Construction of Photoinitiator Functionalized Spherical Nanoparticles Enabling Favorable Photoinitiating Activity and Migration Resistance for 3D Printing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
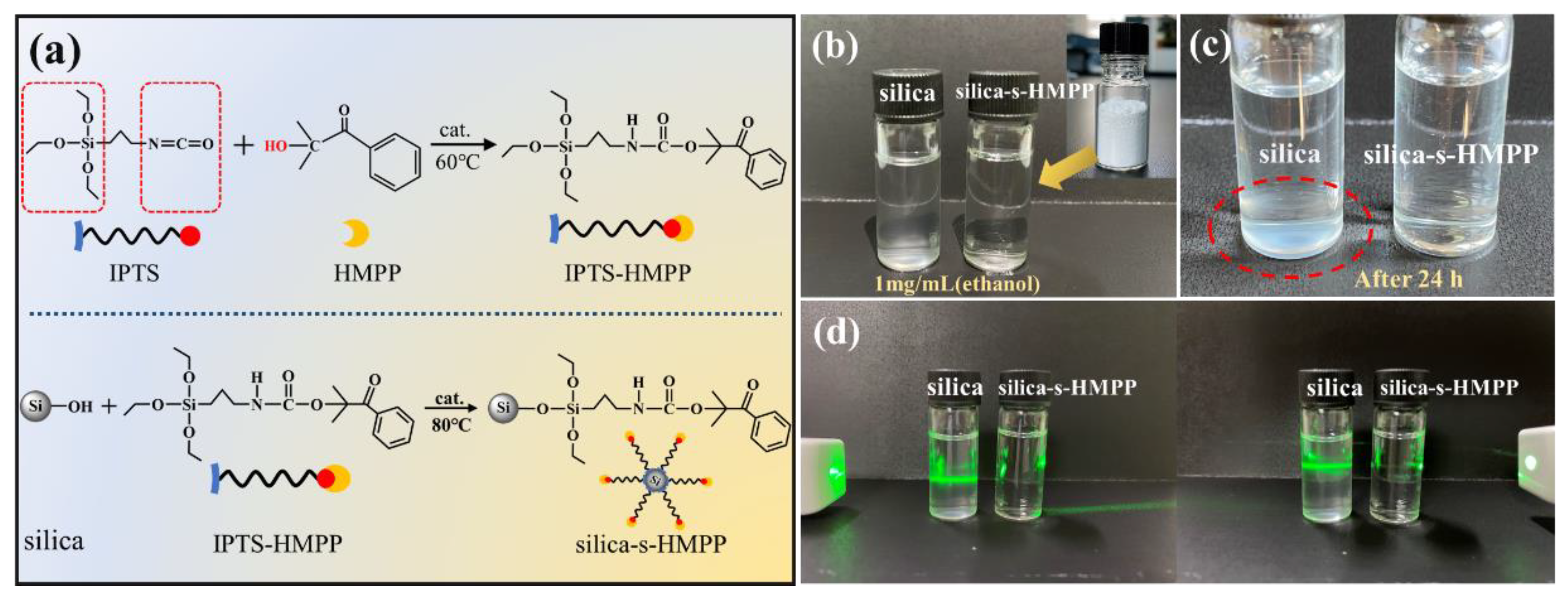
2.2. Preparation of Silica-s-HMPP
2.3. Preparation of the Hybrid Materials
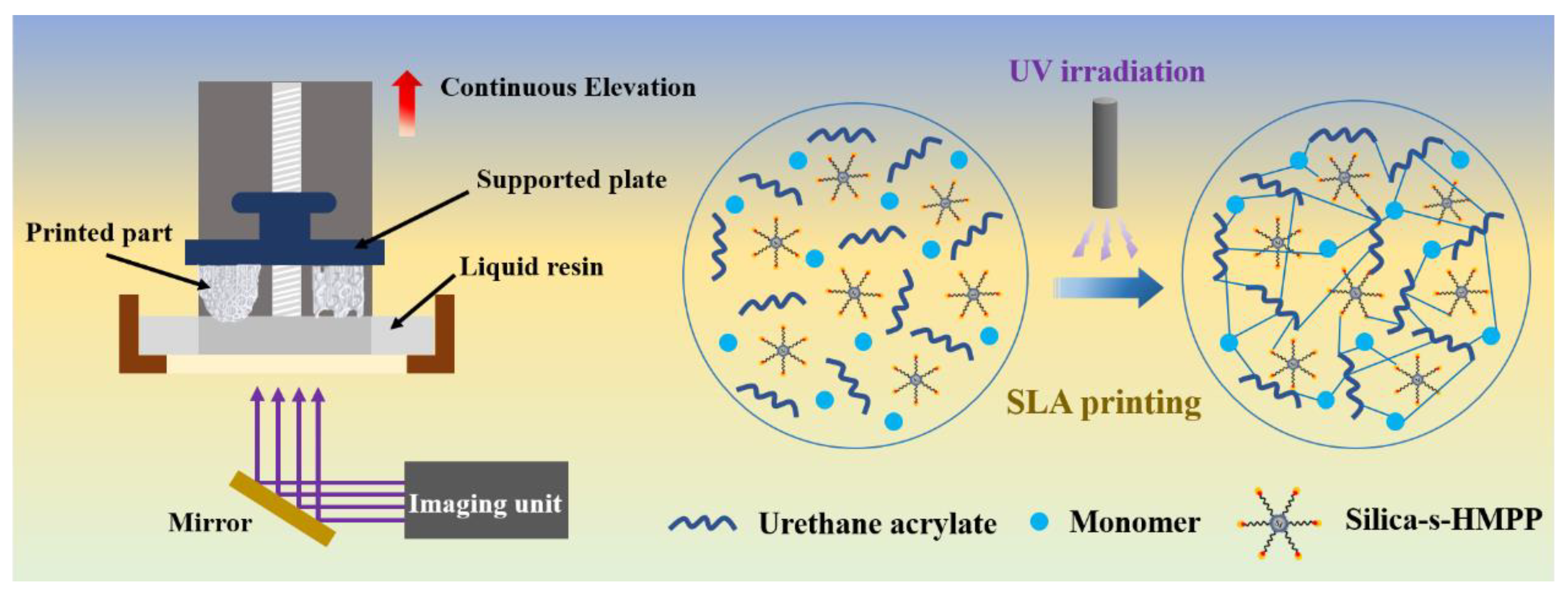
2.4. Stereolithographic 3D Printing
2.5. Characterization
3. Results
3.1. Photoinitiator HMPP Supported onto Silica Surface
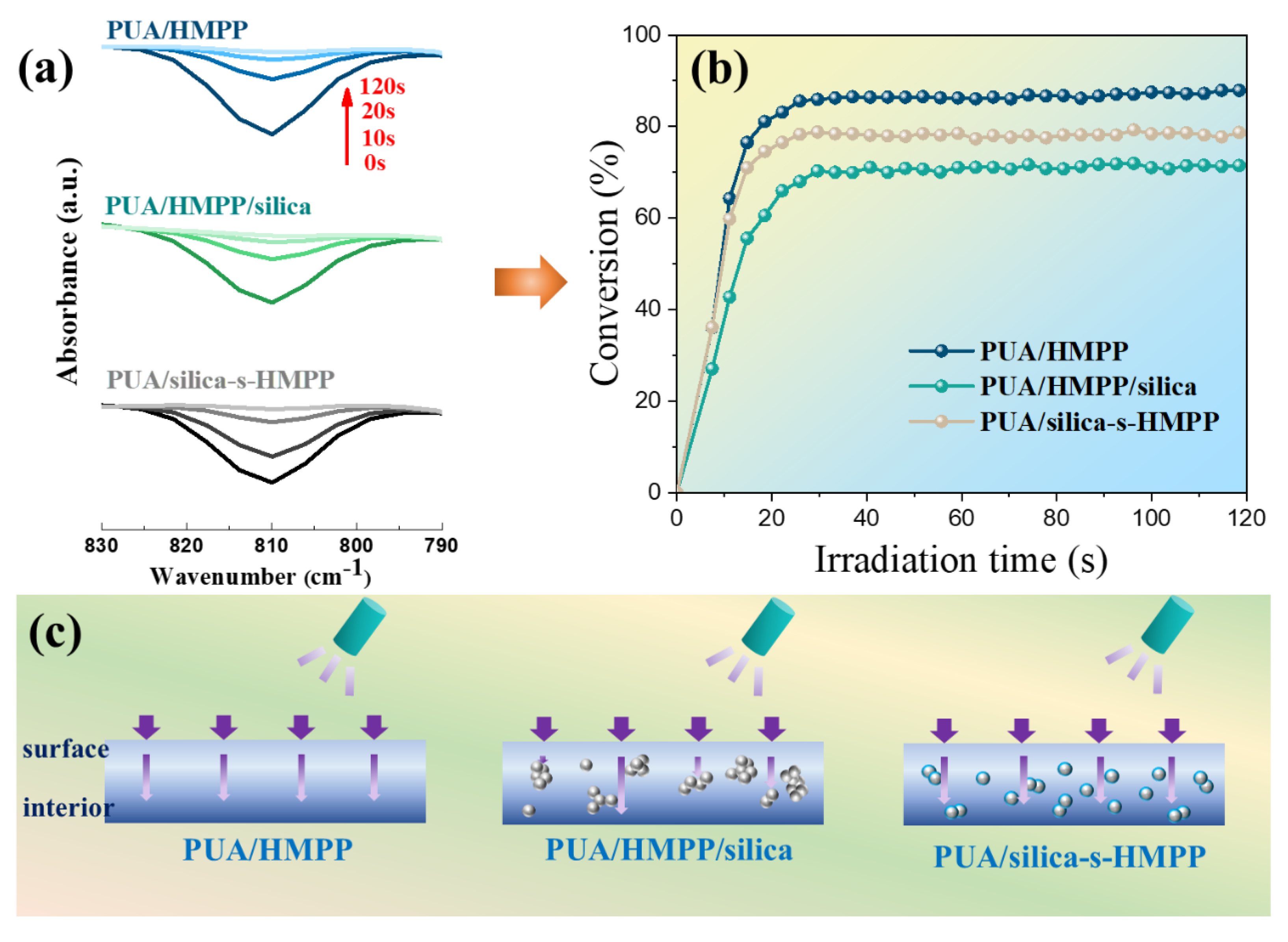
3.2. Kinetic Study of Photopolymerization
3.3. Mechanical Properties and Morphology of Polyurethane Acrylate Composites
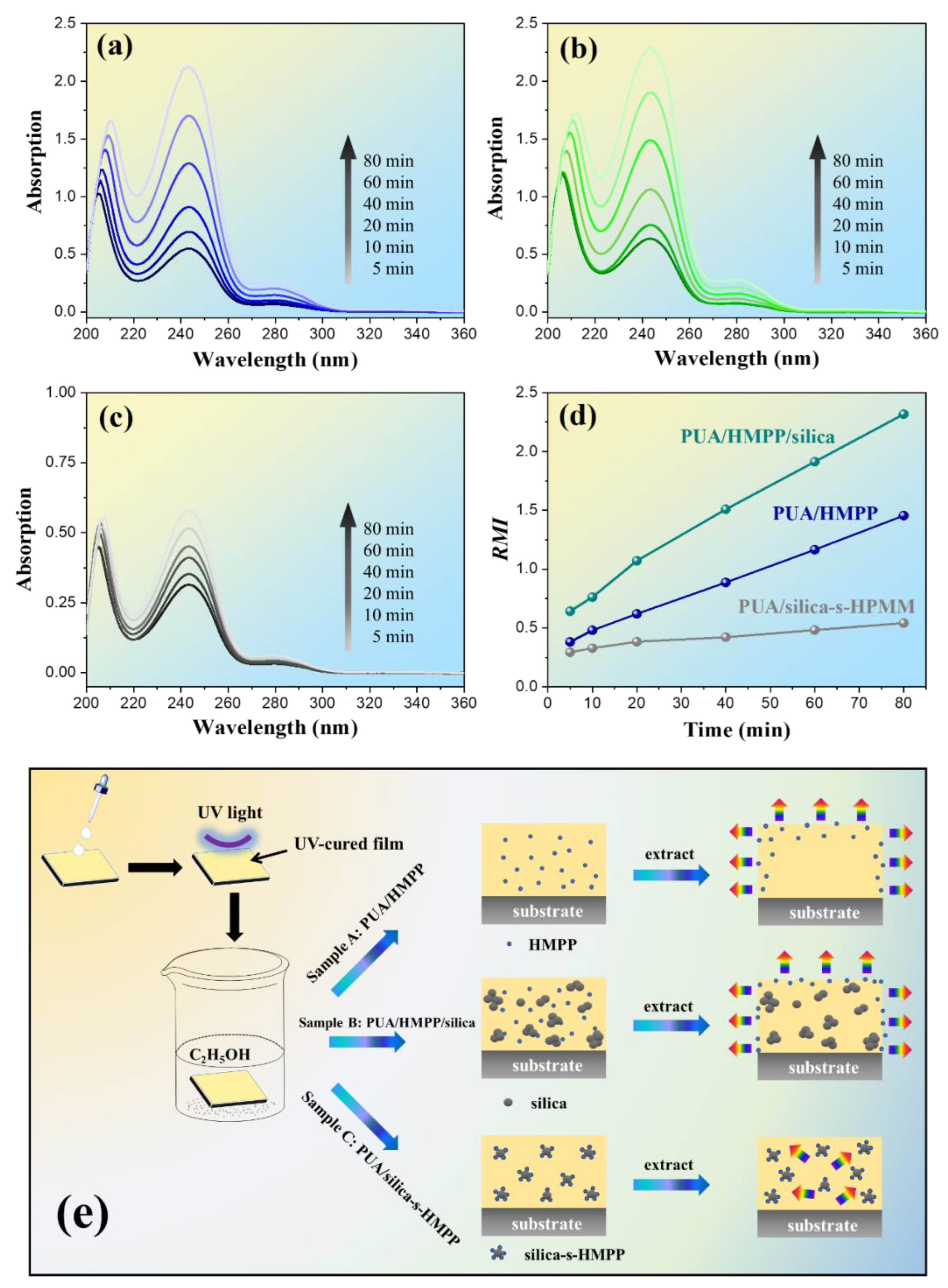
3.4. Migration and Mechanism of the Photoinitiator in the UV-Cured Composites
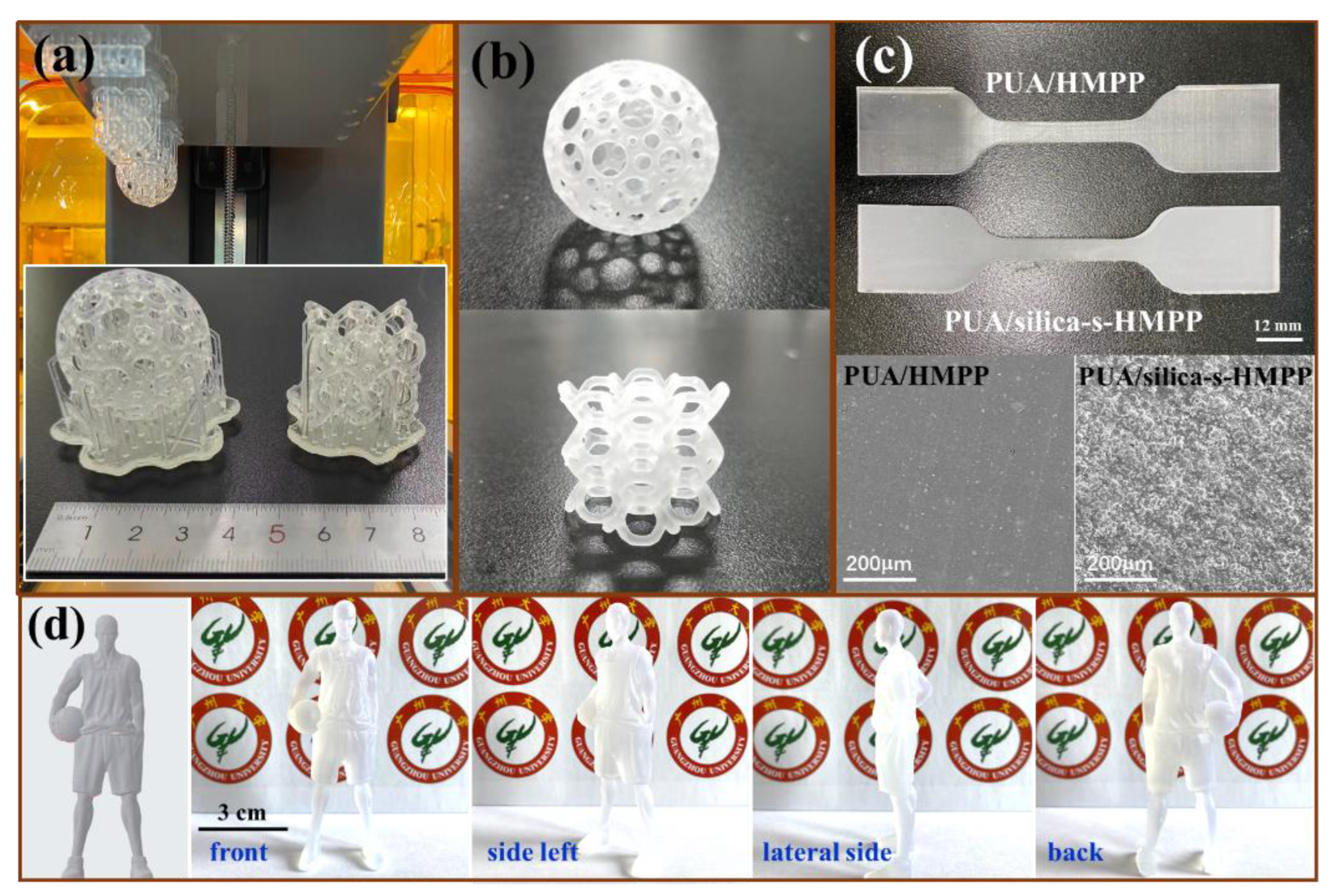
3.5. 3D Printing Resolution and Printed Objects from the PUA/Silica-s-HMPP Ink
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yagci, Y.; Jockusch, S.; Turro, N. Photoinitiated polymerization: Advances, challenges, and opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Isık, T.; Demir, M.; Aydogan, C.; Ciftci, M.; Yagci, Y. Hydrophobic coatings from photochemically prepared hydrophilic polymethacrylates via electrospraying. J. Polym. Sci. Pol. Chem. 2017, 55, 1338–1344. [Google Scholar] [CrossRef]
