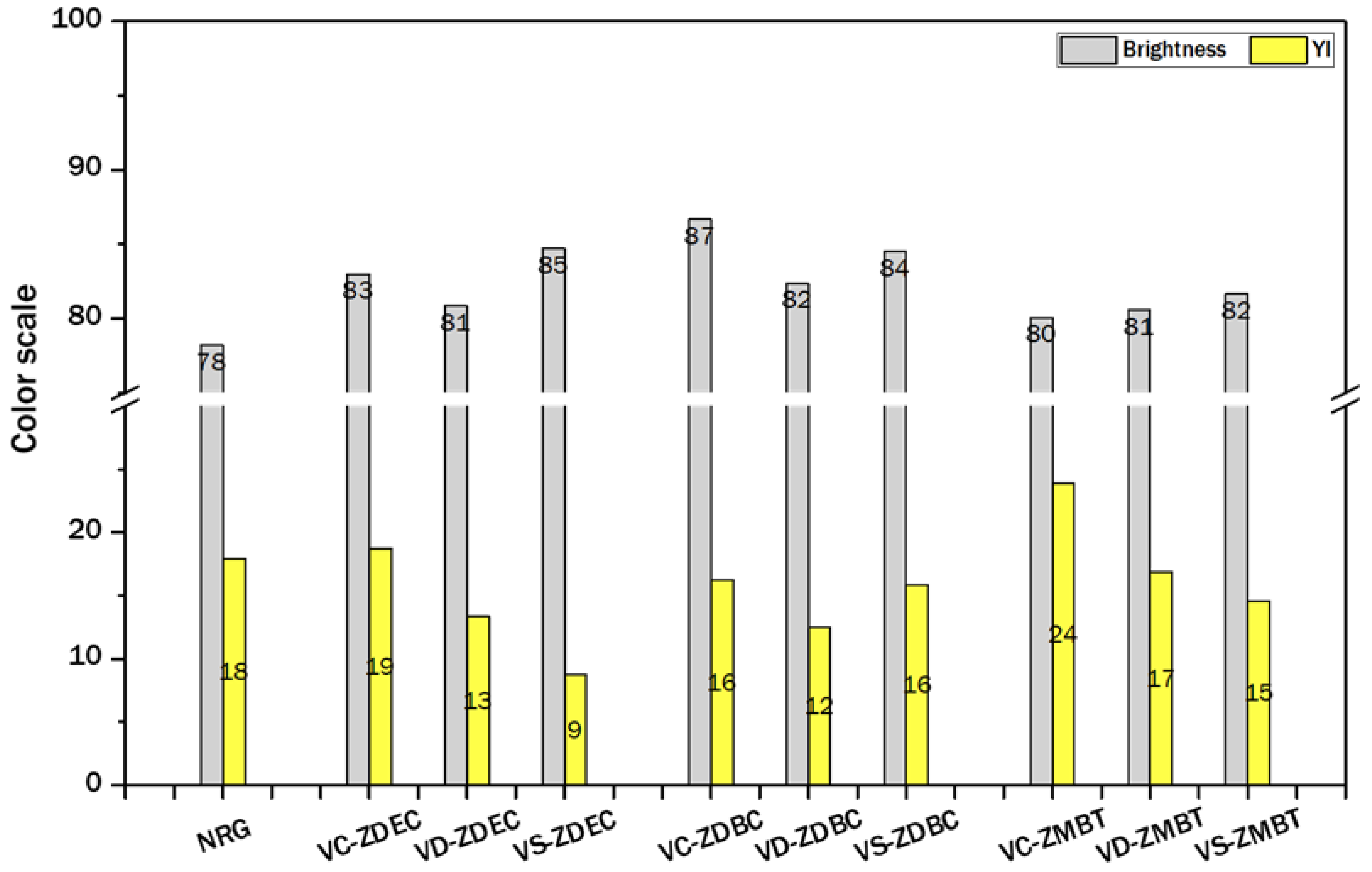
3.4.1. Product Appearance
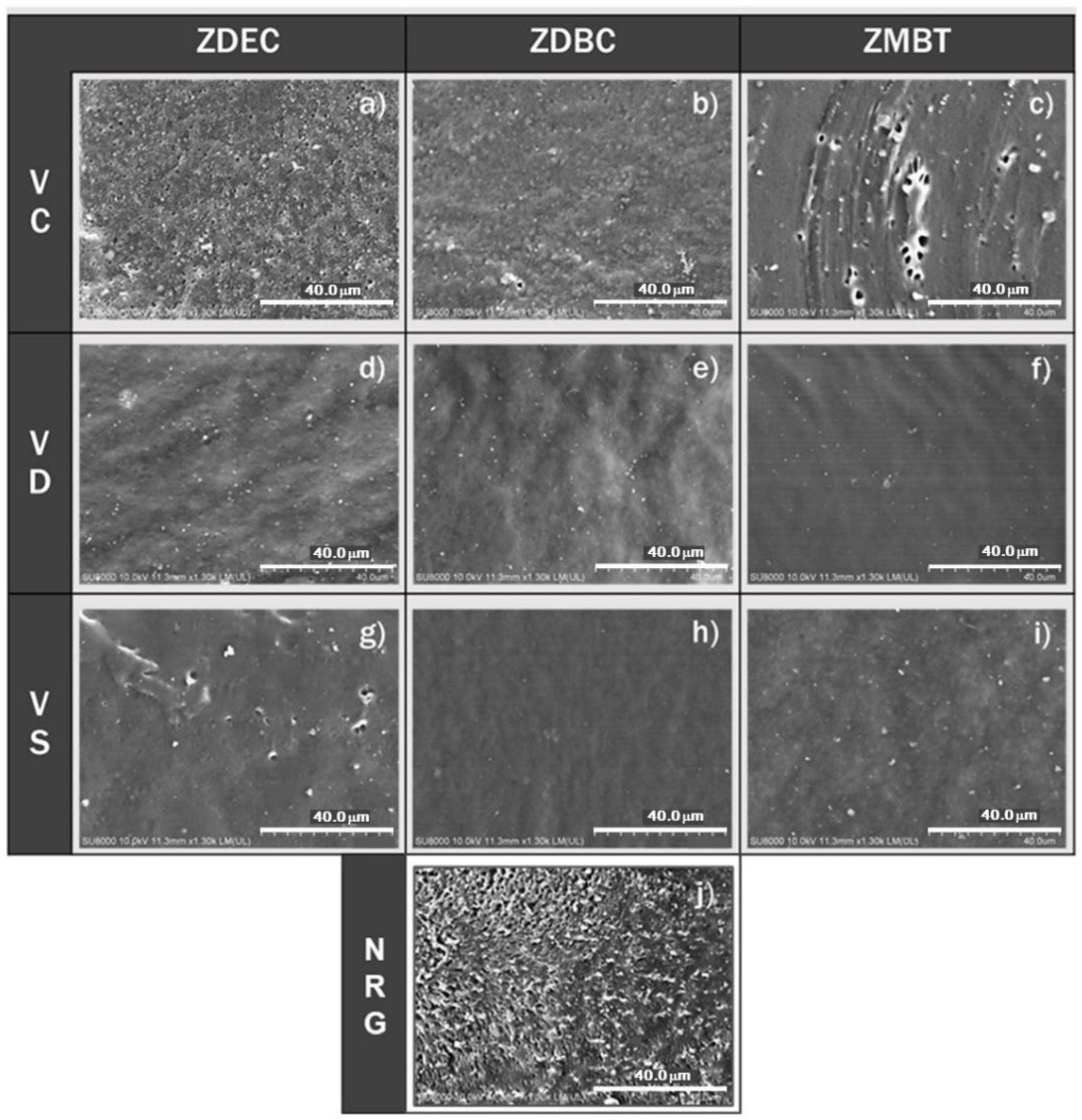
3.4.2. Morphology of Internal Structure
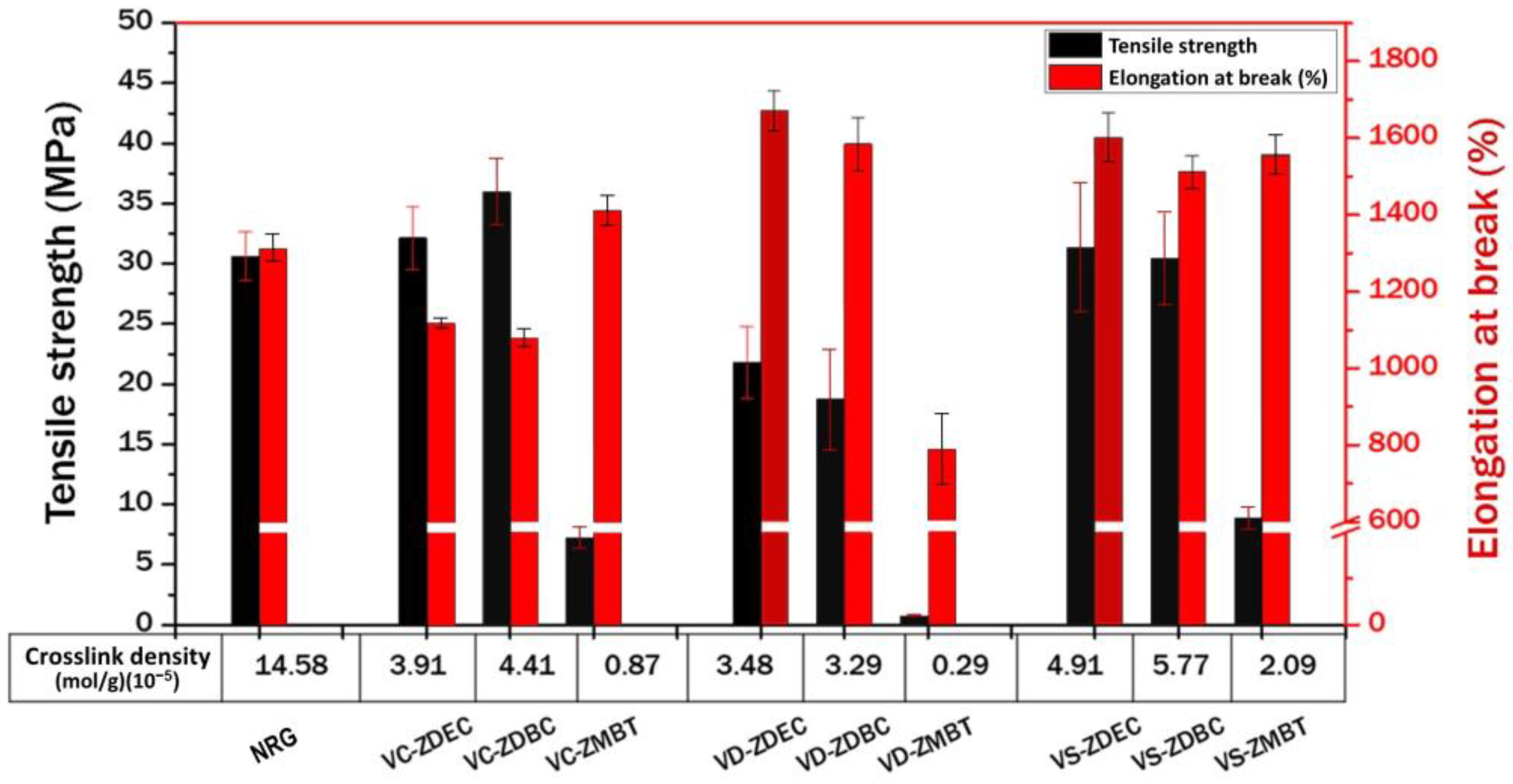
3.4.3. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gnaneswaran, V.; Mudhunuri, B.; Bishu, R.R. A study of latex and vinyl gloves: Performance versus allergy protection properties. Int. J. Ind. Ergon. 2008, 38, 171–181. [Google Scholar] [CrossRef]
- Mylon, P.; Lewis, R.; Carré, M.J.; Martin, N.; Brown, S. A study of clinicians’ views on medical gloves and their effect on manual performance. Am. J. Infect. Control 2014, 42, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Geier, J.; Lessmann, H.; Mahler, V.; Pohrt, U.; Uter, W.; Schnuch, A. Occupational contact allergy caused by rubber gloves–nothing has changed. Contact Dermat. 2012, 67, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Schwensen, J.F.; Menné, T.; Hald, M.; Johansen, J.D.; Thyssen, J.P. Allergic perioral contact dermatitis caused by rubber chemicals during dental treatment. Contact Dermat. 2016, 74, 110–111. [Google Scholar] [CrossRef] [PubMed]
- Sakdapipanich, J.T.; Rojruthai, P. Natural rubber: Biosynthesis, structure, properties and application. In Natural Rubber Materials: Volume 1: Blend and IPNs; Thomas, S., Chan, C.H., Pothen, L.A., Rajisha, K.R., Maria, H.J., Eds.; RSC: Cambridge, UK, 2014; pp. 28–52. [Google Scholar]
- Rojruthai, P.; Kantarum, T.; Sakdapipanich, J. Impact of nonrubber components on the branching structure and the accelerated storage hardening in Hevea rubber. J. Rubber Res. 2020, 23, 353–364. [Google Scholar] [CrossRef]
- Nawamawat, K.; Sakdapipanich, J.; Ho, C.C. Effect of deproteinized methods on the proteins and properties of natural rubber latex during storage. Macromol. Symp. 2010, 288, 95–103. [Google Scholar] [CrossRef]
