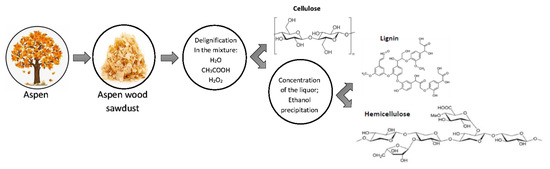
Composition and Structure of Aspen (Pópulus trémula) Hemicelluloses Obtained by Oxidative Delignification
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
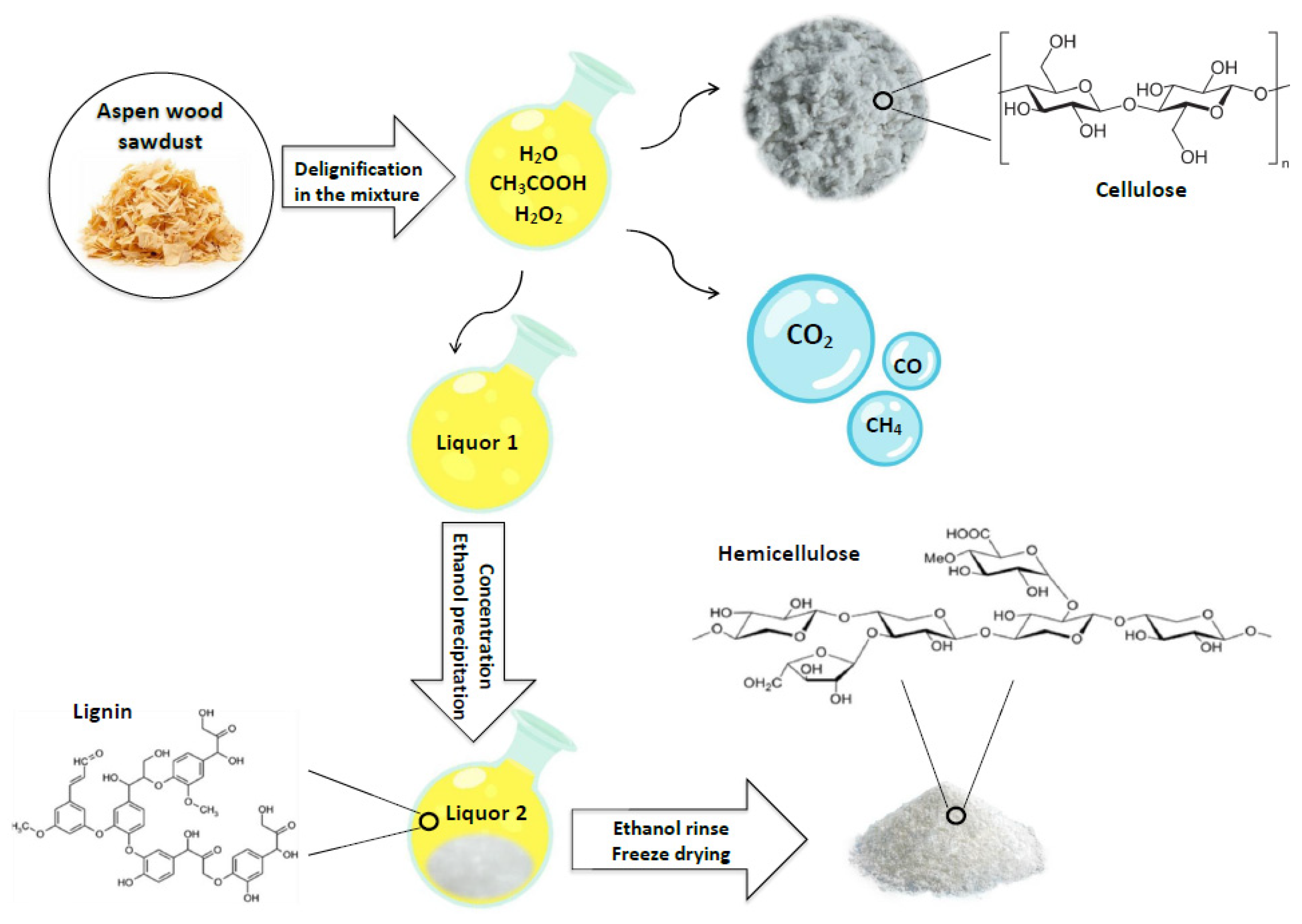
2.2. Aspen Wood Delignification and Extraction of the Hemicelluloses
2.3. Gel Permeation Chromatography
2.4. Analysis of the Monosaccharide Composition
2.5. Fourier-Transform Infra-Red Spectroscopy
2.6. Nuclear Magnetic Resonance
2.7. Thermogravimetric Analysis
2.8. Optimization
2.9. Antioxidant Activity
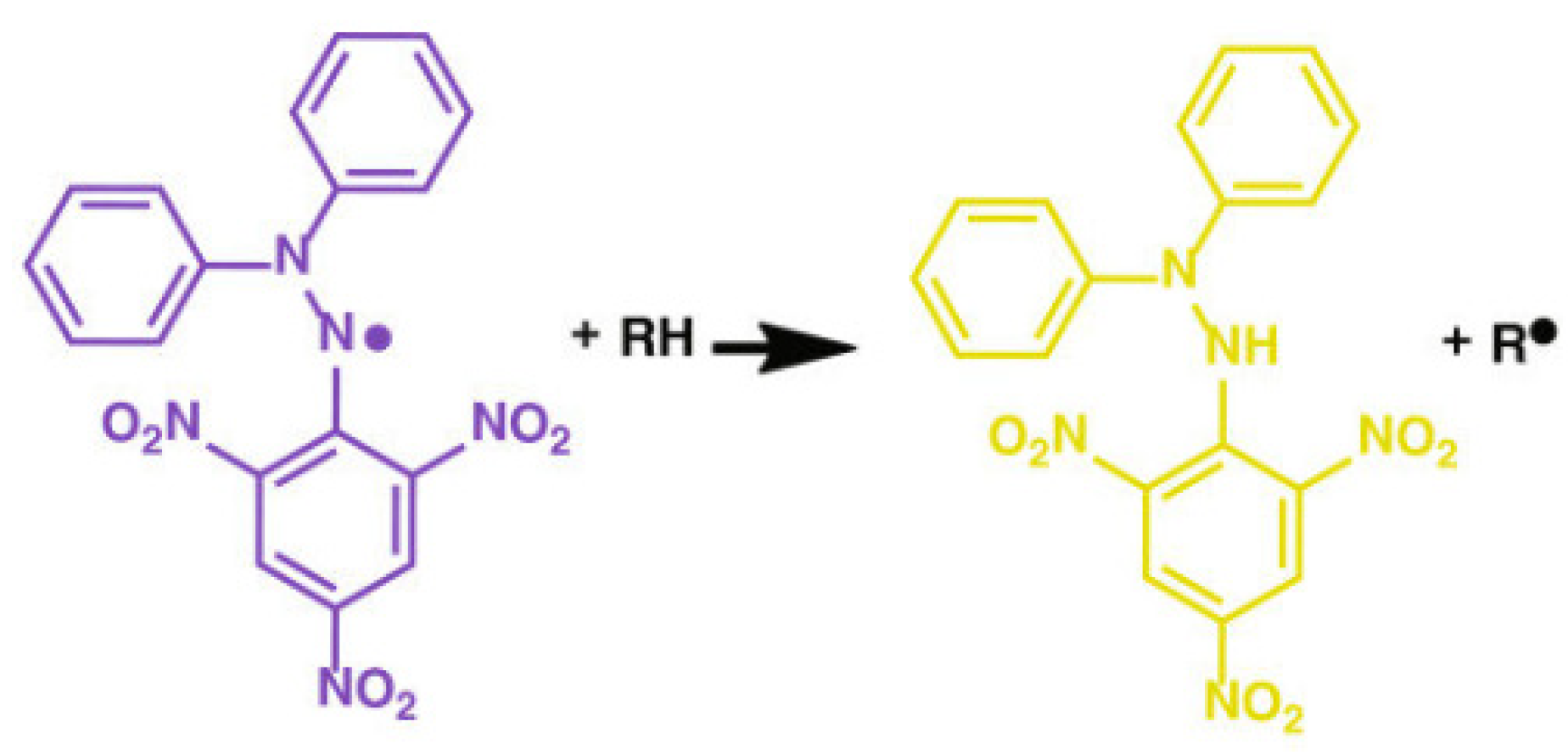
2.9.1. DPPH Radical Scavenging Assay
2.9.2. Hydroxyl Radical Scavenging Assay
3. Results
3.1. Hemicellulose Delignification and Yield
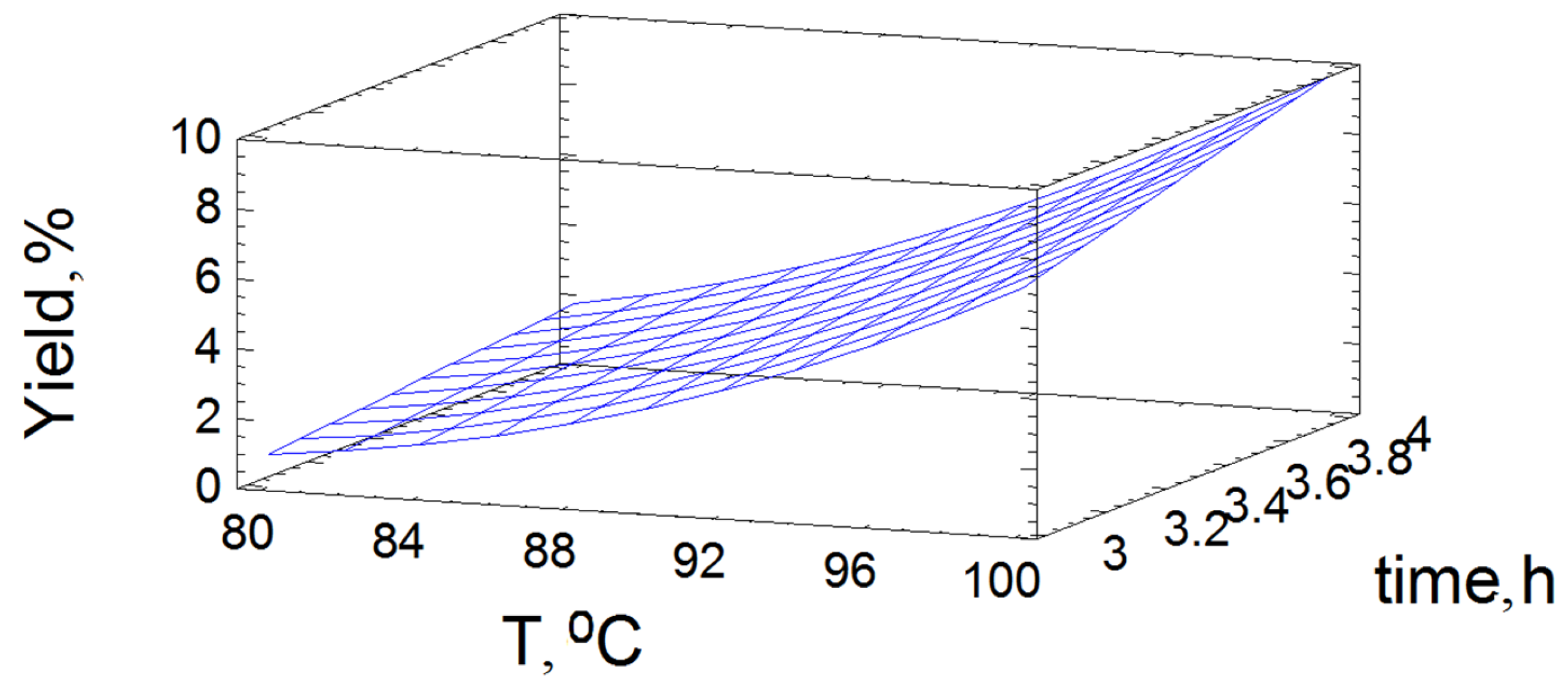
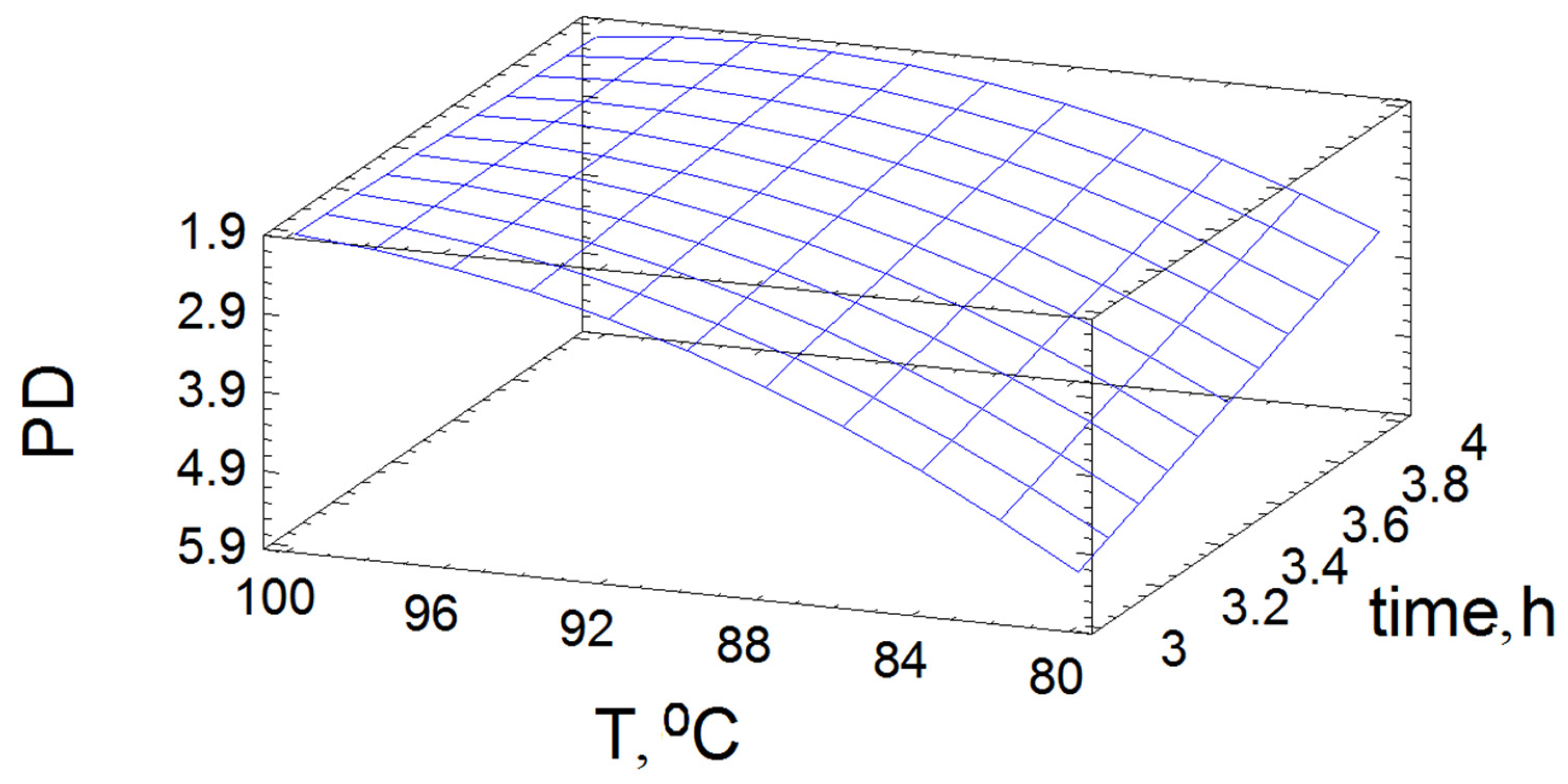
3.2. Optimization of the Oxidative Delignification
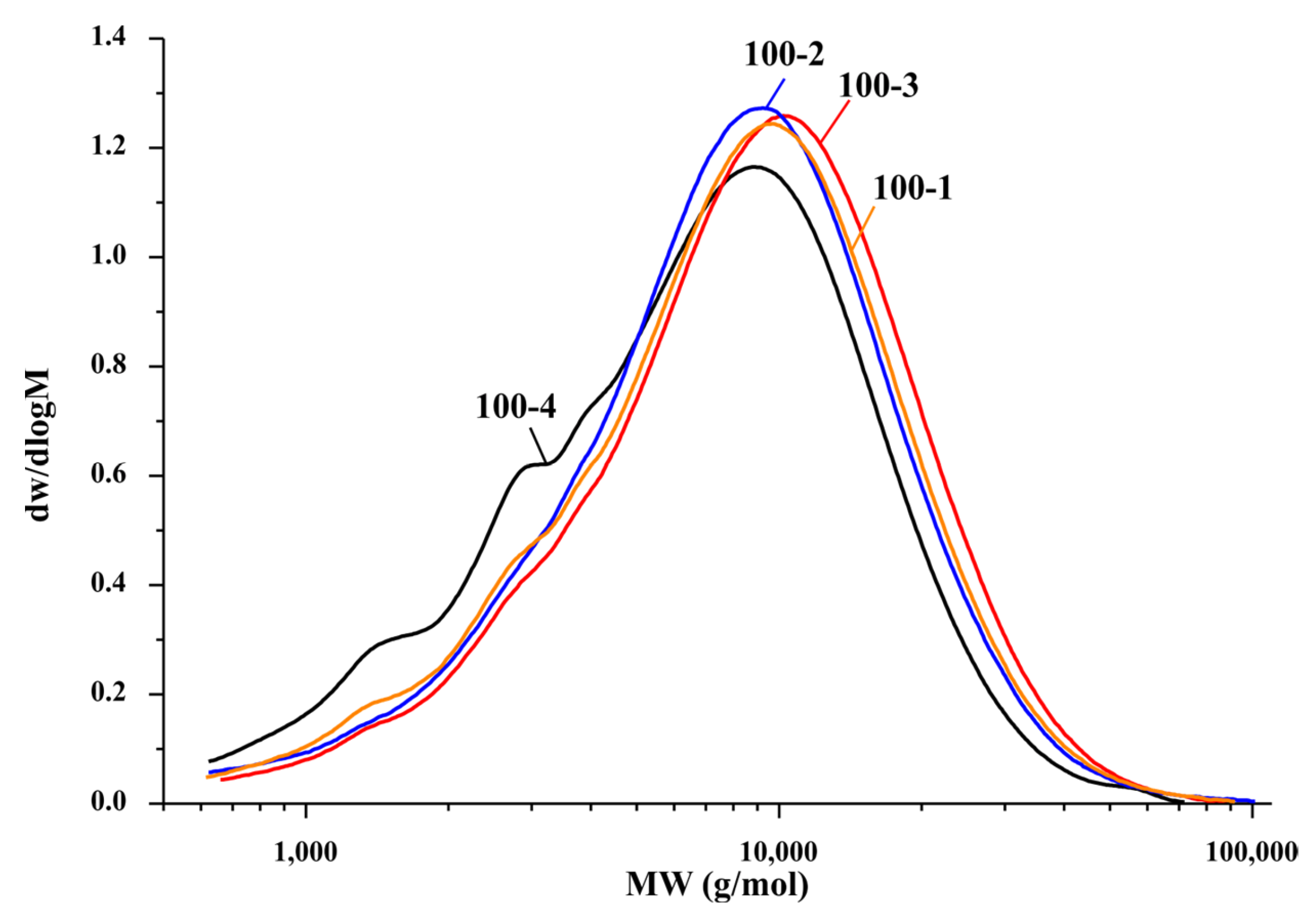
3.3. Gel Permeation Chromatography Study of the Hemicelluloses
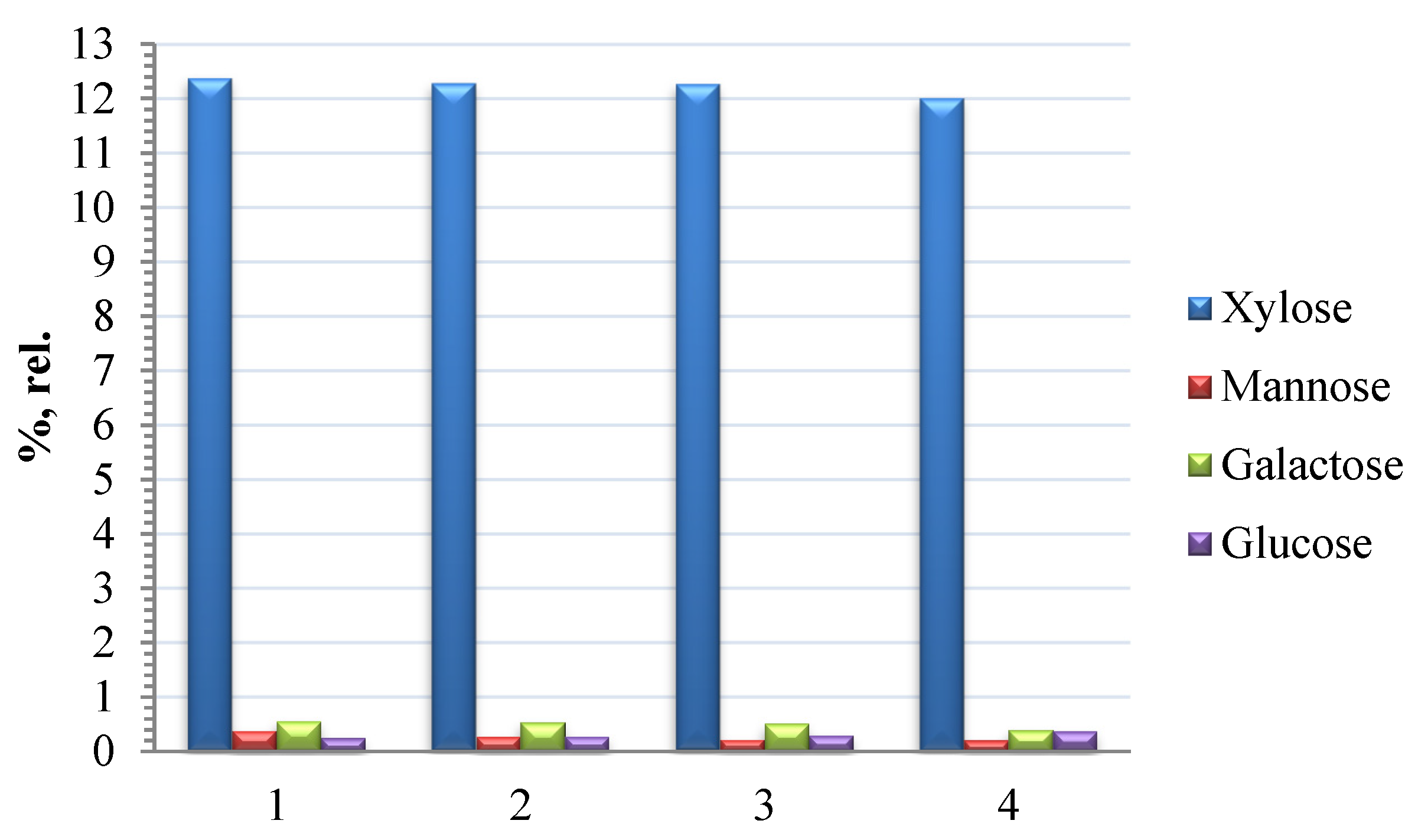
3.4. Analysis of the Monosaccharide Composition of the Hemicelluloses
3.5. Fourier-Transform Infra-Red Spectroscopy
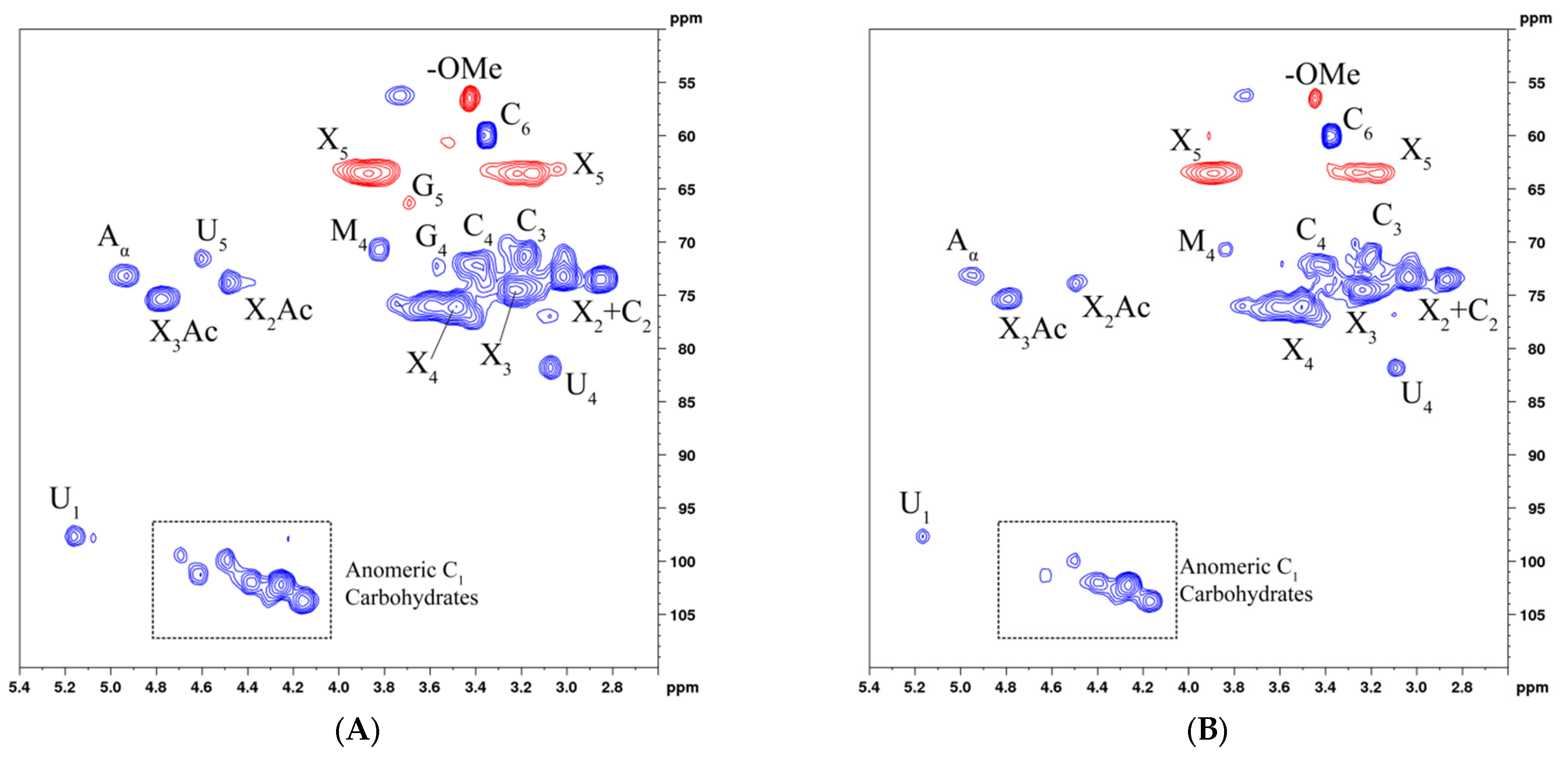
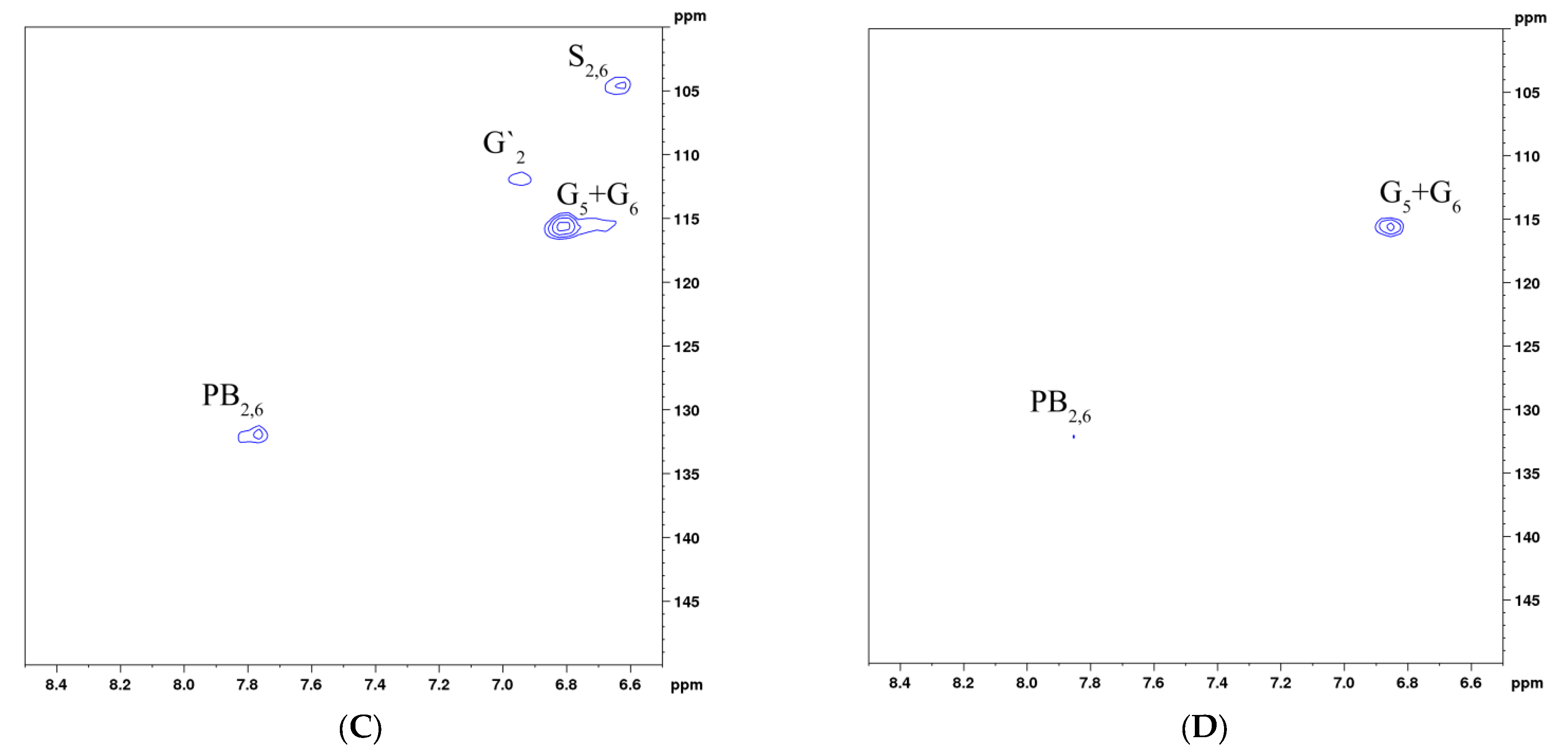
3.6. Nuclear Magnetic Resonance
3.7. Thermogravimetric Analysis
3.8. Analysis of the Antioxidant Activity of the Hemicelluloses
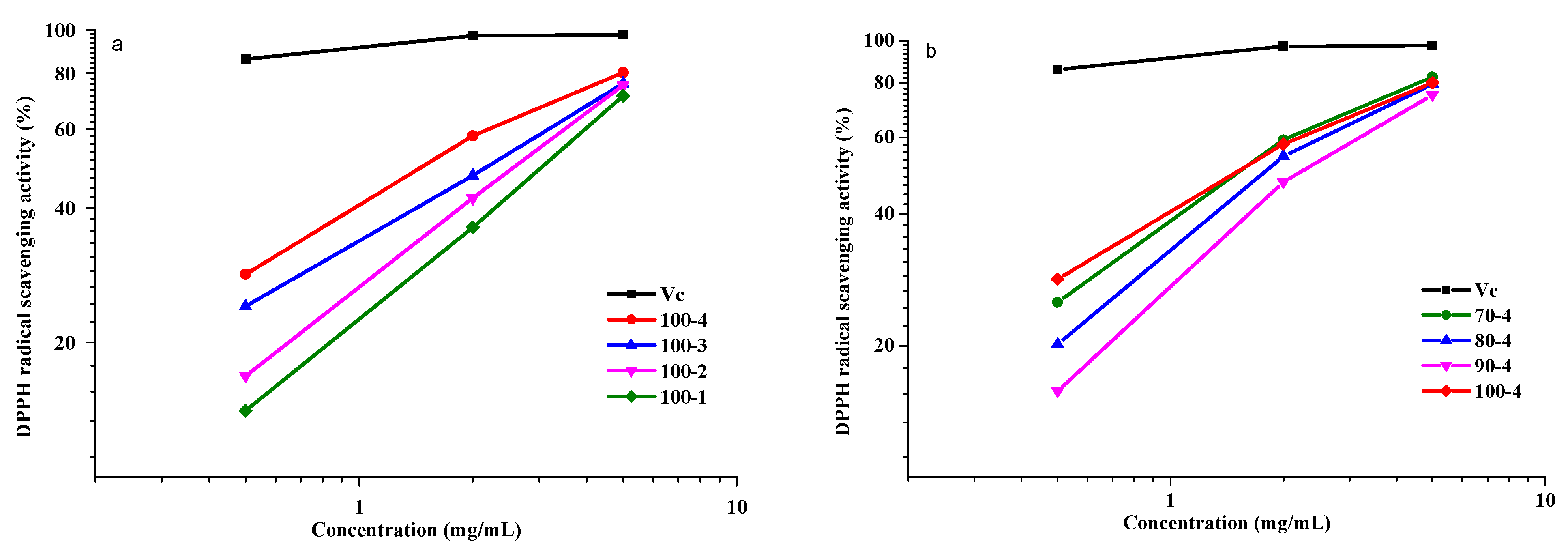
3.8.1. Scavenging Activity of the DPPH Radicals
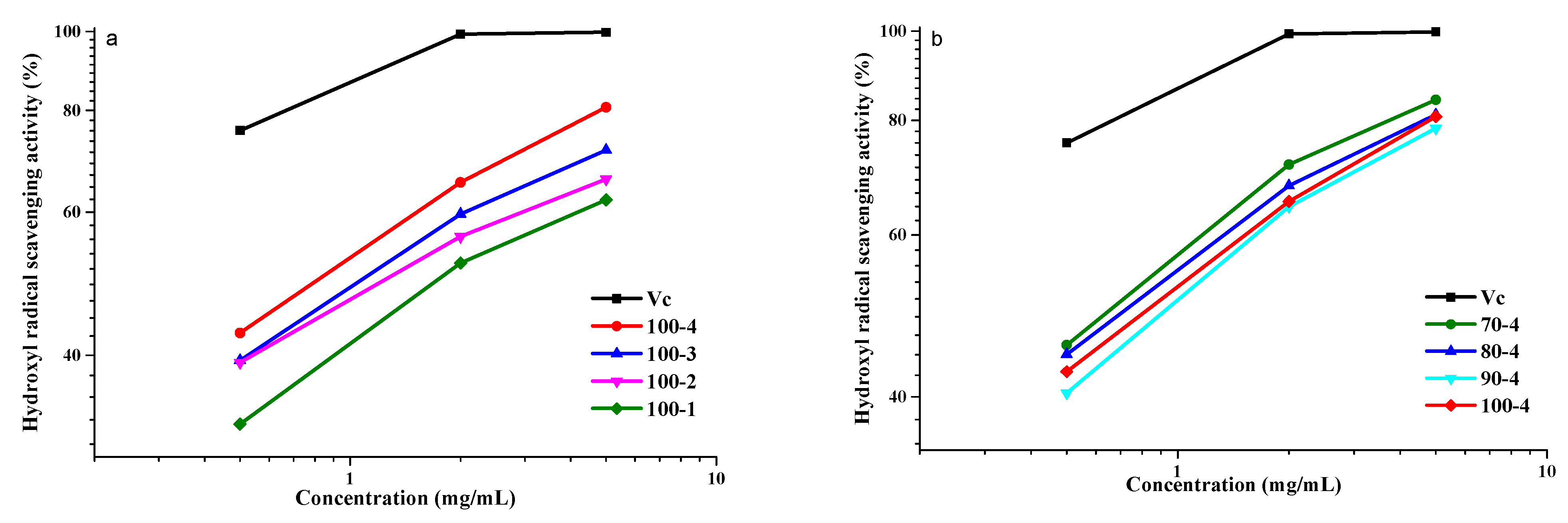
3.8.2. Scavenging Activity of the Hydroxyl Radicals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, S.-F.; Yang, H.-Y.; Yang, J.; Shi, Z.-J. The effect of alkaline extraction of hemicellulose on cocksfoot grass enzymatic hydrolysis recalcitrance. Ind. Crops Prod. 2022, 178, 114654. [Google Scholar] [CrossRef]
- Kocabaş, D.S.; Köle, M.; Yağcı, S. Development and optimization of hemicellulose extraction bioprocess from poppy (Papaver somniferum L.) stalks assisted by instant controlled pressure drop (DIC) pretreatment. Biocatal. Agric. Biotechnol. 2020, 29, 101793. [Google Scholar] [CrossRef]
