Design and Optimization of a Natural Medicine from Copaifera reticulata Ducke for Skin Wound Care
Abstract
1. Introduction
2. Materials and Methods
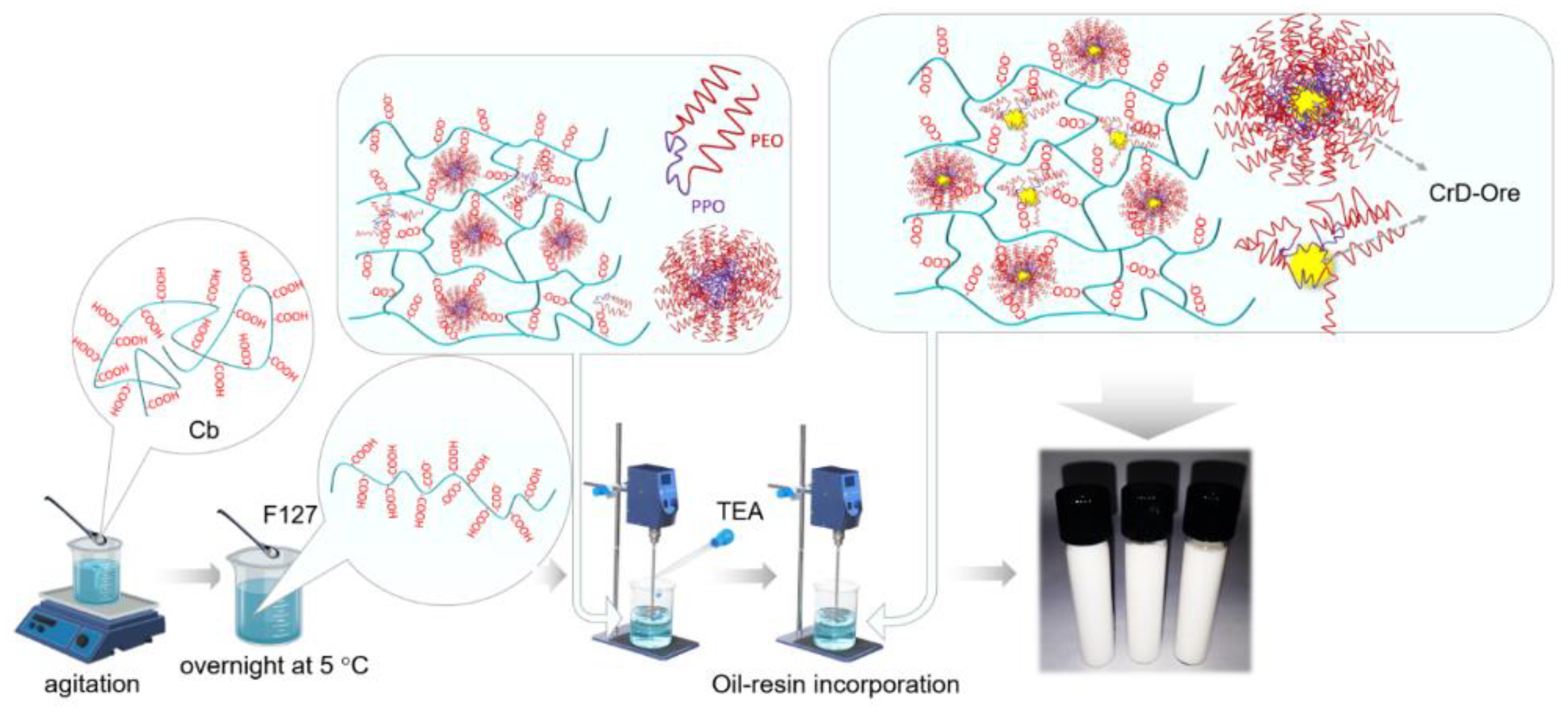
2.1. Bioadhesive Emulsion-Filled Gel Preparation
2.2. Morphological Characterization
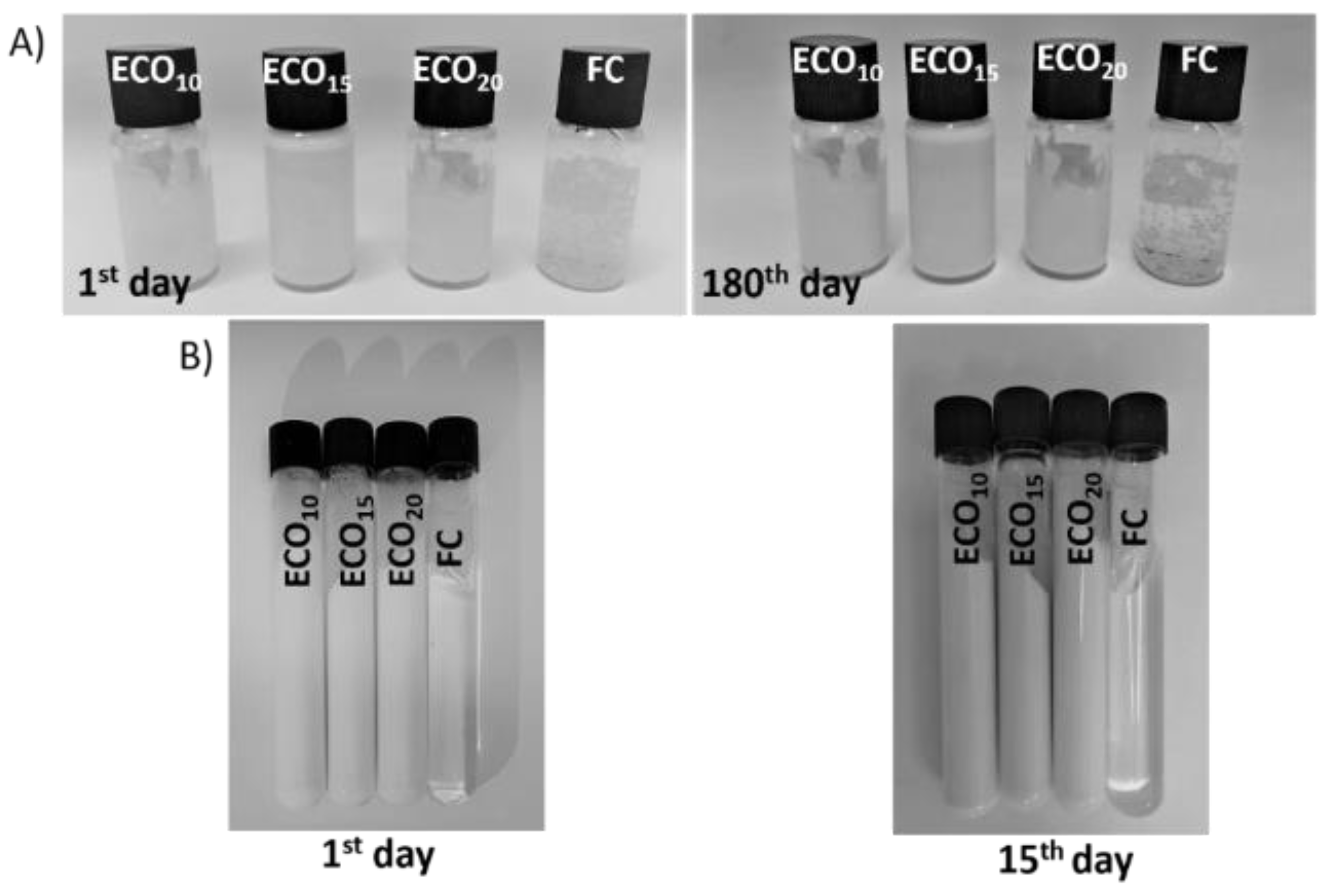
2.3. Accelerated Stability
2.4. Texture Profile Analysis
2.5. Rheological Properties
2.6. Microorganism and Culture Conditions
2.7. Microbiological Analysis
2.8. Statistical Analysis
2.9. EFG Application in a Wound Aggravated by Myiasis: Case Report
3. Results
3.1. ECO: Preliminary Remarks
3.2. Mechanical and Rheological Properties
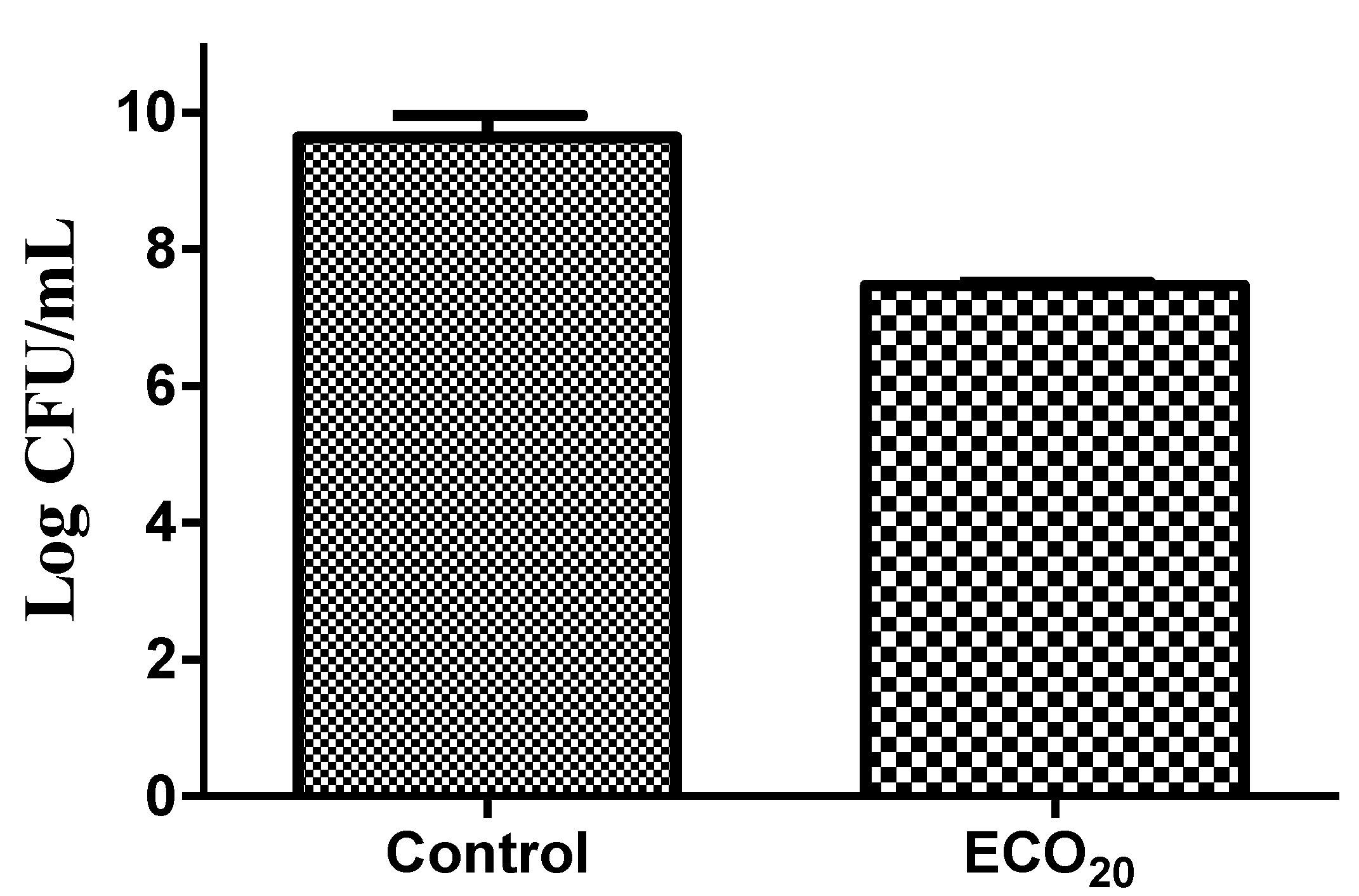
3.3. In Vitro Analysis of the Emulgel
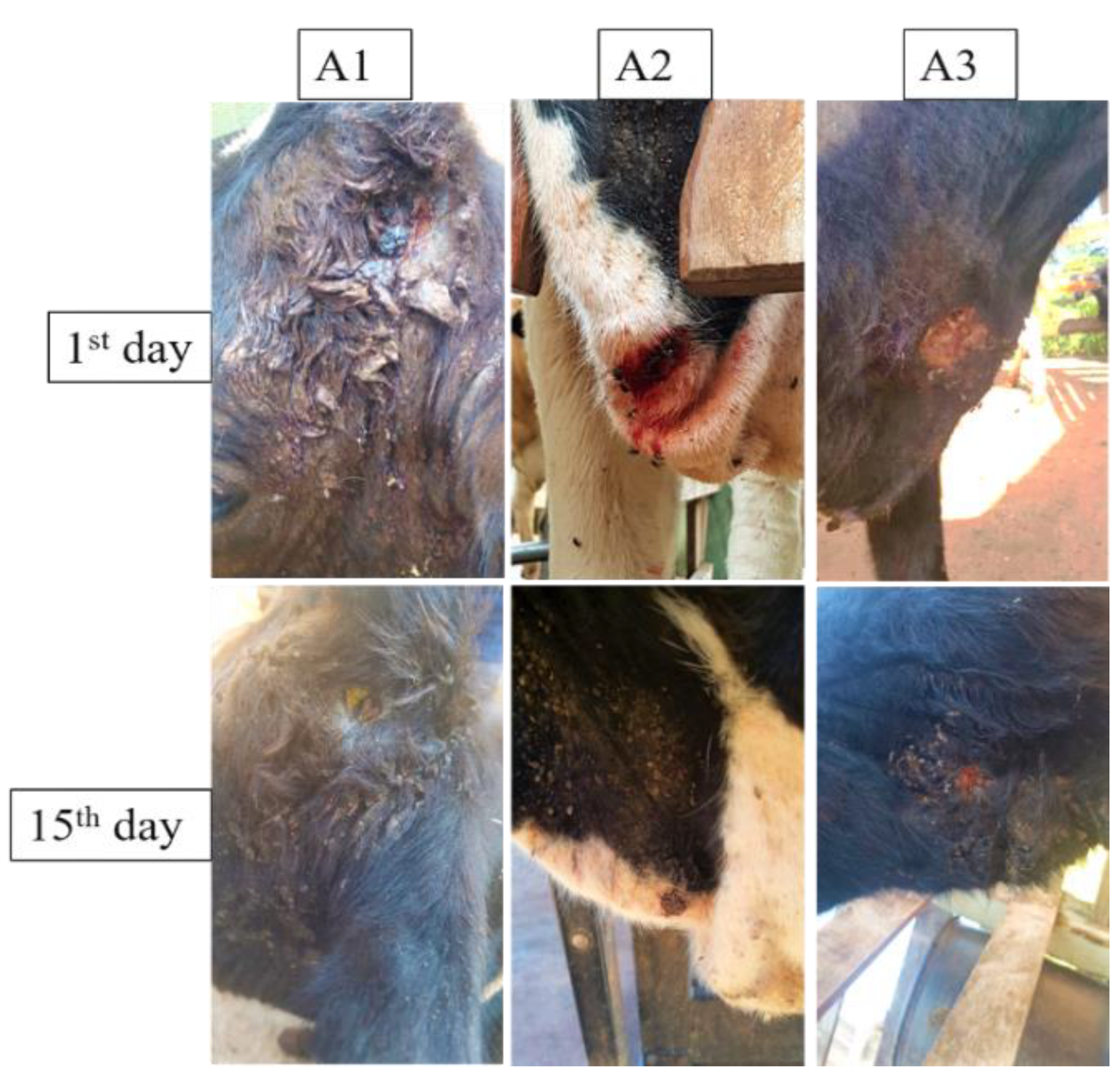
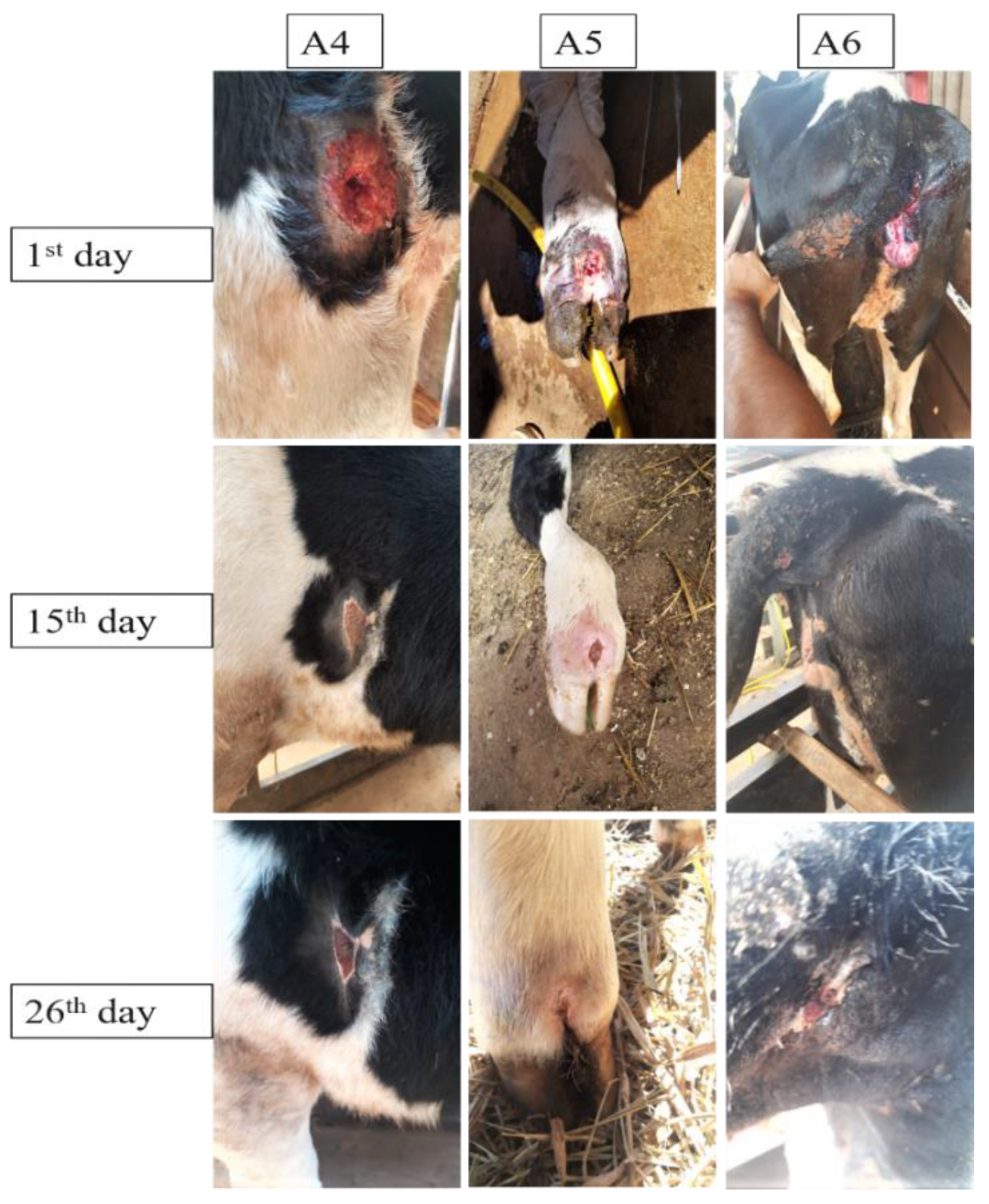
3.4. Wound Treatment Aggravated by Myiasis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Devriendt, N.; de Rooster, H. Initial Management of Traumatic Wounds. Vet. Clin. N. Am. Small Anim. Pr. 2017, 47, 1123–1134. [Google Scholar] [CrossRef]
- Milne, K.E.; Penn-Barwell, J.G. Classification and management of acute wounds and open fractures. Surgery 2020, 38, 143–149. [Google Scholar] [CrossRef]
- Fernández, J.; Ruiz-Ruiz, M.; Sarasua, J.-R. Electrospun Fibers of Polyester, with Both Nano- and Micron Diameters, Loaded with Antioxidant for Application as Wound Dressing or Tissue Engineered Scaffolds. ACS Appl. Polym. Mater. 2019, 1, 1096–1106. [Google Scholar] [CrossRef]
- Jesse, F.; Sadiq, M.; Abba, Y.; Mohammed, K.; Harith, A.; Chung, E.; Bitrus, A.; Lila, M.; Haron, A.; Saharee, A. Clinical Management of Severe Cutaneous Myiasis in a Brangus-Cross Calf. Int. J. Livest. Res. 2016, 6, 82. [Google Scholar] [CrossRef]
- Francesconi, F.; Lupi, O. 31—Myiasis. In Tropical Dermatology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 393–400. [Google Scholar] [CrossRef]
- Al-Gharibi, K.A.; Sharstha, S.; Al-Faras, M.A. Cost-Effectiveness of Wound Care: A concept analysis. Sultan Qaboos Univ. Med. J. 2019, 18, e433–e439. [Google Scholar] [CrossRef]
- Faridnia, R.; Soosaraei, M.; Kalani, H.; Fakhar, M.; Jokelainen, P.; Emameh, R.Z.; Banimostafavi, E.S.; Hezarjaribi, H.Z. Human urogenital myiasis: A systematic review of reported cases from 1975 to 2017. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 235, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, B.; Borges, U.; Pimentel, I. Human vaginal myiasis caused byCochliomyia hominivorax. Int. J. Gynecol. Obstet. 2005, 89, 152–153. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.; Walker, S.L. Diagnostic approach to tropical skin infections. Medicine 2018, 46, 10–15. [Google Scholar] [CrossRef]