- Mekkriengkrai, D.; Sakdapipanich, J.T.; Tanaka, Y. Structural characterization of terminal groups in natural rubber: Origin of nitrogenous groups. Rubber Chem. Technol. 2006, 79, 366–379. [Google Scholar] [CrossRef]
- Kawahara, S.; Klinklai, W.; Kuroda, H.; Isono, Y. Removal of proteins from natural rubber with urea. Polym. Adv. Technol. 2004, 15, 181–184. [Google Scholar] [CrossRef]
- Mahendra, I.P.; Linh, M.; Thang, N.; Thuy, V.; Trang, L.; Thinh, L.; Phuong, N.; Ha, N.; Thuong, N.; Kawahara, S.; et al. Protein removal from natural rubber latex with Fe3O4@Al2O3 nanoparticle. J. Braz. Chem. Soc. 2021, 32, 320–328. [Google Scholar] [CrossRef]
- Moolsin, S.; Prasoppol, P.; Potisuya, P.; Seesook, R. Deproteinized natural rubber film reinforced with silane-modified silica nanoparticles. J. Elastomers Plast. 2021, 53, 31–47. [Google Scholar] [CrossRef]
- Klinklai, W.; Saito, T.; Kawahara, S.; Tashiro, K.; Suzuki, Y.; Sakdapipanich, J.T.; Isono, Y. Hyperdeproteinized natural rubber prepared with urea. J. Appl. Polym. Sci. 2004, 93, 555–559. [Google Scholar] [CrossRef]
- Oliveira, I.P.; Martínez, L. The shift in urea orientation at protein surfaces at low pH is compatible with a direct mechanism of protein denaturation. Phys. Chem. Chem. Phys. 2020, 22, 354–367. [Google Scholar] [CrossRef]
- Su, Z.; Dias, C.L. Molecular interactions accounting for protein denaturation by urea. J. Mol. Liquids 2016, 228, 168–175. [Google Scholar] [CrossRef]
- Yang, Z.; Xiu, P.; Shi, B.; Hua, L.; Zhou, R. Coherent microscopic picture for urea-induced denaturation of proteins. J. Phys. Chem. B 2012, 116, 8856–8862. [Google Scholar] [CrossRef]
- Das, A.; Mukhopadhyay, C. Urea-mediated protein denaturation: A consensus view. J. Phys. Chem. B 2009, 113, 12816–12824. [Google Scholar] [CrossRef]