- Li, B.; Haneklaus, N. The role of renewable energy, fossil fuel consumption, urbanization and economic growth on CO2 emissions in China. Energy Rep. 2021, 7, 783–791. [Google Scholar] [CrossRef]
- Borovkova, V.S.; Malyar, Y.N.; Sudakova, I.G.; Chudina, A.I.; Skripnikov, A.M.; Fetisova, O.Y.; Kazachenko, A.S.; Miroshnikova, A.V.; Zimonin, D.V.; Ionin, V.A.; et al. Molecular Characteristics and Antioxidant Activity of Spruce (Picea abies) Hemicelluloses Isolated by Catalytic Oxidative Delignification. Molecules 2022, 27, 266. [Google Scholar] [CrossRef]
- Sun, X.-F.; Wang, H.-H.; Jing, Z.-X.; Mohanathas, R. Hemicellulose-based pH-sensitive and biodegradable hydrogel for controlled drug delivery. Carbohydr. Polym. 2013, 92, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Z.; Feng, C.; Liu, X.; Qin, F.; Liang, C.; Bian, H.; Qin, C.; Yao, S. Green, efficient extraction of bamboo hemicellulose using freeze-thaw assisted alkali treatment. Bioresour. Technol. 2021, 333, 125107. [Google Scholar] [CrossRef]
- Yuan, T.-Q.; Xu, F.; He, J.; Sun, R.-C. Structural and physico-chemical characterization of hemicelluloses from ultrasound-assisted extractions of partially delignified fast-growing poplar wood through organic solvent and alkaline solutions. Biotechnol. Adv. 2010, 28, 583–593. [Google Scholar] [CrossRef]
- Tu, K.; Ding, Y.; Keplinger, T. Review on design strategies and applications of metal-organic framework-cellulose composites. Carbohydr. Polym. 2022, 291, 119539. [Google Scholar] [CrossRef]
- Han, Z.; Zhu, H.; Cheng, J.-H. Structure modification and property improvement of plant cellulose: Based on emerging and sustainable nonthermal processing technologies. Food Res. Int. 2022, 156, 111300. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites—A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef]
- Gallina, G.; Alfageme, E.R.; Biasi, P.; García-Serna, J. Hydrothermal extraction of hemicellulose: From lab to pilot scale. Bioresour. Technol. 2018, 247, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Gallina, G.; Cabeza, Á.; Biasi, P.; García-Serna, J. Optimal conditions for hemicelluloses extraction from Eucalyptus globulus wood: Hydrothermal treatment in a semi-continuous reactor. Fuel Process. Technol. 2016, 148, 350–360. [Google Scholar] [CrossRef]