- Yusuf, M.A.; Pritt, B.S.; McMichael, J.R. Cutaneous myiasis in an elderly woman in Somaliland. Int. J. Women’s Dermatol. 2019, 5, 187–189. [Google Scholar] [CrossRef]
- Juyena, N.; Tapon, M.; Ferdousy, R.; Paul, S.; Alam, M. A Retrospective Study on Occurrence of Myiasis in Ruminants. Progress. Agric. 2014, 24, 101–106. [Google Scholar] [CrossRef]
- de Oliveira, P.C.; de Almeida, G.P.S.; Cardoso, J.D.; Tavares, R.B.; Fernandes, J.I.; Correia, T.R.; Verocai, G.G.; Scott, F.B. Efficacy of sarolaner on the treatment of myiasis caused by Cochliomyia hominivorax (Diptera: Calliphoridae) in dogs. Vet. Parasitol. 2019, 276, 108966. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.K. Myiasis in domestic animals: New records of calyptrate Diptera. J. Parasit. Dis. 2012, 36, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Colwell, D. Mammalian immune responses to myiasis. Parasitol. Today 1991, 7, 353–355. [Google Scholar] [CrossRef]
- Lionelli, G.T.; Lawrence, W.T. Wound dressings. Surg. Clin. N. Am. 2003, 83, 617–638. [Google Scholar] [CrossRef]
- Jha, S.; Maurya, S.D. Organogels as a potential topical drug delivery system. Int. J. Drug Regul. Aff. 2018, 1, 49–58. [Google Scholar] [CrossRef]
- Sahoo, S.; Kumar, N.; Bhattacharya, C.; Sagiri, S.S.; Jain, K.; Pal, K.; Ray, S.S.; Nayak, B. Organogels: Properties and Applications in Drug Delivery. Des. Monomers Polym. 2011, 14, 95–108. [Google Scholar] [CrossRef]
- Campanholi, K.D.S.S.; Gonçalves, R.S.; da Silva, J.B.; dos Santos, R.S.; de Oliveira, M.C.; Ferreira, S.B.D.S.; de Castro-Hoshino, L.V.; Balbinot, R.B.; Lazarin-Bidóia, D.; Baesso, M.L.; et al. Thermal stimuli-responsive topical platform based on copaiba oil-resin: Design and performance upon ex-vivo human skin. J. Mol. Liq. 2022, 361, 119625. [Google Scholar] [CrossRef]
- Campanholi, K.D.S.S.; da Silva, J.B.; Batistela, V.R.; Gonçalves, R.S.; dos Santos, R.S.; Balbinot, R.B.; Lazarin-Bidóia, D.; Bruschi, M.L.; Nakamura, T.U.; Nakamura, C.V.; et al. Design and Optimization of Stimuli-responsive Emulsion-filled Gel for Topical Delivery of Copaiba Oil-resin. J. Pharm. Sci. 2021. [Google Scholar] [CrossRef]