- Stumpe, M.C.; Grubmller, H. Interaction of urea with amino acids: Implications for urea-induced protein denaturation. J. Am. Chem. Soc. 2007, 129, 16126–16131. [Google Scholar] [CrossRef]
- Nettis, E.; Assennato, G.; Ferrannini, A.; Tursi, A. Type I allergy to natural rubber latex and type IV allergy to rubber chemicals in health care workers with glove-related skin symptoms. Clin. Exp. Allergy 2002, 32, 441–447. [Google Scholar] [CrossRef]
- Warburton, K.L.; Tresukosol, P.; Santhawachart, R.; Jantarat, J.; Sutimuntanakul, S.; Harnirattisai, C.; Suddhasthira, T.; Chaisuriyathepkul, A.M. Patch testing with rubber series in Europe: A critical review and recommendation. Contact Derm. 2016, 76, 195–203. [Google Scholar] [CrossRef]
- Yanpiset, K.; Uter, W.; Geier, J.; Spiewak, R.; Mahler, V.; Crépy, M.; Schuttelaar, M.L.; Bauer, A.; Wilkinson, M. Allergic contact dermatitis of styrenic thermoplastic elastomers and latex sheets in humans. M. Dent. J. 2017, 37, 47–53. [Google Scholar]
- Cao, L.Y.; Taylor, J.S.; Sood, A.; Murray, D.; Siegel, P.D. Allergic contact dermatitis to synthetic rubber gloves: Changing trends in patch test reactions to accelerators. Arch. Dermatol. 2010, 146, 1001–1007. [Google Scholar] [CrossRef]
- De Jong, W.H.; Van Och, F.M.M.; Jager, C.F.D.; Spiekstra, S.W.; Slob, W.; Vandebriel, R.J.; Van Loveren, H. Ranking of allergenic potency of rubber chemicals in a modified local lymph node assay. Toxicol. Sci. 2002, 66, 226–232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Geier, J.; Lessmann, H.; Uter, W.; Schnuch, A. Occupational rubber glove allergy: Results of the information network of departments of dermatology (IVDK), 1995–2001. Contact Dermat. 2003, 48, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Goodier, M.C.; Ronkainen, S.D.; Hylwa, S.A. Rubber accelerators in medical examination and surgical gloves. Dermatitis 2018, 29, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.; Parameswaran, R.; Joseph, R.; Remakumary, P.V. Release of dithiocarbamates into sweat from natural rubber latex surgical gloves. Rubber Chem. Technol. 2005, 78, 674–681. [Google Scholar] [CrossRef]