- Gallina, G.; Cabeza, Á.; Grénman, H.; Biasi, P.; García-Serna, J.; Salmi, T. Hemicellulose extraction by hot pressurized water pretreatment at 160 °C for 10 different woods: Yield and molecular weight. J. Supercrit. Fluids 2018, 133, 716–725. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Malyar, Y.N.; Vasilyeva, N.Y.; Borovkova, V.S.; Issaoui, N. Optimization of guar gum galactomannan sulfation process with sulfamic acid. Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]
- Tang, N.; Tan, X.; Cai, Y.; He, M.-Y.; Xiang, Z.-Y.; Ye, H.; Ma, J.-L. Characterizations and application potentials of the hemicelluloses in waste oil-tea camellia fruit shells from Southern China. Ind. Crops Prod. 2022, 178, 114551. [Google Scholar] [CrossRef]
- Gautam, D.; Kumari, S.; Ram, B.; Chauhan, G.S.; Chauhan, K. A new hemicellulose-based adsorbent for malachite green. J. Environ. Chem. Eng. 2018, 6, 3889–3897. [Google Scholar] [CrossRef]
- Li, Q.; Wang, S.; Jin, X.; Huang, C.; Xiang, Z. The Application of Polysaccharides and Their Derivatives in Pigment, Barrier, and Functional Paper Coatings. Polymers 2020, 12, 1837. [Google Scholar] [CrossRef]
- Wi, S.G.; Cho, E.J.; Lee, D.-S.; Lee, S.J.; Lee, Y.J.; Bae, H.-J. Lignocellulose conversion for biofuel: A new pretreatment greatly improves downstream biocatalytic hydrolysis of various lignocellulosic materials. Biotechnol. Biofuels 2015, 8, 228. [Google Scholar] [CrossRef]
- Chang, V.S.; Holtzapple, M.T. Fundamental Factors Affecting Biomass Enzymatic Reactivity. In Twenty-First Symposium on Biotechnology for Fuels and Chemicals; Humana Press: Totowa, NJ, USA, 2000; Volume 84, pp. 5–37. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, J.S.; Sunwoo, C.; Lee, Y. Pretreatment of corn stover by aqueous ammonia. Bioresour. Technol. 2003, 90, 39–47. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Talebnia, F.; Karakashev, D.B.; Angelidaki, I. Production of bioethanol from wheat straw: An overview on pretreatment, hydrolysis and fermentation. Bioresour. Technol. 2010, 101, 4744–4753. [Google Scholar] [CrossRef] [PubMed]