- Balaguru, S.; Devi, D.R.; Hari, B.N.V. Organogel: An ideal drug delivery carrier for non steroidal anti-inflammatory drugs through topical route. Int. J. Pharm. Qual. Assur. 2015, 6, 32–37. [Google Scholar]
- Esposito, C.L.; Kirilov, P.; Roullin, V.G. Organogels, promising drug delivery systems: An update of state-of-the-art and recent applications. J. Control Release 2018, 271, 1–20. [Google Scholar] [CrossRef]
- Mujawar, N.K.; Ghatage, S.L.; Yeligar, V.C. Organogel: Factors and Its Importance. Int. J. Biol. Chem. Sci. 2014, 4, 758–773. [Google Scholar]
- Barreiro-Iglesias, R.; Alvarez-Lorenzo, C.; Concheiro, A. Incorporation of small quantities of surfactants as a way to improve the rheological and diffusional behavior of carbopol gels. J. Control Release 2001, 77, 59–75. [Google Scholar] [CrossRef]
- Bhat, P.A.; Chat, O.A.; Zhang, Y.; Dar, A.A. An unprecedented dual responsive gelation of Carbopol induced by Pluronic P123 triblock copolymer. Polymer 2016, 102, 153–166. [Google Scholar] [CrossRef]
- Ghosh, S.; Das Mahapatra, R.; Dey, J. Thermoreversible as Well as Thermoirreversible Organogel Formation by l-Cysteine-Based Amphiphiles with Poly(ethylene glycol) Tail. Langmuir 2014, 30, 1677–1685. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, F.; Chen, Y.; Raymond, J.E.; Zhang, S.; Fan, J.; Zhu, J.; Kellie, S.; Seetho, K.; He, X.; et al. Responsive organogels formed by supramolecular self assembly of PEG-block-allyl-functionalized racemic polypeptides into β-sheet-driven polymeric ribbons. Soft Matter 2013, 9, 5951–5958. [Google Scholar] [CrossRef]
- Lin, H.; Sung, K.C. Carbopol/pluronic phase change solutions for ophthalmic drug delivery. J. Control Release 2000, 69, 379–388. [Google Scholar] [CrossRef]
- dos Santos, R.S.; da Silva, J.B.; Rosseto, H.C.; Vecchi, C.F.; Campanholi, K.D.S.S.; Caetano, W.; Bruschi, M.L. Emulgels Containing Propolis and Curcumin: The Effect of Type of Vegetable Oil, Poly(Acrylic Acid) and Bioactive Agent on Physicochemical Stability, Mechanical and Rheological Properties. Gels 2021, 7, 120. [Google Scholar] [CrossRef]
- Alexandridis, P.; Holzwarth, J.F.; Hatton, T.A. Micellization of Poly(ethylene oxide)-Poly(propylene oxide)-Poly(ethylene oxide) Triblock Copolymers in Aqueous Solutions: Thermodynamics of Copolymer Association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Rarokar, N.R.; Saoji, S.D.; Khedekar, P.B. Investigation of effectiveness of some extensively used polymers on thermoreversible properties of Pluronic® tri-block copolymers. J. Drug Deliv. Sci. Technol. 2018, 44, 220–230. [Google Scholar] [CrossRef]