- Zhou, J.; Allonas, X.; Liu, X. Zirconium propoxide: A coupling agent for the synthesis of multifunctional photoinitiators. ChemPhotoChem 2018, 2, 18–21. [Google Scholar] [CrossRef]
- Fertier, L.; Koleilat, H.; Stemmelen, M.; Giani, O.; Joly-Duhamel, C.; Lapinte, V.; Robin, J. The use of renewable feedstock in UV-curable materials-a new age for polymers and green chemistry. Progress Polym. Sci. 2013, 38, 932–962. [Google Scholar] [CrossRef]
- Seo, J.; Kushner, D.; Hickner, M. 3D printing of micropatterned anion exchange membranes. ACS Appl. Mater. Interfaces 2016, 8, 16656–16663. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Ke, Z.; Yang, J.; Zhao, T.; Zeng, Z.; Chen, Y. Low VOC bifunctional photoinitiator based on α-hydroxyalkylphenone structure. Polymer 2006, 47, 4603–4612. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, H. Preparation and characterization of an amphiphilic macrophotoinitiator based on 2-hydroxyl-2-methyl-1-phenylpropanone. J. Appl. Polym. Sci. 2016, 133, 43910. [Google Scholar] [CrossRef]
- Segurola, J.; Allen, N.; Edge, M.; Roberts, I. Photochemistry and photoinduced chemical crosslinking activity of acrylated prepolymers by several commercial type I for UV photoinitiators. Polym. Degrad. Stab. 1999, 65, 153–160. [Google Scholar] [CrossRef]
- Jockusch, S.; Turro, N. Radical addition rate constants to acrylates and oxygen: α-hydroxy and α-amino radicals produced by photolysis of photoinitiators. J. Am. Chem. Soc. 1999, 121, 3921–3925. [Google Scholar] [CrossRef]