- Kazachenko, A.S.; Malyar, Y.N.; Vasilyeva, N.Y.; Fetisova, O.Y.; Chudina, A.I.; Sudakova, I.G.; Antonov, A.V.; Borovkova, V.S.; Kuznetsova, S.A. Isolation and sulfation of galactoglucomannan from larch wood (Larix sibirica). Wood Sci. Technol. 2021, 55, 1091–1107. [Google Scholar] [CrossRef]
- Singh, R.; Shukla, A.; Tiwari, S.; Srivastava, M. A review on delignification of lignocellulosic biomass for enhancement of ethanol production potential. Renew. Sustain. Energy Rev. 2014, 32, 713–728. [Google Scholar] [CrossRef]
- Başar, I.A.; Perendeci, N.A. Optimization of zero-waste hydrogen peroxide—Acetic acid pretreatment for sequential ethanol and methane production. Energy 2021, 225, 120324. [Google Scholar] [CrossRef]
- Chudina, A.I.; Malyar, Y.N.; Sudakova, I.G.; Kazachenko, A.S.; Skripnikov, A.M.; Borovkova, V.S.; Kondrasenko, A.A.; Mazurova, E.V.; Fetisova, O.Y.; Ivanov, I.P. Physicochemical characteristics of polysaccharides from catalytic and noncatalytic acetic acid-peroxide delignification of larch wood. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Zhang, Y.; Virjamo, V.; Sobuj, N.; Du, W.; Yin, Y.; Nybakken, L.; Guo, H.; Julkunen-Tiitto, R. Elevated temperature and CO2 affect responses of European aspen (Populus tremula) to soil pyrene contamination. Sci. Total Environ. 2018, 634, 150–157. [Google Scholar] [CrossRef]
- Sjöström, E.; Alén, R. Analytical Methods of Wood Chemistry, Pulping, and Papermaking; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Sudakova, I.G.; Garyntseva, N.V.; Yatsenkova, O.V.; Kuznetsov, B.N. Optimization of Aspen Wood Delignification by H2O2 with Sulfuric Acid Catalyst. J. Sib. Fed. Univ. 2013, 6, 76–84. [Google Scholar]
- Sun, S.-L.; Wen, J.-L.; Ma, M.-G.; Sun, R.-C. Successive alkali extraction and structural characterization of hemicelluloses from sweet sorghum stem. Carbohydr. Polym. 2013, 92, 2224–2231. [Google Scholar] [CrossRef]