- Santos, A.O.; Ueda-Nakamura, T.; Filho, B.P.D.; Junior, V.F.V.; Pinto, A.C.; Nakamura, C.V. Effect of Brazilian copaiba oils on Leishmania amazonensis. J. Ethnopharmacol. 2008, 120, 204–208. [Google Scholar] [CrossRef]
- Dorneles, F.D.S.; Schafer, D.; Silva, A.; Oliveira, C.B.; Zimmermann, C.E.P.; Rosa, L.D.; Tonin, A.A.; de Oliveira, E.C.P.; Santurio, J.M.; Monteiro, S.G. Susceptibility of Trypanosoma evansi in the copaiba oil: In Vitro test and in mice experimentally infected with the parasite. Acta Sci. Vet. 2013, 41, 1–6. [Google Scholar]
- Menezes, A.C.D.S.; Alves, L.D.B.; Goldemberg, D.C.; de Melo, A.C.; Antunes, H.S. Anti-inflammatory and wound healing effect of Copaiba oleoresin on the oral cavity: A systematic review. Heliyon 2022, 8, e08993. [Google Scholar] [CrossRef] [PubMed]
- Trindade, F.T.T.; Stabeli, R.G.; Pereira, A.A.; Facundo, V.A.; Silva, A. Copaifera multijuga ethanolic extracts, oilresin, and its derivatives display larvicidal activity against Anopheles darlingi and Aedes aegypti (Diptera: Culicidae). Rev. Bras. de Farm. 2013, 23, 464–470. [Google Scholar] [CrossRef]
- Santos, R.C.V.; Alves, C.F.D.S.; Schneider, T.; Lopes, L.; Aurich, C.; Giongo, J.L.; Brandelli, A.; Vaucher, R.D.A. Antimicrobial activity of Amazonian oils against Paenibacillus species. J. Invertebr. Pathol. 2012, 109, 265–268. [Google Scholar] [CrossRef]
- Rodrigues, G.D.M.; Filgueiras, C.T.; Garcia, V.A.D.S.; De Carvalho, R.A.; Velasco, J.I.; Fakhouri, F.M. Antimicrobial Activity and GC-MS Profile of Copaiba Oil for Incorporation into Xanthosoma mafaffa Schott Starch-Based Films. Polymers 2020, 12, 2883. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.C.; Carter, F.L.; Mauldin, J.K. Reticulitermes flavipes (Kollar) (Isoptera: Rhinotermitidae) Responses to Extracts from Six Brazilian Woods. Environ. Entomol. 1983, 12, 458–462. [Google Scholar] [CrossRef]
- Gelmini, F.; Beretta, G.; Anselmi, C.; Centini, M.; Magni, P.; Ruscica, M.; Cavalchini, A.; Facino, R.M. GC–MS profiling of the phytochemical constituents of the oleoresin from Copaifera langsdorffii Desf. and a preliminary in vivo evaluation of its antipsoriatic effect. Int. J. Pharm. 2013, 440, 170–178. [Google Scholar] [CrossRef]
- Ricardo, L.M.; Dias, B.M.; Mügge, F.L.; Leite, V.V.; Brandão, M.G. Evidence of traditionality of Brazilian medicinal plants: The case studies of Stryphnodendron adstringens (Mart.) Coville (barbatimão) barks and Copaifera spp. (copaíba) oleoresin in wound healing. J. Ethnopharmacol. 2018, 219, 319–336. [Google Scholar] [CrossRef]