- Wu, Q.; Tang, K.; Xiong, Y.; Wang, X.; Yang, J.; Tang, H. High-performance and low migration one-component thioxanthone visible light photoinitiators. Macromol. Chem. Phys. 2017, 218, 1600484. [Google Scholar] [CrossRef]
- Su, J.; Liu, X.; Xiong, C.; Huang, H.; Cui, Y. Photoinitiability of triblock copolymer PDMS-b-(PMAEBB-co-PDMAEMA)2 as a macro-photoinitiator prepared via RAFT polymerization. Progress Org. Coat. 2017, 103, 165–173. [Google Scholar] [CrossRef]
- Aparicio, J.; Elizalde, M. Migration of photoinitiators in food packaging: A review. Package Technol. Sci. 2015, 28, 181–203. [Google Scholar] [CrossRef]
- Liang, S.; Yang, Y.; Zhou, H.; Li, Y.; Wang, J. Novel polymerizable HMPP-type photoinitiator with carbamate: Synthesis and photoinitiating behaviors. Progress Org. Coat. 2017, 110, 128–133. [Google Scholar] [CrossRef]
- Chen, L.; Jia, X.; Tang, Y.; Wu, L.; Luo, Y.; Jia, D. Novel functional silica nanoparticles for rubber vulcanization and reinforcement. Compos. Sci. Technol. 2017, 144, 11–17. [Google Scholar] [CrossRef]
- Lalevée, J.; Allonas, X.; Jradi, S.; Fouassier, J. Role of the medium on the reactivity of cleavable photoinitiators in photopolymerization reactions. Macromolecules 2006, 39, 1872–1879. [Google Scholar] [CrossRef]