- Abraham, E.K.; Ramesh, P.; Joseph, R. Release of dithiocarbamates into artificial sweat from latex vulcanizates: Effects of the accelerator type and storage time. J. Appl. Polym. Sci. 2006, 102, 2055–2061. [Google Scholar] [CrossRef]
- Sussman, G.L.; Beezhold, D.H.; Kurup, V.P. Allergens and natural rubber proteins. J. Allergy Clin. Immunol. 2002, 110, S33–S39. [Google Scholar] [CrossRef]
- Chaiear, N.; Sadhra, S.; Jones, M.; Cullinan, P.; Foulds, I.S.; Burge, P.S. Sensitisation to natural rubber latex: An epidemiological study of workers exposed during tapping and glove manufacture in Thailand. Occup. Environ. Med. J. 2001, 58, 386–391. [Google Scholar] [CrossRef]
- Chaiear, N.; Jindawong, B.; Boonsawas, W.; Kanchanarach, T.; Sakunkoo, P. Glove allergy and sensitization to natural rubber latex among nursing staff at Srinagarind hospital, Khon Kaen, Thailand. J. Med Assoc. Thail. 2006, 89, 368–376. [Google Scholar]
- Parisi, C.A.S.; Kelly, K.J.; Ansotegui, I.J.; Gonzalez-Díaz, S.N.; Bilò, M.B.; Cardona, V.; Park, H.; Braschi, M.C.; Macias-Weinmann, A.; Piga, M.A.; et al. Update on latex allergy: New insights into an old problem. World Allergy Organ. J. 2021, 14, 100569. [Google Scholar] [CrossRef]
- Alenius. H.; Turjanmaa. K.; Palosuo, T. Natural rubber latex allergy. Occup. Environ. Med. 2002, 59, 419–424. [Google Scholar] [CrossRef]
- Yeang, H.Y.; Arif, S.A.M.; Raulf-Heimsoth, M.; Loke, Y.H.; Sander, I.; Sulong, S.H.; Lau, C.H.; Hamilton, R.G. Allergenic proteins of natural rubber latex. J. Allergy Clin. Immunol. 2004, 114, 593–598. [Google Scholar] [CrossRef]