- Paiva, L.A.F.; Cunha, K.M.D.A.; Santos, F.A.; Gramosa, N.V.; Silveira, E.R.; Rao, V.S.N. Investigation on the wound healing activity of oleo-resin from Copaifera langsdorffi in rats. Phytotherapy Res. 2002, 16, 737–739. [Google Scholar] [CrossRef] [PubMed]
- Paranhos, S.B.; Ferreira, E.D.S.; Canelas, C.A.d.A.; da Paz, S.P.A.; Passos, M.F.; da Costa, C.E.F.; da Silva, A.C.R.; Monteiro, S.N.; Candido, V.S. Chitosan Membrane Containing Copaiba Oil (Copaifera spp.) for Skin Wound Treatment. Polymers 2022, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.B.; Silva, R.D.B.; Lameira, O.A.; Webber, L.P.; Couto, R.S.D.; Martins, M.D.; Lima, R.R. Copaiba oil-resin (Copaifera reticulata Ducke) modulates the inflammation in a model of injury to rats’ tongues. BMC Complement. Altern. Med. 2017, 17, 313. [Google Scholar] [CrossRef] [PubMed]
- Campanholi, K.D.S.S.; Braga, G.; Da Silva, J.B.; Da Rocha, N.L.; De Francisco, L.M.B.; De Oliveira, L.; Bruschi, M.L.; De Castro-Hoshino, L.V.; Sato, F.; Hioka, N.; et al. Biomedical Platform Development of a Chlorophyll-Based Extract for Topic Photodynamic Therapy: Mechanical and Spectroscopic Properties. Langmuir 2018, 34, 8230–8244. [Google Scholar] [CrossRef]
- ANVISA. Guia de Estabilidade de Produtos Cosméticos—Séries Temáticas, 1st ed.; ANVISA: Brasília, DF, Brazil, 2004. [Google Scholar]
- Cosmetic Europe—The Person Care Association. Guidelines on Stability Testing of Cosmetic. 2004. Available online: https://www.cosmeticseurope.eu/files/5914/6407/8121/Guidelines_on_Stability_Testing_of_Cosmetics_CE-CTFA_-_2004.pdf (accessed on 1 September 2022).
- Maldonado, L.; Sadeghi, R.; Kokini, J. Nanoparticulation of bovine serum albumin and poly-d-lysine through complex coacervation and encapsulation of curcumin. Colloids Surf. B Biointerfaces 2017, 159, 759–769. [Google Scholar] [CrossRef]
- Barnes, H.A.; Hutton, J.F.; Walters, K. An Introduction to Rheology; Rheology Series, Volume 3; U.K. Walters, Department of Mathematics, University College of Wales: Aberystwyth, UK; Amsterdam, The Netherland; London, UK; New York, NY, USA; Tokyo, Japan, 1989; pp. 1–185. [Google Scholar] [CrossRef]
- Menard, K.P. A Practical Introduction. In Dynamic Mechanical Analysis; CRC Press LLC: New York, NY, USA, 1999. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 22 October 2022).