- Roth, M.; Hennen, D.; Oesterreicher, A.; Mostegel, F.; Kappaun, S.; Edler, M.; Griesser, T. Exploring functionalized benzophenones as low-migration photoinitiators for vinyl carbonate/thiol formulations. Eur. Polym. J. 2016, 88, 403–411. [Google Scholar] [CrossRef]
- Sahin, M.; Krawczyk, K.; Roszkowski, P.; Wang, J.; Kaynak, B.; Kern, W.; Schlögl, S.; Grützmacher, H. Photoactive silica nanoparticles: Influence of surface functionalization on migration and kinetics of radical-induced photopolymerization reactions. Eur. Polym. J. 2018, 98, 430–438. [Google Scholar] [CrossRef]
- Dell’ Erba, I.; Arenas, G.; Schroeder, W.; Asmussen, S.; Vallo, C. Hybrid organic-inorganic macromolecular photoinitiator system for visible-light photopolymerization. Progress Org. Coat. 2014, 77, 1848–1853. [Google Scholar] [CrossRef]
- Huber, A.; Kuschel, A.; Ott, T.; Santiso-Quinones, G.; Stein, D.; Bräuer, J.; Kissner, R.; Krumeich, F.; Schçnberg, H.; Levalois-Grützmacher, J.; et al. Phosphorous-functionalized bis (acyl) phosphane oxides for surface modification. Angew. Chem. Int. Ed. 2012, 51, 4648–4652. [Google Scholar] [CrossRef] [PubMed]
- Melinte, V.; Chibac, A.; Buruiana, T.; Buruiana, E. Hybrid nanocomposites prepared by in situ photopolymerization using photoinitiator-modified montmorillonite. Progress Org. Coat. 2017, 104, 125–134. [Google Scholar] [CrossRef]