- Berthelot, K.; Lecomte, S.; Estevez, Y.; Peruch, F. Hevea brasiliensis REF (Hev b 1) and SRPP (Hev b 3): An overview on rubber particle proteins. Biochimie 2014, 106, 1–9. [Google Scholar] [CrossRef]
- Rojruthai, P.; Sakdapipanich, J.T.; Takahashi, S.; Hyegin, L.; Noike, M.; Koyama, T.; Tanaka, Y. In vitro synthesis of high molecular weight rubber by Hevea small rubber particles. J. Biosci. Bioeng. 2010, 109, 107–114. [Google Scholar] [CrossRef]
- Yeang, H.Y.; Arif, S.A.M.; Yusof, F.; Sunderasan, E. Allergenic proteins of natural rubber latex. Methods 2002, 27, 32–45. [Google Scholar] [CrossRef]
- Blackley, D.C. Polymer Latices: Science and Technology Volume 3: Applications of Latices, 2nd ed.; Chapman & Hall: London, UK, 1997. [Google Scholar]
- Havanapan, P.O.; Bourchookarn, A.; Ketterman, A.J.; Krittanai, C. Comparative proteome analysis of rubber latex serum from pathogenic fungi tolerant and susceptible rubber tree (Hevea brasiliensis). J. Proteom. 2016, 131, 82–92. [Google Scholar] [CrossRef]
- Flory, P.J. Statistical Mechanics of Swelling of Network Structures. J. Chem. Phys. 1950, 18, 108–111. [Google Scholar] [CrossRef]
- Rolere, S.; Liengprayoon, S.; Vaysse, L.; Sainte-Beuve, J.; Bonfils, F. Investigating natural rubber composition with Fourier Transform Infrared (FT-IR) spectroscopy: A rapid and non-destructive method to determine both protein and lipid contents simultaneously. Polym. Test. 2015, 43, 83–93. [Google Scholar] [CrossRef]
- Sansatsadeekul, J.; Sakdapipanich, J.; Rojruthai, P. Characterization of associated proteins and phospholipids in natural rubber latex. J. Biosci. Bioeng. 2011, 111, 628–634. [Google Scholar] [CrossRef]
- Cremer, R.; Rihs, H.; Raulf-Heimsoth, M. Natural rubber latex allergy in pediatric patients. Curr. Pediatr. Rev. 2008, 4, 258–265. [Google Scholar] [CrossRef]