- Ruiz-Matute, A.I.; Hernández-Hernández, O.; Rodríguez-Sánchez, S.; Sanz, M.L.; Martínez-Castro, I. Derivatization of carbohydrates for GC and GC–MS analyses. J. Chromatogr. B 2011, 879, 1226–1240. [Google Scholar] [CrossRef]
- Xua, Y.; Songa, S.; Weia, Y.; Wangb, F.; Zhaoa, M.; Guoa, J.; Zhanga, J. Sulfated modification of the polysaccharide from Sphallerocarpusgracilis and its antioxidant activities. Int. J. Biol. Macromol. 2016, 87, 180–190. [Google Scholar] [CrossRef]
- Meng, F.; Li, N.; Yang, H.; Shi, Z.; Zhao, P.; Yang, J. Investigation of hydrogen peroxide-acetic acid pretreatment to enhance the enzymatic digestibility of bamboo residues. Bioresour. Technol. 2022, 344, 126162. [Google Scholar] [CrossRef] [PubMed]
- Palamae, S.; Palachum, W.; Chisti, Y.; Choorit, W. Retention of hemicellulose during delignification of oil palm empty fruit bunch (EFB) fiber with peracetic acid and alkaline peroxide. Biomass Bioenergy 2014, 66, 240–248. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Sudakova, I.G.; Chudina, A.I.; Garyntseva, N.V.; Kazachenko, A.S.; Skripnikov, A.M.; Malyar, Y.N.; Ivanov, I.P. Fractionation of birch wood biomass into valuable chemicals by the extraction and catalytic processes. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- Granato, D.; Reshamwala, D.; Korpinen, R.; Azevedo, L.; Carmo, M.A.V.D.; Cruz, T.M.; Marques, M.B.; Wen, M.; Zhang, L.; Marjomäki, V.; et al. From the forest to the plate—Hemicelluloses, galactoglucomannan, glucuronoxylan, and phenolic-rich extracts from unconventional sources as functional food ingredients. Food Chem. 2022, 381, 132284. [Google Scholar] [CrossRef] [PubMed]
- Guzelgulgen, M.; Ozkendir-Inanc, D.; Yildiz, U.H.; Arslan-Yildiz, A. Glucuronoxylan-based quince seed hydrogel: A promising scaffold for tissue engineering applications. Int. J. Biol. Macromol. 2021, 180, 729–738. [Google Scholar] [CrossRef]
- Qaseem, M.F.; Shaheen, H.; Wu, A.-M. Cell wall hemicellulose for sustainable industrial utilization. Renew. Sustain. Energy Rev. 2021, 144, 110996. [Google Scholar] [CrossRef]