- RStudio Team. R Studio: Integrated Development for R; Version 1.1.463; RStudio, Inc.: Boston, MA, USA, 2015; Available online: https://www.rstudio.com/ (accessed on 22 October 2022).
- Barbosa, A.L.P.; Wenzel-Storjohann, A.; Barbosa, J.D.; Zidorn, C.; Peifer, C.; Tasdemir, D.; Çiçek, S.S. Antimicrobial and cytotoxic effects of the Copaifera reticulata oleoresin and its main diterpene acids. J. Ethnopharmacol. 2019, 233, 94–100. [Google Scholar] [CrossRef]
- Carvalho, J.C.T.; Cascon, V.; Possebon, L.S.; Morimoto, M.S.S.; Cardoso, L.G.; Kaplan, M.A.C.; Gilbert, B. Topical antiinflammatory and analgesic activities of Copaifera duckei dwyer. Phytother. Res. 2005, 19, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, Z.; Spychaj, T.; Story, A.; Story, G. Carbomer microgels as model yield-stress fluids. Rev. Chem. Eng. 2021. [Google Scholar] [CrossRef]
- Baek, G.; Kim, C. Rheological properties of Carbopol containing nanoparticles Published by the The Society of Rheology Carbopol: From a simple to a thixotropic yield stress fluid rheological properties of Carbopol containing nanoparticles. J. Rheol. 2011, 55, 313–330. [Google Scholar] [CrossRef]
- Singh, B.; Dhiman, A.; Rajneesh; Kumar, A. Slow release of ciprofloxacin from β-cyclodextrin containing drug delivery system through network formation and supramolecular interactions. Int. J. Biol. Macromol. 2016, 92, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Lúcia, A.; Milan, K.; Milão, D.; Souto, A.A.; Weber, T.; Corte, F. Estudo da hidratação da pele por emulsões cosméticas para xerose e sua estabilidade por reologia. Braz. J. Pharm. Sci. 2007, 43, 649–656. [Google Scholar]
- Osmałek, T.; Milanowski, B.; Froelich, A.; Górska, S.; Białas, W.; Szybowicz, M.; Kapela, M. Novel organogels for topical delivery of naproxen: Design, physicochemical characteristics and in vitro drug permeation. Pharm. Dev. Technol. 2017, 22, 521–536. [Google Scholar] [CrossRef]
- Bruschi, M.L.; De Freitas, O.; Lara, E.H.G.E.; Panzeri, H.; Gremião, M.P.D.; Jones, D.S. Precursor System of Liquid Crystalline Phase Containing Propolis Microparticles for the Treatment of Periodontal Disease: Development and Characterization. Drug Dev. Ind. Pharm. 2008, 34, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Navarro, R.F. Fundamentos de Reologia de Polímeros; Educs: São Paulo, Brazil, 1997. [Google Scholar]
- Jones, D.S.; Bruschi, M.L.; de Freitas, O.; Gremião, M.P.D.; Lara, E.H.G.; Andrews, G.P. Rheological, mechanical and mucoadhesive properties of thermoresponsive, bioadhesive binary mixtures composed of poloxamer 407 and carbopol 974P designed as platforms for implantable drug delivery systems for use in the oral cavity. Int. J. Pharm. 2009, 372, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Borghi-Pangoni, F.B.; Junqueira, M.V.; Ferreira, S.B.D.S.; Silva, L.L.; Rabello, B.R.; Caetano, W.; Diniz, A.; Bruschi, M.L. Screening and In Vitro Evaluation of Mucoadhesive Thermoresponsive System Containing Methylene Blue for Local Photodynamic Therapy of Colorectal Cancer. Pharm. Res. 2016, 33, 776–791. [Google Scholar] [CrossRef]
- Junqueira, M.V.; Borghi-Pangoni, F.B.; Ferreira, S.B.S.; Rabello, B.R.; Hioka, N.; Bruschi, M.L. Functional Polymeric Systems as Delivery Vehicles for Methylene Blue in Photodynamic Therapy. Langmuir 2015, 32, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Armoškaitė, V. Evaluation of base for optimal drug delivery for iontophoretic therapy: Investigation of quality and stability. Afr. J. Pharm. Pharmacol. 2012, 6, 1685–1695. [Google Scholar] [CrossRef]
- Ferreira, S.B.D.S.; Da Silva, J.B.; Borghi-Pangoni, F.B.; Junqueira, M.V.; Bruschi, M.L. Linear correlation between rheological, mechanical and mucoadhesive properties of polycarbophil polymer blends for biomedical applications. J. Mech. Behav. Biomed. Mater. 2017, 68, 265–275. [Google Scholar] [CrossRef]
- Cassu, S.N.; Felisberti, M.I. Comportamento dinâmico-mecânico e relaxações em polímeros e blendas poliméricas. Química Nova 2005, 28, 255–263. [Google Scholar] [CrossRef]
- Da Silva-Junior, R.C.; Campanholi, K.D.S.S.; De Morais, F.A.P.; Pozza, M.S.D.S.; De Castro-Hoshino, L.V.; Baesso, M.L.; Da Silva, J.B.; Bruschi, M.L.; Caetano, W. Photothermal Stimuli-Responsive Hydrogel Containing Safranine for Mastitis Treatment in Veterinary Using Phototherapy. ACS Appl. Bio Mater. 2020, 4, 581–596. [Google Scholar] [CrossRef]