- Zhou, J.; Allonas, X.; Liu, X. Synthesis and characterization of organozirconiums with type-II photoinitiator ligands as multifunctional photoinitiators for free radical photopolymerization. J. Photochem. Photobiol. A 2018, 356, 580–586. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, T. Stability and rheological properties of nanofluids stabilized by SiO2 nanoparticles and SiO2-TiO2 nanocomposites for oilfield applications. Colloids Surf. A 2018, 539, 171–183. [Google Scholar] [CrossRef]
- Li, Q.; Liao, G.; Zhang, S.; Pang, L.; Tong, H.; Zhao, W.; Xu, Z. Effect of adjustable molecular chain structure and pure silica zeolite nanoparticles on thermal, mechanical, dielectric, UV-shielding and hydrophobic properties of fluorinated copolyimide composites. Appl. Surf. Sci. 2018, 427, 437–450. [Google Scholar] [CrossRef]
- Domun, N.; Hadavinia, H.; Zhang, T.; Sainsbury, T.; Liaghat, G.; Vahid, S. Improving the fracture toughness and the strength of epoxy using nanomaterials-A review of the current status. Nanoscale 2015, 7, 10294–10329. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, H.; Niu, H.; Gestos, A.; Lin, T. Robust, self-healing superamphiphobic fabrics prepared by two-step coating of fluoro-containing polymer, fluoroalkyl silane, and modified silica nanoparticles. Adv. Funct. Mater. 2013, 23, 1664–1670. [Google Scholar] [CrossRef]
- Yu, J.; Huang, X.; Wu, C.; Wu, X.; Wang, G.; Jiang, P. Interfacial modification of boron nitride nanoplatelets for epoxy composites with improved thermal properties. Polymer 2012, 53, 471–480. [Google Scholar] [CrossRef]