- Yunyongwattanakorn, J.; Tanaka, Y.; Sakdapipanich, J.; Wongsasutthikul, V. Highly-purified natural rubber by saponification of latex: Analysis of residual proteins in saponified natural rubber. Rubber Chem. Technol. 2008, 81, 121–137. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, M.; Zhao, K. Insight into the effect mechanism of urea-induced protein denaturation by dielectric spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 32007–32015. [Google Scholar] [CrossRef] [PubMed]
- Than-ardba, B.; Tamura, H.; Furuike, T. Improving deproteinized skim natural rubber latex with a further leaching process. Eng. Appl. Sci. Res. 2019, 46, 64–71. [Google Scholar]
- Fukuhara, L.; Miyano, K.; Yamamoto, Y.; Ishii, H.; Nghia, P.T.; Fukuda, M.; Kawahara, S. Removal of proteins from natural rubber latex and gloves. Kautsch. Gummi Kunstst. 2015, 3, 24–29. [Google Scholar]
- Berthelot, K.; Lecomte, S.; Estevez, Y.; Coulary-Salin, B.; Bentaleb, A.; Cullin, C.; Deffieux, A.; Peruch, F. Rubber elongation factor (REF), a major allergen component in Hevea brasiliensis Latex has amyloid properties. PLoS ONE 2012, 7, e48065. [Google Scholar] [CrossRef]
- Nun-anan, P.; Wisunthorn, S.; Pichaiyut, S.; Nathaworn, C.D.; Nakason, C. Influence of nonrubber components on properties of unvulcanized natural rubber. Polym. Adv. Technol. 2020, 31, 44–59. [Google Scholar] [CrossRef]
- Wititsuwannakul, D.; Chareonthiphakorn, N.; Pace, M.; Wititsuwannakul, R. Polyphenol oxidases from latex of Hevea brasiliensis: Purification and characterization. Phytochemistry 2002, 61, 115–121. [Google Scholar] [CrossRef]
- Sakdapipanich, J.; Insom, K.; Phupewkeaw, N. Composition of color substances of Hevea brasiliensis natural rubber. Rubber Chem. Technol. 2007, 80, 212–230. [Google Scholar] [CrossRef]
- Roslim, R.; Tan, K.S.; Jefri, J. Study on morphological structures and mechanical properties of natural rubber latex films prepared at different prevulcanisation and drying temperatures. J. Rubb. Res. 2018, 21, 1–16. [Google Scholar] [CrossRef]