- Palheta, C.D.S.A.; da Silva, W.M.P.; Couteiro, R.P.; da Silva, P.R.G.; Souza, R.M.T.; Dias, D.V.; Alho, B.C.D.N.; Silva, A.M.F.; Botelho, N.M.; Carneiro, F.R.O. Effect of copaiba oil associated with microneedling on the skin of rats: A comparative study. Surg. Cosmet. Dermatol. 2017, 9, 290–295. [Google Scholar] [CrossRef]
- Fernandes, F.D.F.; Freitas, E.D.P.S. Acaricidal activity of an oleoresinous extract from Copaifera reticulata (Leguminosae: Caesalpinioideae) against larvae of the southern cattle tick, Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Vet. Parasitol. 2007, 147, 150–154. [Google Scholar] [CrossRef]
- Símaro, G.V.; Lemos, M.; da Silva, J.J.M.; Cunha, W.R.; Carneiro, L.J.; Ambrósio, S.R.; Cunha, N.L.; de Andrade, S.F.; Arruda, C.; Banderó-Filho, V.C.; et al. In Vivo study of anti-inflammatory and antinociceptive activities of Copaifera pubiflora Benth oleoresin. Nat. Prod. Res. 2020, 36, 1129–1133. [Google Scholar] [CrossRef]
- Zortéa, T.; Baretta, D.; Volpato, A.; Lorenzetti, W.R.; Segat, J.C.; Maccari, A.P.; Santos, R.C.; Vaucher, R.A.; Stefani, L.M.; Da Silva, A.S. Repellent Effects of Andiroba and Copaiba Oils against Musca domestica (Common House Fly) and Ecotoxicological Effects on the Environment. Acta Sci. Vet. 2017, 45, 8. [Google Scholar] [CrossRef][Green Version]
- dos Santos, A.O.; Costa, M.A.; Ueda-Nakamura, T.; Dias-Filho, B.P.; da Veiga-Júnior, V.F.; Lima, M.M.D.S.; Nakamura, C.V. Leishmania amazonensis: Effects of oral treatment with copaiba oil in mice. Exp. Parasitol. 2011, 129, 145–151. [Google Scholar] [CrossRef]
- dos Santos, A.O.; Ueda-Nakamura, T.; Filho, B.P.D.; Junior, V.F.D.V.; Nakamura, C.V. Copaiba Oil: An Alternative to Development of New Drugs against Leishmaniasis. Evid.-Based Complement. Altern. Med. 2012, 2012, 898419. [Google Scholar] [CrossRef] [PubMed]
- Debone, H.S.; Lopes, P.S.; Severino, P.; Yoshida, C.M.P.; Souto, E.B.; da Silva, C.F. Chitosan/Copaiba oleoresin films for would dressing application. Int. J. Pharm. 2018, 555, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Okur, M.E.; Karantas, I.D.; Şenyiğit, Z.; Okur, N.; Siafaka, P.I. Recent trends on wound management: New therapeutic choices based on polymeric carriers. Asian J. Pharm. Sci. 2020, 15, 661–684. [Google Scholar] [CrossRef]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for Wound Healing and Infection Control. Materials 2019, 12, 2176. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.D.C.; Ferreira, A.M.; Vilhena, J.C.; Almeida, F.B.; Cruz, R.A.; Florentino, A.C.; Souto, R.N.; Carvalho, J.C.; Fernandes, C.P. Development of a larvicidal nanoemulsion with Copaiba (Copaifera duckei) oleoresin. Rev. Bras. Farm. 2014, 24, 699–705. [Google Scholar] [CrossRef]
- Govindarajan, M.; Rajeswary, M.; Hoti, S.L.; Bhattacharyya, A.; Benelli, G. Eugenol, α-pinene and β-caryophyllene from Plectranthus barbatus essential oil as eco-friendly larvicides against malaria, dengue and Japanese encephalitis mosquito vectors. Parasitol. Res. 2016, 115, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Ribas, J.; Carreño, A.M. Avaliação do uso de repelentes contra picada de mosquitos em militares na Bacia Amazônica. An. Bras. Dermatol. 2010, 85, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Leandro, L.M.; Vargas, F.D.S.; Barbosa, P.C.S.; Neves, J.K.O.; da Silva, J.A.; da Veiga-Junior, V.F. Chemistry and Biological Activities of Terpenoids from Copaiba (Copaifera spp.) Oleoresins. Molecules 2012, 17, 3866–3889. [Google Scholar] [CrossRef] [PubMed]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]

 and
and  refer to upward and downward, respectively. Standard deviations are omitted for clarity; however, the relative standard deviation of the replicate analysis was less than 10% in all cases.
refer to upward and downward, respectively. Standard deviations are omitted for clarity; however, the relative standard deviation of the replicate analysis was less than 10% in all cases.
 and
and  refer to upward and downward, respectively. Standard deviations are omitted for clarity; however, the relative standard deviation of the replicate analysis was less than 10% in all cases.
refer to upward and downward, respectively. Standard deviations are omitted for clarity; however, the relative standard deviation of the replicate analysis was less than 10% in all cases.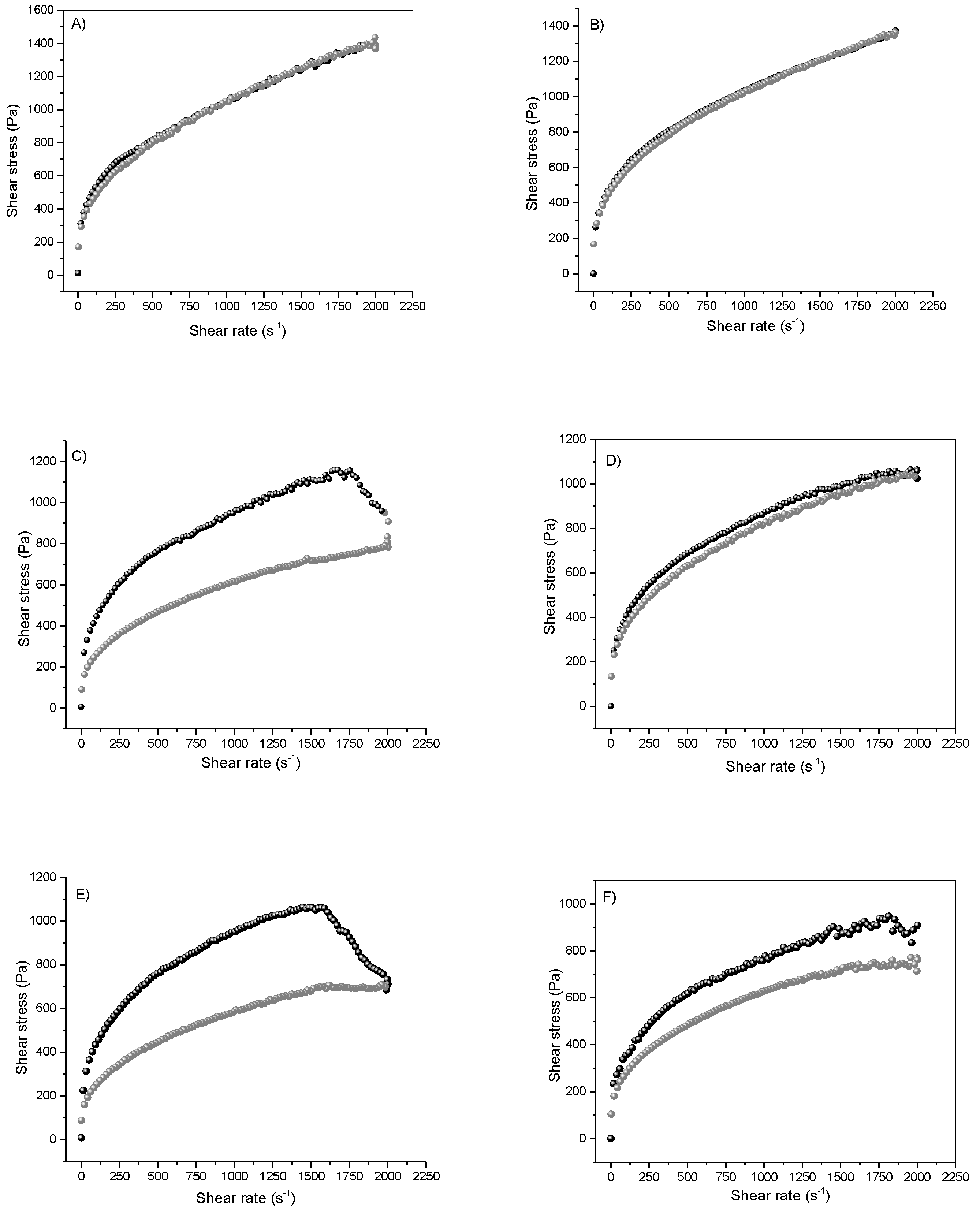
 FC at 25.0 °C,
FC at 25.0 °C,  FC at 37.0 °C,
FC at 37.0 °C,  ECO15 at 25.0 °C,
ECO15 at 25.0 °C,  ECO15 at 37.0 °C,
ECO15 at 37.0 °C,  ECO20 at 25.0 °C,
ECO20 at 25.0 °C,  ECO20 at 37.0 °C. The insert (C) corresponds to the rheological dynamics required to obtain the lag angle.