- Chen, L.; Guo, X.; Jia, Z.; Tang, Y.; Wu, L.; Luo, Y.; Jia, D. High reactive sulphide chemically supported on silica surface to prepare functional nanoparticle. Appl. Surf. Sci. 2018, 442, 673–681. [Google Scholar] [CrossRef]
- Mousavi, A.; Roghani-Mamaqani, H.; Salami-Kalajahi, M.; Shahi, S.; Abdollahi, A. Modification of graphene with silica nanoparticles for use in hybrid network formation from epoxy, novolac, and epoxidized novolac resins by sol-gel method: Investigation of thermal properties. Express Polym. Lett. 2018, 12, 187–202. [Google Scholar] [CrossRef]
- Wu, G.; Ma, L.; Jiang, H.; Liu, L.; Huang, Y. Improving the interfacial strength of silicone resin composites by chemically grafting silica nanoparticles on carbon fiber. Compos. Sci. Technol. 2017, 153, 160–167. [Google Scholar] [CrossRef]
- Zhou, J.; Allonas, X.; Liu, X. Fluorinated organozirconiums: Enhancement of overcoming oxygen inhibition in the UV-curing film. Progress Org. Coat. 2018, 120, 228–233. [Google Scholar] [CrossRef]
- Pan, Z.; Yao, L.; Zhai, J.; Shen, B.; Wang, H. Significantly improved dielectric properties and energy density of polymer nanocomposites via small loaded of BaTiO3 nanotubes. Compos. Sci. Technol. 2017, 147, 30–38. [Google Scholar] [CrossRef]