| Ingredients | phr * |
|---|---|
| 60% CNR, DPNR, or SPNR latex, respectively | 100 |
| 20% KOH solution | 0.3 |
| 20% Potassium laurate solution | 0.2 |
| 50% Sulphur dispersion | 0.5 |
| 50% ZDEC, ZDBC, and ZMBT dispersions, respectively | 0.75 |
| 50% Wingstay® L dispersion | 0.5 |
| 50% ZnO dispersion | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojruthai, P.; Sakdapipanich, J.; Wiriyanantawong, J.; Ho, C.-C.; Chaiear, N. Effect of Latex Purification and Accelerator Types on Rubber Allergens Prevalent in Sulphur Prevulcanized Natural Rubber Latex: Potential Application for Allergy-Free Natural Rubber Gloves. Polymers 2022, 14, 4679. https://doi.org/10.3390/polym14214679
Rojruthai P, Sakdapipanich J, Wiriyanantawong J, Ho C-C, Chaiear N. Effect of Latex Purification and Accelerator Types on Rubber Allergens Prevalent in Sulphur Prevulcanized Natural Rubber Latex: Potential Application for Allergy-Free Natural Rubber Gloves. Polymers. 2022; 14(21):4679. https://doi.org/10.3390/polym14214679
Chicago/Turabian StyleRojruthai, Porntip, Jitladda Sakdapipanich, Jinjutha Wiriyanantawong, Chee-Cheong Ho, and Naesinee Chaiear. 2022. "Effect of Latex Purification and Accelerator Types on Rubber Allergens Prevalent in Sulphur Prevulcanized Natural Rubber Latex: Potential Application for Allergy-Free Natural Rubber Gloves" Polymers 14, no. 21: 4679. https://doi.org/10.3390/polym14214679
APA StyleRojruthai, P., Sakdapipanich, J., Wiriyanantawong, J., Ho, C.-C., & Chaiear, N. (2022). Effect of Latex Purification and Accelerator Types on Rubber Allergens Prevalent in Sulphur Prevulcanized Natural Rubber Latex: Potential Application for Allergy-Free Natural Rubber Gloves. Polymers, 14(21), 4679. https://doi.org/10.3390/polym14214679