- Kotlyarova, I.A. Infrared spectroscopy of wood of pine, birch and oak, modified with monoethanolamine (n→b) threehydroxyborate. Khimiya Rastit. Syrya 2019, 2, 43–49. [Google Scholar]
- Liu, D.; Tang, W.; Xin, Y.; Wang, Z.-X.; Huang, X.-J.; Hu, J.-L.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Isolation and structure characterization of glucuronoxylans from Dolichos lablab L. hull. Int. J. Biol. Macromol. 2021, 182, 1026–1036. [Google Scholar] [CrossRef]
- Thetsrimua, C.; Khammuang, S.; Sarnthima, R. Antioxidant Activity of Crude Polysaccharides from Edible Fresh and Dry Mushroom Fruiting Bodies of Lentinus sp. Strain RJ-2. Int. J. Pharmacol. 2011, 7, 58–65. [Google Scholar] [CrossRef]
- Saito, Y.; Endo, T.; Ando, D.; Nakatsubo, F.; Yano, H. Influence of drying process on reactivity of cellulose and xylan in acetylation of willow (Salix schwerinii E. L. Wolf) kraft pulp monitored by HSQC-NMR spectroscopy. Cellulose 2018, 25, 6319–6331. [Google Scholar] [CrossRef]
- Zhang, M.; Bobokalonov, J.; Dzhonmurodov, A.; Xiang, Z. Optimizing yield and chemical compositions of dimethylsulfoxide-extracted birchwood xylan. J. Bioresour. Bioprod. 2022, 7, 211–219. [Google Scholar] [CrossRef]
- Giummarella, N.; Lawoko, M. Structural Basis for the Formation and Regulation of Lignin–Xylan Bonds in Birch. ACS Sustain. Chem. Eng. 2016, 4, 5319–5326. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Ji, X.; Liu, F.; Ullah, N.; Wang, M. Isolation, purification, and antioxidant activities of polysaccharides from Ziziphus Jujuba cv. Muzao. Int. J. Food Prop. 2018, 21, 1–11. [Google Scholar] [CrossRef]
- Behrendorff, J.B.; Vickers, C.E.; Chrysanthopoulos, P.; Nielsen, L.K. 2,2-Diphenyl-1-picrylhydrazyl as a screening tool for recombinant monoterpene biosynthesis. Microb. Cell Factories 2013, 12, 76. [Google Scholar] [CrossRef]
- Liu, X.-X.; Gu, L.-B.; Zhang, G.-J.; Liu, H.-M.; Zhang, Y.-T.; Zhang, K.-P. Structural characterization and antioxidant activity of polysaccharides extracted from Chinese yam by a cellulase-assisted method. Process Biochem. 2022, 121, 178–187. [Google Scholar] [CrossRef]