- Allen, N.; McIntyre, R.; Kerrod, J.; Hill, C.; Edge, M. Photo-stabilisation and UV blocking efficacy of coated macro and nano-rutile titanium dioxide particles in paints and coatings. J. Polym. Environ. 2018, 26, 4243–4257. [Google Scholar] [CrossRef]
- Christmann, J.; Ley, C.; Allonas, X.; Ibrahim, A.; Croutxé-Barghorn, C. Experimental and theoretical investigations of free radical photopolymerization: Inhibition and termination reactions. Polymer 2018, 160, 254–264. [Google Scholar] [CrossRef]
- Goodner, M.; Bowman, C. Modeling primary radical termination and its effects on autoacceleration in photopolymerization kinetics. Macromolecules 1999, 32, 6552–6559. [Google Scholar] [CrossRef]
- Corcione, C.; Striani, R.; Frigione, M. UV-cured methacrylic-silica hybrids: Effect of oxygen inhibition on photo-curing kinetics. Thermochim. Acta 2014, 576, 47–55. [Google Scholar] [CrossRef]
- Ibrahim, A.; Maurin, V.; Ley, C.; Allonas, X.; Croutxe-Barghorn, C.; Jasinski, F. Investigation of termination reactions in free radical photopolymerization of UV powder formulations. Eur. Polym. J. 2012, 48, 1475–1484. [Google Scholar] [CrossRef]
- Da Silva Prezotto, A.; da Silva, D.; Vitti, R.; Sinhoreti, M.; Brandt, W. Light curing and ratio of glass/fumed silica fillers on degree of conversion and mechanical properties of experimental composite resins. J. Appl. Polym. Sci. 2019, 136, 47008. [Google Scholar] [CrossRef]


| Sample | RJ-426 | RJ-425 | ACMO | HMPP | Silica | Silica-s-HMPP |