ECO20 at 37.0 °C. The insert (C) corresponds to the rheological dynamics required to obtain the lag angle.
 FC at 25.0 °C,
FC at 25.0 °C,  FC at 37.0 °C,
FC at 37.0 °C,  ECO15 at 25.0 °C,
ECO15 at 25.0 °C,  ECO15 at 37.0 °C,
ECO15 at 37.0 °C,  ECO20 at 25.0 °C,
ECO20 at 25.0 °C,  ECO20 at 37.0 °C. The insert (C) corresponds to the rheological dynamics required to obtain the lag angle.
ECO20 at 37.0 °C. The insert (C) corresponds to the rheological dynamics required to obtain the lag angle.
| Compositions | Phytotherapeutic Gels (g) | |||
|---|---|---|---|---|
| FC | ECO10 | ECO15 | ECO20 | |
| Carbopol | 1.2 | 1.2 | 1.2 | 1.2 |
| F127 | 2.4 | 2.4 | 2.4 | 2.4 |
| CrD-Ore | 0 | 10 | 15 | 20 |
| Purified water | 96.4 | 86.4 | 81.4 | 76.4 |
| Properties * | Temperature (°C) | FC | ECO10 | ECO15 | ECO20 |
|---|---|---|---|---|---|
| Hardness (N) | 25 | 0.48 ± 0.01 | 0.41 ± 0.00 | 0.35 ± 0.01 | 0.37 ± 0.01 |
| 37 | 0.47 ± 0.00 | 0.41 ± 0.03 | 0.37 ± 0.01 | 0.40 ± 0.01 | |
| Compressibility (N.mm) | 25 | 2.23 ± 0.05 | 1.84 ± 0.12 | 1.67 ± 0.18 | 1.80 ± 0.01 |
| 37 | 2.26 ± 0.00 | 1.90 ± 0.13 | 1.77 ± 0.10 | 1.89 ± 0.05 | |
| Adhesiveness (N.mm) | 25 | 1.64 ± 0.06 | 1.50 ± 0.12 | 1.33 ± 0.15 | 1.43 ± 0.01 |
| 37 | 1.67 ± 0.01 | 1.51 ± 0.31 | 1.36 ± 0.07 | 1.52 ± 0.06 | |
| Elasticity (mm) | 25 | 0.99 ± 0.00 | 0.99 ± 0.00 | 0.99 ± 0.00 | 1.00 ± 0.00 |
| 37 | 0.99 ± 0.00 | 1.00 ± 0.00 | 0.91 ± 0.00 | 0.99 ± 0.00 | |
| Cohesiveness (Dimensionless) | 25 | 0.89 ± 0.00 | 0.94 ± 0.04 | 0.91 ± 0.00 | 0.93 ± 0.00 |
| 37 | 0.89 ± 0.01 | 0.92 ± 0.08 | 0.88 ± 0.01 | 0.91 ± 0.01 |
| Parameters * | Temperature (°C) | FC | ECO15 | ECO20 |
|---|---|---|---|---|
| Consistency index (K) (Pa.s) | 25 | 78.35 ± 0.96 | 96.34 ± 8.07 | 118.70 ± 6.01 |
| 37 | 79.92 ± 9.88 | 78.17 ± 1.60 | 105.93 ± 10.20 | |
| Power law index (n) (dimensionless) | 25 | 0.38 ± 0.38 | 0.29 ± 0.02 | 0.28 ± 0.01 |
| 37 | 0.37 ± 0.02 | 0.34 ± 0.00 | 0.29 ± 0.00 | |
| Yield value (Pa) | 25 | 126.05 ± 2.76 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 37 | 90.48 ± 9.22 | 40.20 ± 3.36 | 0.00 ± 0.00 | |
| Hysteresis area (Pa/s) | 25.0 | 36,690 ± 4709 | 589,033 ± 23,122 | 591,400 ± 10,182 |
| 37.0 | 45,110 ± 1117 | 105,640 ± 8146 | 439,000 ± 54,164 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campanholi, K.d.S.S.; Silva Junior, R.C.d.; Gonçalves, R.S.; Bassi da Silva, J.; Pedroso de Morais, F.A.; Said dos Santos, R.; Vilsinski, B.H.; Oliveira, G.L.M.d.; Pozza, M.S.d.S.; Bruschi, M.L.; et al. Design and Optimization of a Natural Medicine from Copaifera reticulata Ducke for Skin Wound Care. Polymers 2022, 14, 4483. https://doi.org/10.3390/polym14214483
Campanholi KdSS, Silva Junior RCd, Gonçalves RS, Bassi da Silva J, Pedroso de Morais FA, Said dos Santos R, Vilsinski BH, Oliveira GLMd, Pozza MSdS, Bruschi ML, et al. Design and Optimization of a Natural Medicine from Copaifera reticulata Ducke for Skin Wound Care. Polymers. 2022; 14(21):4483. https://doi.org/10.3390/polym14214483
Chicago/Turabian StyleCampanholi, Katieli da Silva Souza, Ranulfo Combuca da Silva Junior, Renato Sonchini Gonçalves, Jéssica Bassi da Silva, Flávia Amanda Pedroso de Morais, Rafaela Said dos Santos, Bruno Henrique Vilsinski, Gabrielly Lorraynny Martins de Oliveira, Magali Soares dos Santos Pozza, Marcos Luciano Bruschi, and et al. 2022. "Design and Optimization of a Natural Medicine from Copaifera reticulata Ducke for Skin Wound Care" Polymers 14, no. 21: 4483. https://doi.org/10.3390/polym14214483
APA StyleCampanholi, K. d. S. S., Silva Junior, R. C. d., Gonçalves, R. S., Bassi da Silva, J., Pedroso de Morais, F. A., Said dos Santos, R., Vilsinski, B. H., Oliveira, G. L. M. d., Pozza, M. S. d. S., Bruschi, M. L., Saraiva, B. B., Nakamura, C. V., & Caetano, W. (2022). Design and Optimization of a Natural Medicine from Copaifera reticulata Ducke for Skin Wound Care. Polymers, 14(21), 4483. https://doi.org/10.3390/polym14214483