- Hu, Z.; Yu, R.; Sun, J.; Duan, Y.; Zhou, H.; Zhou, W.; Li, G. Static decolorization of polysaccharides from the leaves of Rhododendron dauricum: Process optimization, characterization and antioxidant activities. Process Biochem. 2022, 121, 113–125. [Google Scholar] [CrossRef]
- Peralta, E.; Roa, G.; Hernandez-Servin, J.; Romero, R.; Balderas, P.; Natividad, R. Hydroxyl Radicals quantification by UV spectrophotometry. Electrochim. Acta 2014, 129, 137–141. [Google Scholar] [CrossRef]
- Mutailifu, P.; Nuerxiati, R.; Lu, C.; Huojiaaihemaiti, H.; Abuduwaili, A.; Yili, A. Extraction, purification, and characterization of polysaccharides from Alhagi pseudoalhagi with antioxidant and hypoglycemic activities. Process Biochem. 2022, 121, 339–348. [Google Scholar] [CrossRef]




| Process Temperatures (°C) | 70 | 80 | 90 | 100 |
|---|---|---|---|---|
| Process Times (h) | ||||
| 4 | + | + | + | + |
| 3 | - | + | + | + |
| 2 | - | - | - | + |
| 1 | - | - | - | + |
| Process Temperatures (°C) | 70 | 80 | 90 | 100 |
|---|---|---|---|---|
| Process Times (h) | Hemicellulose Yield (wt%) 1 | |||
| 4 | 0.67 2 | 1.81 3 | 4.79 5 | 9.68 8 |
| 3 | - | 0.92 4 | 2.83 6 | 7.08 9 |
| 2 | - | - | 1.09 7 | 6.30 10 |
| 1 | - | - | - | 4.92 11 |
| Factors and Parameters | Designations | |
|---|---|---|
| In the Text and Figures | In the Equations | |
| Temperature (°C) | T | X1 |
| Time (h) | t | X2 |
| Hemicellulose yield (%) | Yield | Y1 |
| Polydispersity | PD | Y2 |
| Sample | Reaction Temperature (°C) | Reaction Time (h) | Characteristics | |
|---|---|---|---|---|
| Yield, Mass % | PDI | |||
| - | X1 (T) | X2 (t) | Y1 | Y2 |
| 80-3 | 80 | 3 | 0.973 | 5.22 |
| 90-3 | 90 | 3 | 2.833 | 2.94 |
| 100-3 | 100 | 3 | 7.075 | 1.92 |
| 80-4 | 80 | 4 | 1.812 | 3.53 |
| 90-4 | 90 | 4 | 4.785 | 2.10 |
| 100-4 | 100 | 4 | 9.678 | 2.05 |
| Variance Sources | Output Parameters | |||
|---|---|---|---|---|
| Yield Y1, Mass % | Polydispersity, Y2 | |||
| Statistical Characteristics | ||||
| Variance Relations F | Significance Levels p | Variance Relations F | Significance Levels p | |