|---|---|---|---|---|---|---|
| PUA/HMPP | 60 | 40 | 5 | 0.575 | 0 | 0 |
| PUA/HMPP/silica | 60 | 40 | 5 | 0.575 | 4.094 | 0 |
| PUA/silica-s-HMPP | 60 | 40 | 5 | 0 | 0 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Chen, F.; Zhu, W.; Chen, L.; Liang, Y.; Guo, X. Construction of Photoinitiator Functionalized Spherical Nanoparticles Enabling Favorable Photoinitiating Activity and Migration Resistance for 3D Printing. Polymers 2022, 14, 4551. https://doi.org/10.3390/polym14214551
Chen L, Chen F, Zhu W, Chen L, Liang Y, Guo X. Construction of Photoinitiator Functionalized Spherical Nanoparticles Enabling Favorable Photoinitiating Activity and Migration Resistance for 3D Printing. Polymers. 2022; 14(21):4551. https://doi.org/10.3390/polym14214551
Chicago/Turabian StyleChen, Lijuan, Fan Chen, Weixin Zhu, Luoyi Chen, Yingyu Liang, and Xiaohui Guo. 2022. "Construction of Photoinitiator Functionalized Spherical Nanoparticles Enabling Favorable Photoinitiating Activity and Migration Resistance for 3D Printing" Polymers 14, no. 21: 4551. https://doi.org/10.3390/polym14214551
APA StyleChen, L., Chen, F., Zhu, W., Chen, L., Liang, Y., & Guo, X. (2022). Construction of Photoinitiator Functionalized Spherical Nanoparticles Enabling Favorable Photoinitiating Activity and Migration Resistance for 3D Printing. Polymers, 14(21), 4551. https://doi.org/10.3390/polym14214551