| X1: T | 1096.8 | 0.0113 | 4760.1 | 0.0092 |
| X2: Prod | 160.6 | 0.0355 | 800.0 | 0.0225 |
| X12 | 48.8 | 0.0642 | 484.0 | 0.0289 |
| X1X2 | 23.7 | 0.0918 | 690.1 | 0.0242 |
| Number of degrees of freedom | 2 | 2 | ||
| R2adj | 99.6 | 99.9 | ||
| Sample | Mw (g/mol) | PDI |
|---|---|---|
| 70-4 | 33,142 | 6.175 |
| 80-4 | 20,266 | 3.528 |
| 90-4 | 14,149 | 2.102 |
| 100-4 | 8932 | 2.050 |
| Sample | Mw (g/mol) | PDI |
|---|---|---|
| 100-4 | 8932 | 2.050 |
| 100-3 | 11,228 | 1.918 |
| 100-2 | 10,290 | 1.926 |
| 100-1 | 10,484 | 1.988 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borovkova, V.S.; Malyar, Y.N.; Sudakova, I.G.; Chudina, A.I.; Zimonin, D.V.; Skripnikov, A.M.; Miroshnikova, A.V.; Ionin, V.A.; Kazachenko, A.S.; Sychev, V.V.; et al. Composition and Structure of Aspen (Pópulus trémula) Hemicelluloses Obtained by Oxidative Delignification. Polymers 2022, 14, 4521. https://doi.org/10.3390/polym14214521
Borovkova VS, Malyar YN, Sudakova IG, Chudina AI, Zimonin DV, Skripnikov AM, Miroshnikova AV, Ionin VA, Kazachenko AS, Sychev VV, et al. Composition and Structure of Aspen (Pópulus trémula) Hemicelluloses Obtained by Oxidative Delignification. Polymers. 2022; 14(21):4521. https://doi.org/10.3390/polym14214521
Chicago/Turabian StyleBorovkova, Valentina S., Yuriy N. Malyar, Irina G. Sudakova, Anna I. Chudina, Dmitriy V. Zimonin, Andrey M. Skripnikov, Angelina V. Miroshnikova, Vladislav A. Ionin, Alexander S. Kazachenko, Valentin V. Sychev, and et al. 2022. "Composition and Structure of Aspen (Pópulus trémula) Hemicelluloses Obtained by Oxidative Delignification" Polymers 14, no. 21: 4521. https://doi.org/10.3390/polym14214521
APA StyleBorovkova, V. S., Malyar, Y. N., Sudakova, I. G., Chudina, A. I., Zimonin, D. V., Skripnikov, A. M., Miroshnikova, A. V., Ionin, V. A., Kazachenko, A. S., Sychev, V. V., Ponomarev, I. S., & Issaoui, N. (2022). Composition and Structure of Aspen (Pópulus trémula) Hemicelluloses Obtained by Oxidative Delignification. Polymers, 14(21), 4521. https://doi.org/10.3390/polym14214521